Graphical abstract
Keywords: Aflatoxins, Groundnut paste, Risk assessment, Ghana, Mycotoxins, HPLC
Highlights
-
•
Groundnuts and its products from Ghana, meant for consumption on the local markets showed pervasive aflatoxins.
-
•
Majority of samples exceeded permissible limits set by the Ghana Standards Authority (GSA) and the European Union (EU).
-
•
Risk assessment revealed a public health concern and a significance for risk management.
-
•
Aflatoxin exposure and danger chiefly originate from the high consumption of groundnuts.
Abstract
A random assessment and human risk analysis were conducted on 80 groundnut pastes and raw groundnuts from some local markets across the different agroecological zones of Ghana. Total aflatoxins (AFtotal) and aflatoxins (AFB1, AFB2, AFG1, and AFG2) were analyzed using the High-Performance Liquid Chromatography (HPLC) method. Out of 80 samples investigated, 49 (61.25 %) tested positive for AFB1 and ranged from 0.38 ± 0.04–230.21 ± 22.14 μg/kg. The same proportion was recorded for total aflatoxins (AFtotal) and ranged between 0.38 ± 0.02–270.51 ± 23.14 μg/kg. Limits of AFB1 and total aflatoxins (AFtotal) for the Ghana Standards Authority (GSA) (5 and 10 μg/kg) and the European Food Safety Authority (EFSA) (2 and 4 μg/kg), were used as checks. A total of 33 (41.25 %) samples were above the limits for both. Risk assessments recorded for Estimated Daily Intake (EDI), Margin of Exposure (MOE), potency, cancer risk, and population risks ranged 0.087−0.380 μg/Kg.bw/day, 1052.630–4597.700, 0−0.00396 ng Aflatoxins kg−1bwday−1 and, 1.5 × 10−3 - 7.9 × 10-4 respectively for total aflatoxins. While for aflatoxins B1 (AFB1), ranges of values of 0.068−0.300 μg/Kg.bw/day, 1333.33–5882.35, 0−0.00396 ng aflatoxins kg/bw/day and, 1.19 × 10−3 - 6.34 × 10-4 corresponded for Estimated Daily Intake (EDI), Margin of Exposure (MOE), potency, cancer risk, and population risk respectively. There were risks of adverse health effects involved in the consumption of groundnuts for all age groups investigated since MOE values were all below 10,000.
1. Introduction
Groundnuts (Arachis hypogaea) are edible seeds of herbaceous leguminous plants which normally grow to a size of 1.0–1.6 feet [1]. They are eaten as food and treasured widely by almost everyone in Ghana and beyond (globally) owing to their high nutritive, medicinal as well as culinary values ([2,3]). Besides its direct consumption, groundnuts are used extensively for innumerable industrial processes such as abstraction of oils for domestic, industrial uses, soap production, cooking, and the manufacturing of cosmetics [4]. Groundnut is indisputably an important cash crop in Ghana and forms a significant part of the diet of many Ghanaians and as explained by Awuah, [5] and Florkowski and Kolavalli, [6], they form components or used wholly in dishes such as soups, stews, "kuli-kuli", "daakoa", "khebab powder", "aboda”…etc. Groundnuts are rich in proteins so they are used to complement many food staples to combat protein-energy malnutrition in many developing countries [2]. Furthermore, they are used in complementary food formulations for weaning babies. Likewise, used in many animal feed formulations to achieve specific desires.
Fungi, naturally have the penchant to grow very well on different nuts as well as other foods. Previous researchers [7,8] reported that different species of fungi colonize these nuts and specialize in the different phases of activities; cropping, harvesting, handling and transportation so produce an array of mycotoxins that contaminate our foodstuffs. The physical presence of these fungi causes contaminations likewise their mycotoxins.
Mycotoxins are poisonous substances produced by certain fungi found primarily in grains and nut crops, spices, traditional herbal products, alcoholic drinks and other produce [9] and many have been proven by several researchers ([8,10] and [11]) [12,13],) to pose a potential menace to both human and animal health. Contamination due to mycotoxins is indeed a serious food safety and security concern [14] that has had confrontational monetary consequences as well as health penalties in agricultural regions across the globe especially sub-Saharan Africa. The danger involved in their association with food commodities is their obstinate persistence and their ability to stay potent even through rigorous processing and treatment [15] as a majority of them are very fatal and stable thus are of significant importance in food safety. These mycotoxins accrue finally in the liver of human bodies in an active state as a relocation from animal bodies via poisoned animal products.
Aflatoxins (AFs), which are predominantly the most popular of all mycotoxins are produced mainly by fungi of the genus Aspergillus; Aspergillus flavus, Aspergillus parasiticus and rarely by Aspergillus nomius and Aspergillus tamarii [16,17]. Nonetheless, a perfect stage fungal species: Emericella venezuelensis has recently been identified to seldom produce AFB1, AFB2, AFG1 and AFG2 [18]. They are recognized carcinogens to humans and have been categorized aptly as Group 1 carcinogens by the International Agency for Research on Cancer [10]. According to [10] and [11] and Mateo et al., [19], among all of them, AFB1 has been established the most toxic, most carcinogenic [10], principal and most potent hepato carcinogenic natural compound ever categorized. Aflatoxins ominously surge the risk of liver cancer in chronic hepatitis B patients [20] and are considered a risk factor for hepatocellular cancer development. Co-exposure to aflatoxins and hepatitis B virus (HBV) greatly increases hepatocellular cancer (HCC) risk and is common in developing countries. Individuals with both exposures often have an exponentially greater risk of HCC development than those exposed to aflatoxins alone [[20], [21], [22]]. When food contaminated with aflatoxins is ingested, the aflatoxins are then transformed into aflatoxin-8,9-epoxide metabolite in the liver which has been implicated in the numerous hazardous consequences in the body [8,23]. When consumed even at the minutest quantities through the skin, aflatoxins have teratogenic, carcinogenic, hepatotoxic, and mutagenic outcomes on human health [24,25], due to their accumulative potentials. Malnutrition, notably “Kwashiorkor” has been reported to be positively correlated with aflatoxins by several researchers ([2,12,13]) in Africa.
Several countries (at least 100 countries and international organizations) have attempted to curb the exposure to aflatoxin by regulating and monitoring its incidence on food and feed commodities. These regulations differ from country to country and are also reliant on economic as well as health considerations [26]. Strict regulatory limits of 2,4 μg/kg for Aflatoxins B1 and Total aflatoxins respectively for the European Food Safety Authority [27] and 5,10–15 μg/kg by the Ghana Standards Authority [28] have been set to ensure food safety by the European Union as well as the Ghana Government thus non adherence to these may have dire consequences.
The prevention of mycotoxicity (especially with aflatoxin) is one of the most perplexing toxicology issues of recent times. By and large, fungal venoms have become the fulcrum of rife in humans and animals for the past 30 years according to a report by John and Miller [29] and has been the emphasis of vast scientific curiosity and countless investigations pertaining to their harmfulness, frequency in food and agricultural products, production, storage, processing and marketing conditions, etc. The outputs of several international meetings and valuations ([[30], [31], [32],10] and [11]) contain the bulk summary of the researches. As emphasized by WHO in 2018, aflatoxin has been confirmed to have a great impact on a significant fraction of the most prioritized health menaces in developing countries and are probably the most well-researched mycotoxins in the world to the detriment of the other lesser-known but potentially dangerous mycotoxins [33]. Disappointingly, aflatoxin consciousness is still low despite recurrent reports and education of local folks as well as the other stakeholders of aflatoxin contamination of foods spanning over several decades in Ghana (Kpodo et al. 1996, Kumi et al. 2014 [34],) and reviews describing aflatoxin’s toxicological influences in Ghanaians, predominantly among the vulnerable (children and women) (Shuaib et al. 2012 [34,35],). Good manufacturing practices (GMP), knowledge and the awareness of serious adverse health effects presented by these aflatoxins is still poor. Awuah et al. (2008) reiterated and buttressed that most Ghanaians neither have adequate knowledge of aflatoxins nor the health risks posed by these toxins.
The presence of aflatoxins in most of our foods has culminated into the rejection of many food commodities (especially peanut butter, cocoa beans, spices, and edible seeds) that exceed permissible limits at European boundaries (The Rapid Alert System for Food and Feed- RASFF) (RASFF, 2017) and as a result, negatively affected the local trade sector. These regulatory limits may vary between countries as they are influenced by financial affairs [17]. It was hypothesized that no groundnut paste and groundnuts contained aflatoxins above the permissible limits in Ghana. The objectives of this study were to monitor, estimate levels and assess human risk exposure to aflatoxins via the consumption of some groundnut products in Ghana.
2. Materials and methods
2.1. Study area
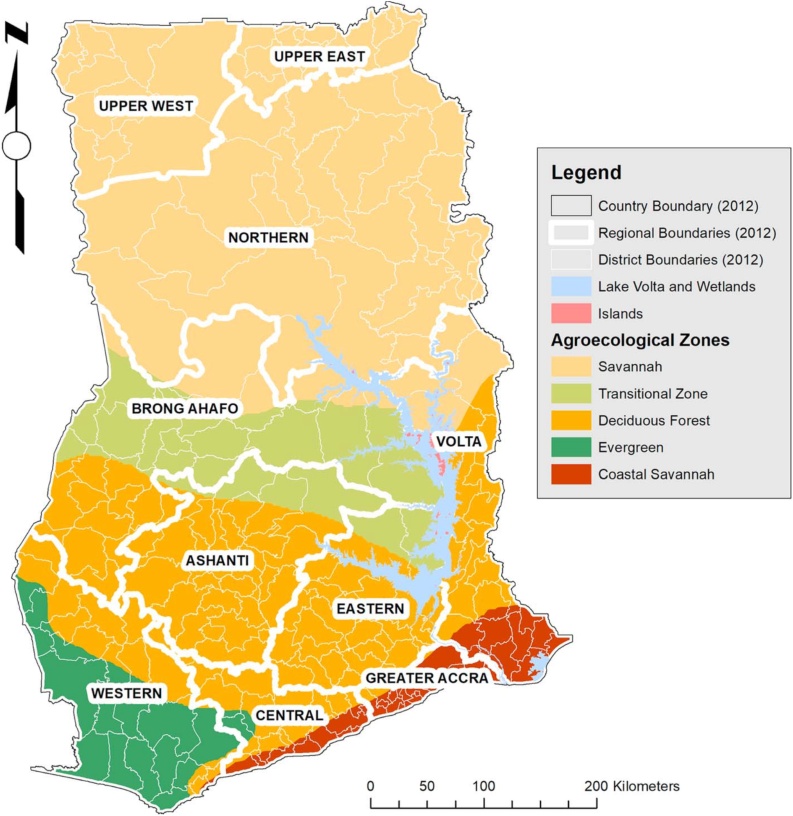
Ghana is geographically positioned on the Gulf of Guinea, West Africa where it covers about 23,884,245 ha of land and water is aptly positioned between latitudes 4 °N and 11 °N and longitudes 4 °W and 2 °E [36]. Ten (10) regions and 216 districts constitute the country and is well defined by five broadly characterized agro-ecological zones namely; Coastal Savannah, Evergreen, Deciduous Forest, Transitional, and Savannah) (Fig. 1). The population of Ghana stands at 24,658,832 people according to the data of Ghana Statistical Service, [37].
Fig. 1.
Map of Ghana showing regions of sampling (Source- Abbam et al, 2018).
2.2. Sample collection
Raw groundnut samples and groundnut paste samples were randomly purchased from different markets across the different regions in Ghana (Table 1) from the period of February to September 2019 and grouped into 2 categories (groundnuts and groundnut paste) (Tables Table 3a, Table 3b, Table 3c, Table 4a, Table 4b, Table 5, Table 6–7). Twenty (20) grams each of the samples were fetched and stored in sterile bags in an ice chest at −4 °C and transported to the laboratory and kept in a deep freezer prior to chemical exploration [38].
Table 1.
Geographical locations and some attributes of the origins of samples.
| Region | No. of samples | Agro-ecological zones | Rainfall (mm) | Temperature (oC) | Coordinates |
|---|---|---|---|---|---|
| Greater Accra | 6/80 | Coastal Savanah | 800−1000 | 26.6 | 5.8143 °N, 0.0747 °E |
| Central | 7/80 | Deciduous Forest | 1400−1600 | 26.7 | 5.5608 °N, 1.0586 °W |
| Western | 10/80 | Evergreen | 1800−2000 | 25.9 | 5.3902 °N, 2.1450 °W |
| Eastern | 9/80 | Deciduous Forest | 1400−1900 | 25.9 | 6.2374 °N, 0.4502 °W |
| Ashanti | 11/80 | Deciduous Forest | 1200−1400 | 26.3 | 6.7470 °N, 1.5209 °W |
| Brong-Ahafo | 7/80 | Transitional zone | 1400−1600 | 23.9 | 7.9559 °N, 1.6761 °W |
| Volta | 9/80 | Coastal Savanah/Deciduous forest | 1000−1400 | 26.2 | 6.5781 °N, 0.4502 °E |
| Northern | 7/80 | Savanah | 1000−1200 | 27.9 | 9.5439 °N, 0.9057 °W |
| Upper East | 7/80 | Savanah | 800−1000 | 28.3 | 10.7082 °N, 0.9821 °W |
| Upper West | 7/80 | Savanah | 1000−1200 | 27.8 | 10.2530 °N, 2.1450 °W |
Table 3a.
Concentration of aflatoxin types in different groundnut paste sampled from different locations of Ghana.
| Sample | Concentrations of Aflatoxins (μg/kg) |
|||||
|---|---|---|---|---|---|---|
| Category | AFB1 | AFB2 | AFG1 | AFG2 | AFTotal | |
| Groundnut paste | GPnsb | 3.99 ± 0.28f | 0.57 ± 0.03f | n.d | n.d | 4.56 ± 0.25e |
| GPnb | n.d | n.d | n.d | n.d | n.d | |
| GPa | 32.20 ± 1.50d | 7.75 ± 0.50bcd | 2.14 ± 0.13a | n.d | 42.16 ± 0.65c | |
| GPb | 44.20 ± 2.50c | 7.90 ± 0.50bc | 1.74 ± 0.02ab | n.d | 53.88 ± 1.02b | |
| GPp | 45.10 ± 2.50bc | 12.50 ± 1.30a | n.d | n.d | 57.67 ± 2.51b | |
| GPd | 22.20 ± 0.43e | 3.93 ± 0.35e | 1.02 ± 0.10c | n.d | 27.20 ± 1.41d | |
| GPe | 17.20 ± 1.21e | 4.89 ± 0.47de | n.d | n.d | 22.11 ± 0.45d | |
| GPf | 31.03 ± 2.50d | 6.97 ± 0.32cd | 1.41 ± 0.06bc | n.d | 39.65 ± 2.50c | |
| GPt | n.d | n.d | n.d | n.d | n.d | |
| GPnk | n.d | n.d | n.d | n.d | n.d | |
| GPnsw | n.d | n.d | n.d | n.d | n.d | |
| GPj | n.d | n.d | n.d | n.d | n.d | |
| GPfr | 3.30 ± 0.22f | 0.82 ± 0.03f | n.d | n.d | 4.12 ± 0.12e | |
| GPbg | 21.00 ± 1.0e | 5.09 ± 0.70cde | n.d | n.d | 26.07 ± 0.36d | |
| GPelm | 4.28 ± 0.43f | 0.72 ± 0.08f | n.d | n.d | 5.00 ± 0.42e | |
| GPho | 52.32 ± 0.94b | 5.16 ± 0.62cde | n.d | n.d | 57.46 ± 1.08b | |
| GPAk | 8.61 ± 0.21f | 2.81 ± 0.13ef | n.d | n.d | 10.68 ± 0.05e | |
| GPfdam | 77.7 ± 1.86+ | 10.5 ± 0.06ab | n.d | n.d | 88.16 ± 1.62a | |
| GPbas | n.d | n.d | n.d | n.d | n.d | |
Means that do not share a letter are significantly different(P < 0.05) n.d- Not detected.
Table 3b.
Concentration of aflatoxin types in different groundnut paste samples from different locations of Ghana.
| Sample | Concentrations of Aflatoxins (μg/kg) |
|||||
|---|---|---|---|---|---|---|
| Category | AFB1 | AFB2 | AFG1 | AFG2 | Total | |
| Groundnut paste | GPagom | 55.11 ± 0.32ef | 12.7 ± 0.98c | n.d | n.d | 67.72 ± 1.67e |
| GPBA2 | 78.20 ± 1.82c | 5.14 ± 0.32efg | n.d | n.d | 83.33 ± 2.12d | |
| GPcm | 65.43 ± 1.71de | 11.5 ± 1.22cd | 9.62 ± 0.24b | 2.45 ± 0.12a | 88.94 ± 3.24d | |
| GPNav | 25.23 ± 0.31hi | 2.53 ± 0.31fg | 1.62 ± 0.35c | n.d | 29.17 ± 0.95fgh | |
| GPTdi | 34.72 ± 2.82gh | 5.13 ± 0.24efg | n.d | n.d | 39.83 ± 3.01f | |
| GPketa | 2.41 ± 0.15k | 0.50 ± 0.03g | 1.02 ± 0.01c | n.d | 3.93 ± 0.22ij | |
| GPbibi | 74.15 ± 1.84cd | 9.6 ± 0.25cde | 32.13 ± 0.41a | n.d | 118.95 ± 2.51c | |
| GPcape | 9.93 ± 0.25jk | 2.97 ± 0.15fg | 2.61 ± 0.92c | n.d | 15.51 ± 1.30hij | |
| GPTam1 | 22.90 ± 0.35i | 7.33 ± 1.80def | n.d | n.d | 30.23 ± 2.15fgh | |
| GPAsafo | 175.33 ± 2.15a | 45.4 ± 2.25a | 7.57 ± 1.83b | 2.66 ± 0.71a | 230.63 ± 8.33a | |
| GPKane | 43.5 ± 0.51fg | 12.7 ± 0.9c | n.d | n.d | 56.3 ± 1.42e | |
| GPkojo | 16.72 ± 0.81ij | n.d | n.d | n.d | 16.7 ± 0.84ghi | |
| GPazugyr | 1.51 ± 0.06k | n.d | n.d | n.d | 1.51 ± 0.06j | |
| GPBolga | 5.19 ± 0.26jk | 0.69 ± 0.01g | n.d | n.d | 5.93 ± 0.27ij | |
| GPMakola | 5.96 ± 0.26jk | 1.78 ± 0.05g | n.d | n.d | 7.74 ± 0.32ij | |
| GPwow | 2.88 ± 0.12k | 0.59 ± 0.04g | n.d | n.d | 3.75 ± 0.6ij | |
| GPAgtime | 106.23 ± 7.21b | 29.8 ± 0.40b | n.d | n.d | 135.81 ± 2.12b | |
| GPAboa | 23.7 ± 0.39hi | 6.95 ± 0.33def | n.d | n.d | 30.65 ± 2.14fg | |
Means that do not share a letter are significantly different(P < 0.05) n.d- Not detected.
Table 3c.
Concentrations of aflatoxin types in different groundnut samples from different locations of the country.
| Sample | Concentrations of Aflatoxins (μg/kg) |
|||||
|---|---|---|---|---|---|---|
| Category | AFB1 | AFB2 | AFG1 | AFG2 | Total | |
| GPhuni | 4.24 ± 0.27f | 1.37 ± 0.06c | n.d | n.d | 5.61 ± 0.8ef | |
| GPEjisu1 | n.d | n.d | n.d | n.d | n.d | |
| GPEjusi 2 | 36.92 ± 0.25b | 12.8 ± 0.39a | n.d | n.d | 49.75 ± 0.65b | |
| GPDonko1 | 24.61 ± 0.71d | 2.91 ± 0.14bc | 20.92 ± 0.5a | n.d | 48.44 ± 0.6b | |
| GPDonko2 | 45.1 ± 0.65a | 12.50 ± 0.64a | n.d | n.d | 57.67 ± 1.1a | |
| GPJF | 28.5 ± 0.71c | 5.66 ± 0.91b | 5.62 ± 0.82b | n.d | 39.74 ± 0.65c | |
| Gsini | n.d | n.d | n.d | n.d | n.d | |
| GPKoft | 0.38 ± 0.04g | n.d | n.d | n.d | 0.38 ± 0.02g | |
| GPefij | n.d | n.d | n.d | n.d | n.d | |
| GPSuh | n.d | n.d | n.d | n.d | n.d | |
| GPkint | n.d | n.d | n.d | n.d | n.d | |
| GPhoh | 2.12 ± 0.73fg | n.d | n.d | n.d | 2.12 ± 0.18fg | |
| GPSoga | 7.36 ± 0.45e | 1.00 ± 0.22c | n.d | n.d | 8.36 ± 0.85e | |
| GPFosu | 28.2 ± 0.52c | n.d | 2.73 ± 0.56c | n.d | 30.93 ± 4.50d | |
| GPAseswa | n.d | n.d | n.d | n.d | n.d | |
Means that do not share a letter are significantly different.
Table 4a.
Concentrations of aflatoxin types in different groundnut samples from different locations of the country.
| Sample | Concentrations of Aflatoxins (μg/kg) |
|||||
|---|---|---|---|---|---|---|
| Category | AFB1 | AFB2 | AFG1 | AFG2 | Total | |
| Groundnuts | GNn | n.d | n.d | n.d | n.d | n.d |
| GNk | n.d | n.d | n.d | n.d | n.d | |
| GNesi | 230.21 ± 22.14a | 38.85 ± 1.06a | 2.14 ± 0.23c | n.d | 270.51 ± 23.14a | |
| GNbibi | n.d | n.d | n.d | n.d | n.d | |
| GNmor | n.d | n.d | n.d | n.d | n.d | |
| GNkaye | n.d | n.d | n.d | n.d | n.d | |
| GNsav | n.d | n.d | n.d | n.d | n.d | |
| GNkwah | n.d | n.d | n.d | n.d | n.d | |
| GNashT | n.d | n.d | n.d | n.d | n.d | |
| GNoffin | n.d | n.d | n.d | n.d | n.d | |
| GNdonko | n.d | n.d | n.d | n.d | n.d | |
| GNkwae | 3.18 ± 0.22b | n.d | n.d | n.d | 3.18 ± 0.22b | |
| GNaboab | n.d | n.d | n.d | n.d | n.d | |
| GNnkwa | n.d | n.d | n.d | n.d | n.d | |
| GNTam3 | n.d | n.d | n.d | n.d | n.d | |
| GNsagn | n.d | n.d | n.d | n.d | n.d | |
| GNguru | 1.62 ± 0.17b | n.d | n.d | n.d | 1.62 ± 0.17b | |
Means that do not share a letter are significantly different(P < 0.05) n.d- Not detected.
Table 4b.
Concentrations of aflatoxin types in different groundnut samples from different locations of the country.
| Category | Food Sample | Concentrations of Aflatoxins (μg/kg) |
||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| Groundnuts | GNtfc | n.d | n.d | n.d | n.d | n.d |
| GNnulu | n.d | n.d | n.d | n.d | n.d | |
| GNkpan | n.d | n.d | n.d | n.d | n.d | |
| GNadido | n.d | n.d | n.d | n.d | n.d | |
| GNlaw | 1.77 ± 0.12a | n.d | n.d | n.d | 1.77 ± 0.12a | |
| GNtumu | 1.61 ± 0.13a | n.d | n.d | n.d | 1.61 ± 0.13a | |
| GNsab | n.d | n.d | n.d | n.d | n.d | |
| GNnkor | n.d | n.d | n.d | n.d | n.d | |
| GNbere | 3.01 ± 0.43b | 2.55 ± 0.44 | n.d | n.d | 5.56 ± 0.93b | |
Means that do not share a letter are significantly different(P < 0.05) n.d- Not detected.
Table 5.
Proportions of samples that exceeded AFTotal and AFB1 and limits of Ghana Standard Authority (GSA) and the European Food Safety Authority (EFSA).
| Samples | Total samples | Exceeding GSA regulation |
Exceeding EFSA regulation |
|||
|---|---|---|---|---|---|---|
| Yes (%) | Range | Yes (%) | Range | |||
| AFTotal | ||||||
| Group 1 | 54 | 27 (50) | 10.68−230.63 | 34 (62.96) | 4.1−230.63 | |
| Group 2 | 26 | 1 (3.85) | 270.5 | 1 (3.85) | 270.5 | |
| Total | 80 | 28 (53.85) | 10.68−270.5 | 35 (66.81) | 4.1−270.5 | |
| AFB1 | ||||||
| Group 1 | 54 | 31 (57) | 5.19−175.0 | 38 (70.37) | 2.12−175.0 | |
| Group 2 | 26 | 1 (3.85) | 230.2 | 3 (11.53) | 3.01−230.2 | |
| Total | 80 | 32 (60.85) | 5.19−230.2 | 41 (81.9) | 2.12–230.2 | |
European Union Food Safety (EFSA) limit for AFTotal = 4 μg/kg.
European Union Food Safety (EFSA) limit for AFB1 = 2 μg/kg.
Ghana Standards Authority(GSA) limit = 10 μg/kg.
Ghana Standards Authority(GSA) limit = 5 μg/kg.
Table 6.
Evaluation of risk for Total Aflatoxins via consumption of groundnuts and groundnut paste.
| Age | Weight (kg) | References (weight) | EDI | MOE | Pav | Population Risk |
|---|---|---|---|---|---|---|
| Infants (6−52mths) | 7 | Glover-Amengor et al [39] | 0.38 | 1052.63 | 0.00396 | 1.51 × 10−3 |
| Abeshu et al [40] | ||||||
| Children (5−11y) | 24−28 | Biritwum et al [41] | 0.20 | 2000.00 | 0.00396 | 7.9 × 10−4 |
| WHO [42] | ||||||
| Adolescents (12−18yrs) | 38.5−54 | Afrifa-Anane et al [43] | 0.11 | 3636.36 | 0.00396 | 4.5 × 10−4 |
| Adults (18−60yrs) | 60.7 | Walpole et al [44] | 0.087 | 4597.70 | 0.00396 | 3.45 × 10−4 |
EDI – Estimated Daily Intake - (μg/Kg.bw/day).
MOE- Margin of Exposure.
Pav – Average Potency (ng Aflatoxins kg−1bwday−1).
*Mean aflatoxins- 60.62 μg/kg.
*Daily intake of groundnuts for infants was halved (0.5 × 0.087).
Table 7.
Evaluation of risk for Aflatoxin B1 (AFB1) via consumption of groundnuts and groundnut paste among age groups in Ghana.
| Age | Weight (kg) | References (weight) | EDI | MOE | Pav | Population Risk |
|---|---|---|---|---|---|---|
| Infants (6−52mo) | 2.5−11.65 | Glover-Amengor et al [39] | 0.30 | 1333.33 | 0.00396 | 1.19 × 10−3 |
| Abeshu et al [40] | ||||||
| Children (5−11y) | 24−28 | [41] | 0.16 | 2500.00 | 0.00396 | 6.34 × 10−4 |
| WHO [42] | ||||||
| Adolescents (12−18y) | 38.5−54 | Afrifa-Anane et al [43] | 0.09 | 4444.44 | 0.00396 | 3.56 × 10−4 |
| Adults (18−60y) | 60.7 | Walpole et al [44] | 0.068 | 5882.35 | 0.00396 | 2.69 × 10−4 |
EDI – Estimated Daily Intake - (μg/Kg.bw/day).
MOE- Margin of Exposure.
Pav – Average Potency (ng Aflatoxins kg−1bwday−1).
*Mean of AFB1−47.45 μg/kg.
*Daily intake of groundnuts for infants was halved (0.5 × 0.087).
2.3. Extraction of samples
Procedure outlined by The European Committee for Standardization (CEN) [45] official method EN14123 for the determination of AFB1, AFB2, AFG, and AFG2 from samples were followed accordingly. For the extraction, methanol in water (200 mL) (8 + 2) and 5 g NaCl were used for 20 g of samples. Materials with greater than 50 % fat contents, 100 mL of Hexane was added and the mixture homogenized for 3 min at 3000 rpm (2 min) and 3500 rpm (1 min). Filtration of extracts was achieved following the addition of 10 ml–60 ml of phosphate buffer saline (PBS) for solid-phase extraction using a pre-conditioned immunoaffinity column specific for AFB1, AFB2, AFG1, and AFG2. The 70 mL filtrate-PBS mixture was loaded onto the pre-conditioned column and allowed to elute by gravity at a flow rate of 1 mL min−1. A cleanup with 15 mL distilled water at a flow rate of 5 mL min−1. Aflatoxins were eluted in two steps into a 5 mL volumetric flask with 0.5 mL followed by 0.75 mL of methanol (HPLC grade) and permitted to elute by gravity. Deionized water was added up to a volume of eluates to 5 mL and eluate vortexed and 2 mL pipetted into HPLC vials for quantification.
2.4. HPLC parameters
Injection volume: 10 μl flow rate: 1 mL/min, column temperature: 35 ℃, excitation wavelength: 360 nm, emission wavelength: 440 nm, mobile phase composition: water/acetonitrile/MeOH (65:15:20 v/v/v), post-column derivatization: Kobra cells. HPLC Column Specification Spherisorb ODS1- Excel
(4.6 mmx25 cm), 5 μm particle size, 250 A pore size
LOD = limit of detection
LOQ = limit of quantification
ACN = Acetonitrile
MeOH = Methanol
Supplier of Column R- Biopharm, Block 10 campus, West Scotland
Science Park, Acre Road, Glasgow, Scotland G20 OXA.
Analysis of samples
High-Performance Liquid Chromatography HPLC (Agilent 1260 Series, OpenLab software, X-bridge column) (250 mm x 4.6 mm, i.d., 5 μm), USA with fluorescence detector and post-column derivatization using Kobra cells to produce bromine electrochemically was used to evaluate the aflatoxins at the CSIR- Food Research Institute, Ghana. LOD for AFB1, AFB2, AFG1 and AFG2 were 0.20 μg/kg, 0.17 μg/kg, 0.26 μg/kg and 0.36 μg/kg respectively (Table 2).
Table 2.
Limits of Detection and Quantification (LOD & LOQ) of aflatoxins AFB1, AFB2, AFG1, AFG2 and Total aflatoxins (μg/kg) measured by HPLC.
| Aflatoxin | Limits | Amount (μg/kg) |
|---|---|---|
| AFB1 | LOD | 0.20 |
| LOQ | 0.60 | |
| AFB2 | LOD | 0.17 |
| LOQ | 0.51 | |
| AFG1 | LOD | 0.26 |
| LOQ | 0.78 | |
| AFG2 | LOD | 0.36 |
| LOQ | 1.08 |
LOD-Limit of Detection.
LOQ-Limit of Quantification.
3. Limit of detection/quantification (LOD/LOQ)
Limit of detection and quantification (LOD/LOQ) of the HPLC were estimated by making a calibration curve around the least standard used for spiking, 5 μ/kg (lowest concentration range of calibration curve). Blank did not produce any signal, so the LOD and LOQ were calculated as;
| LOD = 3 X standard deviation/slope. | (1) |
| LOQ = 3 X LOD. | (2) |
4. Measurement accuracy
Spiking of pure aflatoxin standard solution was done to ensure measurement accuracy of analysis [46]. Three levels spiking were done at the lower, mid and upper concentration range of the calibration curve concentrations (5 ppb, 15 ppb and 30 ppb). Spike volumes of pure standards were calculated as;
| (3) |
Spike volumes were distributed evenly on aflatoxins free sample (blank) and spiked sample analyzed for percentage recovery which was calculated as;
| (4) |
Cs-concentration measured in spike
Cb-concentration measured in blank
Sa- spiked amount
5. Measurement precision
Repeatability and intermediate precision analyses of an internal reference material (IRM) was used to ensure measurement precision of the method [46]. For repeatability analysis, 10 parallel extractions of the IRM was done by the same analyst at the same time using the same HPLC and the relative standard deviation among results calculated. For intermediate precision, 10 extractions of the IRM were done at different days by different analysts and the relative standard deviation among results calculated. The relative standard deviations were calculated as;
| [Standard deviation / mean] x 100. |
6. Required performance criteria for accuracy and precision
Repeatability: Relative standard deviation among repeatable results should be less than 15 %.
Intermediate Precision: Relative standard deviation among results obtained under intermediate precision conditions should be less than 20 %.
Recovery: Percent recovery of measurement procedure should be in a range of 80–120 %.
Limit of Detection: The limit of detection should be less than 1 ug/kg for all aflatoxins.
Limit of Quantification: The limit of Quantification should be less than 3 ug/kg for all aflatoxins.
Linearity: Linearity from regression curve should be 0.99 (B1, B2, G1) and 0.98 (G2).
7. Experimental data
Repeatability: Relative standard deviation was;
B1 = 5.5 %; B2 = 6.7 %; G1 = 7.4 %; G2 = 12.1 % and Total aflatoxins = 5.2 %.
Intermediate Precision (Reproducibility): Relative standard deviation was;
B1 = 13.2 %; B2 = 13.4 %; G1 = 13.7 %; G2 = 12.2 % and Total aflatoxins = 11.9 %.
Recovery: Percent recovery of measurement procedure was;
Low concentration: B1 = 107 %; B2 = 87.2 %; G1 = 113.4 %; G2 = 112.8 % and Total aflatoxins = 108.2 %
High concentration: B1 = 102.6 %; B2 = 101.6 %; G1 = 104.2 %; G2 = 104.4 % and Total aflatoxins = 103.3 %
Linearity: Linearity from regression curve was;
B1 = 0.991; B2 = 0.997, G1 = 0.994; G2 = 0.995
8. Assessment of Human risk due to aflatoxins via consumption of groundnuts
8.1. Exposure estimation
Estimated Daily Intake (EDI) was arrived at by using the average quantities of aflatoxins in groundnut products, the daily ingestion of the same samples, and the average body weight. Eq. (5) was used for the calculation of EDI as described by Dos Santos et al., (2013) for mean aflatoxins and articulated as μg/kg of body weight/day (μg/kg b.w/day)
| (5) |
Weekly intake of groundnuts in Ghana is 0.61 kg/week ([5], Jolly et al. 2008, [6]). Daily intake of groundnuts in Ghana is, therefore, 0.61/7 = 0.087 kg/day
8.2. Population risk characterization for aflatoxins
The Margin of Exposures (MOEs) is the most appropriate method for the evaluation of health risk for aflatoxins (genotoxic and oncogenic biomolecules). This was determined by dividing the Benchmark dose lower limit (BMDL) for aflatoxins- 400 ngkg−1bwday−1 by EDI values (EFSA 2020) as expressed in Eq. (6).
| (6) |
A public health concern is indicated in situations where MOEs were lesser than 10,000. Conversely, it implies that aflatoxin exposures above 0.04 ngkg−1bwday−1 (as obtained by dividing 400 ngkg−1bwday−1 by 10,000) would be an indication of a risk of public health concern ([8,47,48]).
8.3. Liver Cancer risk valuation via consumption of Groundnuts
The liver cancer risk valuation for Ghanaian adult consumers was worked out for aflatoxins owing to the potential initiation and development of liver cancer [49,50] through the consumption of the toxin. Reckoning the population cancer risk per 10,000 was obtained as a product of the EDI and the mean hepatocellular carcinoma (HCC) potency figure from individual potencies of HBsAg-positive and for HBsAg negative groups.
The Joint Expert Committee for Food and Agriculture (JECFA) predictable potency values for AFB1 which tallied with 0.3 cancers year−1 10,000−1 population/ ngkg−1bwday−1 (uncertainty range: 0.05−0.5) in HBs Ag positive individuals and 0.001 cancers year−110,000−1population/ ngkg−1bwday−1 (uncertainty range: 0.002−0.03) in HBsAg-negative individuals (EFSA, 2020; [50]) were assumed. Also, the HBsAg + prevalence rate of 10.2 % for Ghana [51] was assumed and 89.8 % (100 to 10.2 %) was deduced for HBsAg-negative groups. Hereafter the average potency for cancer in Ghana was valued in Eq. (7) as follows:
| Average potency = (0.03 × 0.102) + (0.001 × 0.898) | (7) |
= 0.003958 cancers per year per 10,000 population per ng Aflatoxins kg−1bwday−1.
Accordingly, the population risk was assessed with Eq. (8):
| Population risk = Exposure (EDI) × Average potency | (8) |
8.4. Statistical analysis
Regression analysis were used to estimate the quantities of aflatoxins computed from the curves resulting from the plotted aflatoxins standards using Excel for Microsoft Windows (version 10). Analysis of variance (single factor ANOVA) and separation of means were determined post-hoc with Duncan’s multiple range test (DMRT). A 5% level of significance (p < 0.05) was used. Data was reported as means + standard deviations and analyzed using SPSS 22 (Chicago, USA).
9. Results
Good linearity or coefficients of correlations (R2 > 0.990) within the tested zone was attained in most of the samples tested. For the recovery analysis, one groundnut and groundnut paste samples previously analyzed to guarantee the absence of studied mycotoxins, were used in the validation procedure. The Limits of Detection for AFB1 and AFB2 likewise AFG1 and AFG2 ranged between 0.13−0.15 while Limits of Quantification ranged between 0.26−0.30 respectively for both (Table 2).
The number of groundnut samples contaminated with AFB1, AFB2, AFG1, AFG2 and AF Total (Total Aflatoxins) are presented in Table 3a, Table 3b, Table 3c. The degree of toxicity was in a decreasing order of AFG2< AFG1< <AFB2<AFB1. As explained by Quinto et al., [52], the life-threatening furan moiety of AFB1 is the critical point accountable the degree of biological activity of this group of fungal toxins. Out of eighty (80) samples investigated, 49 (61.25 %) tested positive for AFB1 and the contamination levels of AFB1 ranged from 0.38 ± 0.04–230.21 ± 22.14 μg/kg for GNac and GPkof1 respectively. For AFB2, 40 (50 %) samples tested positive and had levels ranging from 0.50 ± 0.03–38.85 ± 1.06 μg/kg for GPketa and GPAsafo respectively. AFG1 was present in 14 (17.5 %) samples at a range of 1.02 ± 0.10–32.13 ± 0.41 μg/kg for GPd and GPbibiani respectively while, AFG2 was detected in only 3 (3.75 %) samples, and their values ranged between 2.45 ± 0.12–2.66 ± 0.71 μg/kg for GPcm and GPAsafo respectively. Lastly, AFTotal ranged between 0.38 ± 0.02–270.51 ± 23.14 μg/kg for GPFR and GNac respectively. The total aflatoxin determinations were obtained from 49 (61.25 %) samples.
The total aflatoxin yields of 270.51 ± 23.14 and 230.63 ± 8.33 μg/kg obtained from Esiama (Western Region) and Asafo (Ashanti Region) markets respectively, were significantly (p < 0.05) higher than all other samples studied.
Toxin quantity limits prescribed by the Ghana Standards Authority [28] which is an extension of European Union [49] limits, are 5 and 10 μg/kg respectively for AFB1 and Total aflatoxins. Out of the total samples investigated, 33 (41.25 %) were above the limits for both.
Results from the study were compared to the European Food Safety Authority (EFSA) and Ghana Standards Authority (GSA) regulatory concentration limits for total aflatoxins (AFTotal) and Aflatoxins (AFB1) (Table 5). A half (50 %) of the 54 samples analyzed for total aflatoxins in group one (groundnut paste) exceeded the limits of GSA. These groundnuts paste samples had AFTotal ranging from 10.68 to 230.63 μg/kg. Only 1(3.85 %) out of 26 groundnut samples (Group two) was found to exceed the GSA limit. For AFB1 57 % of Group one and 3.9 % of Group two samples exceeded GSA standard (Table 5).
Nearly 63 % of groundnut paste and 3.9 % groundnuts exceeded the tolerable limit of the ESFA, for AFtotal, whereas 70.4 and 11.5 % of samples respectively from the two groups exceeded EFSA for AFB1 (Table 5).
9.1. Risk assessment
The Estimated Daily Intakes (EDI) of the total aflatoxins in the groundnut samples were 0.38, 0.2, 0.1 and 0.087 μg/Kg.bw/day for infants, children, adolescents, and adults respectively. For the Margin of Exposure (MOE), values of 1052.63, 2000.00, 3636.36 and 4597.70 were recorded respectively. The average potency of the aflatoxins was 0.00396 ng Aflatoxins kg−1bwday−1 and produced a population risk of 1.51 × 10−3, 7.9 × 10-4, 4.5 × 10-4, 3.45 × 10-4 respectively (Table 6). For AFB1, the EDIs for infants, children, adolescents and adults were 0.30, 0.16, 0.09 and 0.068 μg/Kg.bw/day respectively. MOE values recorded were 1333.33, 2500.00, 4444.44 and 5882.35 respectively. The average potency was the same as total aflatoxins while the population risk was respectively 1.19 × 10−3, 6.34 × 10-4, 3.56 × 10-4 and 2.69 × 10-4 (Table 7).
10. Discussion
Maize and groundnuts are key primary crops for the bulk of the population [53] in Ghana. According to the Ministry of Food and Agriculture [53], the upper regions (the Northern, Upper East, and Upper West regions) of Ghana is where well over 90 % of groundnuts are produced and generates income and livelihood for many farmers. This sector lies within the Southern Guinea Savanna (SGS) and Derived Savanna (DS) Agro-Ecological Zones (AEZ) [53]. The above mentioned two food crops (maize and groundnuts) are combined in most cases in the design of complementary feed formulae for babies or adults to fight protein-energy malnutrition (PEM) [2]. It is conjectured that the consumption of groundnuts results in high exposure to aflatoxins in Ghana since most of the nuts are contaminated from the field, storage and handling that have survived most of the processing methods. In 2009, Ghana produced approximately 495,000 metric tonnes of groundnuts on 346,900 ha by Ghanaian farmers and its production tripled the record for over ten years period between 1995 and 2005 [54] due to its high demand for consumption. The Northern, Upper West and Upper East regions of Ghana are the major regions of production where about a fifth of farmers named groundnut as one of their two most important crops (GSS, 2011). Florkowski and Kolavalli, [6], as well as Awuah [5], estimated average weekly consumption of 0.61 kg/week for Ghanaians.
The results obtained in this study were higher than the values reported in several related works. Recently, Asare- Bediako et al. [38] and Agbetiameh et al. [55] reported mean values of 928.7 ng/g and 145.6 ppb in groundnut samples from the Upper West and Brong Ahafo regions of Ghana respectively. Sugri et al. also reported 250 ppb for samples from Bantanarigu in the Upper Regions of Ghana. Awuah & Kpodo [56] previously reported relatively low levels of total aflatoxins (50 % infection rate – 0.1 μg/kg to 12.2 μg/kg) in undamaged kernels and a range of 5.7 μg/kg to 22,168 μg/kg in damaged kernels. Also, Florkowski and Kovalli (2013), reported 288.78 ppb in groundnut samples purchased from an Accra market, Ghana. Abizari et al. [57] also reported a range of 1.0–7.45 from the Northern regions of Ghana. A study by Kumi et al. [58] in the Ejura- Sekyedumase District of Ghana, food samples locally made into infant foods from a relational blend of groundnut, beans, and maize contained aflatoxins within a range of 7.9−500 ppb in 36 samples out of which 30 (83 %) of the samples had exceeded the 20 ppb limit.
Studies from other parts of Africa have shown high levels of aflatoxin contamination in staple foods. Greater quantities of aflatoxin have been reported for groundnut samples from Kenya (Max; 250 μgkg−1), and Nigeria (216.1 μgkg−1) ([4,59,60]). Adetunji et al. [8] from Nigeria reported a range of 29–33.78 ng/g. From the Gambia, a range of 2.2–459 μgkg−1 was recorded by some scientists (Turner et al. 2000, 2003, 2007, Diallo et al. 1995, Wild and Hall, 2000)
Aflatoxin B1 (AFB1) which is known to be linked with the total aflatoxins, is of high relevance to food safety. Results obtained in this study followed a similar trend as the total. Mean values reported by Willliams et al. (2004) and Kpodo [61] were 12.8 μgkg−1 and 3300 μgkg−1 respectively from groundnut paste samples in Ghana. From Mali, Waliyar et al. [62] reported values of range 171.2–530.2 μg/kg from different districts (Kolokani, Kayes, and Kita) and were within the range of aflatoxin levels of >500 μg/kg from groundnut produce of farmers in Andhra Pradesh, India [63]. Gachomo et al. [60] from Kenya, reported AFB1 values of 159 ppb in groundnut samples. Related results from Sudan reveal a range of 0.2–345 μgkg−1 in some groundnuts and groundnut pastes samples ([64,65], Idris et al., 2010). However, a rather surprising low values of <10 μgkg−1 of AFB1 was recorded by Mutegi et al. [66] from the Western Province of Kenya. Oh et al. [67] reported that the exposure level of total aflatoxins (B1+B2+G1+G2) through food (including nuts) intake marketed in Korea was 0.04 ng/kg BW/day
Our results obtained for AFG1 and AFG2, disagreed with results reported by Kooprasertying et al. (2016) as they found no (nil) AFG1 and AFG2 in groundnut samples but agreed with results (within range of values) reported by Anyebuno et al. (2018).
Early life introduction to aflatoxin is concomitant with compromised growth, mainly stunting. This early introduction is a probable risk for synergistic interactions with other toxins as subjects develop in future. [68,69]. It is noteworthy that no little quantity of aflatoxins is considered safe for human and animal consumption as these toxins are potential bio accumulators in the liver [70].
Albeit a substantial fraction of samples from each region was seemingly regarded as safe for consumption in Ghana (≤20 ng/g), although there is evidence of some components exceeding 20 ng/g which makes it riskier. The high aflatoxin incidence in the samples investigated were obtained from the deciduous forests to the evergreen rainforests, might be due to unfitting postharvest drying which may lead to fungal infestation and aflatoxin buildup in store. It can be conjectured that most of the groundnut samples used for the study were collected during the dry season (October and November 2019) and stockpiled for some time before sampling. Groundnuts usually piled by farmers for field drying/curing prior to home drying (Guchi, 2015) and subsequently harvested are exposed to warm and moist conditions usually during the period of storage leading to an accelerated fungal colonization and subsequent aflatoxin accumulation in the store as a traditional practice by farmers. Aflatoxin contamination worsens in some locations in the Northern, Upper East, and Upper West regions of Ghana by a distinctive late-season drought accompanied with high temperature during the planting season [71]. Pre-harvest contamination by aflatoxigenic Aspergilli is suggested by Torres et al. (2014) to be influenced by plant stress ensuing from high soil temperatures and drought affects. The highly contaminated samples (> 20 ng/g) occurring in the fields, have the tendency to contaminate clean samples in times of handling, processing and storage [6].
Earlier studies done by Mintah and Hunter [72] pointed to a surge in aflatoxin levels in groundnut obtained in the Northern and Volta regions that were marketed in Ghana’s capital Accra, there were some uncertainties regarding the mode of contamination. There were uncertainties regarding the mode (during transportation and storage before commercialization) and period (prior or after) of contamination with aflatoxins in the producing areas. Several years afterwards in this current study, a trend of high aflatoxin levels were also detected in both groundnut paste and groundnut from the Western and Ashanti regions; it can be inferred that there is still the persistence of this problem of contamination in Ghana in spite of the numerous education and warnings given by stakeholders to farmers and this observation may be attributed to several reasons.
There is sufficient evidence from pertinent literature ([5,38,2,58]) points that post-harvest aflatoxin contamination of groundnuts is still a major problem in Ghana and probably in most West African groundnut-producing countries too. Worthy of note, there was an observed comparatively high toxin levels in groundnut paste as found in kernels, and this could be attributed to the inferior quality of kernels used for preparing groundnut paste.
It is conjectured that non-compliance to Good Agricultural Practices (GAP) might have resulted in the aflatoxins contamination of these groundnuts and products. Additionally, the contamination process could have ensued during transport and/or storage before sale to consumers. A suggested implementation of proper post-harvest handling measures by small retailers/traders in Sub-Saharan Africa although difficult because of the prevalence of the informal/unorganized market system (Hell and Mutegi, 2011), could be a solution. The provision of efficient market policy implementation mechanisms in most Sub-Saharan African countries as well as enclosed instead of open-air market systems where the pods become prone to pod spoilage owing to the occurrence of abrupt rainstorms that wets the pods, can be avoided.
10.1. Risk assessment
As explained by Kuiper-Goodman [73], risk estimations are modeled to envisage the health problems linked with mycotoxin contact and guide food regulators to set thresholds for these toxic substances in foodstuffs. Risk assessment results obtained in this study were comparable to published findings of Lee et al. (2009) on aflatoxins in Korea as they reported values of range 0.00−0.2 ng/kg bw/day and 0.022 representing exposure or EDI and potency respectively. Kooprasertying et al. (2016) reported an estimated average ingestion of aflatoxins of 0.49, 0.40 and 2.13 ng/kg bw/day for raw, roasted and ground peanuts, respectively in Thailand. Thus, a probable menace for cancer was projected at 0.01−0.12 cancer−1 year−1100,000 persons health among some community in Thailand, could be harmed by aflatoxins intoxication via consumption of peanuts and products. In a related study, Tahghizadeh et al. (2018) recorded an estimated daily intake (EDI) of 0.013 ng/kg bw/day of pistachio (nuts) in an Iranian population and further calculated a HI value less than 1 which indicated that ingestion of investigated pistachios, posed no momentous health hazard.
A Swedish population appraisal pointed at a mean dietary intake of aflatoxins amounting to 1750 μg/ person/day and 3.5 μg/ person/day for groundnuts and almonds respectively. Thuvander et al., [74] also reported that out of ten percent nut samples investigated, AFTotal levels were greater than the maximum thresholds prescribed by the EU. From neighboring Nigeria, Adetunji et al. [8] reported a range of EDIs of 25.13–29.28 ng/kg bw/day (mean 27.20 ng/kg bw/day) and also, with an average potency of 0.04944 arrived at a cancer risk of range 1.24–1.45 (mean 1.35) from the consumption of groundnuts.
Conversely, some researchers ([60,4,59]) reported greater aflatoxin quantities of maximum; 250 μgkg−1, 10.3 μgkg−1 and 216.1 μgkg−1 for groundnut samples from Kenya, Iraq, and Nigeria respectively. The previous study from Malaysia by Chin et al. [75] reported contact with high concentrations of 24.3- 34.0 ngkg−1bwday−1 of aflatoxins in groundnuts owing to a greater daily ingestion rate in their diets.
Our findings support the reports of Adetunji et al. [8] with EDI values of range 25.3–29.28 μgkg−1 corresponding to MOE values of range 5.81–6.76 which indicated a high risk due to groundnut consumption and was of public health concern.
Likewise, high PDI value of 91.2 ngkg−1bwday−1 was reported by Oyedele et. al. (2017) from Nigeria for groundnut patrons in the damp forest zones and was accompanied with risks which needed public health attention.
It is worthy to note that no amount of aflatoxin above the zero level is regarded as safe. “Reduction to As Low As Reasonably Achievable” is the endorsement of JECFA regarding the safe level of aflatoxins in foods following the significant genotoxic carcinogenic probability of this toxin [30,76].
11. Conclusion
The present study investigated the persistence of aflatoxins in groundnuts and its products sold on some local markets across Ghana. It showed that both total aflatoxins and AFB1, a total of 33 (41.25 %) samples were above the stipulated thresholds of the Ghana Standards Authority (GSA) and the European Food Safety Authority (EFSA) and could be a basis for rejection during export. Human health risk valuation from aflatoxins contact via groundnut and its paste consumption from the markets by infants, children, adolescents, and adults showed a significant adverse health risk to humans since all calculated values for MOE were below 10,000.
Good agricultural practices (GAP), good manufacturing practices (GMP) as well as good hygiene practices (GHP) are vital so as to avert the formation of aflatoxins in the field and during storage. By precluding the aflatoxins formation in groundnut, both public health is protected and economic losses can be avoided. Monitoring groundnuts for presence of mycotoxins in a consistent manner is prudent to assess public level of awareness.
Author contribution
NKK, TA, and PTA performed the experiments and wrote the manuscript. TA and HAA handled analysis of AFB1, AFB2, AFG1 and AFG2. NKK, MW-K, HAA, and GA helped conceive the experiments and prepare the manuscript. PTA, NKK, and GA conceived the original study and supported in the analysis and interpretation of the data. MA-A, GA and HAA led the sampling and study in Ghana. All authors made inputs and permitted the final manuscript.
Funding information
Not applicable.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We are indebted to the CSIR-Food Research Institute for their contribution of aflatoxin analysis and the University of Health and Allied Sciences and the immense support for a successful completion of this research work. Our heartfelt thanks to the families of the authors for their never-ending backing.
Edited by Dr. A.M Tsatsaka
References
- 1.Geetha K., Ramarao N., Kiran R.S., Srilatha K., Mamatha P., Rao V.U. An overview on Arachis hypogaea plant. Int. J. Pharm. Sci. Res. 2013;4(12):4508–4518. doi: 10.13040/IJPSR.0975-8232.4(12).4508-4518. [DOI] [Google Scholar]
- 2.Achaglinkame M.A., Opoku N., Amagloh F.K. Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: A review. J. Nutr. Intermed. Metab. 2017;10:1–7. doi: 10.1016/j.jnim.2017.09.001. [DOI] [Google Scholar]
- 3.Bhat R.V., Vasanthi S. vol. 3. 2003. (Food Safety in Food Security and Food Trade. Mycotoxin Food Safety Risk in Developing Countries IFPRI. Brief). [Google Scholar]
- 4.Abdulla N.Q.F. Evaluation of fungal flora and mycotoxin in some important nut products in Erbil local markets. Res. J. Environ. Earth Sci. 2013;5:330–336. [Google Scholar]
- 5.Awuah R.T. Aflatoxigenic fungi and aflatoxin contamination of groundnut and groundnut-based products in Ghana: implications and concerns. In: Awuah R.T., Ellis W.O., editors. Proceedings of a National Workshop on Groundnut and Groundnut Aflatoxins, 19–21 September 1999; Kumasi, Ghana: Kwame Nkrumah University of Science and Technology; 2000. pp. 17–26. [Google Scholar]
- 6.Florkowski W.J., Kolavalli S. 2013. Aflatoxin Control Strategies in the Groundnut Value Chain in Ghana. Ghana Strategy Support Program. International Food Policy Research Institute. Working Paper 33.www.ifpri.org Internet- [Google Scholar]
- 7.Reddy K.R.N., Reddy C.S., Kumar P.N., Reddy C.S., Muralidharan K. Genetic variability of aflatoxin B1 producing Aspergillus flavus strains isolated from discolored rice grains. World J. Microbiol. Biotechnol. 2009;25:33–39. [Google Scholar]
- 8.Adetunji M.C., Alika O.P., Awa N.P., Atanda O.O., Mwanza M. Microbiological quality and risk assessment for aflatoxins in groundnuts and roasted cashew nuts meant for human consumption. J. Toxicol. 2018:1–11. doi: 10.1155/2018/1308748. Article ID 1308748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odamtten G.T., Nartey L.K., Wiafe-Kwagyan M., Anyebuno G., Kyei-Baffour V. Resident microbial load, toxigenic potential and possible quality control measures of six imported seasoning powders on the ghanaian market. J. Nutr. Health Food Eng. 2018;8(1):00252. doi: 10.15406/jnhfe.2018.08.00252. [DOI] [Google Scholar]
- 10.International Agency for Research on Cancer (IARC) vol. 56. IARC Press; Lyon, France: 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins; pp. 245–395.http://monographs.iarc.fr/ENG/Monographs/vol82/mono82.pdf (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). Available from. [Google Scholar]
- 11.International Agency for Research on Cancer. (IARC) Vol. 82. IARC monographs on the evaluation of carcinogenic risks to humans; 2002. pp. 1–556. (Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene). [PMC free article] [PubMed] [Google Scholar]
- 12.Kimanya M.E., De Meulenaer B., Roberfroid D., Lachat C., Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- 13.Kimanya M.E. The health impacts of mycotoxins in the eastern Africa region. Curr. Opin. Food Sci. 2015;6:7–11. [Google Scholar]
- 14.Ali N. Aflatoxins in rice: worldwide occurrence and public health perspectives. Toxicol. Rep. 2019;6:1188–1197. doi: 10.1016/j.toxrep.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saalia F.K., Phillips R.D. Degradation of aflatoxins by extrusion cooking: effects on nutritional quality of extrudates. Lwt - Food Sci. Technol. 2011;44(2011):1496–1501. doi: 10.1016/j.lwt.2011.01.021. [DOI] [Google Scholar]
- 16.Iqbal S.Z., Asi M.R., Ari˜no A., Akram N., Zuber M. Aflatoxin contamination in different fractions of rice from Pakistan and estimation of dietary intakes. Mycotoxin Res. 2012;28:175–180. doi: 10.1007/s12550-012-0131-1. [DOI] [PubMed] [Google Scholar]
- 17.Tarannum N., Nipa M.N., Das S., Parveen S. Aflatoxin M1 detection by ELISA in raw and processed milk in Bangladesh. Toxicol. Rep. 2020;7:1339–1343. doi: 10.1016/j.toxrep.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al- Zoreky F.A., Saleh N.S. Limited survey on aflatoxin contamination in rice. Saudi J. Biol. Sci. 2017:1–7. doi: 10.1016/j.sjbs.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateo E.M., Gil-Serna J., Patino B., Jimenez M. Aflatoxins and ochratoxin A in stored barley grain in Spain and impact of PCR-based strategies to assess the occurrence of aflatoxigenic and ochratoxigenic Aspergillus spp. Int. J. Food Microbiol. 2011;149:118–126. doi: 10.1016/j.ijfoodmicro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Groopman J.D., Kensler T.W., Wild C.P. Protective interventions to prevent aflatoxin induced Carcinogenesis in developing countries. Ann. Rev Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- 21.Scholl P.F., Groopman J.D. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography- Fluorescence. Cancer Epidemiol. Biomarkers Prev. 2008;17:1436–1439. doi: 10.1158/1055-9965.EPI-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Wu F. Global burden of Aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 2010;118(6):818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton D.L., Gallagher E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 24.Zain M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15:129–144. [Google Scholar]
- 25.Wen J., Kong W., Hu Y., Wang J., Yang M. Multi-mycotoxins analysis in ginger and related products by UHPLC-FLR detection and LC-MS/MS confirmation. Food Control. 2014;43:82–87. [Google Scholar]
- 26.Heshmati A., Nejad A.S.M. Ochratoxin A in dried grapes in Hamadan province, Iran. Food Addit. Contam. Part B. 2015;8(4):255–295. doi: 10.1080/19393210.2015.1074945. [DOI] [PubMed] [Google Scholar]
- 27.EFSA Risk assessment of aflatoxins in food. EFSA panel on contaminants in the food chain. Efsa J. 2020;18(3):1–20. doi: 10.2903/j.efsa.2020.6040. e06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghana Standards Authority (GSA) Ghana Standards Authority; Accra, Ghana: 2001. Nuts - Specification for Groundnut. [Google Scholar]
- 29.John I.P., Miller J.D. A conscise history of mycotoxin research. J. Agric. Food Chem. 2017;65:7021–7033. doi: 10.1021/acs.jafc.6b04494. [DOI] [PubMed] [Google Scholar]
- 30.FAO . FAO Food and Nutrition Paper, 81. FAO; Rome: 2004. Food and Agriculture Organization of the United Nations. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. [Google Scholar]
- 31.FAO . 2007. Available: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 32.FAO/WHO . FAO; Rome: 2006. Food and Agriculture Organization of the United Nations/World Health Organization. Food Safety Risk Analysis. A Guide for National Food Safety Authorities: FAO Food and Nutrition Paper, 87. [PubMed] [Google Scholar]
- 33.WHO . 2018. Mycotoxins.https://www.who.int/news-room/fact-sheets/detail/mycotoxins [Google Scholar]
- 34.Kortei N.K., Agyekum A.A., Akuamoa F., Kyei- Baffour V., Alidu H.W. Risk assessment and levels of naturally occurring aflatoxins in packaged cereals and cereal-based foods on the Ghanaian market. Toxicol. Rep. 2019;6:34–41. doi: 10.1016/j.toxrep.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afum C., Cudjoe L., Hills J., Hunt R., Padilla L.A., Elmore S., Afriyie A., Opare-Sem O., Phillips T., Jolly P.E. Association between aflatoxin M1 and liver disease in HBV/HCV infected persons in Ghana. Int. J. Environ. Res. Public Health. 2016;13:377. doi: 10.3390/ijerph13040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FAO . FAO Land and Water Bulletin; 1997. Irrigation Potential of Africa: a Basin Approach; p. 4.http://www.fao.org/3/w4347e/w4347e00.htm#Contents Accessed: December 3, 2020. [Google Scholar]
- 37.Ghana Statistical Service (GSS) 2016. 2015 Labor Force Report.https://www.statsghana.gov.gh/gssmain/fileUpload/Demography/LFS%20REPORT_fianl_21-3-17.pdf [Google Scholar]
- 38.Asare- Bediako K., Dzidzienyo D., Ofori K., Offei S.K., Asibuo J.Y., Adu Amoah R., Obeng J. Prevalence of fungi and aflatoxin contamination in stored groundnut in Ghana. Food Control. 2019;104:152–156. doi: 10.1016/j.foodcont.2019.04.034. [DOI] [Google Scholar]
- 39.Glover-Amengor M., Agbemafle I., Hagan L.L., Mboom F.P., Gamor G., Larbi A., Irmgard Hoeschle- Zeledon I. Nutritional status of children 0–59 months in selected intervention communities in northern Ghana from the Africa RISING project in 2012. Arch. Public Health. 2016;74(12):1–7. doi: 10.1186/s13690-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abeshu M.A., Lelisa A., Geleta B. Complementary feeding: review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries – lessons from Ethiopia. Front. Nutr. 2016;3(2016):41. doi: 10.3389/fnut.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biritwum R.B., Gyapong J., Mensah G. The epidemiology of obesity in Ghana. Ghana Med. J. 2005;39(3):82–85. PMID: 17299549. [PMC free article] [PubMed] [Google Scholar]
- 42.WHO . 2006. WHO Child Growth Standards: length/height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age: Methods and Development.https://www.who.int/childgrowth/standards/Technical_report.pdf [Google Scholar]
- 43.Afrifa-Anane E., Agyemang C., Codjoe N.A., Ogedegbe G., de-Graft Aikins A. The association of physical activity, body mass index and the blood pressure levels among urban poor youth in Accra, Ghana. BMC Public Health. 2015;15(269):1–9. doi: 10.1186/s12889-015-1546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walpole S.C., Prieto-Merino D., Edwards P., Cleland J., Stevens G., Roberts Ian. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12(1):439. doi: 10.1186/1471-2458-12-439. PMID 22709383. Retrieved 14 November 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.European Committee for Standardisation (EN 14123) 2003. Foodstuffs — Determination of Aflatoxin B1 and the Sum of Aflatoxin B1, B2, G1 and G2 in Peanuts, Pistachios, Figs, and Paprika Powder — High Performance Liquid Chromatographic Method With Postcolumn Derivatization and Immunoaffinity Column Clean-up. [Google Scholar]
- 46.Stroka J., Anklam E. Method for the determination of aflatoxins, ochratoxin a and Patulin in foodstuffs of vegetable origin. Based on JAOAC. 1997;1991(74):81–84. [Google Scholar]
- 47.World Health Organization . 2008. The Global Burden of Disease:2004 Update, 2008.http://www.who.int/healthinfo/globalburdendisease/2004reportupdate/en/index.html [Google Scholar]
- 48.EFSA Outcome of a public consultation on the draft risk assessment of aflatoxins in food. Efsa Support. Publ. 2020;17(3):1798E. [Google Scholar]
- 49.EFSA European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. Efsa J. 2007;446:1–127. [Google Scholar]
- 50.Shephard G.S. Risk assessment of aflatoxins in food in Africa. Food Additives Contam. Part A Chem. Analysis Control Expos. Risk Assessment. 2008;25(10):1246–1256. doi: 10.1080/02652030802036222. [DOI] [PubMed] [Google Scholar]
- 51.Ofori-Asenso R., Agyeman A.A. Hepatitis B in Ghana: a systematic review & meta-analysis of prevalence studies (1995-2015) BMC Infect. Dis. 2016;16:130. doi: 10.1186/s12879-016-1467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinto M., Spadaccino G., Palermo C., Centonze D. Determination of aflatoxins in cereal flours by solid-phase microextraction coupled with liquid chromatography and post- column photochemical derivatization-fluorescence detection. J. Chromatogr. A. 2009;1216:8636–8641. doi: 10.1016/j.chroma.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 53.Ministry of Food and Agriculture (MoFA) Produced by the Statistics, Research and Information Directorate (SRID), Ministry of Food and Agriculture; Accra: 2011. Agriculture in Ghana: Facts and Figures. [Google Scholar]
- 54.Yaw A.J., Akromah R., Safo-Kantanka O., Adu-Dapaah H.K., Ohemeng-Dapahh S., Agyeman A. Chemical composition of groundnut, Arachis hypogaea (L) landraces. Afr. J. Biotechnol. 2008;7(13):2203–2208. [Google Scholar]
- 55.Agbetiameh D., Ortega Beltran A., Awuah R.T., Atehnkeng J., Cotty P.J., Bandyopadhyay R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018;102:764–772. doi: 10.1094/PDIS-05-17-0749-RE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Awuah R.T., Kpodo K.A. High incidence of Aspergillus flavus and aflatoxins in stored groundnut in Ghana and the use of a microbial assay to assess the inhibitory effects of plant extracts on aflatoxin synthesis. Mycopathologia. 1996;134:109–114. doi: 10.1007/BF00436873. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 57.Abizari A.R., Garti H.A., Gajate-Garrido G., Hoffman V., Hotz C., Magnan N., Opoku N. Technical report from CSIR-SARI; 2016. Technological and Market Interventions for Aflatoxin Control in Ghana: Final Report. [Google Scholar]
- 58.Kumi J., Dotse E., Asare G.A., Ankrah N.-A. Urinary aflatoxin M1 exposure in Ghanaian children weaned on locally prepared nutritional food. Afr. J. Sci Res. 2015;4(6):28–32. [Google Scholar]
- 59.Oyedele O.A., Ezekiel C.N., Sulyok M. Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 2017;251:24–32. doi: 10.1016/j.ijfoodmicro.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Gachomo E., Mutitu E.W., Kotchoni O.S. Diversity of fungal species associated with peanuts in storage and the levels of aflatoxins in infected samples. Int. J. Agric. Biol. 2004;6(6):955–959. [Google Scholar]
- 61.Kpodo K.A. United States Agency for International Development (USAID); Washington D.C., USA: 1997. Mycotoxin Management in Peanut by Prevention of Contamination and Monitoring. Final Report of the Food Research Institute (FRI) Texas A&M Peanut Collaborative Research Support Programme (Peanut CRSP) Project. [Google Scholar]
- 62.Waliyar F., Osiru M., Ntare B.R., Vijay Krishna Kumar K., Sudini H., Traore A., Diarra B. Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin J. 2015;8(2):245–252. [Google Scholar]
- 63.Waliyar F., Reddy S.V., Subramaniam K., Reddy T.Y., Rama Devi K., Craufurd P.Q., Wheeler T.R. Importance of mycotoxins in food and feed in India. Asp. Appl. Biol. 2003;68:147–154. [Google Scholar]
- 64.Yousif I.M., Mariod A.A., Elnour I.A., Mohamed A.A. Determination of aflatoxin levels in Sudanese edible oils. Food Chem. Toxicol. 2010;48(8):2539–2541. doi: 10.1016/j.fct.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Kabbashi E.B.M., Ali S.E.E. Aflatoxins in groundnut paste in Khartoum State, Sudan. US Open Food Sci. Technol. J. 2014;1(3):1–8. [Google Scholar]
- 66.Mutegi C.K., Ngugi H.K., Hendriks S.L., Jones R.B. Factors associated with the incidence of Aspergillus section Flavi and aflatoxin contamination of peanuts in the Busia and Homa bay districts of western Kenya. Plant Pathol. 2012;61(6):1143–1153. [Google Scholar]
- 67.Oh K.S., Suh J.H., Sho Y.S. Exposure assessment of total aflatoxin in foods. Korean J. Food Technol. 2007;39:25–28. [Google Scholar]
- 68.Ali N., Hashim N., Saad B., Safan K., Nakajima M., Yoshizawa T. Evaluation of a method to determine the natural occurrence of aflatoxins in commercial traditional herbal medicines from Malaysia and Indonesia. Food Chem. Toxicol. 2005;43:1763–1772. doi: 10.1016/j.fct.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 69.Makun H.A., Dutton M.F., Njobeh P.B., Mwanza M., Kabiru A.Y. Natural multi-occurrence of mycotoxins in rice from Niger State. Nigeria. Mycotoxin Res. 2011;27:97–104. doi: 10.1007/s12550-010-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahlberg S., Randolph D., Okoth S., Lindahl J. Aflatoxin binders in foods for human consumption—can this be promoted safely and ethically? Toxins. 2019;11(410):1–11. doi: 10.3390/toxins11070410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugri I., Osiru M., Abudulai M., Abubakari M., Yahaya Asieku Y., Lamini S., Zakaria M. Integrated peanut aflatoxin management for increase income and nutrition in Northern Ghana. Cogent Food Agric. 2017;3:1312046. doi: 10.1080/23311932.2017.1312046. [DOI] [Google Scholar]
- 72.Mintah S., Hunter R.B. The incidence of aflatoxin found in groundnuts (Arachis hypogaea L.) purchased from markets in and around Accra, Ghana. Peanut Sci. 1978;5(1):13–16. [Google Scholar]
- 73.Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: aflatoxin, ochratoxin, and zearalenone. Can. J. Physiol. Pharmacol. 1990;68:1017–1024. doi: 10.1139/y90-155. [CrossRef] [PubMed] [DOI] [PubMed] [Google Scholar]
- 74.Thuvander A., Möller T., Enghardt Barbieri H., Jansson A., Salomonsson A.-C., Olsen M. Dietary intake of some important mycotoxins by the Swedish population. Food Addit. Contam. 2001;18:696–706. doi: 10.1080/02652030121353. [DOI] [PubMed] [Google Scholar]
- 75.Chin C.K., Abdullah A., Sugita-Konishi Y. Dietary intake of aflatoxins in the adult Malaysian population - an assessment of risk. Food Addit. Contam. Part B Surveill. 2012;5(4):286–294. doi: 10.1080/19393210.2012.713028. [DOI] [PubMed] [Google Scholar]
- 76.Hathout A.S., Abbel-Fattah S.M., Abou-Sree Y.H., Fouzy A.S.M. Incidence and exposure assessment of aflatoxins and ochratoxins A in Egyptian wheat. Toxicol. Rep. 2020;7:867–873. doi: 10.1016/j.toxrep.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]