Abstract
Glioblastoma, as an invasive tumor, is one of the most common primary malignant brain tumors. Despite maximum aggressive treatment, patients with glioblastoma have a dismal prognosis. Thymoquinone (TQ) has been found to show anti-cancer effects on different types of cancer. There are a few in vitro studies on the effect of TQ on glial tumors. However, the molecular mechanism of TQ's anti-cancer effect has not been fully elucidated. In the present study, we aimed to investigate the genotoxic, apoptotic, and cytotoxic effects of TQ on C6 rat glioma cells.
C6 glioma cells were analyzed after 24 h of exposure to different concentrations of TQ by the ATP cell viability assay for cytotoxicity, comet assay for genotoxicity, 2′,7′dichlorodihydrofluorescein diacetate (H2DCF-DA) for intracellular reactive oxygen species (iROS) generation, 3.3′dihexyloxacarbocyanine iodide (DiOC6(3)) for mitochondrial membrane potential, GSH/GSSG-Glo Assay for glutathione level and Fura-2AM for intracellular calcium levels. Apoptosis induction was studied by acridine orange/ethidium bromide double staining, flow cytometry, and western blotting analyses. Caspase-3, Caspase-9, Bax, Bcl-2, and pSTAT3 protein levels were determined by the western blotting method.
Cytotoxicity was enhanced by TQ in C6 glioma cells in a concentration-dependent manner. TQ also induced DNA damage, apoptosis, and increased iROS. Also, MMP and GSH levels were decreased by TQ. It inhibited pSTAT3, resulting in apoptosis induction through the regulation of anti-apoptotic and pro-apoptotic proteins.
Our results suggest that TQ would be an effective treatment in glioma. Further studies should support these findings.
Keywords: Thymoquinone, Glioblastoma, Genotoxicity, Cytotoxicity, Apoptosis, Reactive oxygen species
1. Introduction
Glioblastoma is one of the most aggressive and fatal cancer types in the central nervous system [1]. They are characterized by the rapid growth rate, high invasion capacity, and resistance to treatment. In recent years, it has been reported as a steady increase in glioma. [2]. According to the statistics, malignant gliomas are responsible for 2.5 % of the global cancer death rate [3,4]. The standard treatment method for the patient with glioblastoma includes surgical resection, radiotherapy, and adjuvant chemotherapy. Despite all of these treatment methods, the median survival from the first diagnosis for patients is approximately 15 months. Researchers have been studying on other treatment options for glioblastoma, but no ideal treatment has yet been found. Thymoquinone (TQ) is the most active ingredient of Nigella sativa. It has a preventive effect on pathologies, such as inflammation, oxidative stress, and hypertension [1,5,6]. In addition, it inhibits cancer cells by apoptotic and autophagic pathways, while its toxicity to healthy cells is very low compared to cancer cells [7]. Recent studies show that toxic effects of TQ against various cancers [8] have been demonstrated, such as breast-adenocancer [9], ovarian adenocancer [10], colorectal cancer [11], pancreatic cancer [12], osteosarcoma [13], melanoma [14], and lung cancers [15]. It also reduces the toxicity of chemotherapeutics and their side effects [16]. Meanwhile, studies have shown that the use of high concentration TQ was found to be safe in mice and rats. Our group has shown for the first time that TQ is useful in an established intracranial tumor model in mice using B16F10 melanoma through inducing apoptosis by inhibiting pSTAT3 [17].
For the current study, the most important feature of TQ is that it crosses through the blood-brain barrier because of its dimension and lipophilicity [18,19]. Anti-cancer effects of TQ is based on inhibitory and excitatory impulses on genes, pathways, and proteins [20]. The mechanism of tumorigenesis and the proliferation of tumor cells is also considered as a reason to avoid apoptosis. Abnormal cells’ elimination mechanism is based on decreased Bcl-2 and increased Bax levels [21]. The proteins that inhibit apoptosis are up-regulated, and the proteins that activate apoptosis are downregulated in recurrent glioblastoma. These protein expressions show that glioma cells have their selection for being resistant to apoptosis [22]. Despite all research, the anti-tumoral mechanism of TQ is still obscure [23]. In our study, we investigate the different effects of TQ on the C6 glioma cell line by examining the levels of apoptosis, intracellular calcium (iCa2+), reactive oxygen species (iROS), genotoxicity, and cytotoxicity.
2. Materials and methods
2.1. Chemical and reagents
TQ, horse serum (HS), F12 K medium, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), 3,3′-dihexyloxacarbocyanine iodide, penicillin/streptomycin (P/S) (10,000 U/mL), ethidium bromide (EB), Fura-2AM, trypsin-EDTA, acridine orange (AO) were purchased from Merck, Fluka, Sigma-Aldrich and Gibco. Annexin-V FITC apoptosis assay kit was purchased from Ebioscience (Carlsbad, CA 92,008 USA). All chemicals used in our study are analytical grade. The final concentration of DMSO in the prepared stock TQ concentrations was less than 0.1 % for all experiments, and low concentrations were diluted with non-serum F12 K medium. The remaining reagents were prepared fresh before each experiment.
2.2. In vitro cell culture
C6 cell line (ATCC ® CCL-107™ Rattus norvegicus brain glioma) were purchased from the American Type Culture Collection (ATCC, Middlesex, UK). Glioma cells were cultured in a complete medium, including F12 K medium, 10 % HS, and 1 % P/S under 5 % CO2 incubator at 37 °C equilibrium. Before the experiments, we checked cell viability with trypan blue. 2.5–200 μM dose range of TQ was used for cytotoxicity and iROS levels. After the IC50 was found by cytotoxicity test, IC50 and below doses were studied in all other experiments. There is no statistically significant difference between the 12, 24, and 48 h incubation times of TQ in glioma cells. Therefore, 24 h of incubation was chosen for all experiments.
2.3. ATP assay (Cytotoxicity test)
The cytotoxic effect of TQ in glioma cells was examined by the luminometric ATP method. Glioma cells seeded into 96 opaque-white plate at density of 104 cells/well for 24 h in the incubator (37 °C in 5 % CO2) to adhere. After incubation, the fresh medium was replaced and incubated with different concentrations of TQ (2.5–200 μM) on glioma cells for 24 h. Thereafter, 100 μL of ATP kit (Cell Titer-Glo Luminescent Cell Viability Assay, Promega) solution was added to each well without removing the culture medium and incubated for 10 min at room temperature. The results were evaluated in luminometry (Varioskan Flash Multimode Reader, Thermo, Waltham, MA). Luminescence emitted from wells is directly proportional to ATP, i.e., cell viability. The results were calculated as % relative luminescence according to the control. The IC50 value of TQ was calculated according to the concentration-response curve by non-linear regression analysis. All concentrations (including the control group) were repeated four independent times, and the standard deviation was within 5 %.
2.4. Measurement of intracellular reactive oxygen species production
We used a fluorescence probe, H2DCF-DA for measuring the levels of intracellular reactive oxygen species. As the iROS level increases, the diacetate in the probe is separated, and fluorescence irradiation occurs. The generation of iROS is correlated with emitted fluorescence. Glioma cells were incubated with different concentrations of TQ (2.5–200 μM) for 24 h. After incubation, the media were washed with cold 1xdPBS. After incubating with 100 μL, 100 μM H2DCF-DA for 30 min in an incubator, aspirated, and 100 μL 1xdPBS was added. The results were evaluated in a fluorescence plate reader (Varioskan Flash Multimode Reader, Thermo, Waltham, MA) Ex/Em: 485/535 nm. The results of all concentrations were given as % relative fluorescence according to the control. Each concentration (including the control group) was run in 4 independent replicates.
2.5. Measurement of intracellular glutathione (GSH) level
The effect of TQ in glioma cells on glutathione levels was measured with the luminometric GSH kit (GSH/GSSG-Glo™ Assay, Promega; Madison, Wisconsin, USA). Glioma cells seeded into 96 opaque-white plate at density of 104 cells/well for 24 h in the incubator (37 °C in 5 % CO2) to adhere. After incubation, the fresh medium was replaced and incubated with different concentrations below IC50 of TQ on glioma cells for 24 h. Thereafter, the medium was removed, and 50 μL Total or Oxidized Glutathione Reagent was added for lysing the cells. The plate was incubated in a shaker for 5 min at room temperature. After incubation, luciferin generation reagent was added 50 μL and incubated for 30 min at room temperature. Finally, Luciferin Detection Reagent was added 100 μL to all wells for equilibrating 15 min. The luminometer (Varioskan Flash Multimode Reader, Thermo, Waltham, MA) was used to measure GSH levels in glioma cells. Luminescence emitted from wells is directly proportional to glutathione. The results were calculated as % relative luminescence according to the control. All concentrations (including the control group) were repeated four independent times, and the standard deviation was within 5 %.
2.6. Determination of mitochondrial membrane potential (MMP, DΨm)
The effect of TQ on mitochondrial membrane potential in glioma cells was measured in flow cytometry with DiOC6(3) fluorescence molecular probe (Ex/Em: 483/501 nm) [24]. In glioma cells, this fluorescent probe accumulates in the matrix under the influence of the mitochondrial potential. Glioma cells were seeded into the 6-well plate at density of 5 × 104 cells/well for 24 h in the incubator (37 °C in 5 % CO2) to adhere. After incubation, the fresh medium was replaced and incubated with different concentrations below IC50 of TQ on glioma cells for 24 h for the determination of mitochondrial membrane potential. Afterward, cells were removed by trypsin-EDTA and centrifuged with 1xdPBS at 400xg for 5 min at +4 °C. The supernatant was thrown, cell debris was suspended with DiOC6(3) and incubated at 37 °C for 20 min. After incubation, the cell suspension was analyzed with flow cytometry (BD, FACS Canto II).
2.7. Effect of TQ on intracellular calcium
Intracellular calcium level is associated with the mechanism of apoptosis. The effect of TQ on intracellular calcium level in glioma cells was measured using the Fura-2AM fluorescence probe. The Fura-2AM probe is oxidized in the cell where calcium is and emits fluorescence radiation. Glioma cells were incubated with different concentrations under IC50 of TQ (2.5–200 μM) for 24 h. After incubation, the media were aspirated and washed with cold 1xdPBS. After incubating with 5 μM Fura-2AM for 30 min in an incubator. The results were evaluated in a fluorescence plate reader (Varioskan Flash Multimode Reader, Thermo, Waltham, MA) Ex/Em: 488/520 nm. The results of all concentrations were given as % relative fluorescence according to the control. Each concentration (including the control group) was run in 4 independent replicates.
2.8. Detection of apoptosis
To measure the apoptotic effects of TQ on glioma cells by different methods detailed below. Glioma cells were seeded to 5 × 104 cells/well in 6 well plates overnight. Then it was incubated with under IC50 concentrations of TQ for another 24 h. Cells were removed with 0.25 % Trypsin-EDTA, washed with 1xdPBS at +4 °C, and the following methods were applied.
2.9. Apoptosis detection by fluorescence microscope (AO/EB Dye)
Apoptosis detection using AO/EB dye is a method developed by McGahon et al. that detects apoptosis according to morphological changes in apoptotic cells [25]. Cells that undergo apoptosis are morphologically different from healthy cells. Acridine orange (AO) and ethidium bromide (EB) are DNA-specific dyes. AO dye penetrates both living and dead cells and is a cationic dye that stains double and single-strand nucleic acids. EB enters necrotic and apoptotic cell membranes with impaired cell permeability and stains nucleic acids. AO/EB double staining gives green in live cells, orange in apoptotic cells, and red in necrotic cells under a fluorescence microscope [26]. After washing, the cell suspension and AO/EB dye were mixed in a 1:1 ratio and evaluated by a fluorescence microscope (Leica DM 1000, Solms, Germany). Multiple images were obtained in randomly selected areas, and a minimum of 100 cells were counted. All concentrations (including control) were performed four times.
2.10. Determination of anti-apoptotic and pro-apoptotic proteins level
After washing, the cells were incubated on ice with lysis solution (NP-40 lysis solution: 0.2 % protease inhibitor cocktail, 150 mM NaCl, 2 mM Tris-Cl pH 7.5, 10 % glycerol) for 30 min. The lysed cells were centrifuged at 15000xrpm for 15 min at +4 °C, and supernatants (including cytosolic fraction) were separated. Protein determination was performed from the separated supernatant by the Bradford method [27]. The proteins in the supernatant were incubated at 100 °C for 15 min to denature. Proteins were separated by 8–12 % SDS-PAGE and transferred to PVDF-membrane. PVDF-membrane was incubated with phospho-signal transducer and activator of transcription 3 (pSTAT3), Bax, Caspase-3, Bcl-2, Caspase-9, and β-actin proteins primary and secondary antibodies (Cell Signaling Technology). Protein bands were visualized with Clarity Western ECL Substrate (Biorad, California-USA), and band densities were calculated with the Image-J program. Protein expressions were normalized to β-actin.
2.11. Apoptosis detection by flow cytometry (Annexin V-FITC)
The annexin-V FITC kit was used to detect apoptosis. The method principle shows Annexin-V anti-phospholipase activity and binds to phosphatidylserine. FITC labeling provides simple direct detection with FACS analysis. Counter-staining with propidium iodide allows the differentiation of apoptotic cells. After washing, the procedure was carried out according to the manufacturer's instructions. After the cell suspension was incubated with annexin-V, FITC, and PI. Apoptosis of glioma cell was analyzed with flow cytometry (BD, FACS Canto II; Ex/Em: 488/525 nm).
2.12. Measurement of DNA damage
The effect of TQ on DNA damage on glioma cells was evaluated by alkaline single cell gel electrophoresis (Comet assay method) modified by Singh et al. [28]. Glioma cells seeded into 6-well plate at density of 5 × 104 cells/well for 24 h in the incubator (37 °C in 5 % CO2) to adhere. After incubation, the fresh medium was replaced and incubated with different concentrations below IC50 of TQ on glioma cells for 24 h for the determination of DNA Damage of TQ. Afterward, cells were removed by 0.25 % trypsin-EDTA and centrifuged with 1xdPBS at 400xg for 5 min at +4 °C. The supernatant was thrown, cell debris was suspended and used in the method described below.
85 μL of 0.6 % low melting agarose (LMA) and 10 μL of cell suspension were mixed and placed onto slides with coated 1 % normal melting agarose (NMA). Slides were left in lysis solution for 1 h at +4 °C and then washed with cold 1xdPBS. Slides placed in the electrophoresis tank to open bonds in DNA were incubated for 40 min at +4 °C of alkaline electrophoresis buffer, and electrophoresis (25 V, 300 mA, and 25 min at +4 °C) was performed. Slides were rinsed 3 times with neutralized buffer, and then slides were treated with EB dye (2 μg/mL). All slides were taken under a Leica fluorescence microscope at 200x magnification. We evaluated DNA damage in images with the Comet Assay IV analysis program. All concentrations (including control) were repeated 4 times [29].
2.13. Statistical analysis
All results were given as four independent replicates, mean ± standard deviation (mean ± SD). The statistical significance level of all experimental data was investigated with variance analysis (One Way ANOVA). The statistical value of p < 0.05 was considered significant. The IC50 value of TQ in rat glioma cells were calculated by non-linear regression analysis. The Pearson correlation coefficient showed the relationships between cytotoxicity and iROS, DNA Damage, glutathione, iCa2+, MMP, and apoptosis. The statistical value of p < 0.05 was considered significant. All statistical analyses were stuck with the IBM SPSS version 23 statistical program.
3. Results
3.1. Cell viability toward glioma cells
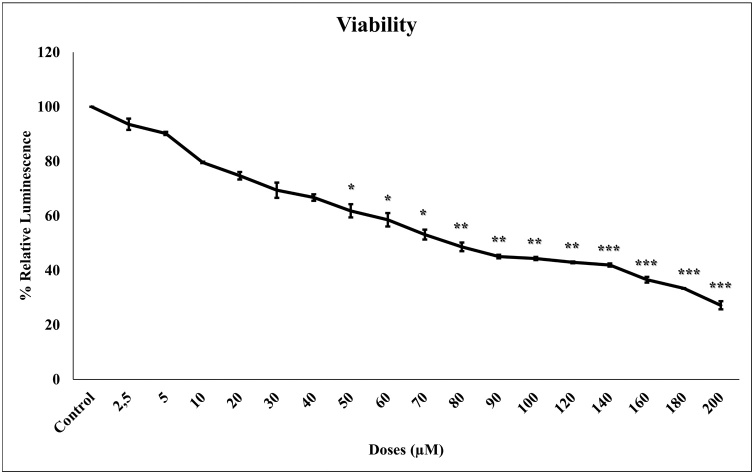
To evaluate the effect of TQ on cell viability, the concentration-response cell viability ATP test was performed after 24 h incubation into glioma cells. Glioma cell viability decreased with increasing TQ concentrations (2.5–200 μM) (Fig. 1). The percentage of cytotoxic activity increased statistically significantly (p < 0.001) depending on the control.
Fig. 1.
The effect of increasing concentrations (2.5 to 200 μM) of TQ on glioma cell viability. Glioma cells were incubated for 24 h with different concentration of TQ. Luminometric ATP assay was used to measure viability. The results were calculated by taking relative to control. All concentrations were studied at least four times and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
The IC50 concentration of TQ on glioma cells was calculated by a concentration-response graph and was found to be 72 μM. All data indicate that TQ is cytotoxic at all concentrations.
3.2. Concentration-dependent reactive oxygen generating activity
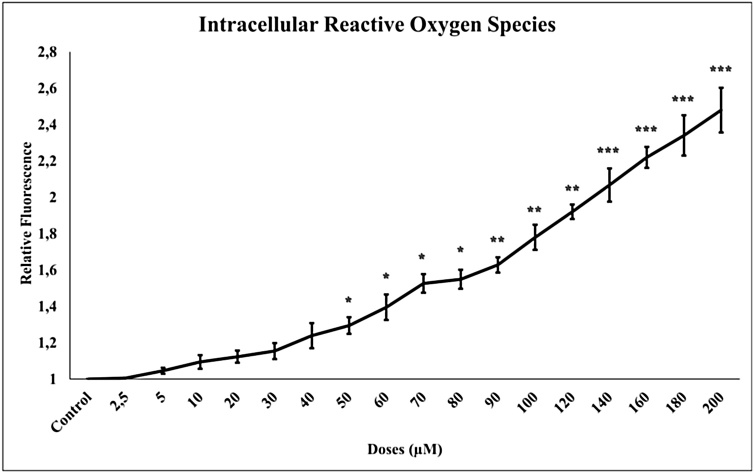
We measured the iROS level shown in Fig. 2 using the H2DCF-DA fluorescence dye. 24 h TQ (2.5–200 μM) incubation in glioma cells increased the iROS levels statistically significantly compared to control (p < 0.001).
Fig. 2.
TQ increased intracellular ROS formation in glioma cells. The increased TQ concentration increased from (2.5 to 200 μM) iROS levels. All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
3.3. TQ reduces glutathione level
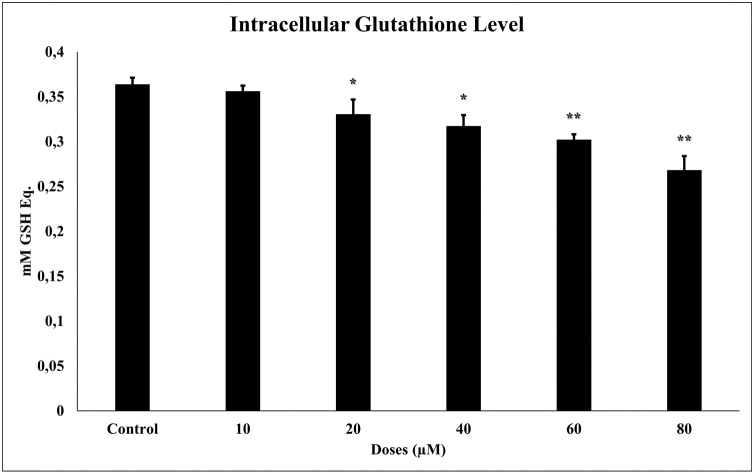
We measured glutathione levels in glioma cells by the luminometric method (GSH/GSSG-Glo Assay). 24 h TQ (10–80 μM) incubation in glioma cells significantly reduced glutathione levels (p ≤ 0.001). The reduced glutathione shown in Fig. 3 is glutathione relative luminescence values. There is a negative correlation between TQ concentration and glutathione levels.
Fig. 3.
Glutathione levels in glioma cells after 24 h of TQ exposure (10–80 μM). All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
3.4. TQ leads to decreased DΨm in glioma cell
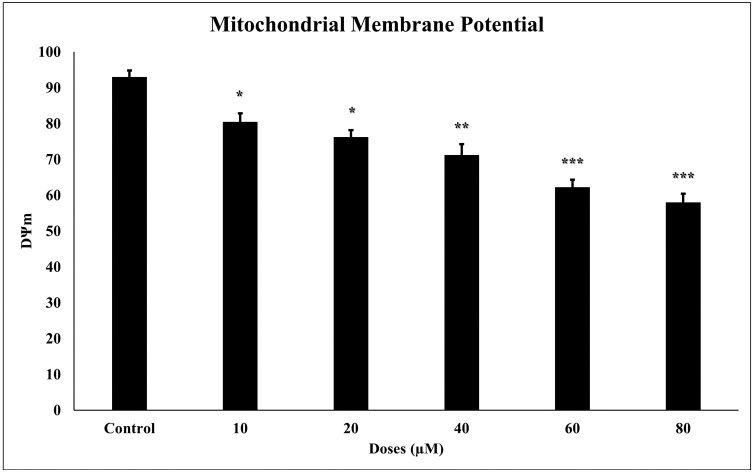
Mitochondrial pathways have been explored to demonstrate the mechanisms underlying the apoptotic effect of TQ in the glioma cell. DΨm levels were measured since the decrease in mitochondrial membrane potential caused apoptosis. According to Flow Cytometry analysis results, 24 h TQ incubation in glioma cells statistically significantly reduced (p < 0001) the mitochondrial membrane potential (Fig. 4). Reduced mitochondrial membrane potential correlates positively with increased apoptosis and cytotoxicity.
Fig. 4.
The effect of TQ on mitochondrial membrane potential in glioma cells. Loss of mitochondrial membrane potential in glioma cells developed in response to 24 h TQ incubation. All concentrations (10–80 μM) were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
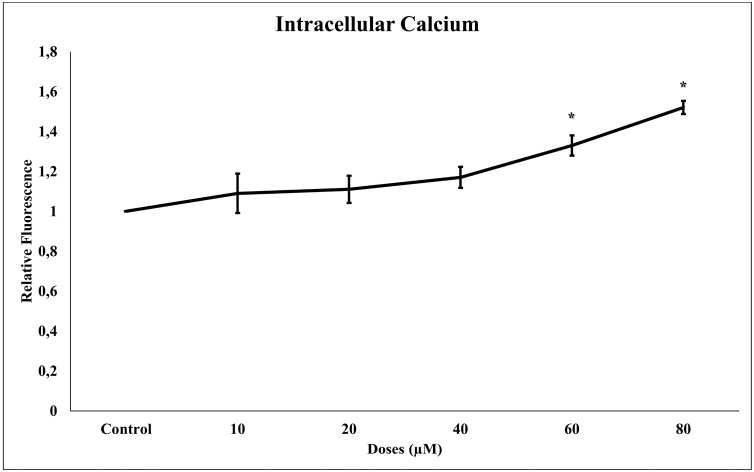
3.5. Effect of TQ on intracellular calcium level
The determination of the intracellular calcium level was implemented using the iCa2+ selective fluorescent indicator probe Fura-2AM. The 24 h incubation of TQ on glioma cells increased the intracellular calcium level statistically (p < 0.001). Increasing calcium activates the apoptotic mechanisms of the cell, causing apoptosis (Fig. 5). There is a significant positive correlation between increased TQ concentration and intracellular calcium levels.
Fig. 5.
Intracellular calcium levels in glioma cells after 24 h of TQ exposure (10–80 μM). All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
3.6. TQ induces apoptosis
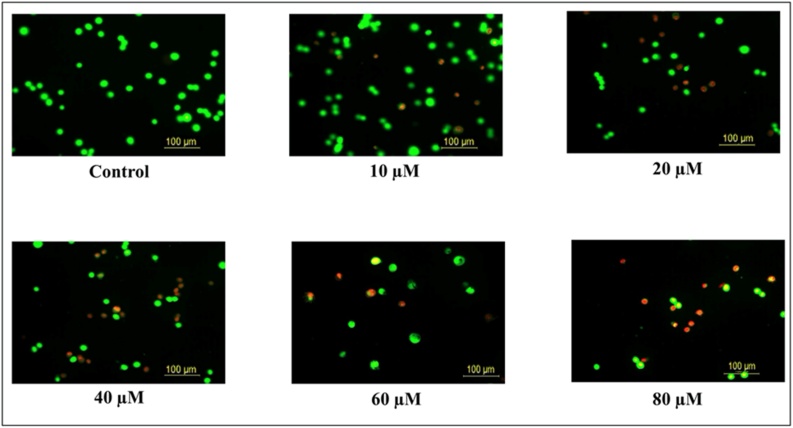
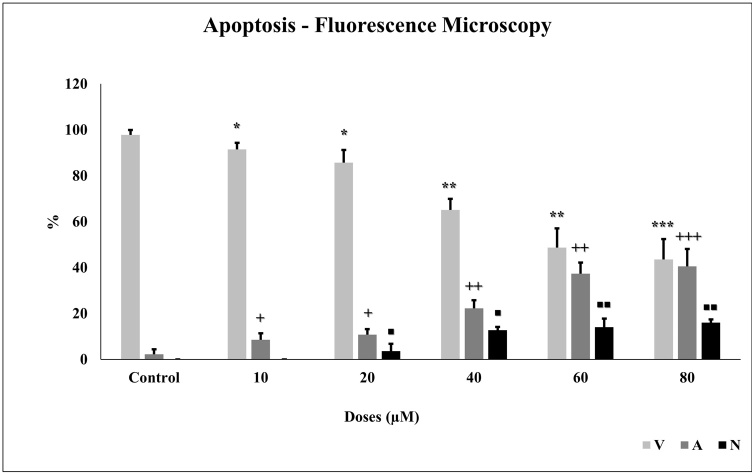
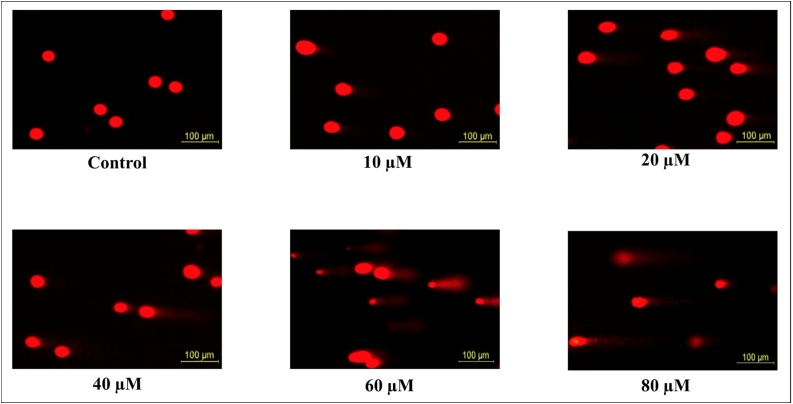
Apoptosis is important for tumor formation and treatment resistance. Apoptosis was measured by using protein expression with western blot and fluorescence microscope using the AO/EB dye. To confirm the morphological characteristics of apoptosis, after the glioma cells were incubated with different concentrations of TQ for 24 h, cells were stained with AO/EB dye and observed under fluorescence microscopy (Fig. 6). As the TQ concentration increased, the number of apoptotic cells increased statistically significantly (p < 0.001) in glioma cells (Fig. 7).
Fig. 6.
Effect of 24 h TQ incubation on glioma cells. Increasing TQ concentration causes apoptotic and necrotic cells to form in glioma cells.
Fig. 7.
TQ induces apoptosis in glioma cells. We visualized the apoptosis caused by TQ (10 to 80 μM) under a fluorescence microscope using AO/EB dye. All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001; + p < 0.05, ++ p < 0.01, +++ p < 0.001, and ◼ p < 0.05, ◼◼ p < 0.01, ◼◼◼ p < 0.001 according to the control.
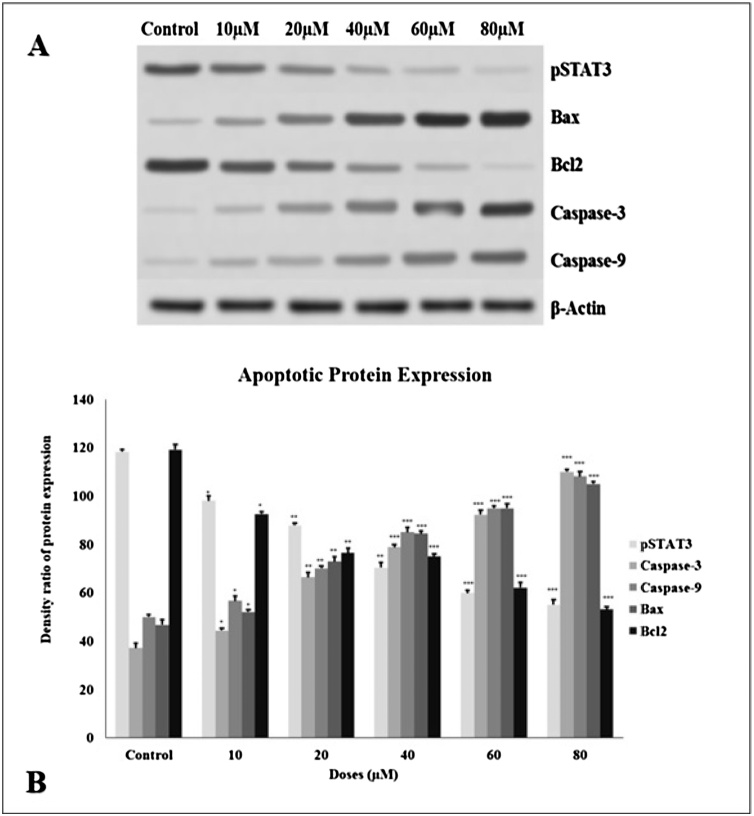
The anti- and pro-apoptotic effects of TQ were evaluated. Expressions of anti-apoptotic (Bcl-2, pSTAT3) and pro-apoptotic (Caspase-9, Caspase-3, and Bax) proteins were examined by the western blot method. After 24 h of TQ incubation in glioma cells, expressions of anti-apoptotic proteins such as Bcl-2 and pSTAT3 decreased, whereas Bax, Caspase-3, and Caspase-9 protein expressions increased concentration-dependent. β-actin has been used as a control (Fig. 8).
Fig. 8.
A. TQ therapy in the glioma cell regulates apoptosis pathways. Expressions of pro-apoptotic and anti-apoptotic proteins were normalized to β-actin. B. We measured the protein expression blot density with the image j program. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
3.7. Higher concentration of TQ induces apoptotic activity by Annexin-V FITC
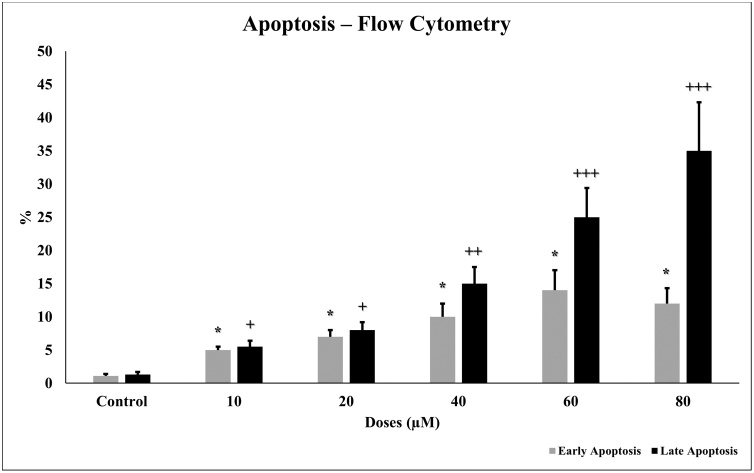
Apoptosis after 24 h TQ incubation in glioma cells was measured in flow cytometry. Annexin-V FITC stain represents early and late apoptosis and necrosis. Increased TQ concentration (10–80 μM) on glioma cells induced apoptosis. Early and late apoptosis in the glioma cells increased statistically significantly (p < 0.001) in a concentration-dependent manner (Fig. 9).
Fig. 9.
Apoptotic effect of TQ in glioma cells for 24 h. TQ increases early and late apoptosis in glioma cells. IC50 concentrations (10 to 80 μM) were analyzed in flow cytometry using Annexin-V FITC dye. All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 and + p < 0.05, ++ p < 0.01, +++ p < 0.001 according to the control.
3.8. TQ induces DNA damage
Since increased levels of iROS can cause damage to DNA, the effect of TQ treatment on DNA damage in glioma cells were examined. The single-cell alkaline comet assay method was studied to measure the 24 h genotoxic activity of TQ on glioma. In this method, DNA damage is measured. When the DNA is damaged, a shiny head and scattered tail are formed. If there is no damage to the DNA, a circular structure without tail appears (Fig. 10).
Fig. 10.
The 24 h incubation of TQ in glioma cells caused DNA damage. The increased TQ induced DNA Damage and comet formation occurred.
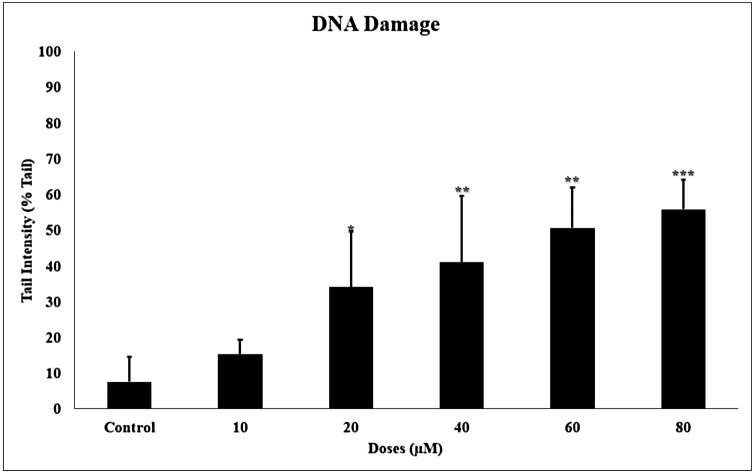
Each figure shows the DNA of the cells. The observed cells have the appearance of either a compact shape or a comet. The comet images of the DNA formed as a result of the 24 h incubation of TQ on glioma cells, which are shown in Fig. 3. Head density decreased after 24 h of TQ incubation, while tail density increased. The genotoxicity of TQ on glioma increased statistically significantly (p < 0001) by damaging the DNA in a concentration-dependent manner (Fig. 11).
Fig. 11.
TQ increases DNA damage in glioma cells. A comet image appears in the DNA. The data are given as % Tail intensity. All concentrations were made in four independent repeats and expressed as mean ± standard deviation. Statistical differences were shown as * p < 0.05, ** p < 0.01, *** p < 0.001 according to the control.
4. Discussion
Brain tumors are extremely difficult to diagnose, and the least treatable [30]. Glioblastoma is the primary brain tumor frequently encountered and has a high mortality rate considering extensive efforts to improve new therapies [31]. Therapeutic methods such as radiotherapy, and immunotherapy used in cancer treatment have limited effectiveness and have many side effects [32,33]. Due to the insufficiency of available chemotherapeutics, the search for a common target that could provide an overall strategy for cancer treatment has increased, regardless of tumor type. For this reason, the effective treatment options for glioblastoma are lacking. There is a need for new substances that can show cytotoxic, genotoxic, and apoptotic effects, decrease intracellular glutathione and mitochondrial function, and increase calcium levels. There has been an increasing number of in vitro and in vivo scientific studies on the relationship between TQ and cancer in recent years. In our study, we have shown different therapeutic effects of TQ on glioma cells in a concentration-dependent manner through various mechanisms, as described in other studies [6,[34], [35], [36]].
The anti-cancer activity of TQ brings with it the necessity of being selective to cancer cells in clinical applications. In a study, cytotoxic effects of TQ on brain cancer cells SW1088 and A172 and cortical neuronal cells-2 (HCN2), which are accepted as healthy cells, were compared, and it was shown that the cytotoxicity of TQ in HCN2 was much less than that of cancer cells [37]. In another study, Gli36, T98 G, and U87MG human glioblastoma cells proliferation inhibitory doses were found to be lower than normal astrocyte (NHA) cells in application with TQ. At least 8 μM TQ with some easily observable cell growth was not affected by NHA proliferation, even at TQ concentrations of up to 16 μM. By comparison, 2 μM of TQ was sufficient to dramatically inhibit Gli36 cell growth, with no detectable cells at 4 μM. However, T98 G cells were resistant (IC50 = 10.3 μM), and intermediate sensitivity (IC50 = 8.3 μM) for U87MG cells [38]. ATP assay was performed by examining the TQ ability to suppress glioma cell growth. Due to the astrocytic properties of C6 glioma cells, it is protective against the modulation of polyphenols on neuroglial plasticity and unsteady ROS/RNS regulation [39,40]. The agents used in the treatment of glioma are also neurotoxic, but herbal agents such as TQ are generally anti-oxidant and anti-inflammatory and can be resolved with pro-oxidant effects at high doses in treatment [41,42]. According to our results, it also demonstrates that TQ-treated C6 glioma cells exhibit with a significant decrease in cell viability in a concentration-dependent manner. The IC50 value for TQ occurred on glioma cancer cells was 72 μM.
Intracellular reactive oxygen species are related to mitochondrial protein release and apoptosis due to playing essential roles in different physiological and pathological processes. Studies in the literature have shown that TQ increases cytotoxicity and apoptosis by increasing iROS in various cancer cells [[43], [44], [45], [46]]. However, the absence of a study which includes the TQ-iROS correlation in C6 glioma cells constitutes a gap in the literature. In a study, the level of generation of mitochondrial and cytosolic ROS was investigated in C6 glioma cells with TQ. It was observed that the development of cytosolic ROS in glioma cells was increased at a concentration of TQ greater than 20 μM. Maximum iROS production has been detected at 50–70 μM TQ in cells [47]. In our study, we showed that TQ increases iROS generation on glioma cells; therefore, it affects cell viability and apoptosis. In addition, iROS production also causes in cancer cells due to increased metabolism, gene mutation, and relative hypoxia [48].
Because of the high metabolic activity of cancer cells, they may be more vulnerable to agents that increase iROS levels or that may decrease glutathione levels [49,50]. Studies have shown increased levels of oxidative damage products in clinical cancer cell lines. Furthermore, the levels of the iROS-scavenging enzyme such as glutathione peroxidase have been shown to modify significantly in malignant tumor cells. Thus, pro-oxidant agents such as TQ can be considered a viable way to selectively induce cancer cell death while protecting normal cells. To date, several intracellular iROS modulating and GSH-reducing agents have demonstrated anti-tumor activity in preclinical and clinical studies [47,51]. From our obtained results of GSH, it is observed that TQ also decreases the GSH levels on C6 glioma cells in comparison to the control.
It is associated with apoptosis due to decreased mitochondrial membrane potential, translocation of Bax protein, and release of cytochrome C into the cytoplasm [52]. As Salim and his colleagues showed in their study of leukemia, we showed one of the mechanisms underlying apoptosis by measuring the mitochondrial membrane potential in glioma cell cells. Dψ shows mitochondrial membrane potential, and loss of Dψ indicates mitochondrial damage, and it is an essential and premature marker for apoptosis. According to our flow cytometric analysis, the mitochondrial apoptosis demonstrated that TQ increased the loss of Dψ and apoptotic induction.
Intracellular calcium (iCa2+) homeostasis is one of the signaling mechanisms that control many cellular functions. Cytosolic Ca2+ concentrations are dependent on Ca2+ ions from the cell membrane or endoplasmic reticulum via special ion channels and are sensitive to changes [53]. Increasing Ca2+ concentrations in cells triggers the pro-apoptotic mechanism [54]. The obtained results showed that TQ on C6 glioma cells increase the intracellular calcium level statistically in comparison to the control. Increasing calcium activates the apoptotic mechanisms of the cell, causing apoptosis. A significant positive correlation was found between increased TQ concentration and intracellular calcium levels. This is the first research to show that excessively increased intracellular calcium was present in C6 glioma cells, and that TQ evoked robust Ca2+ influx. An acute rise in Ca2+ induces an excess of mitochondrial Ca2+ and thus activates apoptosis. The production of high levels of intracellular calcium is thought to play a significant role in triggering apoptotic mechanisms. Increased levels of Ca2+ result in mitochondrial Ca2+ overload within the cell, leading to the release of caspase factors (caspase 3, caspase 9) and other regulators (Bax, Bcl-2). A further Ca2+ gradient between the cytoplasm and other organelles is formed within the intracellular space, including the endoplasmic reticulum and mitochondria [55]. The importance of intracellular calcium levels at concentrations of 60–80 μM TQ in the study indicates that calcium is an essential regulator for apoptosis. Taken together, our study suggests that TQ is a safe, potential medication that could provide a new approach to gliomas management.
One of the most important findings for the anti-tumor effect of TQ is the induction of apoptosis on C6 glioma cells, which was observed with TQ treatment in a concentration-dependent manner. We assessed whether TQ leads C6 glioma cells to undergo apoptosis, like other studies in different cancer models [7,19,20,[56], [57], [58], [59]]. Anti-apoptotic proteins, such as Bcl-2, can be induced by chemotherapeutic agents [60]. Also, studies have proven that abnormal activity of STAT3 is related to malignancies like gastric, breast, and lung cancer [61]. We investigated the expressions of anti- and pro-apoptotic proteins after glioma cells exposed to different TQ concentrations. We sought to measure the extent to which apoptosis might govern the anti-proliferative effect of TQ by expressional analysis of Bax and Caspase 3&9, Bcl-2, and pSTAT3 proteins. In our results, 24 h incubation of TQ on glioma cells increased the expression of Caspase-3, Caspase-9, and Bax along with reducing the expression of Bcl-2 and pSTAT3 in a concentration-dependent manner. The results of our study coincide with other studies in the literature [60,62].
Apoptosis is important for tumor formation and treatment resistance. Preceding studies have shown that the incidence and development of gliomas are associated with apoptosis [63,64]. Apoptosis caused by TQ was measured and visualized under a fluorescence microscope using the AO/EB dye. With an increase in TQ treatment concentration, the number of normal cells constantly decreased while the number of apoptotic cells increased significantly in C6 glioma cells, which emitted red fluorescence in the cytoplasm or nucleus. According to the findings, TQ induces apoptosis in glioma cells. The results were coherent with mitochondrial membrane potential.
Consequently, we measured the damage of DNA caused by TQ. Similar to other studies, we found that TQ prompts DNA damage within increasing concentrations [65]. According to our findings, TQ can be used as an anti-cancer agent, but in vivo studies must be performed for further investigation.
An additional limitation for our study is that we do not have pretentious information about the interaction of TQ with chemotherapeutic agents. In that event, drug interactions with TQ also should be investigated. Both proteomic and genomic in vitro and in vivo studies should be conducted to understand the anti-cancer properties of TQ better. The results from these studies will allow us to better understand the effect of TQ on various cancer cells. In addition, the use of a single cell besides the use of a Rattus norvegicus cell line instead of human are also limitations of our study.
5. Conclusions
In our study, we have shown that natural anti-cancer TQ induces cytotoxicity and apoptosis by increasing intracellular calcium and iROS depending on the concentration against glioma cancer cells. Overproduction of iROS causes oxidative stress, which affects DNA damage and apoptosis key proteins. Our results suggest that TQ may be the treatment option for glioma. Further investigation with in vivo and preclinical studies is warranted. These features of TQ can guide new anti-cancer studies.
Role of funding source
Bezmialem Vakif University Scientific Research Project Unit supported our work with project number 5.2016/16.
Conflict of interest
The authors declare no conflict of interest.
Edited by Dr. A.M. Tsatsaka
Contributor Information
Eray Metin Guler, Email: eraymetinguler@gmail.com.
Behice Hande Sisman, Email: handesisman@gmail.com.
Abdurrahim Kocyigit, Email: abdurrahimkocyigit@yahoo.com.
Mustafa Aziz Hatiboglu, Email: azizhatiboglu@yahoo.com.
References
- 1.Perry James, Okamoto Masahiko, Guiou Michael, Shirai Katsuyuki, Errett Allison, Chakravarti Arnab. Novel therapies in glioblastoma. J. Neurol. Res. Int. 2012;2012 doi: 10.1155/2012/428565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloizou Athina-Maria, Pateraki Georgia, Siokas Vasileios, Mentis Alexios-Fotios A., Liampas Ioannis, Lazopoulos George, Kovatsi Leda, Mitsias Panayiotis D., Bogdanos Dimitrios P., Paterakis Konstantinos. The role of MiRNA-21 in gliomas: hope for a novel therapeutic intervention? Toxicol. Rep. 2020 doi: 10.1016/j.toxrep.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel R.L., Torre L.A., Jemal A. ’Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries (vol 68, pg 394, 2018)’. CA. 2020;70 doi: 10.3322/caac.21492. 313-13. [DOI] [PubMed] [Google Scholar]
- 4.Silantyev Artemiy S., Falzone Luca, Libra Massimo, Gurina Olga I., Kardashova Karina Sh, Nikolouzakis Taxiarchis K., Nosyrev Alexander E., Sutton Christopher W., Mitsias Panayiotis D., Tsatsakis Aristides. Current and future trends on diagnosis and prognosis of glioblastoma: from molecular biology to proteomics. Cells. 2019;8:863. doi: 10.3390/cells8080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rooney S., Ryan M.F. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005;25:2199–2204. [PubMed] [Google Scholar]
- 6.El-Mahdy M.A., Zhu Q., Wang Q.E., Wani G., Wani A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer. 2005;117:409–417. doi: 10.1002/ijc.21205. [DOI] [PubMed] [Google Scholar]
- 7.Gali-Muhtasib H., Ocker M., Kuester D., Krueger S., El-Hajj Z., Diestel A., Evert M., El-Najjar N., Peters B., Jurjus A., Roessner A., Schneider-Stock R. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell. Mol. Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imran Muhammad, Rauf Abdur, Khan Imtiaz Ali, Shahbaz Muhammad, Qaisrani Tahira Batool, Fatmawati Sri, Abu-Izneid Tareq, Imran Ali, Rahman Khaliq Ur, Gondal Tanweer Aslam. Thymoquinone: a novel strategy to combat cancer: a review. Biomed. Pharmacother. 2018;106:390–402. doi: 10.1016/j.biopha.2018.06.159. [DOI] [PubMed] [Google Scholar]
- 9.Aumeeruddy M..Zakariyyah, Mahomoodally M..Fawzi. Combating breast cancer using combination therapy with 3 phytochemicals: piperine, sulforaphane, and thymoquinone. Cancer. 2019;125:1600–1611. doi: 10.1002/cncr.32022. [DOI] [PubMed] [Google Scholar]
- 10.Taha M.M.E., Sheikh B.Y., Salim L.Z.A., Mohan S., Khan A., Kamalidehghan B., Ahmadipour F., Abdelwahab S.I. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell. Mol. Biol. 2016;62:97–101. [PubMed] [Google Scholar]
- 11.Ballout Farah, Monzer Alissar, Fatfat Maamoun, Ouweini Hala El, Jaffa Miran A., Abdel-Samad Rana, Darwiche Nadine, Abou-Kheir Wassim, Gali-Muhtasib Hala. Thymoquinone induces apoptosis and DNA damage in 5-Fluorouracil-resistant colorectal cancer stem/progenitor cells. Oncotarget. 2020;11:2959. doi: 10.18632/oncotarget.27426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karki Namrata, Aggarwal Sita, Laine Roger A., Greenway Frank, Losso Jack N. Cytotoxicity of juglone and thymoquinone against pancreatic cancer cells. Chem. Biol. Interact. 2020;327 doi: 10.1016/j.cbi.2020.109142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarman H., Bayram R., Benek S.B. ’Anticancer drugs with chemotherapeutic interactions with thymoquinone in osteosarcoma cells’. Eur. Rev. Med. Pharmacol. Sci. 2016;20:1263–1270. [PubMed] [Google Scholar]
- 14.Hatiboglu Mustafa Aziz, Kocyigit Abdurrahim, Guler Eray Metin, Akdur Kerime, Nalli Arife, Karatas Ersin, Tuzgen Saffet. ’Thymoquinone induces apoptosis in B16-F10 melanoma cell through inhibition of p-STAT3 and inhibits tumor growth in a murine intracerebral melanoma model’. World Neurosurg. 2018;114:e182–e190. doi: 10.1016/j.wneu.2018.02.136. [DOI] [PubMed] [Google Scholar]
- 15.Samarghandian Saeed, Azimi‐Nezhad Mohsen, Farkhondeh Tahereh. ’Thymoquinone‐induced antitumor and apoptosis in human lung adenocarcinoma cells’. J. Cell. Physiol. 2019;234:10421–10431. doi: 10.1002/jcp.27710. [DOI] [PubMed] [Google Scholar]
- 16.Schneider-Stock R., Fakhoury I.H., Zaki A.M., El-Baba C.O., Gali-Muhtasib H.U. ’Thymoquinone: fifty years of success in the battle against cancer models’. Drug Discov. Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Hatiboglu M.A., Kocyigit A., Guler E.M., Akdur K., Nalli A., Karatas E., Tuzgen S. Thymoquinone induces apoptosis in B16-F10 melanoma cell through inhibition of p-STAT3 and inhibits tumor growth in a murine intracerebral melanoma model. World Neurosurg. 2018;114:e182–e190. doi: 10.1016/j.wneu.2018.02.136. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H., Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 19.Al-Majed A.A., Al-Omar F.A., Nagi M.N. NEuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006;543:40–47. doi: 10.1016/j.ejphar.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Kaseb A.O., Chinnakannu K., Chen D., Sivanandam A., Tejwani S., Menon M., Dou Q.P., Reddy G.P. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007;67:7782–7788. doi: 10.1158/0008-5472.CAN-07-1483. [DOI] [PubMed] [Google Scholar]
- 21.Gurung R.L., Lim S.N., Khaw A.K., Soon J.F., Shenoy K., Mohamed Ali S., Jayapal M., Sethu S., Baskar R., Hande M.P. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krakstad C., Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol. Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racoma I.O., Meisen W.H., Wang Q.E., Kaur B., Wani A.A. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells’. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rottenberg Hagai, Wu Shaolong. ’Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells’. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1998;1404:393–404. doi: 10.1016/s0167-4889(98)00088-3. [DOI] [PubMed] [Google Scholar]
- 25.McGahon Anne J., Martin Seamus J., Bissonnette Reid P., Mahboubi Artin, Shi Yufang, Mogil Rona J., Nishioka Walter K., Green Douglas R. ’The end of the (cell) line: methods for the study of apoptosis in vitro’. Methods Cell Biol. 1995;46:153–185. doi: 10.1016/s0091-679x(08)61929-9. [DOI] [PubMed] [Google Scholar]
- 26.Ribble Deborah, Goldstein Nathaniel B., Norris David A., Shellman Yiqun G. ’A simple technique for quantifying apoptosis in 96-well plates’. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger Nicholas J. The Bradford method for protein quantitation. Basic Protein Peptide Protocols. 1994:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 28.Singh Narendra P., McCoy Michael T., Tice Raymond R., Schneider Edward L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann A., Agurell E., Beevers C., Brendler-Schwaab S., Burlinson B., Clay P., Collins A., Smith A., Speit G., Thybaud V. ’ReCommendations for conducting the in vivo alkaline comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 30.Tuaeva Natalia O., Falzone Luca, Porozov Yuri B., Nosyrev Alexander E., Trukhan Vladimir M., Kovatsi Leda, Spandidos Demetrios A., Drakoulis Nikolaos, Kalogeraki Alexandra, Mamoulakis Charalampos. Translational application of circulating DNA in oncology: review of the last decades achievements. Cells. 2019;8:1251. doi: 10.3390/cells8101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shergalis Andrea, Bankhead Armand, Luesakul Urarika, Muangsin Nongnuj, Neamati Nouri. ’Current challenges and opportunities in treating glioblastoma’. Pharmacol. Rev. 2018;70:412–445. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christofi Theodoulakis, Baritaki Stavroula, Falzone Luca, Libra Massimo, Zaravinos Apostolos. Current perspectives in cancer immunotherapy. Cancers. 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falzone Luca, Salomone Salvatore, Libra Massimo. ’Evolution of cancer pharmacological treatments at the turn of the third millennium’. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gali‐Muhtasib Hala, Ocker Matthias, Kuester Doerthe, Krueger Sabine, El‐Hajj Zeina, Diestel Antje, Evert Matthias, El‐Najjar Nahed, Peters Brigitte, Jurjus Abdo. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell. Mol. Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arafa El-Shaimaa A., Zhu Qianzheng, Shah Zubair I., Wani Gulzar, Barakat Bassant M., Racoma Ira, El-Mahdy Mohamed A., Wani Altaf A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. Mol. Mech. Mutagen. 2011;706:28–35. doi: 10.1016/j.mrfmmm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Najjar Nahed, Chatila Manal, Moukadem Hiba, Vuorela Heikki, Ocker Matthias, Gandesiri Muktheshwar, Schneider-Stock Regine, Gali-Muhtasib Hala. ReActive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 37.Shahein Samar A., Aboul-Enein Ahmed M., Higazy Iman M., Abou-Elella Faten, Lojkowski Witold, Ahmed Esam R., Mousa Shaker A., AbouAitah Khaled. Targeted anticancer potential against glioma cells of thymoquinone delivered by mesoporous silica core-shell nanoformulations with pH-dependent release. Int. J. Nanomedicine. 2019;14:5503. doi: 10.2147/IJN.S206899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Racoma Ira O., Meisen Walter Hans, Wang Qi-En, Kaur Balveen, Wani Altaf A. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quincozes-Santos André, Bobermin Larissa Daniele, Latini Alexandra, Wajner Moacir, Souza Diogo Onofre, Gonçalves Carlos-Alberto, Gottfried Carmem. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park Se-Ho, Lee Jae-Yeul, Jhee Kwang-Hwan, Yang Seun-Ah. ’Amyloid-ß peptides inhibit the expression of AQP4 and glutamate transporter EAAC1 in insulin-treated C6 glioma cells’. Toxicol. Rep. 2020;7:1083–1089. doi: 10.1016/j.toxrep.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahmoud Yasmina K., Abdelrazek Heba M.A. Cancer: thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019;115 doi: 10.1016/j.biopha.2019.108783. [DOI] [PubMed] [Google Scholar]
- 42.Erdal Serap, McCarthy Bridget J., Gurule Natalia, Berwick Marianne, Gonzales Emily, Byrd Johanna, Flores Kristina, Shimek Jo Anna, Il’yasova Dora, Ali-Osman Francis. Application of mutagen sensitivity assay in a glioma case-control study. Toxicol. Rep. 2018;5:183–188. doi: 10.1016/j.toxrep.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taha M.M., Sheikh B.Y., Salim L.Z., Mohan S., Khan A., Kamalidehghan B., Ahmadipour F., Abdelwahab S.I. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell. Mol. Biol. (Noisy-le-grand) 2016;62:97–101. [PubMed] [Google Scholar]
- 44.El-Najjar N., Chatila M., Moukadem H., Vuorela H., Ocker M., Gandesiri M., Schneider-Stock R., Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 45.Koka P.S., Mondal D., Schultz M., Abdel-Mageed A.B., Agrawal K.C. ’STudies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: role of reactive oxygen species’. Exp. Biol. Med. (Maywood) 2010;235:751–760. doi: 10.1258/ebm.2010.009369. [DOI] [PubMed] [Google Scholar]
- 46.Hussain A.R., Ahmed M., Ahmed S., Manogaran P., Platanias L.C., Alvi S.N., Al-Kuraya K.S., Uddin S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic. Biol. Med. 2011;50:978–987. doi: 10.1016/j.freeradbiomed.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Krylova N.G., Drobysh M.S., Semenkova Galina N., Kulahava T.A., Pinchuk S.V., Shadyro O.I. Cytotoxic and antiproliferative effects of thymoquinone on rat C6 glioma cells depend on oxidative stress. Mol. Cell. Biochem. 2019;462:195–206. doi: 10.1007/s11010-019-03622-8. [DOI] [PubMed] [Google Scholar]
- 48.Perillo Bruno, Di Donato Marzia, Pezone Antonio, Di Zazzo Erika, Giovannelli Pia, Galasso Giovanni, Castoria Gabriella, Migliaccio Antimo. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020:1–12. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumari Seema, Badana Anil Kumar, Malla Rama Rao. ’ReActive oxygen species: a key constituent in cancer survival’. Biomark. Insights. 2018;13 doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wondrak Georg T. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trachootham Dunyaporn, Alexandre Jerome, Huang Peng. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 52.Fauzi Agustine Nengsih, Norazmi Mohd Nor, Yaacob Nik Soriani. ’Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines’. Food Chem. Toxicol. 2011;49:871–878. doi: 10.1016/j.fct.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Berridge Michael J. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Hajnóczky György, Csordás György. Calcium signalling: fishing out molecules of mitochondrial calcium transport. Curr. Biol. 2010;20:R888–R891. doi: 10.1016/j.cub.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honda Hidemi, Kondo Takashi, Zhao Qing-Li, Feril Loreto B., Jr., Kitagawa Hiroshi. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med. Biol. 2004;30:683–692. doi: 10.1016/j.ultrasmedbio.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Jafri S.H., Glass J., Shi R., Zhang S., Prince M., Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J. Exp. Clin. Cancer Res. 2010;29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attoub S., Sperandio O., Raza H., Arafat K., Al-Salam S., Al Sultan M.A., Al Safi M., Takahashi T., Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013;27:557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 58.Banerjee S., Kaseb A.O., Wang Z., Kong D., Mohammad M., Padhye S., Sarkar F.H., Mohammad R.M. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–5583. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]
- 59.Lei X., Lv X., Liu M., Yang Z., Ji M., Guo X., Dong W. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem. Biophys. Res. Commun. 2012;417:864–868. doi: 10.1016/j.bbrc.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 60.Zhu W.Q., Wang J., Guo X.F., Liu Z., Dong W.G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016;22:4149–4159. doi: 10.3748/wjg.v22.i16.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong A., Yang Z., Shen Y., Zhou J., Shen Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers (Basel) 2014;6:926–957. doi: 10.3390/cancers6020926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo C.C., Hsu A., Kumar A.P., Sethi G., Tan K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisele G.ünter, Roth Patrick, Hasenbach Kathy, Aulwurm Steffen, Wolpert Fabian, Tabatabai Ghazaleh, Wick Wolfgang, Weller Michael. APO010, a synthetic hexameric CD95 ligand, induces human glioma cell death in vitro and in vivo. Neurooncology. 2011;13:155–164. doi: 10.1093/neuonc/noq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konkankit Veerauo V., Kim Won, Koya Richard C., Eskin Ascia, Dam Mai-Anh, Nelson Stanley, Ribas Antoni, Liau Linda M., Prins Robert M. Decitabine immunosensitizes human gliomas to NY-ESO-1 specific T lymphocyte targeting through the Fas/Fas ligand pathway. J. Transl. Med. 2011;9:1–13. doi: 10.1186/1479-5876-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Racoma Ira O., Meisen Walter Hans, Wang Qi-En, Kaur Balveen, Wani Altaf A. Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. J. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072882. [DOI] [PMC free article] [PubMed] [Google Scholar]