Abstract
Objectives
Chronic kidney disease (CKD) is associated with an increased complication rate after cardiac interventions. Although CKD has a high prevalence among atrial fibrillation patients, the impact of CKD on periprocedural complications and the outcome after an interventional left atrial appendage closure (LAAC) is unclear. The present study, therefore, aimed to investigate whether CKD influences the procedure’s effectiveness and safety.
Methods
LAARGE is a prospective, non-randomised registry. LAAC was conducted with different standard commercial devices, and the follow-up period was one year. CKD was defined by an eGFR < 60 mL/min/1.73 m2, and subgroups were further analysed (i.e. eGFR < 15, 15–29, and 30–59 mL/min/1.73 m2, respectively).
Results
Two hundred ninety-nine of 623 patients (48.0%) revealed a CKD. The prevalence of cardiovascular comorbidity, CHA2DS2-VASc score (4.9 vs. 4.2), and HAS-BLED score (4.3 vs. 3.5) was significantly higher in CKD patients (each p < 0.001). Implantation success was similarly high across all GFR groups (97.9%). Periprocedural MACCE (0.7 vs. 0.3%), and other major complications (4.7 vs. 3.7%) were comparably infrequent. Survival free of stroke was significantly lower among CKD patients within 1 year (82.0 vs. 93.0%; p < 0.001; consistent after adjustment for confounding factors), without significant accentuation in advanced CKD (i.e. eGFR < 30 mL/min/1.73 m2; p > 0.05 vs. eGFR 30–59 mL/min/1.73 m2). Non-fatal strokes were absolutely infrequent during follow-up (0 vs. 1.1%). Severe non-fatal bleedings were observed only among CKD patients (1.4 vs. 0%; p = 0.021).
Conclusions
Despite an increased cardiovascular risk profile of CKD patients, device implantation was safe, and LAAC was associated with effective stroke prevention across all CKD stages.
Electronic supplementary material
The online version of this article (10.1007/s00392-020-01638-5) contains supplementary material, which is available to authorized users.
Keywords: Atrial fibrillation, Chronic kidney disease, Left atrial appendage, Left atrial appendage closure, LAARGE
Introduction
Stroke and systemic embolisation are prognostically relevant complications of atrial fibrillation (AF) [1]. In patients with non-valvular AF, > 90% of thrombi originate from the left atrial appendage (LAA), which is located in front of the left atrium, and has intensively trabeculated walls [2]. While the use of non-vitamin K antagonist oral anticoagulants (NOAC) is the recommended standard for prophylaxis in patients with non-valvular AF and a high thromboembolic risk [1], some patients reveal contraindications for a long-term use of such substances [3, 4]. For these patients, the left atrial appendage closure (LAAC) has evolved as an interventional alternative and was proven to be effective and safe in high-risk patients even without a post-procedural period with continued anticoagulation [1, 5].
While the prevalence of AF is high among patients with impaired renal function, these patients are prone to an increased thromboembolic risk compared to AF patients with normal renal function [6, 7]. Clinically relevant chronic kidney disease (CKD) is defined by an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 [8]. Besides the increased thromboembolic risk, bleeding complications are more frequent in patients with AF and concomitant CKD, particularly in patients who are anticoagulated [6]. This is also depicted by the integration of an impaired renal function as a risk factor in the HAS-BLED score [9]. Moreover, the use of NOACs should be avoided in patients with severely impaired renal function, i.e. eGFR < 15 mL/min/1.73 m2, because insufficient data are available, and warfarin use is associated with conflicting outcome results [1].
Especially in combination with other cardiovascular risk factors, CKD might render AF patients ineligible for long-term OAC and might favour LAAC in many of these patients. However, renal failure was also shown to increase the rate of periprocedural complications in cardiac interventions and to worsen the outcome [10, 11]. Currently, outcome data on LAAC in CKD patients are limited [12]. The present subanalysis of the Left-Atrium-Appendage occluder Register—GErmany (LAARGE, ClinicalTrials.gov Identifier: NCT02230748), therefore, aimed to investigate on the intra-hospital outcome as well as the effectiveness and safety during one-year follow-up in patients with AF and CKD.
Methods
The Registry
LAARGE is a prospective, non-randomised, multicentre real-world registry that encompasses patients from 37 voluntary participating centres. Its main objective is to represent the LAAC procedure’s clinical reality. For this reason, the protocol neither influenced indication nor clinical management, but it claimed consecutive enrolment to avoid a recruitment bias. Devices should be implanted according to current recommendations. Recruitment in the registry started in July 2014 and ended in January 2016.
For the present subanalysis, patients with started procedure and documented renal function were selected from the whole database. The study was carried out according to the principles of the declaration of Helsinki and was approved by the ethics committee of the State Chamber of Medicine in Rhineland-Palatinate, Germany. Written informed consent was obtained from all study patients.
Definition of chronic kidney disease
The eGFR was calculated using The Modification of Diet in Renal Disease (MDRD) Study formula [8]. According to the guidelines, patients with eGFR < 60 mL/min/1.73 m2 were categorised as having a clinical relevant impaired renal function [13]. Patients with CKD were categorised into three groups (i.e. eGFR < 15 mL/min/1.73 m2, eGFR 15–29 mL/min/1.73 m2, and 30–59 mL/min/1.73 m2).
Procedure
As described previously [14], the preprocedural screening, the conduction of the implantation procedure as well as the postprocedural management including the antithrombotic treatment were at the discretion of the operating physician. Different standard commercial devices were implanted taking into consideration the specific manufacturer’s recommendations.
Data acquisition
All participating centres reported procedural data and intra-hospital complications as well as discharge medication via an electronic case report form. Patients were contacted directly or via phone call one year after the implantation procedure to assess the survival, the occurrence of complications, and the antithrombotic treatment. If no contact could be established with a patient, information was obtained from the registration offices. For the purpose of data validation, all relevant events were reviewed and evaluated by an Endpoint Adjudication Committee, if necessary based on the original medical documents.
Outcome measures
The effectiveness was primarily assessed by the absence of all-cause death and stroke during follow-up, secondarily by the absence of transient ischemic attacks (TIA) and systemic embolism. The implantation success was defined as a stable device anchorage. Complications including para-device leaks > 5 mm, device dislocations, severe and moderate bleedings during hospitalisation and during follow-up as well as thromboembolism in the venous system represented the safety outcome measure.
Statistics
Statistical analyses were performed with SAS® version 9.4 (SAS Institute, Cary, NC, USA). Continuous data are presented as means with standard deviation or as medians with interquartile ranges (25th and 75th percentiles), categorical data as frequencies with group-related percentages. Trends across the patient groups were assessed by a Cochran-Armitage test regarding categorical variables, or by an exact Cochran-Armitage test in case of rare events, and by a Jonckheere–Terpstra test regarding metrical variables, as indicated in the tables. In addition, CKD patients were compared to non-CKD patients using the Pearson Chi-squared test or Mann–Whitney–Wilcoxon test for categorical and metrical variables, respectively. These statistics were based on the available cases.
The one-year mortality after the implantation procedure and the incidence of the combined event of death or stroke were evaluated by methods of survival analysis (Kaplan–Meier curves, log-rank test). Hazard ratios with 95% confidence intervals (CI) were estimated using Cox regression without adjustment and adjusted for baseline characteristics significantly associated with CKD and known as clinically relevant risk factors: age (linear), sex, body mass index > 25 kg/m2, arterial hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, and LVEF ≤ 40%. The expected annual rates of major bleeding and stroke were calculated from the individual HAS-BLED [9] and CHA2DS2-VASc score, respectively [15]. The follow-up duration was defined as the time span from the index discharge to the date of the follow-up contact. p values ≤ 0.05 (two-tailed) were considered significant.
Results
Baseline characteristics
623 patients were included in the present analysis. 299 (48.0%) revealed a CKD (Table 1). The median eGFR value was calculated at 41.1 vs. 78.8 mL/min/1.73 m3 (p < 0.001 for the comparison to non-CKD patients). CKD patients were significantly older (77.8 ± 7.5 vs. 74.4 ± 7.8 years, p < 0.001) and revealed a significantly higher stroke (CHA2DS2-VASc score 4.9 ± 1.5 vs. 4.2 ± 1.5, p < 0.001) and bleeding risk (HAS-BLED score 4.3 ± 1.0 vs. 3.5 ± 1.0, p < 0.001), whereby an HAS-BLED score ≥ 3, corresponding to a high bleeding risk, was significantly more frequent in CKD patients (97.6 vs. 84.7%, p < 0.001). CKD patients also revealed a more pronounced cardiovascular risk profile.
Table 1.
Baseline characteristics
| eGFR < 15 mL/min | eGFR 15–29 mL/min | eGFR 30–59 mL/min | No CKD | p value for trend* | |
|---|---|---|---|---|---|
| Total cohort, n (% of all patients) | 15 (2.4) | 45 (7.2) | 239 (38.4) | 324 (52.0) | |
| Male, n (%) | 12 (80.0) | 26 (57.8) | 124 (51.9) | 218 (67.3) | 0.069 |
| Age [years], median (IQR) | 75 (69; 79) | 80 (76; 82) | 79 (74; 83) | 76 (71; 80) | < 0.001 |
| Body mass index [kg/m2], median (IQR) | 25 (23; 32) | 28 (25; 30) | 27 (24; 31) | 26 (24; 30) | 0.038 |
| CHA2DS2-VASc score, mean ± SD | 5.1 ± 1.7 | 5.3 ± 1.6 | 4.8 ± 1.4 | 4.2 ± 1.5 | < 0.001 |
| HAS-BLED score, mean ± SD | 4.6 ± 1.1 | 4.8 ± 0.9 | 4.2 ± 1.0 | 3.5 ± 1.0 | < 0.001 |
| Type of AF, each n (%) | |||||
| Paroxysmal | 7 (46.7) | 16 (35.6) | 99 (41.4) | 144 (44.4) | 0.39 |
| Persistent | 3 (20.0) | 10 (22.2) | 42 (17.6) | 57 (17.6) | 0.59 |
| Permanent | 5 (33.3) | 19 (42.2) | 98 (41.0) | 123 (38.0) | 0.66 |
| Congestive heart failure, n (%) | 6 (40.0) | 22 (48.9) | 74 (31.0) | 69 (21.3) | < 0.001 |
| Arterial hypertension, n (%) | 14 (93.3) | 43 (95.6) | 222 (92.9) | 301 (92.9) | 0.69 |
| Diabetes mellitus, n (%) | 10 (66.7) | 24 (53.3) | 96 (40.2) | 84 (25.9) | < 0.001 |
| Prior cerebrovascular event, each n (%) | |||||
| TIA | 1 (6.7) | 4 (8.9) | 14 (5.9) | 33 (10.2) | 0.22 |
| Stroke | 3 (20.0) | 11 (24.4) | 46 (19.2) | 72 (22.2) | 0.72 |
| Coronary heart disease, n (%) | 11 (73.3) | 22 (48.9) | 133 (55.6) | 123 (38.0) | < 0.001 |
| Prior CABG, n (%) | 2 (13.3) | 7 (15.6) | 35 (14.6) | 29 (9.0) | 0.056 |
| Peripheral arterial disease, n (%) | 6 (40.0) | 17 (37.8) | 66 (27.6) | 74 (22.8) | 0.012 |
| Prior major bleeding, n (%) | 6 (40.0) | 18 (40.0) | 94 (39.3) | 131 (40.4) | 0.87 |
| Indication for LAAC, each n (%) | |||||
| Prior bleeding | 14 (93.3) | 37 (82.2) | 199 (83.3) | 247 (76.2) | 0.022 |
| Prior cerebrovascular event despite OAC | 4 (26.7) | 13 (28.9) | 52 (21.8) | 98 (30.2) | 0.2 |
| Absolute contraindication against any OAC | 3 (20.0) | 7 (15.6) | 48 (20.1) | 62 (19.1) | 0.88 |
| Labile INR | 1 (6.7) | 6 (13.3) | 27 (11.3) | 20 (6.2) | 0.061 |
| Incompliance with OAC | 0 (0.0) | 5 (11.1) | 15 (6.3) | 13 (4.0) | 0.2 |
| Patient preference | 3 (20.0) | 5 (11.1) | 54 (22.6) | 88 (27.2) | 0.028 |
| Other reason | 2 (13.3) | 5 (11.1) | 20 (8.4) | 31 (9.6) | 0.82 |
| Medication at presentation, each n (%) | |||||
| Anticoagulants | 9 (60.0) | 27 (60.0) | 153 (64.0) | 193 (59.6) | 0.6 |
| Antiplatelet agent | 6 (40.0) | 19 (42.2) | 92 (38.5) | 102 (31.5) | 0.056 |
AF atrial fibrillation, CKD chronic kidney disease, eGFR estimated glomerular filtration rate, INR international normalised ratio, IQR interquartile range, LAAC left atrial appendage closure, MDRD modification of diet in renal disease, OAC oral anticoagulation, SD standard deviation, TIA transitory ischemic attack
*Tested by Cochran–Armitage or Jonckheere–Terpstra test; p < 0.05 is indicating a significant difference (printed in bold type)
Participating centres could document more than one indication for LAAC in the same patient (Table 1). Across all predefined GFR groups, the main indication was a prior bleeding event (79.8%; p = 0.022 for trend).
Supplemental Table 1 shows data from cardiac imaging procedures. While left atrial diameters were larger in CKD patients (p = 0.024 for trend), this finding did not correspond with the LAA diameters (each p > 0.05 for trend).
Procedural data and intra-hospital outcome
Technical success was high across all groups (97.9%; p = 0.76 for trend; supplemental Table 2), and no peri-device leak > 5 mm was present. Three interventions had to be interrupted prematurely (p = 0.87 for trend). A stable device anchorage could not be achieved in additional three patients. Device selection and dimensions as well as procedural parameters did not differ significantly (each p > 0.05 for trend).
Table 2.
Intra-hospital outcome
| eGFR < 15 mL/min | eGFR 15–29 mL/min | eGFR 30–59 mL/min | No CKD | p value for trend* | |
|---|---|---|---|---|---|
| Total cohort, n (% of all patients) | 15 (2.4) | 45 (7.2) | 239 (38.4) | 324 (52.0) | |
| MACCE, n (%) | 0 (0.0) | 2 (4.4) | 0 (0.0) | 1 (0.3) | 0.097 |
| Death, n (%) | 0 (0.0) | 2 (4.4) | 0 (0.0) | 0 (0.0) | 0.028 |
| Myocardial infarction, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0.62 |
| Stroke, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0.62 |
| Other major complication, n (%) | 2 (13.3) | 2 (4.4) | 10 (4.2) | 12 (3.7) | 0.27 |
| Severe bleeding, n (%) | 1 (6.7) | 0 (0.0) | 3 (1.3) | 3 (0.9) | 0.43 |
| AV fistula or pseudoaneurysm, n (%) | 0 (0.0) | 1 (2.2) | 2 (0.8) | 3 (0.9) | 1.0 |
| Pericardial effusion requiring action, each n (%) | |||||
| Surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.6) | 0.38 |
| Intervention | 1 (6.7) | 1 (2.2) | 5 (2.1) | 6 (1.9) | 0.44 |
| Device dislodgement requiring action, each n (%) | |||||
| Surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Additional intervention | 1 (6.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0.028 |
| Moderate complications, n (%) | 4 (26.7) | 5 (11.1) | 20 (8.4) | 29 (9.0) | 0.18 |
| Moderate bleeding, n (%) | 1 (6.7) | 2 (4.4) | 2 (0.8) | 6 (1.9) | 0.4 |
| TIA, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Successful cardiopulmonary resuscitation, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 2 (0.6) | 0.71 |
| Access site infection, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1.0 |
| Pericardial effusion with conservative treatment, n (%) | 0 (0.0) | 0 (0.0) | 4 (1.7) | 7 (2.2) | 0.31 |
| Device dislodgement handled by immediate retraction, n (%) | 0 (0.0) | 0 (0.0) | 3 (1.3) | 2 (0.6) | 1.0 |
AV arteriovenous, CKD chronic kidney disease, eGFR estimated glomerular filtration rate, MACCE major adverse cardiac and cerebrovascular events, TIA transitory ischemic attack
*Tested by exact Cochran–Armitage test; p < 0.05 is indicating a significant difference (printed in bold type)
Intra-hospital complications, and particularly major adverse and cerebrovascular events (MACCE) were generally rare (each p > 0.05 for trend; Table 2). Correspondingly, time to discharge was generally short (p = 0.097 for trend). Two intra-hospital deaths among the CKD patients were due to either an unknown or of cardiovascular aetiology, respectively. Seven dislodged devices could be retrieved catheter-based (each p > 0.05 for trend). Antithrombotic discharge medication did not differ significantly (each p > 0.05 for trend; supplemental Fig. 1), provided that 12.2% of patients stayed on anticoagulation when leaving the hospital (p = 0.57 for trend).
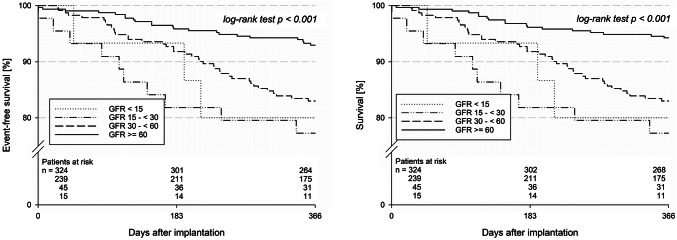
Fig. 1.
One-year incidence of all-cause death and stroke after left atrial appendage closure (LAAC). Left figure: freedom from all-cause death and stroke after left atrial appendage closure; right figure: freedom from all-cause death after LAAC
Follow-up
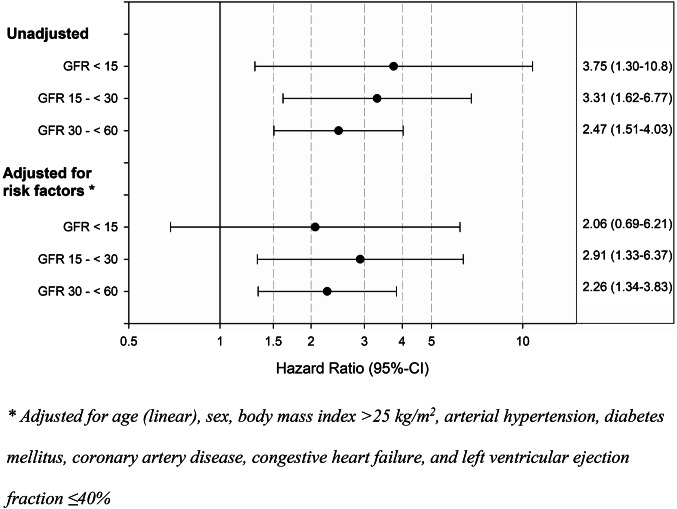
A total of 608 patients (97.9%) could be followed-up (p = 0.85 for trend; Table 3). Limited to 365 days after the procedure, the combined primary effectiveness outcome measure was reached in 82.0% among CKD patients and 93.0% among patients without impaired renal function (p < 0.001; Fig. 1). Even after adjustment for relevant risk factors, this effect was still present (Fig. 2), but there was no statistically significant difference when comparing the patient groups with an eGFR < 60 mL/min/1.73 m2 among each other (p = 0.76). Only three non-fatal strokes were observed in the total cohort (p = 0.25 for trend), which all were ischemic. Moreover, rates of TIA and systemic embolism were low cross all GFR groups (each p > 0.05 for trend). Severe (p = 0.021 for trend) and moderate bleedings (p = 0.52 for trend) were infrequent across all groups. Despite only 6.0% of patients received anticoagulation after one year (p = 0.13 for trend), only two deep vein thromboses were registered (p = 1.00 for trend). 89.6% of patients were completely content with the intervention, and 96.6% of patients felt safe during hospital stay (each p > 0.05 for trend).
Table 3.
Follow-up data
| eGFR < 15 mL/min | eGFR 15–29 mL/min | eGFR 30–59 mL/min | No CKD | p value for trend* | |
|---|---|---|---|---|---|
| Discharged alive, n | 15 | 43 | 239 | 324 | |
| Documented follow-up, n (%) | 15 (100.0) | 42 (97.7) | 234 (97.9) | 317 (97.8) | 0.85 |
| Death, n (% of patients with documented vital status) | 3 (20.0) | 8 (19.0) | 39 (16.7) | 18 (5.7) | < 0.001 |
| Events in survivors of total follow-up | |||||
| Stroke, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.1) | 0.25 |
| TIA, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.4) | 1.0 |
| Systemic embolism, n (%) | 0 (0.0) | 1 (3.4) | 0 (0.0) | 0 (0.0) | 0.08 |
| Major adverse events | |||||
| Device dislodgement requiring action, each n (%) | |||||
| Surgery | 0 (0.0) | 1 (3.6) | 0 (0.0) | 2 (0.7) | 1.0 |
| Additional intervention | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1.0 |
| Pericardial effusion requiring action, each n (%) | |||||
| Surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Intervention | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0.64 |
| Pulmonary embolism, n (%) | 0 (0.0) | 1 (3.4) | 5 (2.8) | 0 (0.0) | 0.04 |
| Severe bleeding, n (%) | 1 (9.1) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 0.021 |
| Moderate adverse events | |||||
| Deep vein thrombosis, n (%) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.4) | 1.0 |
| Moderate bleeding, n (%) | 1 (9.1) | 1 (3.4) | 8 (4.5) | 10 (3.6) | 0.52 |
| Antithrombotic medication, each n (%) | |||||
| Anticoagulants | 1 (9.1) | 4 (13.8) | 11 (6.1) | 14 (5.1) | 0.13 |
| Antiplatelet agents | 8 (72.7) | 27 (93.1) | 152 (84.9) | 232 (83.8) | 0.74 |
| Subjective feeling of treatment success, each n (%) | |||||
| Completely | 7 (87.5) | 23 (92.0) | 141 (91.0) | 210 (88.6) | 0.53 |
| Partly | 1 (12.5) | 1 (4.0) | 8 (5.2) | 16 (6.8) | 0.77 |
| Not | 0 (0.0) | 1 (4.0) | 4 (2.6) | 11 (4.6) | 0.33 |
| Subjective feeling of safety during index hospitalisation, n (%) | 9 (100.0) | 25 (96.2) | 153 (96.2) | 237 (96.7) | 0.96 |
CKD chronic kidney disease, eGFR estimated glomerular filtration rate, TIA transitory ischemic attack
*Tested by exact Cochran–Armitage (events) or asymptotic Cochran–Armitage test; p < 0.05 is indicating a significant difference (printed in bold type)
Fig. 2.
Adjustment of the primary efficacy outcome measure for relevant risk factors
Discussion
This subanalysis of the multicentre LAARGE registry confirmed an excellent procedural success and could demonstrate that LAAC was associated with effective stroke prevention also in patients with CKD.
Almost half of the patients (48.0%) were affected by a renal impairment. Despite an accentuated cardiovascular risk profile of CKD patients, and in contrast to prior published data [6, 7], patients were affected by a similar number of prior strokes across all stages. CKD patients are also known to be at higher risk for bleeding independent from the use of OAC [6, 7]. In our analysis, this was reflected by significantly more patients in the CKD group who were indicated for LAAC due to prior bleedings.
Independent from the renal function, the implantation success was high (97.9%). Periprocedural MACCE and other major complications were infrequent in both, patients with and without renal impairment, and rates were comparable to other recently published data [16]. This observation differentiates the LAAC procedure from interventions in the arterial system, as these cardiac procedures were shown to be associated with higher periprocedural complication rates and a worse outcome in CKD patients [10, 11, 17]. A fact which might be explained by intra-arterial administration being an independent risk factor for contrast-induced acute kidney injury [18]. Such low complication rates in contrast with the initial PROTECT-AF trial, reporting 8.9% of major adverse events, might also reflect the growing experience with the LAAC procedure [19].
Even after adjustment for relevant risk factors, the combined incidence of all-cause death and stroke was higher in the CKD group during follow-up, but was not accentuated in patients with an advanced renal insufficiency (i.e. eGFR < 30 mL/min/1.73 m2). Cases of death accounted for the vast majority of all these events (100 and 85.7%, respectively). An excess mortality among renally impaired patients is certainly not unexpected in a patient collective that is prone to a pre-existing and well described higher baseline risk.
Despite an increased risk of thromboembolic events, as reflected by a CHA2DS2-VASc score of 4.9 vs. 4.2, and thus despite a collective at noticeably higher risk than in the initial trials [19, 20], non-fatal strokes were extremely infrequent in both, patients with and without renal impairment (0 vs. 1.1%, respectively), standing for a dramatic reduction compared to the estimated annual stroke risk of 6.3 and 5.3%, respectively [15]. By stating that the majority of patients would otherwise not have been anticoagulated, this is a remarkable result, in particular in the more vulnerable CKD patients who comparably benefited.
The observed annual major bleeding rate was low, too, but, nonetheless, all major bleedings appeared in CKD patients. A finding which is not surprising given frequent analogous reporting in literature [6]. Against such a backcloth, it is all the more remarkable that the observed rates were much lower than the expected annual major bleeding rates based on the HAS-BLED score of 9.2 and 6.4%, respectively [9]. Moderate bleedings were infrequent across all stages of renal function. Despite only 6.0% of patients who were anticoagulated after one year, only 3.2 and 0.4% of patients suffered a thromboembolic event in the venous system. Thus, the LAAC procedure was shown to be a safe alternative for AF patients with renal impairment, while NOACs, which are also recommended for this subpopulation [1, 21], are associated with conflicting safety results particularly concerning bleeding events in CKD patients [21, 22].
These achievements may have contributed substantially to the fact that the intervened patients were highly content with the procedure (91.0 vs. 88.6%) and felt safe during the index hospitalisation (96.4 vs. 96.7%), which highlights the not only theoretical but also practical impact on the quality of life.
Study limitations
These analyses were based on observational registry data with the inherent limitations of this study type. The conduction of the intervention was not influenced by the study protocol and based on the operators’ discretions as well as the relevant recommendations, which respected the observational character of the registry. This individualised decision algorithm may have had impact on the outcome measures but surely reflects the clinical practice. The implantation volume per centre and per operator was naturally heterogeneous, which also meant a good mixture of experience. Though a separate group with renal replacement therapy was envisaged, there were not enough cases to perform an individual analysis on such patients. Despite these limitations, this registry is surely serving as a data source for a little-studied topic.
Conclusions
Despite an increased cardiovascular risk profile of CKD patients, a consistently high implantation success with low complication rates was seen in all stages of renal function. The observed stroke rates were comparably low in all groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open Access funding provided by Projekt DEAL.
Abbreviations
- AF
Atrial fibrillation
- CI
Confidence interval
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- LAA
Left atrial appendage
- LAAC
Left atrial appendage closure
- LAARGE
Left-Atrium-Appendage occluder Register GErmany
- MACCE
Major adverse cardiac and cerebrovascular events
- MDRD
Modification of diet in renal disease
- (N)OAC
(Non-vitaminK antagonist) oral anticoagulants
- TIA
Transient ischemic attack
Funding
The LAARGE registry was funded by the Stiftung Institut für Herzinfarktforschung (IHF; Ludwigshafen am Rhein, Germany). The present work was supported by an unrestricted grant from Boston Scientific (Marlborough, MA, USA).
Compliance with ethical standards
Conflict of interest
Johannes Brachmann reports grants and personal fees from Biotronik (Berlin, Germany), Medtronic (Dublin, Ireland), Pfizer (New York City, NY, USA), and St. Jude Medical (Saint Paul, MN, USA). Horst Sievert reports study honoraria to institution, reimbursement of travel expenses, and consulting fees (personal honoraria had not been paid) from Abbott (Chicago, IL, USA), Boston Scientific (Marlborough, MA, USA), Lifetech (Nanshan District, Shenzhen, China), and Occlutech (Jena, Germany). Matthias Hochadel, Steffen Schneider, and Jochen Senges report unrestricted grants from Boston Scientific (Marlborough, MA, USA) for performing statistical analyses. Christian Fastner, Thorsten Lewalter, Uwe Zeymer, Martin Borggrefe, Christoph A. Nienaber, Christian Weiß, Sven T. Pleger, Hüseyin Ince, Jens Maier, Stephan Achenbach, Holger H. Sigusch, and Ibrahim Akin do not report any relevant conflicts of interest.
References
- 1.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 2.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 3.Alamneh EA, Chalmers L, Bereznicki LR. Suboptimal use of oral anticoagulants in atrial fibrillation: has the introduction of direct oral anticoagulants improved prescribing practices? Am J Cardiovasc Drugs. 2016 doi: 10.1007/s40256-016-0161-8. [DOI] [PubMed] [Google Scholar]
- 4.D'Ancona G, Safak E, Ince H. Left atrial appendage occlusion in patients with atrial fibrillation and high risk of fall: a clinical dilemma or a budgetary issue? Clin Res Cardiol. 2019;108(12):1406–1407. doi: 10.1007/s00392-019-01476-0. [DOI] [PubMed] [Google Scholar]
- 5.Reddy VY, Mobius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology) J Am Coll Cardiol. 2013;61(25):2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 7.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM, Committee RAS Investigators Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127(2):224–232. doi: 10.1161/circulationaha.112.107128. [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 10.Gupta T, Goel K, Kolte D, Khera S, Villablanca PA, Aronow WS, Bortnick AE, Slovut DP, Taub CC, Kizer JR, Pyo RT, Abbott JD, Fonarow GC, Rihal CS, Garcia MJ, Bhatt DL. Association of chronic kidney disease with in-hospital outcomes of transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10(20):2050–2060. doi: 10.1016/j.jcin.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Hayashida K, Mouillet G, Hovasse T, Chevalier B, Oguri A, Watanabe Y, Dubois-Rande JL, Morice MC, Lefevre T, Teiger E. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;62(10):869–877. doi: 10.1016/j.jacc.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 12.Kefer J, Tzikas A, Freixa X, Shakir S, Gafoor S, Nielsen-Kudsk JE, Berti S, Santoro G, Aminian A, Landmesser U, Nietlispach F, Ibrahim R, Danna PL, Benit E, Budts W, Stammen F, De Potter T, Tichelbacker T, Gloekler S, Kanagaratnam P, Costa M, Cruz-Gonzalez I, Sievert H, Schillinger W, Park JW, Meier B, Omran H. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int J Cardiol. 2016;207:335–340. doi: 10.1016/j.ijcard.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 14.Fastner C, Nienaber CA, Park JW, Brachmann J, Zeymer U, Goedde M, Sievert H, Geist V, Lewalter T, Krapivsky A, Kaunicke M, Maier J, Ozdemir B, Hochadel M, Schneider S, Senges J, Akin I. Impact of left atrial appendage morphology on indication and procedural outcome after interventional occlusion: results from the prospective multicentre German LAARGE registry. EuroIntervention. 2018;14(2):151–157. doi: 10.4244/eij-d-17-00866. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM, Bergmann MW, Investigators E Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–2474. doi: 10.1093/eurheartj/ehv730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedaghat A, Vij V, Streit SR, Schrickel JW, Al-Kassou B, Nelles D, Kleinecke C, Windecker S, Meier B, Valglimigli M, Nietlispach F, Nickenig G, Gloekler S. Incidence, predictors, and relevance of acute kidney injury in patients undergoing left atrial appendage closure with Amplatzer occluders: a multicentre observational study. Clin Res Cardiol. 2019 doi: 10.1007/s00392-019-01524-9. [DOI] [PubMed] [Google Scholar]
- 18.Pistolesi V, Regolisti G, Morabito S, Gandolfini I, Corrado S, Piotti G, Fiaccadori E. Contrast medium induced acute kidney injury: a narrative review. J Nephrol. 2018;31(6):797–812. doi: 10.1007/s40620-018-0498-y. [DOI] [PubMed] [Google Scholar]
- 19.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet (Lond, Engl) 2009;374(9689):534–542. doi: 10.1016/s0140-6736(09)61343-x. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DR, Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Georg Haeusler K, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018 doi: 10.1093/europace/euy054. [DOI] [PubMed] [Google Scholar]
- 22.Ando G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol. 2017;231:162–169. doi: 10.1016/j.ijcard.2016.11.303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.