Abstract
Residual amblyopia is seen in 40% of amblyopic patients treated with part-time patching. Amblyopic patients with infantile onset strabismus or anisometropia can develop fusion maldevelopment nystagmus syndrome (FMNS). The purpose of this study was to understand the effects of presence of FMNS and clinical subtype of amblyopia on visual acuity and stereo-acuity improvement in children treated with part-time patching. Forty amblyopic children who had fixation eye movement recordings and at least 12 months of follow-up after initiating part-time patching were included. We classified amblyopic subjects per the fixational eye movements characteristics into those without any nystagmus, those with FMNS and patients with nystagmus without any structural anomalies that do not meet the criteria of FMNS or idiopathic infantile nystagmus. We also classified the patients per the clinical type of amblyopia. Patching was continued until amblyopia was resolved or no visual acuity improvement was noted at two consecutive visits. Children with anisometropic amblyopia and without FMNS have a faster improvement and plateaued sooner. Regression was only seen in patients with strabismic/mixed amblyopia particularly those with FMNS. Patients with FMNS had improvement in visual acuity but poor stereopsis with part-time patching and required longer duration of treatment.
Subject terms: Medical research, Eye diseases, Ocular motility disorders
Introduction
Amblyopia arises due to the disruption in the correlated activity of the two eyes during the critical periods of vision development1,2. Neurophysiologic studies suggest that the effects of the de-correlated binocular signals on the visual cortex are most significant if they occur at the emergence of stereoacuity in early infancy3–5. Non-human primate model studies have revealed that the loss of horizontal binocular connections within area V1 in infancy results in the development of latent nystagmus6–10, now referred to as Fusion Maldevelopment Nystagmus Syndrome (FMNS)11. FMNS is commonly associated with infantile strabismus, but monocular deprivation and high anisometropia that causes binocular de-correlation in early infancy can produce FMNS7,12.
Patching therapy is commonly employed, but up to 40% of treated children have residual amblyopia, and 25% have regression13,14. Some risk factors associated with residual/recurrent amblyopia include severe amblyopia and older age at the time of diagnosis, strabismic amblyopia, lower (younger) age at the end of treatment, and abrupt cessation, particularly of full-time patching15–19. Other possible causes that could be associated with treatment response include the presence of FMNS and increased fixation instability seen in amblyopia patients20–26. The patching therapy was considered to be contraindicated in patients with FMNS because the intensity (amplitude × frequency) of FMNS increases under monocular viewing conditions27,28. A successive study in a small cohort of patients showed that a significant improvement in visual acuity could be obtained in patients with FMNS with full-time patching during all waking hours29. Simonsz et al. recorded eye movements in five patients with FMNS before and after 2 days of full-time patching of the fellow eye. They found a reduction in slow phase velocity when the amblyopic eye was fixing post occlusion30. Thus, these studies provide evidence that full-time patching therapy can improve visual acuity in FMNS patients. Birch et al. have described an association between abnormal stereo-acuity and patients with FMNS. They found that 67% of all children with abnormal stereo-acuity had FMNS waveform, and nearly all children with nil stereo-acuity had FMNS waveforms26. Bosworth and Birch31 have found that the risk for persistent amblyopia was greater among children with nil stereo-acuity than those with measurable stereoacuity at treatment onset. The current standard of amblyopia treatment comprises of part-time patching ranging from 2 to 6 h/day per the amblyopia severity as per the guidelines from the landmark PEDIG Amblyopia Treatment Studies32–34. Little is known to date about the association between stereo-acuity and part-time patching treatment in FMNS.
In the current manuscript, we measured fixation eye movements at the end of part-time patching treatment and analyzed the rate of improvement of visual acuity and stereoacuity improvement in amblyopia patients with and without FMNS. We hypothesize that the presence of FMNS, would be associated with a slower rate of visual acuity improvement in amblyopic patients treated with patching therapy which requires monocular viewing compared to patients without FMNS. We also hypothesize that the presence of FMNS will be associated with minimal/no change in stereoacuity, as FMNS is a hallmark of binocular maldevelopment in early infancy. We also analyzed the regression of amblyopia in patients after stopping part-time patching.
Results
Clinical type of amblyopia
The study subjects were categorized based on the clinical type (anisometropia: n = 15, strabismic n = 3, mixed n = 22). The age of the start of patching treatment was similar in patients with mixed/strabismic amblyopia compared to anisometropic amblyopia (anisometropic: 75 ± 17 months vs mixed/strabismic: 64 ± 29 months, p = 0.36). Similarly, there was no difference in visual acuity at the time of diagnosis per the clinical types of amblyopia (anisometropic = 0.64 ± 0.42 logMAR, strabismic/mixed = 0.53 ± 0.26 logMAR, p = 0.54), while there was an expected significant difference in stereoacuity (anisometropic = 2.09 ± 0.66 logarcsec vs. strabismic/mixed = 2.82 ± 0.87 logarcsec, p = 0.002). Eight patients required strabismus surgery (Table 1). There was no difference in age (surgery vs no surgery: 54 ± 33 months vs 66 ± 21 months, Mann Whitney U test p = 0.47), visual acuity (surgery vs no surgery: 0.60 ± 0.30 log MARvs 0.58 ± 0.34 logMAR, Mann Whitney U Test p = 0.48) and stereoacuity (surgery vs no surgery: 3.05 ± 0.85 logarcsec vs 2.45 ± 0.85 logarcsec, p = 0.693) at the time of diagnosis between patients with strabismus requiring surgery versus those that did not require surgery.
Table 1.
Demographics, ophthalmic exam and strabismus surgery data of the enrolled subjects.
| ID | Gender | Age at patching (duration) (months) | Category at time of patching | Visual Acuity at time of patching (LogMAR) | Stereoacuity at time of patching (arc second) | Eye movement characteristics | Refraction (RE; LE) (diopters) | Strabismus (near; distance) (prism diopters) | Surgery and age (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 27 (30) | Strabismic | 1.4 | Nil | None | + 6.5 | ET 30 | BMR |
| Severe | 0.3 | + 6.25 | ET 30 | REC | |||||
| Age 3 | |||||||||
| 2 | M | 69 (26) | Mixed | 0.5 | 200 | None | + 5 | ET 35 | R&R RE |
| Moderate | 0 | + 1 | ET 35 | Age 6 | |||||
| 3 | F | 82 (18) | Mixed | 0.4 | 100 | None | + 3.0 + 1.25 × 65 | ET 12 | |
| Moderate | 0.1 | + 1.25 + 0.25 × 115 | E(T) 4–6 | ||||||
| 4 | M | 44 (48) | Mixed | 0.2 | 140 | None | + 2.5 | Ortho with glasses | |
| Moderate | 0.5 | + 4.5 | |||||||
| 5 | M | 84 (9) | Mixed | 0 | 100 | None | Plano + 0.50 × 95 | XT 20 | |
| Moderate | 0.4 | − 0.75 + 3.5 × 85 | XT 30 | ||||||
| 6 | M | 46 (75) | Mixed | 0 | 140 | None | + 6.5 + 2.00 × 70 | Ortho with glasses | |
| Severe | 0.8 | + 0.5 + 0.5 × 90 | |||||||
| 7 | F | 41 (22) | Mixed | 0.1 | 50 | None | + 5.5 + 1.00 × 100 | Ortho with glasses | |
| Moderate | 0.3 | + 6.5 + 1.0 × 80 | |||||||
| 8 | F | 77 (21) | Mixed | 0 | Nil | None | + 4.50 + 2.00 × 90 | E(T) 8 | |
| Moderate | 0.3 | + 5.5 + 2.25 × 90 | E(T) 10 | ||||||
| 9 | M | 79 (6) | Anisometropic | 0 | Nil | None | Plano + 0.75 × 95 | Ortho | |
| Moderate | 0.5 | + 4.25 + 2.00 × 90 | |||||||
| 10 | F | 66 (14) | Anisometropic | 0.5 | 80 | None | + 5 + 0.50 × 100 | Ortho | |
| Moderate | 0.1 | + 3 + 0.50 × 80 | |||||||
| 11 | F | 60 (19) | Anisometropic | 0.7 | 50 | None | + 7.5 | Ortho | |
| Severe | 0.1 | + 5.0 + 0.50 × 180 | |||||||
| 12 | M | 53 (15) | Anisometropic | 0.55 | 40 | None | + 4 + 0.50 × 105 | Ortho | |
| Moderate | 0.2 | + 0.5 + 0.5 × 85 | |||||||
| 13 | F | 117 (6) | Anisometropic | 0 | 40 | None | − 0.25 + 0.5 × 90 | Ortho | |
| Mild | 0.2 | Plano + 2 × 85 | |||||||
| 14 | F | 81 (6) | Anisometropic | 0.4 | 60 | None | − 2.75 + 4.25 × 95 | Ortho | |
| Moderate | 0 | + 1.5 | |||||||
| 15 | F | 53 (21) | Anisometropic | 0.1 | 60 | None | + 0.5 + 1.00 × 90 | Ortho | |
| Moderate | 0.6 | + 3.5 + 1.00 × 90 | |||||||
| 16 | F | 63 (6) | Anisometropic | 0.4 | 40 | None | + 4.25 + 1.0 × 95 | Ortho | |
| Moderate | 0 | + 1.75 + 0.25 × 80 | |||||||
| 17 | M | 90 (30) | Anisometropic | 0.2 | 50 | None | Plano + 0.50 × 85 | Ortho | |
| Moderate | 0.5 | + 5.25 + 2.00 × 105 | |||||||
| 18 | M | 90 (22) | Anisometropic | 1.2 | 140 | None | + 7.00 + 0.50 × 60 | Ortho | |
| Severe | 0 | + 1.00 + 0.25 × 50 | |||||||
| 19 | M | 39 (21) | Strabismic | 0 | 140 | Nystagmus No | + 2.75 + 0.50 × 180 | ET 35 | BMR |
| Moderate | 0.3 | FMNS | + 2.75 + 0.50 × 180 | ET 35 | REC | ||||
| Age 3 | |||||||||
| 20 | F | 83 (17) | Mixed | 0.2 | 100 | Nystagmus No | − 1.75 + 3 × 85 | Ortho with glasses | |
| Moderate | 0.6 | FMNS | − 10.00 + 3.75 × 85 | ||||||
| 21 | F | 66 (11) | Mixed | 0.3 | Nil | Nystagmus No | + 2.25 + 0.75 × 80 | Ortho with glasses | |
| Severe | 0.7 | FMNS | + 3.5 + 0.5 × 135 | ||||||
| 22 | M | 63 (27) | Mixed | 0.2 | Nil | Nystagmus No | + 1.25 + 0.75 × 110 | Ortho with glasses | |
| Mild | 0 | FMNS | + 0.25 + 2.0 × 80 | ||||||
| 23 | F | 95 (28) | Mixed | 0.4 | 200 | Nystagmus No | − 11.5 + 0.75 × 75 | XT 14 | RLR |
| Moderate | 0.1 | FMNS | − 6.5 + 1.0 × 105 | XT 25 | REC | ||||
| Age 8 | |||||||||
| 24 | M | 102 (28) | Mixed | 0.1 | 100 | Nystagmus No | + 6 + 2.0 × 90 | Ortho with glasses | |
| Mild | 0.2 | FMNS | + 7 + 1.75 × 90 | ||||||
| 25 | M | 33 (31) | Mixed | 0.2 | Nil | Nystagmus No | + 1.50 | LE(T) 8 | |
| Severe | 0.7 | FMNS | + 4 | LE(T) 10 | |||||
| 26 | F | 84 (20) | Mixed | 0.1 | 200 | Nystagmus No | − 0.75 + 0.5 × 75 | XT 20 | RLR |
| Moderate | 0.4 | FMNS | + 1.5 + 1.00 × 90 | XT 25 | REC | ||||
| Age 8 | |||||||||
| 27 | M | 85 (6) | Mixed | 0 | 80 | Nystagmus no | + 1.25 + 1.5 × 100 | Flick X(T) | |
| Moderate | 0.6 | FMNS | + 4.00 + 2.00 × 70 | 6 LX(T) | |||||
| 28 | M | 63 (34) | Anisometropic | 0.1 | 140 | Nystagmus No | + 0.25 + 0.5 × 90 | Ortho | |
| Severe | 1.9 | FMNS | − 10.75 + 2.0 × 50 | ||||||
| 29 | M | 80 (39) | Anisometropic | 0.2 | 100 | Nystagmus No | + 7.25 + 1.5 × 90 | Ortho | |
| Moderate | 0.4 | FMNS | + 8.25 + 1.5 × 100 | ||||||
| 30 | M | 75 (31) | Anisometropic | 0.8 | 200 | Nystagmus No | + 6.75 + 3 × 90 | Ortho | |
| Severe | 0 | FMNS | + 0.5 | ||||||
| 31 | F | 71 (16) | Anisometropic | 0.4 | 100 | Nystagmus No | + 4 + 1.25 × 85 | Ortho | |
| Moderate | 0 | FMNS | + 1.5 + 0.5 × 85 | ||||||
| 32 | F | 81 (25) | Anisometropic | 0 | 80 | Nystagmus No | + 1.00 + 0.5 × 90 | Ortho | |
| Moderate | 0.5 | FMNS | + 3.75 | ||||||
| 33 | F | 14 (45) | Strabismic | 0.7 | Nil | FMNS | + 3.50 + 1.75 × 90 | ET 45 | BMR |
| Severe | 0.2 | + 3.50 + 1.75 × 90 | ET 45 | REC | |||||
| Age 1 | |||||||||
| 34 | M | 72 (20) | Mixed | 0.2 | Nil | FMNS | + 5.00 + 0.50 × 90 | Ortho with glasses | |
| Severe | 0.7 | + 6.25 + 1.00 × 95 | |||||||
| 35 | M | 80 (6) | Mixed | 0.55 | Nil | FMNS | − 9.5 + 2.5 × 165 | ET 4 | |
| Moderate | 0 | plano + 0.75 × 45 | ET 4 | ||||||
| 36 | F | 67 (38) | Mixed | 0 | Nil | FMNS | + 5 + 1.5 × 80 | Ortho with glasses | |
| Moderate | 0.6 | + 6 + 1.5 × 95 | |||||||
| 37 | M | 83 (25) | Mixed | 0.4 | Nil | FMNS | − 6.75 + 3.75 × 90 | XT 25 | BLR |
| Severe | 0.8 | − 9.0 + 3.75 × 90 | XT 45 | REC | |||||
| Age 8 | |||||||||
| 38 | M | 18 (54) | Mixed | 0.3 | Nil | FMNS | + 4.5 | E(T) 30 | BMR |
| Severe | 0.2 | + 3.5 | ET 25 | REC | |||||
| Age 3 | |||||||||
| 39 | F | 60 (38) | Mixed | 0.3 | 400 | FMNS | + 4 | XT 20 | |
| Moderate | 0.1 | + 2.25 | XT 20 | ||||||
| 40 | M | 30 (79) | Mixed | 0.5 | 200 | FMNS | + 8.00 + 1.5 × 90 | ET 6–8 | |
| Moderate | 0.3 | + 7.25 + 0.5 × 90 | ET 4 |
BLR bilateral lateral recti muscles, BMR bilateral medial recti muscles, CC with correction, ET esotropia, E(T) intermittent esotropia, F female, FMNS fusion maldevelopment nystagmus syndrome, LE left eye, M male, RE right eye, REC recession, R&R recession and resection, RLR right lateral rectus recession, XT exotropia, X(T) intermittent exotropia.
Eye movement characteristics
The fixational eye movement traces obtained at the end of treatment were evaluated, and amblyopic patients were classified based on the presence or absence of nystagmus (Fig. 1)35. Patients without nystagmus (no nystagmus—Fig. 1A) exhibited alternating fixational saccades with inter-saccadic drifts, similar to healthy subjects23,36,37. Patients with nystagmus were further classified into those with FMNS (Fig. 1B) versus those that did not meet the criteria of FMNS (Fig. 1C). The presence of FMNS was determined based on the classic reversal in the quick phase of nystagmus with linear/decreasing velocity nasally directed slow phase observed during monocular viewing conditions28. Patients with nystagmus/nystagmus like movements who did not exhibit the classic reversal in the direction of quick phases were characterized as Nystagmus without FMNS (Nyst no FMN). These patients had jerk nystagmus with dynamic overshoots of quick phases and differed from Infantile Nystagmus Syndrome patients in that their velocity was decreasing or linear, unlike the increasing eye velocity characteristics seen in patients with Infantile Nystagmus Syndrome. Also, patients with nystagmus but no FMNS did not have the Dissociated Vertical Deviation frequently seen in FMNS patients. The fixational eye movements were evaluated, and amblyopic patients were classified into those with no nystagmus (n = 18), those with FMNS (n = 8), and those with nystagmus but without the classic reversal in the quick phase of nystagmus seen in FMNS (n = 14). There was no difference in the age of the start of patching per the fixational eye movement characteristics (No nystagmus = 68 ± 22 months, Nystagmus no FMNS = 73 ± 19 months, FMNS = 60 ± 41 months, p = 0.38). Per the fixation eye movement characteristics, there was no difference in visual acuity at the time of diagnosis (None = 0.52 ± 0.23 logMAR, Nystagmus no FMNS = 0.62 ± 0.43 logMAR and FMNS = 0.50 ± 0.16 logMAR, p = 0.9), while there was a significant difference in stereoacuity (None = 2.05 ± 0.56 logarcsec, Nystagmus no FMNS = 2.56 ± 0.84 logarcsec and FMNS = 3.49 ± 0.65 logarcsec, p = 0.001).
Figure 1.
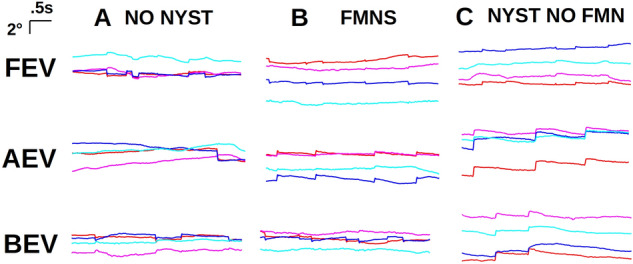
Eye movement records. Eye positions vs time for patients without nystagmus (A), FMNS (B), and with nystagmus but not FMNS (C), during fellow eye viewing (top row), amblyopic eye viewing (middle row), and both eyes viewing (bottom row). Waveforms are plotted with a common scale indicated at upper left. Red: right horizontal, blue: left horizontal, magenta: right vertical, cyan: left vertical. The positive vertical axis corresponds to rightward and upward eye movements. Note the reversal in direction between FEV and AEV in FMNS patients that is not present in nystagmus without FMN patient. Note also the greater intensity during AEV and the absence of acceleration during slow phases in nystagmus patients both with and without FMNS.
Improvement and type of amblyopia
Figure 2A,B plot the average and stdev of visual acuity and stereo-acuity in the anisometropic and mixed/strabismic groups at baseline and within the first year after initiating patching treatment. To determine statistical significance per different groups, we employed mixed-effect model with random intercepts. We found that the visual acuity change within the first year of patching treatment was found to be significantly different between the anisometropic versus strabismic/mixed amblyopia type (mixed effect model: F = 5.9, p = 0.016). Both groups had improvement in visual acuity (indicated by negative values). The average beta coefficient of visual acuity change ((log MAR visual acuity/3 months of patching treatment) was greater in patients with anisometropia (− 0.025 ± 0.01) compared to the strabismic/mixed amblyopia group (− 0.012 ± 0.02). We also found that the change of stereo-acuity within the first year of patching treatment was significantly different between anisometropic versus strabismic/mixed groups (mixed effect model: F = 8.8, p = 0.005). The average beta coefficient of stereo-acuity change (log arcsec/3 months of patching treatment) was greater in the anisometropic group (− 0.05 ± 0.08) compared to strabismic/mixed amblyopia type (0.003 ± 0.04). A positive value indicates a lack of improvement in the strabismic/mixed group.
Figure 2.
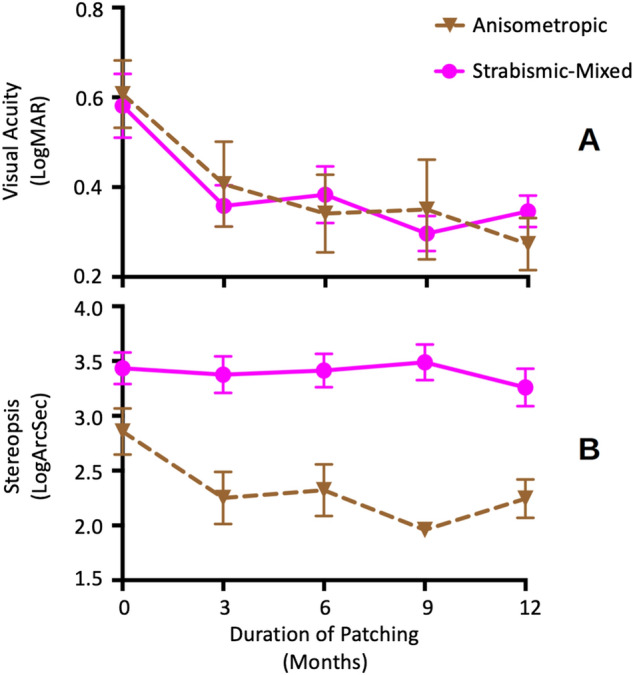
Visual acuity and stereoacuity per type of amblyopia. The mean and standard error of mean of visual acuity (A) and stereoacuity (B) change sub-grouped by the type of amblyopia at baseline and during the first year after initiating patching treatment. The lower scores indicate better visual acuity (log MAR) and stereoacuity (log arc sec). visual acuity and stereoacuity improvement was greater in anisometropic than strabismic/mixed group. Brown triangles—dashed line: anisometropic; magenta circles—full line: strabismic/mixed.
Improvement and fixational eye movements characteristics
Figure 3A,B plots the average and stdev of visual acuity and stereo acuity for each subgroup at baseline and within the first year after initiating patching treatment. To determine statistical significance between different groups, we employed a mixed-effect model with random intercepts. We found that the change of visual acuity within the first year of patching treatment was found to be significantly different between no nystagmus, nystagmus without FMNS, and FMNS groups (mixed effect model: F = 4.3, p = 0.04). All three groups had improvement in visual acuity (indicated by negative values). The average beta coefficient of visual acuity change (log MAR visual acuity/3 months of patching treatment) was greater in patients with no nystagmus (− 0.025 ± 0.01) compared to nystagmus no FMNS (− 0.018 ± 0.02) and FMNS (− 0.01 ± 0.01) groups. We also found that the change of stereoacuity within the first year of patching treatment was found to be significantly different between no nystagmus, nystagmus without FMNS, and FMNS groups (mixed effect model: F = 5.8, p = 0.02). The average beta coefficient of stereoacuity change (log arcsec/3 months of patching treatment) was greater in no nystagmus group (− 0.049 ± 0.09) compared to nystagmus without FMNS (− 0.015 ± 0.03) group with no improvement in the FMNS group (0.02 ± 0.04). A positive value indicates a lack of improvement in the FMNS group.
Figure 3.
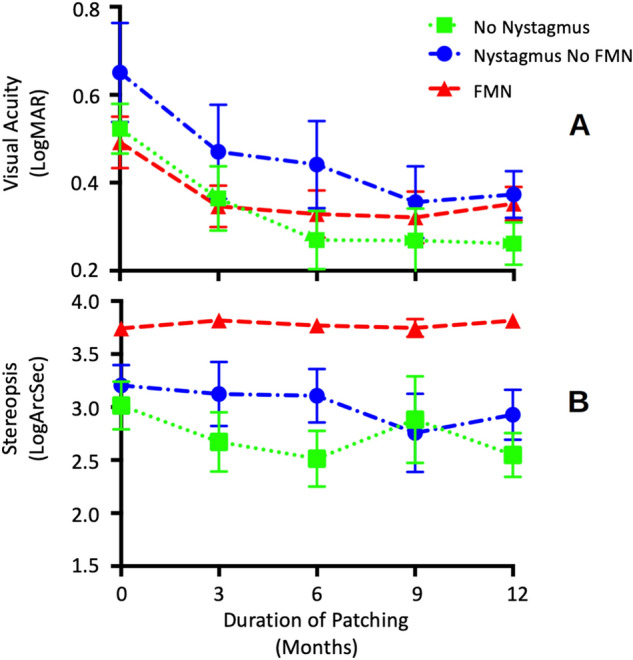
Visual acuity and stereoacuity per fixational eye movements characteristcs. The mean and standard error of mean of visual acuity (A) and stereoacuity (B) change sub-grouped by the fixation eye movement characteristics at baseline and during the first year after initiating patching treatment. The lower scores indicate better visual acuity (log MAR) and stereoacuity (log arc sec). Visual acuity and stereoacuity improvement was greater in patients without nystagmus. No improvement of stereoacuity was recorded in FMNS group. Green squares: no nystagmus; blue circles: nystagmus no FMNS; red triangles: FMNS.
No improvement and regression
In our cohort, 17% of patients had regression with a decrease in visual acuity after stopping patching treatment. All of the patients who experienced regression had strabismic/mixed amblyopia and had received patching treatment for at least 6 months before the treatment was discontinued due to a plateau in visual acuity or the resolution of amblyopia (defined as inter-ocular visual acuity difference of < 2 lines). Regression was noted in 2/18 patients without nystagmus, 1/14 patient with nystagmus without FMNS, and 4/8 patients with FMNS. Of the two patients without nystagmus who experienced regression (Subject 3 and 4), both patients regained their visual acuity on restarting the patching treatment and had mild residual amblyopia with some stereoacuity at the end of treatment. The patients with nystagmus without FMNS (Subject 22) and FMNS (Subjects 33, 38, 39, and 40) experienced regression and regained the visual acuity after restarting treatment but did not have any stereoacuity at the end of treatment.
10% of our cohort had no improvement in visual acuity post patching treatment. These include one subject with no nystagmus (Subjects 2), two with nystagmus without FMNS (Subject 23 and 27), and one with FMNS (Subject 35). Patching was started after age 5 in all four subjects, and all of them had high anisometropia (2 aniso-myopia, 2 with aniso-hyperopia). The two aniso-hyperopic patients opted for atropine penalization treatment.
Visual acuity improvement and treatment plateau
The improvement in visual acuity and stereoacuity was analyzed between groups per the fixation eye movement characteristics and as a function of the clinical type of amblyopia. Patients who did not have any improvement in visual acuity with patching treatment were excluded from this analysis. Similar visual acuity improvement levels were found in patients with and without FMNS (no nystagmus: 0.39 ± 0.28, nystagmus no FMNS: 0.33 ± 0.36, FMNS: 0.29 ± 0.16 LogMAR, F = 0.30, p = 0.74). A similar analysis was performed as a function of the clinical type of amblyopia. The results were comparable between anisometropic versus strabismic/mixed groups (visual acuity improvement: anisometropia: 0.41 ± 0.31 and strabismic/mixed: 0.29 ± 0.26 LogMAR, t-test p = 0.29). Children with no nystagmus plateaued sooner in terms of visual acuity improvement compared to the no nystagmus group (no nystagmus: 9.3 ± 6.5; nystagmus no FMNS: 14.2 ± 8.2; FMNS: 30.2 ± 23.2 months, one way ANOVA, F = 6.4, p = 0.005). Children with anisometropic amblyopia plateaued sooner in terms of visual acuity improvement than strabismic/mixed amblyopia groups (anisometropia: 9.1 ± 6.8 months, strabismic/mixed: 22.1 ± 17 months, unpaired t test p = 0.01).
Stereoacuity improvement and treatment plateau
21/40 patients had improvement in stereoacuity. The majority of patients without nystagmus had improvement in stereoacuity (14/18) compared to nystagmus no FMNS (6/14) and FMNS (1/8). The extent of stereoacuity improvement was analyzed in patients with and without nystagmus—we excluded FMNS patients from this analysis as only 1 FMNS patient had improvement in stereoacuity after 29 months of treatment. Of the patients that had improvement in stereoacuity, there was a trend that no nystagmus had greater improvement (1.3 ± 0.76 log arcsec) compared to nystagmus no FMNS group (0.96 ± 0.57 log arcsec, p = 0.15, Mann–Whitney U test). There was no difference in the time to reach the best possible stereoacuity in patients without nystagmus (26 ± 19 months) versus those with nystagmus without FMNS (20.5 ± 9.5 months, p = 0.5, Mann Whitney U test).
Of the 21 patients with improvement in stereoacuity, 14 had anisometropic amblyopia, and 7 had strabismic/mixed amblyopia. The extent of stereoacuity improvement was similar irrespective of amblyopia type (anisometropia: 1.2 ± 0.82 versus mixed/strabismic: 1.4 ± 0.30 log arcsec, Mann–Whitney U test p = 0.5). Like visual acuity improvement, patients with anisometropic amblyopia (19.7 ± 12.5 months) reached the best possible stereoacuity sooner with treatment than patients with strabismic/mixed amblyopia (34 ± 19.5 months, Mann Whitney test p = 0.02).
Discussion
In this retrospective study, we characterized fixational eye movements at the end of treatment in amblyopic patients treated with part-time patching therapy and evaluated the rate of improvement in visual acuity and stereoacuity. We found that the presence of FMNS was associated with a slower rate of visual acuity improvement and poor recovery of stereopsis. Amblyopic patients with nystagmus without the reversal in the direction of the quick phase, as seen in FMNS, had a similar rate of improvement in visual acuity but less improvement in stereoacuity compared to patients without nystagmus. The velocity waveform of nystagmus differs from that seen in patients with idiopathic infantile nystagmus syndrome. Nystagmus that is not FMNS or INS, has been previously reported in patients with monocular vision loss in early childhood38,39. Amblyopic patients have increased drifts. Thus, reduced visual acuity due to amblyopia and the increased drifts interrupted by corrective saccades could result in the development of nystagmus beats in the absence of FMNS/INS. We found that the rate of visual acuity and stereoacuity improvement was faster in anisometropic amblyopia and plateaued sooner with part-time patching than strabismic/mixed amblyopia patients. Similarly, patients without nystagmus (both anisometropic and strabismic, or mixed, amblyopia) plateaued sooner than those with nystagmus. The patients who experienced regression had strabismic/mixed amblyopia and had received part-time patching treatment for at least 6 months before the treatment was discontinued. The risk of regression was greater in FMNS patients and required longer durations of treatment than amblyopic patients without FMNS.
Effect of patching on visual acuity
A few studies describe the visual acuity improvement as a function of patching duration. Stewart et al. found an average visual acuity improvement of 0.35 logMAR from a cumulative dose of patching for 150–250 h irrespective of the type of amblyopia and a flattening of the dose/response curve after 400 h of treatment40. ATS2A and ATS2B32,33 did not find any differences in the extent of visual acuity improvement at 17 weeks in groups per the clinical type of amblyopia- however, they found that patients with worse initial visual acuity and age at treatment with children < 5 years of age had greater visual acuity improvement. In a large retrospective study of 877 patients recruited per the PEDIG ATS2 A and B inclusion criteria34, the authors found similar levels of visual acuity improvement, as reported in PEDIG studies. However, the treatment duration was longer, probably due to the differences in motivation for compliance and follow-ups between patients included in trials versus in real-world clinical practice. We have previously described the results of visual acuity and stereoacuity measurements obtained at the end of part-time patching therapy per the clinical type of amblyopia and per the presence of FMNS41. We found that anisometropic patients had less severe residual amblyopia at the end of part-time patching treatment. We found that the visual acuity of patients with FMNS improved with part-time patching but required longer treatment duration with poor stereoacuity at the end of the treatment. In the current study, we found although visual acuity improved in patients with and without FMNS within the first year of treatment, but the presence of FMNS was associated with a lower rate of visual acuity improvement. The treatment plateau occurs sooner in patients without nystagmus (average 39 weeks) compared to those with nystagmus without FMNS (60 weeks) and FMNS patients (> 2 years). We computed the treatment duration, which included the time when patching had to resume patching due to regression of the amblyopic eye visual acuity. We also had 4 (10%) children < 3 years of age. These could potentially result in greater treatment durations reported in our study than other studies32–34.
In our cohort, four patients did not have any visual acuity improvement after part-time patching. All these patients had mixed amblyopia with high anisometropia (> 4 diopters) and initiated patching treatment at age 5.5 years or older. These results agree with other studies that have reported high anisometropia and late age at therapy as risk factors for no improvement in visual acuity post patching treatment26,34,42–47.
Effect of patching on stereoacuity
We analyzed the stereoacuity improvement in patients with and without FMNS and per the clinical type of amblyopia. We found that patients without nystagmus and anisometropic amblyopia had better stereoacuity at baseline, and both the visual acuity and stereoacuity improved with treatment. On the other hand, we found that patients with FMNS typically had no stereoacuity improvement despite the reduction of visual acuity deficit in the amblyopic eye with part-time patching treatment. This is in agreement with an observational study by Birch et al., where they reported that none of the amblyopic patients with normal stereoacuity had FMNS, whereas 67% of children with abnormal stereoacuity had FMNS. In contrast, all the children with nil stereoacuity had FMNS waveforms26. Also, in patients without FMNS, the stereoacuity improvement rate was slower, and the plateau time was higher than that of visual acuity improvement. This is likely due to the delayed development of fine stereoacuity, which is thought to still be immature at 5 years of age and with adult levels reaching between 6 and 9 years of age48–51.
Regression
In our cohort, we found a regression risk of 17%. A few other studies have reported similar regression rates17,18. The PEDIG study 2004 has reported regression of 24% following patching therapy, with 6% of patients patching for more than 8 h/day52. Another PEDIG study reported regression of 7% within the first year of treatment cessation in older children between the ages of 7–12 years53. Other studies have found a regression rate of 24–27% in children after full-time occlusion therapy with a gradual taper19 versus an abrupt taper54. Studies have found that the risk of regression inversely correlates with the patient’s age at termination of treatment19. Other factors reported to be associated with regression are better visual acuity at the time of cessation of patching, greater visual acuity improvement during treatment, or previous regression55,56. In our cohort, one of our patients had experienced previous regression. All the patients except one who experienced regression were < 6 years of age. Overall we did not see a systematic trend between the risks of regression versus the level of visual acuity improvement. The differences between regression rates between our and other studies could be due to the varying ages of children in our cohort and the strictly part-time patching employed in our study. In our cohort, we found a significantly higher proportion of regression in patients with FMNS (50%) than in other groups (10%), with regression occurring only in those with strabismic/mixed form of amblyopia.
Increased risk of recurrence has been reported previously in patients with mixed amblyopia15,17. Nilsson et al. have reported the presence of microstrabismus alone as a risk factor of recurrence18. Holmes et al. and Rutstein and Fuhr found that excellent stereoacuity does not preclude the recurrence of amblyopia55,57. On the other hand, Bosworth and Birch reported the risk for persistent amblyopia was 2.2 times greater among children with nil stereoacuity31. Birch et al. have also described a higher rate of persistent amblyopia in patients affected by infantile esotropia (up to 60%) than accommodative esotropia26. In our cohort, we found that patients with strabismic or mixed amblyopia and FMNS both had greater chances to develop regression, whereas patients with strabismus without FMNS had similar levels of visual acuity and stereoacuity improvement as anisometropic amblyopes with a lower risk of regression.
Animal model studies have shown that disruption of binocularity during infancy is invariably associated with gaze instabilities, most often FMNS6,58. Tychsen and colleagues have shown in experiments that the prevalence and severity of FMNS increases with the longer duration of binocular decorrelation with 100% prevalence of FMNS in primates who are exposed to periods of binocular decorrelation that is equivalent to 3 months in humans. Tychsen has proposed that the binocular maldevelopment of the striate cortex is passed on to downstream extrastriate regions, namely the medial superior temporal area that drive conjugate gaze. The disruption results in a nasalward bias that is pathognomic of FMNS. Thus, animal model studies suggest that the development of FMNS is strongly associated with abnormal visual experience in infancy and can be used as a surrogate marker of the presence of amblyogenic risk factors/strabismus in the first year of life59.
Amblyopic patients both with and without nystagmus have fixation eye movement abnormalities compared to controls23,37,60. We have found that patients without nystagmus have a reduced frequency of physiologic microsaccades in the amblyopic eye compared to the fellow eye with increased inter-saccadic drifts in both the fellow and amblyopic eye. Patients with nystagmus with and without FMNS have increased slow phase velocities compared to inter-saccadic drift velocities in patients without nystagmus. We have also found that the slow phase velocities of FMNS patients are greater compared to patients with nystagmus without FMNS60. In the current paper, the analysis shows that FMNS is associated with a slower visual acuity rate of improvement, poor stereoacuity recovery, and higher regression rate with part-time patching treatment. We also found that patients with nystagmus but not FMNS tended to respond better to patching treatment than those with FMNS, even though eye movement abnormalities are still present. Thus, we speculate that patients with FMNS are likely to have early onset of amblyopia than those without FMNS, resulting in differences between the treatment outcomes in this cohort. The findings from our paper highlight the utility of fixational eye movement recordings in amblyopic patients in order to advance our understanding and field of knowledge of residual/recurrent amblyopia and to improve amblyopia therapy for specific types of amblyopia.
The study's main limitations are that the eye movement recordings were obtained at the end of treatment and that the treatment effect was determined based on a retrospective chart review. To reduce the impact of inaccurate data and individual testing biases, we excluded patients with incomplete data or that were not interpreted the same by at least two independent reviews. In our experience, these patients were noncompliant with treatment and did not follow up as frequently in our office per the recommendations. When evaluating the efficacy of patching treatment, it is essential to consider the effects of compliance. While we could not measure objective compliance, we did extract data obtained from clinical history to determine subjective compliance and included patients thought to be at least 50% compliant. Since the study is a longitudinal follow-up over a period of years, the visual acuity testing method differs depending on the techniques judged appropriate for the child's maturity. Regression was determined by two separate measurements with the same testing method to reduce the bias. The study reflects real-world scenario mimicking as encountered in clinical practice.
In summary, we examined the association between the presence of FMNS (confirmed on eye movement recordings) and the rate of improvement of visual acuity and stereoacuity and regression in amblyopia patients treated with part-time patching. We found that patients without nystagmus have a faster improvement of visual acuity and stereoacuity and plateaued sooner to reach their best possible visual acuity. FMNS is seen in patients with strabismic/mixed amblyopia, and the presence of FMNS was associated with a slower rate of improvement in visual acuity with poor/absent recovery of stereoacuity and a higher risk of regression. Thus, these results collectively highlight the link between the lack of binocular function and recurrent amblyopia. The current study's data suggest that eye movement characterization and quantification can play an essential role in amblyopia management. Children with FMNS and amblyopia should be observed closely with long-term follow-up and with a careful taper of the patching treatment. Future prospective studies, that measure FEMs at the time of treatment initiation will allow us to directly probe and understand the association of severity of visual acuity and stereoacuity deficits at the time of diagnosis and effects of different amblyopia treatments in patients with FMNS and other FEM abnormalities.
Methods
Study participants
Eye movement recordings were obtained in 80 amblyopic patients without any structural anomalies of the eye or neurologic disorders. The Cleveland Clinic Institutional Review Board approved the protocol and written informed consent was obtained from each participant or parent/legal guardian in accordance with the Declaration of Helsinki. The clinical parameters were extracted from a retrospective chart review for all the enrolled subjects. After review, we recruited 40 patients who had at least 12 months of follow up after initiating patching treatment and three sets of measurements, first at baseline, the second measurements between 3 and 6 months and third measurement between 9 and 12 months after initiating treatment, were included. Patients deemed to be at least 50% compliant were included in the study.
We categorized them based on the clinical type of amblyopia61 and on the fixational eye movements waveform characteristics (Table 1). Patients with manifest strabismus were treated according to the American Academy of Ophthalmology Preferred Practice Pattern62.
Eye movement recording and analysis
A high-resolution video-based eye tracker (EyeLink 1000®, SR Research, Ontario, Canada) was used to measure binocular horizontal and vertical eye positions during binocular, fellow and amblyopic eye viewing conditions. All eye movement recordings were obtained at the end of patching treatment. An infrared permissive filter that blocked the visible light but allowed eye movement measurements of the non-viewing eye was used. Monocular calibration and validation were performed per the manufacturer’s guidelines. The subjects fixated their gaze on a circular target projected on the LCD screen on a white background (luminance 144 cd/m2) in a completely dark room for 45 s. The eye position data was analyzed after removal of blinks. The eye position signal was differentiated using MatlabTM (Mathworks, Natick, MA, USA) differential function and was further smoothened with the Savitzkey–Golay filter to measure eye velocity22,35. Fixational saccades and quick phases of nystagmus were identified using an unsupervised clustering method35. Drifts and slow phases were defined as epochs between fixational saccades and quick phases, respectively.
Measurement of visual acuity, stereoacuity and strabismus
The clinical parameters were extracted from a retrospective chart review. The ages at the start of treatment and at follow up visits, visual acuity of the fellow and amblyopic eye and stereoacuity, cycloplegic refraction, strabismus angle measurements, and treatment compliance was noted. Visual acuity was measured in each eye monocularly, starting from the right eye, using the participant’s optimal spectacle correction with Snellen linear optotype. For patients younger than 6 years of age, per the child’s ability to perform the test, crowding bars HOTV optotypes were preferred and used over picture optotypes (Allen optotypes with crowding bars presented with commercially available computer-based system Accomodata Stimuli™). Visual acuity was measured at 20 feet distance, and the value was considered only if the patient could read all the letters (or symbols) of the line. Stereoacuity was measured with the Titmus Stereo Test at 40 cm. For analyses, visual acuity scores were converted into logMAR values, and stereoacuity scores in seconds of arc were converted to log arcsec values. For the purpose of analysis, subjects with no detectable (nil) stereoacuity were assigned a value of 7000″. There were only four patients that were diagnosed before their ability to perform any optotype and stereo-testing—they all had manifest strabismus with strong fixation preferencee (Table 1, patients n. 1, 25, 33, 38).
These four patients were all assigned as having severe amblyopia with absent stereoacuity. The strabismus was assessed in the primary position at distance and near measured by alternate and simultaneous prism cover tests and Hirschberg and Krimsky tests in younger patients. The clinical categorization of amblyopia subtype and severity at the time of diagnosis was based on PEDIG studies32,33,47,63.
Amblyopia treatment and measurement
The treatment comprised of part-time occlusion (2–6 h/day), prescribed per the severity of amblyopia32,64. Strabismic patients were diagnosed before other groups (anisometropic vs strabismic vs mixed: 71.7 ± 15.9 vs 23.7 ± 13.4 vs 59.7 ± 15.1 months, Kruskal–Wallis Test p = 0.016), while no differences in presentation time to start of patching treatment were observed grouped per fixational eye movement characteristics (no nystagmus vs nystagmus no FMNS vs FMNS: 68.2 ± 21.1 vs 62 ± 23.9 vs 49.8 ± 28.1 months, Kruskal–Wallis Test p = 0.346). Investigators judged patching compliance to be good (> 50%), fair (26–50%), or poor (≤ 25%), based on discussions with the parents documented in the chart comparing the number of hours prescribed and the ones declared by the parents including the number of daily and weekly hours of patching treatment65.
Visual acuity and stereoacuity from the start to the end of treatment were computed as a function of the clinical type and fixation eye movements characteristics. We also calculate the rate of visual acuity and stereoacuity change within the first year. The rate of improvement was analyzed as a function of the clinical type of amblyopia and fixational eye movements characteristics. Patients who did not have any improvement in visual acuity on two consecutive visits were not included in this analysis as they were considered to be non-responsive to patching treatment. These patients opted for either atropine penalization or stopped the treatment. The patching treatment was continued beyond the first year per the clinical management. Patching was discontinued if the visual acuity had stabilized with no further improvement or deterioration ≥ 2 consecutive visits ≥ 6 weeks apart in patients with at least 50% compliance. Patients who were patching 6 h/day were gradually weaned of treatment as the visual acuity improved52. After the patching treatment was discontinued, the visual acuity and stereoacuity measurements obtained at 3 months interval were recorded to detect regression. Regression was defined as a drop in visual acuity by 2 lines as obtained by two separate measurements (on the same or different day) from the previous visit, and treatment was restarted in these patients52. The duration of patching treatment (treatment plateau in months) required to reach the best possible visual acuity and stereoacuity with no further improvement or regression was analyzed as a function of clinical subtype and fixational eye movements characteristics.
Statistical analysis
All analyses were performed in SPSS and GraphPad Prism 7 (La Jolla, CA, USA). A t test, Kruskal–Wallis Test, Mann Whitney U Test and one-way ANOVA were used to compare the demographics and baseline characteristics amongst the groups.
Mixed-effects regression models with random intercepts to test the hypothesis of a difference in the rate of change in the visual acuity and stereoacuity between patients per fixational eye movements characteristics was used. The hypothesis was assessed by comparing the slope of change in the LogMAR visual acuity over the 12-month period per the fixational eye movement characteristics with a negative slope reflecting visual acuity improvement. The slope of change in the log arc seconds stereoacuity over the 12-month period per the fixational eye movement characteristics was compared with a negative slope reflecting stereoacuity improvement. We also performed linear regression separately for each patient (visual function versus treatment duration) and extracted the beta coefficient (degree of visual function change for every 3 months of patching treatment). We also report the average beta coefficient values for a given subgroup. A similar analysis per the different clinical types of amblyopia: anisometropic and strabismic/mixed was done (patients with strabismus and mixed amblyopia were pooled together as there were few strabismic patients n = 3). One-way ANOVA was used to compare the total improvement of visual acuity and stereoacuity and treatment duration grouped per the fixational eye movement characteristics. A t test was used to analyze these parameters as a function of the clinical type of amblyopia (strabismic/mixed versus anisometropic patients).
Acknowledgements
Supported by Grants from Blind Children’s Center, RPB Unrestricted Grant CCLCM-CWRU, CTSC Pilot Grant Program, Research to Prevent Blindness Walt and Lilly Disney Award for Amblyopia Research and Cleveland Clinic RPC Grant (FG) and Departmental NEI T32 Grant (JM).
Author contributions
M.S. has made substantial contributions in the acquisition, analysis, and interpretation of data, has drafted the work, has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. J.M. has made substantial contributions in the acquisition, analysis, and interpretation of data, has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. P.J. has revised the article, has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. A.G.S. has made substantial contributions to the conception or design of the work, in the creation of new software used in the work, has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. F.F.G. has made substantial contributions to the conception or design of the work, in the acquisition, analysis, or interpretation of data, in the creation of new software used in the work, has drafted the work, has approved the submitted version and has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.von Noorden G, Campos E. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. Maryland Heights: C.V. Mosby Co; 2002. [Google Scholar]
- 2.Wong AMF. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can. J. Ophthalmol. 2012;47:399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Löwel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science (80–) 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 4.Löwel, S. & Engelmann, R. Neuroanatomical and neurophysiological consequences of strabismus: Changes in the structural and functional organization of the primary visual cortex in cats with alternating fixation and strabismic amblyopia. In Strabismus vol. 10 95–105 (Strabismus, 2002). [DOI] [PubMed]
- 5.Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J. Neurosci. 2001;21:3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tychsen L. Advances in Translational Neuroscience of Eye Movement Disorders. Berlin: Springer; 2019. Fusion maldevelopment (Latent) nystagmus: How insights from nonhuman primate experiments have benefitted clinical practice; pp. 255–270. [Google Scholar]
- 7.Tychsen L, et al. The neural mechanism for Latent (fusion maldevelopment) nystagmus. J. Neuroophthalmol. 2010;30:276–283. doi: 10.1097/WNO.0b013e3181dfa9ca. [DOI] [PubMed] [Google Scholar]
- 8.Tychsen L. Causing and curing infantile esotropia in primates: The role of decorrelated binocular input (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2007;105:564–593. [PMC free article] [PubMed] [Google Scholar]
- 9.Tychsen L. Adler’s Physiology of the Eye; Clinical Application. Maryland Heights: Mosby; 1992. Binocular vision. [Google Scholar]
- 10.Hasany A, Wong A, Foeller P, Bradley D, Tychsen L. Duration of binocular decorrelation in infancy predicts the severity of nasotemporal pursuit asymmetries in strabismic macaque monkeys. Neuroscience. 2008;156:403–411. doi: 10.1016/j.neuroscience.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee, N. E. I./N. I. H. A Classification of Eye Movement Abnormalities and Strabismus (CEMAS). Report of a National Eye Institute Sponsored Workshop. (2001).
- 12.Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann. N. Y. Acad. Sci. 2002;956:346–360. doi: 10.1111/j.1749-6632.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 13.Repka MX, et al. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch. Ophthalmol. 2005;123:149–157. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Holmes JM, et al. Risk of amblyopia recurrence after cessation of treatment. J. AAPOS. 2004;8:420–428. doi: 10.1016/S1091-8531(04)00161-2. [DOI] [PubMed] [Google Scholar]
- 15.Levartovsky S, Oliver M, Gottesman N, Shimshoni M. Factors affecting long term results of successfully treated amblyopia: Initial visual acuity and type of amblyopia. Br. J. Ophthalmol. 1995;79:225–228. doi: 10.1136/bjo.79.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace DK, et al. Time course and predictors of amblyopia improvement with 2 hours of daily patching. JAMA Ophthalmol. 2015;133:606–609. doi: 10.1001/jamaophthalmol.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacagni DJ, Stewart CE, Moseley MJ, Fielder AR. Factors affecting the stability of visual function following cessation of occlusion therapy for amblyopia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007;245:811–816. doi: 10.1007/s00417-006-0395-2. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson J, Baumann M, Sjöstrand J. Strabismus might be a risk factor for amblyopia recurrence. J. AAPOS. 2007;11:240–242. doi: 10.1016/j.jaapos.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 19.Bhola R, Keech RV, Kutschke P, Pfeifer W, Scott WE. Recurrence of amblyopia after occlusion therapy. Ophthalmology. 2006;113:2097–2100. doi: 10.1016/j.ophtha.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 20.González EG, Wong AMF, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest. Ophthalmol. Vis. Sci. 2012;53:5386–5394. doi: 10.1167/iovs.12-9941. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Invest. Ophthalmol. Vis. Sci. 2013;54:1998–2003. doi: 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaikh AG, Otero-Millan J, Kumar P, Ghasia FF. Abnormal fixational eye movements in amblyopia. PLoS One. 2016;11:20. doi: 10.1371/journal.pone.0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, S. L., Beylergil, S. B., Otero-Millan, J., Shaikh, A. G. & Ghasia, F. Fixational eye movement waveforms in amblyopia: Characteristics of fast and slow eye movements. J. Eye Mov. Res. (2019) (in press). [DOI] [PMC free article] [PubMed]
- 24.Chen D, Otero-Millan J, Kumar P, Shaikh AG, Ghasia FF. Visual search in amblyopia: Abnormal fixational eye movements and suboptimal sampling strategies. Invest. Ophthalmol. Vis. Sci. 2018;59:4506–4517. doi: 10.1167/iovs.18-24794. [DOI] [PubMed] [Google Scholar]
- 25.Birch EE, Subramanian V, Weakley DR. Fixation instability in anisometropic children with reduced stereopsis. J. AAPOS. 2013;17:287–290. doi: 10.1016/j.jaapos.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birch EE. Amblyopia and binocular vision. Prog. Retin. Eye Res. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duke-Elder S, Wybar KC. System of Ophthalmology. Maryland Heights: Mosby CV; 1973. Ocular motility and strabismus; p. 824. [Google Scholar]
- 28.Abadi RV, Scallan CJ. Waveform characteristics of manifest latent nystagmus. Invest. Ophthalmol. Vis. Sci. 2000;41:3805–3817. [PubMed] [Google Scholar]
- 29.von Noorden GK, Avilla C, Sidikaro Y, LaRoche R. Latent nystagmus and strabismic amblyopia. Am. J. Ophthalmol. 1987;103:87–89. doi: 10.1016/S0002-9394(14)74174-1. [DOI] [PubMed] [Google Scholar]
- 30.Simonsz HJ. The effect of prolonged monocular occlusion on latent nystagmus in the treatment of amblyopia. Doc. Ophthalmol. 1989;72:375–384. doi: 10.1007/BF00153506. [DOI] [PubMed] [Google Scholar]
- 31.Bosworth RG, Birch EE. Binocular function and optoype-grating acuity discrepancies in amblyopic children. Invest. Ophthalmol. Vis. Sci. 2003;44:e3183. [Google Scholar]
- 32.Holmes JM, et al. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Repka MX, et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch. Ophthalmol. 2003;121:603. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 34.Buckle M, Billington C, Shah P, Ferris JD. Treatment outcomes for amblyopia using PEDIG amblyopia protocols: A retrospective study of 877 cases. J. AAPOS. 2019;23(98):e1–98.e4. doi: 10.1016/j.jaapos.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Otero-Millan J, Castro JLA, Macknik SL, Martinez-Conde S. Unsupervised clustering method to detect microsaccades. J. Vis. 2014;14:18–18. doi: 10.1167/14.2.18. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Conde S. Fixational eye movements in normal and pathological vision. Prog. Brain Res. 2006;154:151–176. doi: 10.1016/S0079-6123(06)54008-7. [DOI] [PubMed] [Google Scholar]
- 37.Shaikh AG, Ghasia FF. Fixational saccades are more disconjugate in adults than in children. PLoS One. 2017;12:20. doi: 10.1371/journal.pone.0175295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider RM, et al. Neurological basis for eye movements of the blind. PLoS One. 2013;8:e56556. doi: 10.1371/journal.pone.0056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felius J, et al. Nystagmus and related fixation instabilities following extraction of unilateral infantile cataract in the Infant Aphakia Treatment Study (IATS) Investig. Ophthalmol. Vis. Sci. 2014;55:5332–5337. doi: 10.1167/iovs.14-14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart CE, Stephens DA, Fielder AR, Moseley MJ. Modeling dose-response in amblyopia: Toward a child-specific treatment plan. Investig. Ophthalmol. Vis. Sci. 2007;48:2589–2594. doi: 10.1167/iovs.05-1243. [DOI] [PubMed] [Google Scholar]
- 41.Scaramuzzi M, et al. Progress in Brain Research. New York: Elsevier; 2019. Fixation instability in amblyopia: Oculomotor disease biomarkers predictive of treatment effectiveness; pp. 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen PL, et al. Anisometropic amblyopia treated with spectacle correction alone: Possible factors predicting success and time to start patching. Am. J. Ophthalmol. 2007;143:54–60. doi: 10.1016/j.ajo.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Holmes JM, et al. Effect of age on response to amblyopia treatment in children. Arch. Ophthalmol. (Chicago, Ill. 1990) 2011;129:1451–1457. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pediatric Eye Disease Investigator Group A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmology. 2003;110:1632–1638. doi: 10.1016/S0161-6420(03)00500-1. [DOI] [PubMed] [Google Scholar]
- 45.Weakley DR. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–171. doi: 10.1016/S0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 46.Weakley DR. Transactions of the American Ophthalmological Society. New York: American Ophthalmological Society; 1999. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus; pp. 987–1021. [PMC free article] [PubMed] [Google Scholar]
- 47.Cotter SA, et al. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giaschi D, Narasimhan S, Solski A, Harrison E, Wilcox LM. On the typical development of stereopsis: Fine and coarse processing. Vis. Res. 2013;89:65–71. doi: 10.1016/j.visres.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vis. Res. 2005;45:827–837. doi: 10.1016/j.visres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Hayward J, Truong G, Partanen M, Giaschi D. Effects of speed, age, and amblyopia on the perception of motion-defined form. Vis. Res. 2011;51:2216–2223. doi: 10.1016/j.visres.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 51.Hadad B-S, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Dev. Sci. 2011;14:1330–1339. doi: 10.1111/j.1467-7687.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- 52.Pediatric Eye Disease Investigator Group Risk of amblyopia recurrence after cessation of treatment. J. AAPOS. 2004;8:420–428. doi: 10.1016/S1091-8531(04)00161-2. [DOI] [PubMed] [Google Scholar]
- 53.Hertle RW, et al. Stability of visual acuity improvement following discontinuation of amblyopia treatment in children aged 7 to 12 years. Arch. Ophthalmol. 2007;125:655–659. doi: 10.1001/archopht.125.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh LA, Hahn EK, LaRoche GR. The method of treatment cessation and recurrence rate of amblyopia. Strabismus. 2009;17:107–116. doi: 10.1080/09273970903126709. [DOI] [PubMed] [Google Scholar]
- 55.Holmes JM, Melia M, Bradfield YS, Cruz OA, Forbes B. Factors associated with recurrence of amblyopia on cessation of patching. Ophthalmology. 2007;114:1427–1432. doi: 10.1016/j.ophtha.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saxena R, et al. Factors predicting recurrence in successfully treated cases of anisometropic amblyopia. Indian J. Ophthalmol. 2013;61:630–633. doi: 10.4103/0301-4738.123144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutstein RP, Fuhr PS. Efficacy and stability of amblyopia therapy. Optom. Vis. Sci. 1992;69:747–754. doi: 10.1097/00006324-199210000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Upadhyaya S, et al. Fixational saccades and their relation to fixation instability in strabismic monkeys. Investig. Ophthalmol. Vis. Sci. 2017;58:5743–5753. doi: 10.1167/iovs.17-22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tychsen L. Can ophthalmologists repair the brain in infantile esotropia? Early surgery, stereopsis, monofixation syndrome, and the legacy of Marshall Parks. J. AAPOS. 2005;9:510–521. doi: 10.1016/j.jaapos.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Scaramuzzi M, et al. Part time patching treatment outcomes in children with amblyopia with and without fusion maldevelopment nystagmus: An eye movement study. PLoS One. 2020;15:e0237346. doi: 10.1371/journal.pone.0237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manh VM, et al. A randomized trial of a binocular ipad game versus part-time patching in children aged 13 to 16 years with amblyopia. Am. J. Ophthalmol. 2018;186:104–115. doi: 10.1016/j.ajo.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Academy of Ophthalmology. Pediatric Ophthalmology/Strabismus Summary Benchmarks. https://www.aao.org/summary-benchmark-detail/pediatric-ophthalmology-strabismus-summary-benchma (2019).
- 63.Holmes JM, et al. A randomized trial of binocular dig rush game treatment for amblyopia in children aged 7 to 12 years. Ophthalmology. 2019;126:456–466. doi: 10.1016/j.ophtha.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Repka MX, et al. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch. Ophthalmol. (Chicago, Ill. 1960) 2005;123:149–157. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- 65.Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch. Ophthalmol. 2002;120:268. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]


