Abstract
Parkinson's disease (PD) is an incapacitating neurodegenerative disease. Patients with PD and their caregivers may have interactive effects on each other’s psychological well-being. This study aimed to assess the dyadic dynamics of resilience, fatigue, and suicidal ideation on the depression severity of PD patients and their caregivers. In total, 175 PD patients and 175 caregivers were recruited at a medical center from August 2018 to May 2020. Structural equation modeling (SEM) was used to examine the actor/partner effects on the psychological well-being of both the PD patients and their caregivers. The most common psychiatric diagnoses of both the PD patients (28.6%) and their caregivers (11.4%) were depressive disorders. The PD patients’ and their caregivers’ fatigue, suicidal ideation, and lack of resilience were significantly associated with the severity of their depression, respectively. Interactive effects existed between psychological well-being of individuals with PD and their caregivers. Clinicians must be aware of, and manage, these contributing factors between PD patients and their caregivers in order to prevent them from worsening each other’s depression.
Subject terms: Diseases, Health care
Introduction
Parkinson’s disease (PD) is primarily a prevalent disease among elderly individuals1. PD is characterized by tremors, bradykinesia, rigidity, and postural instability; and affects approximately 1 million individuals in the US2. Besides motor disability, PD patients also exhibit non-motor symptoms such as anxiety, apathy, cognitive dysfunction, and depression3. The impacts of untreated depression extend far beyond mood symptoms; this includes greater functional disability, faster physical and cognitive deterioration, poorer quality of life, and increased mortality rates4,5. The estimated prevalence of depression among patients with PD varied widely among different studies (from 2.7 to 90%). Around 35% of these patients displayed clinically significant symptoms of depression. It is, however, difficult to point out a representative figure across the different study cohorts6,7. Studies using the DSM-IV’s criteria and a structured clinical interview, reported a prevalence of major depression ranging from 20 to 25% in all PD patients8.
With the disease’s progression, PD patients require increasingly more assistance in their everyday life, often delivered by caregivers9. These caregivers subsequently experience distress in the physical, mental, and social aspects of their lives10,11. This fact is reiterated in the findings of prior studies, which indicate that caring for a person with PD is associated with an increased risk of psychological distress, anxiety and depression12. Thus, clinicians should concern about the caregivers of PD patients, especially regarding their mood condition. Previous studies revealed that the rate of depression of caregivers of PD patients ranged from 14 to 35%13,14. Compared to studies used self-rated questionnaires to detect depression, the study used a structured clinical interview to diagnose the caregivers of PD patients showed a lower rate of depression (11.1%) among caregivers15.
Previous studies have investigated fatigue16,17, resilience18,19, and suicidal ideation20,21 in PD patients. Fatigue is characterized as a sensation ranging from tiredness to exhaustion, and is a frequent non-motor complaint of patients with PD (37–56%)16,17. Resilience is the ability to quickly overcome adversity and stress, as well as regain a normal psycho-emotional state18. Resilience correlates with lesser disability, a better quality of life in PD patients, and plays a critical role in adjusting to the disease18,19. The combining effect of motor disabilities, depression (and other psychiatric disorders), along with the neuropathology of PD may put these patients at risk of suicide22,23. The prevalence of suicidal ideation in PD patients ranged from 10.2% ~ 14.4%20,21. Furthermore, few studies that have detected fatigue15,24, resilience25,26, and suicidal ideation27 among caregivers of PD patients. One study demonstrated that caregivers of PD patients experienced daily physical health problems including: muscle strain, headaches, and fatigue (17%)24. One study found that resilience modulated the inverse relation between perceived stress and QOL25. Furthermore, resilience partially influenced the effects of social support on the mitigation of mental health symptoms26. In a large register-based cohort study, PD patient is associated with a higher risk of death by external causes, including an almost two-fold higher risk of suicide27. Furthermore, fatigue, degrees of resilience, and suicidal ideation were all associated with depression among PD patients and their caregivers in prior studies15,22,28,29.
The actor/partner interdependence model (APIM) is a model that simultaneously estimates the effect of a person’s own variable (actor effect) and the corresponding variable from the partner (partner effect) on an outcome variable30. The APIM has been widely applied for analyses of dyadic data in the social sciences31,32. Previous studies have examined the dyadic effects on QOL, the caregivers’ burdens, and sleep disturbances in PD patients and their caregivers33,34. Only one paper detected the impact of dispositional mindfulness in a stress-health model among dyads consisting of PD patients and their caregivers35. To our knowledge, there are no studies on the detection of the interactive effects that resilience, fatigue, and suicidal ideation for depression between PD patients and their caregivers using an APIM.
In sum, the hypothesis of this study was that interactive effects existed between psychological well-being of individuals with PD and their caregivers. The first aim of this study was to use an APIM to investigate the effects of fatigue, resilience, and suicidal ideation for depression in PD patients and their caregivers. The second aim of this study was to compare the prevalence of depression between PD patients and their caregivers using a standardized structured interview.
Results
Of the 175 PD patients who successfully completed the study, 64% (n = 112) were males. The average age of these PD patients was 65.3 ± 9.3 years. Their mean education level was 10.5 ± 4.8 years, 90.3% were married, and 17.7% were currently employed. The average duration of disease was 8.8 ± 6.4 years (Table 1). Of the 175 caregivers that successfully completed the study, 68.6% (n = 120) were females. The average age of caregiver was 59.5 ± 12.3 years. Their mean education level was 11.4 ± 4.3 years, 86.3% were married, and 33.7% were currently employed. The average duration of caring was 7.8 ± 5.3 years (Table 2).
Table 1.
Demographic and clinical characteristics of the patients with Parkinson’s disease (N = 175).
| Characteristics | Depression N = 50 |
Non-depression N = 125 |
Total N = 175 |
z/x2 | p |
|---|---|---|---|---|---|
| Gender | 1.09 | 0.30 | |||
| Male | 29 (58.0) | 83 (66.4) | 112 (64.0) | ||
| Female | 21 (42.0) | 42 (33.6) | 63 (36.0) | ||
| Age | 66.0 ± 9.0 | 65.0 ± 9.4 | 65.3 ± 9.3 | − 0.38 | 0.70 |
| Age of onset | 57.6 ± 11.1 | 56.3 ± 11.6 | 56.7 ± 11.5 | − 0.61 | 0.54 |
| Duration of PD | 8.6 ± 5.5 | 8.8 ± 6.8 | 8.8 ± 6.4 | − 0.09 | 0.93 |
| Education | 0.02 | 0.89 | |||
| Less than high school (< 12) | 21 (42.0) | 54 (43.2) | 75 (42.9) | ||
| More than college (≧12) | 29 (58.0) | 71 (56.8) | 100 (57.1) | ||
| Years of education | 10.5 ± 4.9 | 10.4 ± 4.8 | 10.5 ± 4.8 | − 0.22 | 0.83 |
| Marital Status | 0.42 | 0.52 | |||
| Unmarried | 6 (12.0) | 11 (8.8) | 17 (9.7) | ||
| Married | 44 (88.0) | 114 (91.2) | 158 (90.3) | ||
| Unemployment | 46 (92.0) | 98 (78.4) | 144 (82.3) | 4.53 | 0.03* |
| Comorbid with other diseases | 33 (66.0) | 84 (67.2) | 117 (66.9) | 0.02 | 0.88 |
| Past psychiatric history | 3.87 | 0.05* | |||
| No psychiatric history | 37 (74.0) | 108 (86.4) | 145 (82.9) | ||
| Depressive disorder | 11 (22.0) | 8 (6.4) | 19 (10.9) | ||
| Anxiety disorder | 1 (2.0) | 4 (3.2) | 5 (2.9) | ||
| Insomnia | 1 (2.0) | 7 (5.6) | 8 (4.6) | ||
| Suicide history | 1 (2.0) | 1 (0.8) | 2 (1.1) | 2.91 | 0.23 |
| Family psychiatric history | 1.03 | 0.79 | |||
| No psychiatric history | 48 (96.0) | 119 (95.2) | 167 (95.4) | ||
| Depressive disorder | 1 (2.0) | 4 (3.2) | 5 (2.9) | ||
| Anxiety disorder | 1 (2.0) | 1 (0.8) | 2 (1.1) | ||
| Family suicide history | 1 (2.0) | 4 (3.2) | 5 (2.9) | 0.19 | 0.67 |
| Anxiolytics/Hypnotics use | 24 (48.0) | 27 (21.6) | 51 (29.1) | 12.05 | 0.001* |
| UPDRS scores | 43.6 ± 17.3 | 35.8 ± 13.6 | 37.8 ± 15.0 | − 2.55 | 0.01* |
| H&Y staging | 2.4 ± 0.7 | 2.1 ± 0.5 | 2.2 ± 0.5 | − 1.96 | 0.05* |
| BHS | 8.7 ± 5.2 | 5.0 ± 3.8 | 6.1 ± 4.6 | − 4.33 | < 0.001* |
| FSS | 37.2 ± 17.3 | 23.6 ± 15.2 | 27.5 ± 16.9 | − 4.80 | < 0.001* |
| CDRISC | 24.7 ± 9.7 | 32.5 ± 8.8 | 30.3 ± 9.7 | − 4.45 | < 0.001* |
| HAMD | 15.2 ± 5.5 | 4.0 ± 2.6 | 7.2 ± 6.3 | − 10.04 | < 0.001* |
PD Parkinson’s disease, UPDRS Unified Parkinson's Disease Rating Scale, BHS Beck Hopelessness Scale, FSS Fatigue Severity Scale, CORISC Connor-Davidson Resilience Scale, HAMD Hamilton Depression Rating Scale, H&Y staging Hoehn and Yahr staging, *p < 0.05.
Table 2.
Demographic and clinical characteristics of the caregivers (N = 175).
| Characteristics | Depression N = 20 |
Non-depression N = 155 |
Total N = 175 |
z/x2 | p |
|---|---|---|---|---|---|
| Gender | 0.43 | 0.51 | |||
| Male | 5 (25.0) | 50 (32.3) | 55 (31.4) | ||
| Female | 15 (75.0) | 105 (67.7) | 120 (68.6) | ||
| Age | 57.5 ± 9.3 | 59.7 ± 12.6 | 59.5 ± 12.3 | − 1.57 | 0.12 |
| Duration of caring | 10.7 ± 6.5 | 7.4 ± 5.1 | 7.8 ± 5.3 | − 2.40 | 0.02* |
| Education | 0.06 | 0.81 | |||
| Less than high school (< 12) | 7 (35.0) | 50 (32.3) | 57 (32.6) | ||
| More than college (≧12) | 13 (65.0) | 105 (67.7) | 118 (67.4) | ||
| Years of education | 11.2 ± 3.8 | 11.4 ± 4.4 | 11.4 ± 4.3 | − 0.44 | 0.66 |
| Marital status | 0.26 | 0.61 | |||
| Unmarried | 2 (10.0) | 22 (14.2) | 24 (13.7) | ||
| Married | 18 (90.0) | 133 (85.8) | 151 (86.3) | ||
| Unemployment | 12 (60.0) | 104 (67.1) | 116 (66.3) | 0.40 | 0.53 |
| Comorbid with other diseases | 11 (55.0) | 76 (49.0) | 87 (49.7) | 0.25 | 0.62 |
| Past psychiatric history | 13.23 | < 0.001* | |||
| No psychiatric history | 14 (70.0) | 146 (94.2) | 160 (91.4) | ||
| Depressive disorder | 3 (15.0) | 3 (1.9) | 6 (3.4) | ||
| Anxiety disorder | 2 (10.0) | 1 (0.6) | 3 (1.7) | ||
| Insomnia | 3 (15.0) | 6 (3.9) | 9 (5.1) | ||
| Suicide history | 0 | 1 (0.6) | 1 (0.6) | 0.13 | 0.72 |
| Family psychiatric history | 0.76 | 0.68 | |||
| No psychiatric history | 18 (90.0) | 147 (94.8) | 165 (94.3) | ||
| Depressive disorder | 1 (5.0) | 6 (3.9) | 7 (4.0) | ||
| Anxiety disorder | 1 (5.0) | 2 (1.3) | 3 (1.7) | ||
| Family suicide history | 1 (5.0) | 5 (3.2) | 6 (3.4) | 0.17 | 0.68 |
| Anxiolytics/Hypnotics use | 6 (30.0) | 11 (7.1) | 17 (9.7) | 10.60 | 0.001* |
| UPDRS of caring patients | 41.8 ± 22.2 | 37.7 ± 12.2 | 37.9 ± 13.1 | − 0.38 | 0.71 |
| H&Y staging | 2.6 ± 0.9 | 2.1 ± 0.4 | 2.2 ± 0.4 | − 0.61 | 0.54 |
| BHS | 5.1 (0–14) | 3.1 (0–15) | 3.3 (0–15) | − 2.08 | 0.04* |
| FSS | 33.4 ± 13.4 | 23.0 ± 13.4 | 24.1 ± 13.8 | − 3.24 | 0.001* |
| CDRISC | 23.4 ± 7.2 | 31.8 ± 7.6 | 30.8 ± 8.0 | − 4.33 | < 0.001* |
| HAMD | 13.0 (3–26) | 2.5 (0–13) | 3.7 (0–26) | − 7.03 | < 0.001* |
UPDRS Unified Parkinson's Disease Rating Scale, BHS Beck Hopelessness Scale, FSS Fatigue Severity Scale, CORISC Connor–Davidson Resilience Scale, HAMD Hamilton depression rating scale, H&Y staging Hoehn and Yahr staging, *p < 0.05.
The results showed that 67% of PD patients and 50% of the caregivers had one or more physical illnesses. Seventeen percent of patients and 9% of the caregivers had a past psychiatric history; and 29% of patients and 9.7% of caregivers had used hypnotics in the past (Tables 1 and 2). The average UPDRS of the people with PD was 37.8 ± 15.0, and their average H&Y staging was 2.2 (± 0.5) (Table 1).
The most common psychiatric diagnoses of the PD patients were depressive disorder (28.6%), followed by rapid eye movement (REM) sleep behavior disorder (9.7%), insomnia disorder (8.0%), anxiety disorder not otherwise specified (NOS) (2.9%), and adjustment disorder (2.9%). Among the depressive disorders, the most prevalent was depressive disorder NOS (14.3%), followed by major depressive disorder (MDD) (12.0%), and dysthymia (2.3%). Of the PD patients, 55% had a psychiatric diagnosis (Table 3).
Table 3.
Psychiatric diagnoses of patients (N = 175) and caregivers (N = 175).
| MINI diagnoses | Patients N = 175 |
Caregivers N = 175 |
|---|---|---|
| Depressive disorders | 50 (28.6) | 20 (11.4) |
| Major depressive disorder | 21 (12.0) | 6 (3.4) |
| Depressive disorder NOS | 25 (14.3) | 12 (6.9) |
| Dysthymia | 4 (2.3) | 2 (1.1) |
| Adjustment disorder | 5 (2.9) | 6 (3.4) |
| Anxiety disorder NOS | 5 (2.9) | 7 (4.0) |
| Panic disorder | 2 (1.1) | 1 (0.6) |
| Insomnia disorder | 14 (8.0) | 13 (7.4) |
| REM sleep behavior disorder | 17 (9.7) | 0 |
| Others | 13 (7.4) | 4 (2.3) |
| No diagnosis | 79 (45.1) | 127 (72.6) |
MINI Mini International Neuropsychiatric Interview, Anxiety disorder NOS Anxiety disorder not otherwise specified, Depressive disorder NOS Depressive disorder not otherwise specified, REM sleep behavior disorder Rapid eye movement sleep behavior disorder, Others Depressive disorder not otherwise specified history, Major depressive disorder history.
The most common psychiatric diagnoses of the caregivers were depressive disorder (11.4%), followed by insomnia disorder (7.4%), anxiety disorder NOS (4.0%), and adjustment disorder (3.4%). Among the depressive disorders, the most prevalent was depressive disorder NOS (6.9%), followed by MDD (3.4%), and dysthymia (1.1%). Of the caregivers, 27% had a psychiatric diagnosis (Table 3).
In the univariate analyses of the 175 PD patients, factors significantly associated with depressive disorders included more unemployment (92.0% vs 78.4%, p < 0.05), higher UPDRS scores (43.6 ± 17.3 vs 35.8 ± 13.6, p < 0.05), more habitual hypnotics use (48.0% vs 21.2%, p < 0.05), greater suicidal risk (8.7 ± 5.2 vs 5.0 ± 3.8, p < 0.01), higher fatigue scores (37.2 ± 17.3 vs 23.6 ± 15.2, p < 0.05), and lower CDRISC scores (24.7 ± 9.7 vs 32.5 ± 8.8, p < 0.001) (Table 1).
In the univariate analyses of the 175 caregivers, factors significantly associated with depressive disorders included the duration of caring (10.7 ± 6.5 vs 7.4 ± 5.1, p < 0.05), psychiatric past history (× 2 = 13.23, p < 0.001), more habitual hypnotics use (30.0% vs 7.1%, p < 0.05), greater suicidal risk [5.1(0–14) vs 3.1(0–15), p < 0.05], higher fatigue scores (33.4 ± 13.4 vs 23.0 ± 13.4, p < 0.05), and lower CDRISC scores (23.4 ± 7.2 vs 31.8 ± 7.6, p < 0.001) (Table 2).
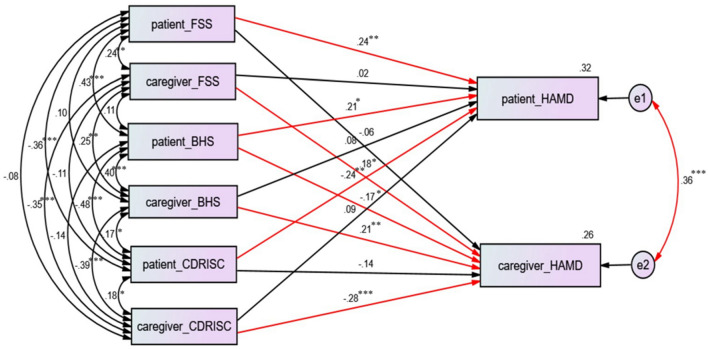
Using SEM, we found that patients’ fatigue severity (β = 0.24, p < 0.01), patients’ suicidal ideation severity (β = 0.21, p < 0.05), and patients’ resilience severity (β = − 0.24, p < 0.01) were significantly linked with depression severity in patients with PD (Fig. 1). Furthermore, we found that caregivers’ fatigue severity (β = 0.18, p < 0.05), caregivers’ suicidal ideation severity (β = 0.21, p < 0.05), caregivers’ resilience severity (β = − 0.28, p < 0.001), and patients’ suicidal ideation severity (β = − 0.17, p < 0.05) were significantly linked with depression severity in the caregivers (Fig. 1). Patients’ depression severity and caregivers’ depression severity had significant interactive effects (β = 0.36, p < 0.001).
Figure 1.
Structural equation modeling (SEM) of factors linked to depression in patients PD and their caregivers. Model summary: chi-square = 0; df = 0; p = \p. The model fit: AGFI = \AGFI; RMSEA = \ RMSEA; AIC = 72.00. *p < 0.05; **p < 0.01;***p < 0.001.
Moreover, we conducted qualitative interview to elucidate the reasons underlying the association of suicide idea and depression among PD patients and their caregivers. Two narratives of PD patients and their caregivers recorded as follows: Patient A: “I hope that I can die soon because my health is getting worse and worse.” Caregiver A: “Once he dies, I can do anything I want.” Patient B: “I have no future. My wife cannot understand my discomfort. She doesn’t come to me at once when I feel distressed.” Caregiver B: “I think he is a whiner. His illness is not that serious. How can I take care of him if I feel downcast like him? There is a long way to go.”
Discussion
We found depressive disorder (28.6%) is the most common psychiatric comorbidity in PD patients. However, the comorbidity rate herein is lower than which reported in previous studies on depression morbidity of PD patients (35% ~ 90%)10,11. Moreover, we found that depressive disorder is the most frequent psychiatric diagnosis in caregivers of PD patients(11.4%), which is lower than prior studies on depression prevalence in caregivers of PD patients (14% ~ 35%)13,14. The possible explanation for depression morbidity being lower, both in PD patients and their caregivers, in our results than in previous studies is that our study used structured diagnostic interviews, rather than self- rated depression questionnaires which renders lower false positive cases of depression. The advantage of using a standardized clinical interview is that it provides a clinical indicator for whether the individuals should receive proper management.
Our study found that the morbidity of depression in PD patients was twice as prevalent as in their caregivers. Patients with PD experienced motor disability, drug side effects, and cognitive impairment which might aggravate their mood condition12. Nevertheless, caregivers still attend PD patients through the disease process, despite having a high depression morbidity that might interfere with their care ability and quality of life15. Pharmacotherapy and cognitive-behavioral therapy are currently first-line treatments for depression in patients with PD36. Future studies are warranted to elucidate whether treatments for depression in both PD patients and their caregivers are beneficial for them each other.
We found that both PD patients’ and their caregivers’ fatigue, resilience, and suicidal ideation were associated with depression. The aforementioned clinical characteristics, which correlated with depression, were elucidated in previous studies and described in the introduction section16–21,24–27. In order to establish models of potential mechanisms underlie the linkage of associated factors and depression in patients with PD and their caregivers, SEM was used to examine APIM. The actor effects were as follows: patients’ fatigue, patients’ death ideation, and patients’ resilience were significantly linked with the depression severity of PD patients; caregivers’ fatigue, caregivers’ suicidal ideation, and caregivers’ resilience were significantly linked with the depression severity of the caregivers. Two partner effects were found, the first was that the patients’ suicidal ideation (β = − 0.17, p < 0.05) was significantly linked with the depression severity of their caregivers; the second was that patients’ depression severity and their caregivers’ depression severity had a significant interactive effect. Of note, we found that patients’ suicidal ideation negatively influenced their caregivers’ depression. Based on the above mentioned narratives, we can speculate that many caregivers cope with PD persons’ negative thinking and even hopelessness well. This result can partially explain why caregivers’ depression morbidity is lower than PD patients’ depression morbidity.
Based on the results of this study, we assume that caregivers of PD patients have established a high benefit finding (BF), positive life changes resulting from the struggle to cope with a stressful life event such as illness. They can overcome negative thinking from taking care of their patients37. Navarta-Sánchez et al.38 investigated factors influencing psychosocial adjustment and the QOL in PD patients and informal caregivers. They found that coping was a significant predictor of psychosocial adjustment in patients and caregivers. Macchi et al.39 reported that patients’ depression and quality of life contribute to caregiver burden in persons living with PD. Lindsay Penny Prizer et al.40 suggest that presence of a caregiver may be an important modifying variable on patient outcomes. This finding might partially support our aforementioned hypothesis.
In addition to the study of Navarta-Sánchez, a study by Karlstedt et al. (2017) tests the determinants of a dyadic relationship and its psychosocial impact among PD patients and their spouses. They found that high levels of mutuality experienced by the PD patient was associated with their QOL; and that non-motor symptoms contributed to a larger extent to the mutual relationship of PD affected dyads than motor disabilities did. However, the limited case number is the main disadvantage of this research33. There are few dyadic studies that use APIM analysis in different medical conditions31,32. This is probably the first study to examine fatigue, resilience and suicidal ideation on PD patients and their caregivers using an APIM.
The UPDRS has been the most commonly used scale to assess impairment longitudinally and disability of PD patients. The UPDRS made up of five parts with non-motor symptoms, ADL, motor symptoms, complications of therapy, and Hoehn & Yahr staging. Our results showed that UPDRS’ total score was associated with depression. We further analyzed the five parts of UPDRS and found that the score of subscales correlated to depression except on the motor symptoms subscale. Our results suggested that the motor symptoms of PD patients might be less influential on the severity of depression.
The strengths of this study are: (1) the high response rate (91.1%), (2) the use of a structured clinical interview by psychiatrists, and (3) APIM was used to clarify the interaction of PD patients and their caregivers. However, there are several limitations to this study that should be mentioned: (1) our study design involved consecutive sampling, which may have led to a sampling bias. However, a response rate of over 90% of the caregivers compromised the effect of this limitation. (2) Our samples were from a general hospital, which may not be representative of the general population. (3) This was a cross-sectional study, which does not allow for the exploration of PD patients’ and their caregivers’ psychiatric disorders through the course of the disease and their caregiving. Therefore, further follow-up studies should be conducted to understand the precise nature of depression morbidity in PD patients and their caregivers, as well as any associated factors involved.
The clinical implications of this study are: (1) The morbidity of depression in PD patients is more prevalent than the morbidity of depression in their caregivers. (2) Fatigue, resilience, and suicidal ideation might contribute to the depression severity of PD patients and their caregivers. (3) Patients’ suicidal ideation negatively influenced their caregivers’ severity of depression. (4) It is crucial that clinicians are aware of, and manage these contributing factors in PD patients and their caregivers in order to prevent these two groups from worsening each other’s depression. Future studies are warranted to elucidate whether treatment for depression in both PD patients and their caregivers is beneficial for them both.
Methods
Participants
This study used a cross-sectional design with consecutive sampling. Participants were recruited from the neurology ward or neurology outpatient clinic at a general hospital from August 2018 to May 2020. Inclusion criteria of patients: (1) Individuals have been diagnosed with PD by an expert neurologist; (2) Individuals are able to understand the study procedure and can provide the written informed consent. Exclusion criteria of patients: (1) Individuals with a diagnosis of delirium, or atypical parkinsonism (e.g. Dementia with Lewy bodies, progressive supranuclear palsy, multiple system atrophy, corticobasal syndrome) or secondary parkinsonism; (2) Individuals who are too weak to complete the questionnaire or clinical interview.
Inclusion criteria of caregivers: (1) Individuals are patient’s principal caregivers. Principal caregivers are defined as “family members who living with the patients and taking care of their daily needs”; (2) Individuals are able to understand the study procedure and can provide written informed consent. Exclusion criteria of caregivers: Individuals who are too weak to complete the questionnaire or clinical interview.
In total, 192 PD patients and caregivers were invited to take part in this study initially; data collection was completed for 175 PD patients and caregivers (response rate: 91.1%). Among all the PD patients, three of them were too weak to complete the questionnaire or clinical interview, and 14 refused to partake in the interview. Among the 175 caregivers, 133 (76%) were spouses, 32 (18.3%) were children, and 10 (5.7%) were parents, siblings, or friends.
Assessments
Unified Parkinson's Disease Rating Scale (UPDRS) (scores range from 0 to 144)
The UPDRS is the most commonly used scale in the clinical study of PD, and is used to follow the longitudinal course of PD41. The UPDRS is made up of the following sections: Part I: evaluation of behavioral problems such as intellectual decline, hallucinations, and depression; Part II: self-evaluation of activities of daily living (ADLs); Part III: clinician-scored monitored motor evaluation; Part IV: complications of therapy; Part V: Hoehn and Yahr staging of severity of PD42.
Fatigue Severity Scale (FSS) (scores range from 9 to 63)
The Fatigue Severity Scale (FSS) is a nine-item scale that measures the impact of fatigue on motivation, exercise, physical functioning, and interference with professional, familial, and social life41. The FSS is widely used to identify features of fatigue related to medical conditions, including multiple sclerosis, systemic lupus erythematosus, cancer, and PD43. The FSS can be applied to examine fatigue severity in caregivers44. The Chinese version of the FSS was validated for assessing fatigue-related impairment in Chinese-speaking persons with major depressive disorder45.
Connor–Davidson resilience scale (CD-RISC) (scores range from 0 to 40)
The original Connor-Davidson Resilience scale (CD-RISC) was a self-reported 25-item scale with scores ranging from 0 to 4 per item. Higher scores mean greater resilience, i.e., a greater ability to cope with stress. An abbreviated version of the CD-RISC comprising 10 items was developed on the basis of factor analysis, providing a rapid and brief method to quantify resilience46. Good reliability and validity were confirmed among different populations and diseases47.
Beck hopelessness scale (BHS)
The Beck Hopelessness Scale is a 20-item, self-reported tool designed to measure three major aspects (affective, motivational and cognitive) of hopelessness: feelings about the future, loss of motivation, and expectations48. Scores range from 0 to 20, with higher scores indicative of higher levels of hopelessness and higher suicide risk. The Chinese version of the BHS was translated and validated in Taiwan49.
Mini international neuropsychiatric interview (MINI)
The MINI is a short, structured clinical interview which assists researchers in executing diagnoses of psychiatric disorders, especially depressive disorders and anxiety disorders, based on DSM-IV or ICD-10 criteria50. The MINI was designed for epidemiological studies and has achieved satisfactory levels of validity and reliability51. Approximately 15–20 min are needed to conduct the interview.
Hamilton depression rating scale (HAM-D)
It is used to probe mood, feelings of guilt, suicidal ideation, insomnia, agitation, or retardation, anxiety, weight loss, and somatic symptoms52. The HAM-D has been widely applied to assess the severity of depression, though it has been criticized for over-emphasis on neuro-vegetative symptoms. The HAM-D is administered by clinicians or researchers. The reliability and validity of the Chinese version of the 17-item HAM-D has been verified, and it can be used in clinical and research settings53.
Procedures
Ethical approval was obtained from the human research ethics committee of Chang Gung Memorial Hospital (201702186B0). Study procedures were as follows: (1) Once our research assistant received a referral from the neurological ward or neurological outpatient clinic from the in-charge doctor, the research assistant went to the to contact the person with PD and his/her caregiver. After explaining the study procedure and aims, only those patients and their caregivers who agreed to sign an informed consent form were enrolled in the study. (2) Both the PD patients and their caregivers received the BHS, FSS, CD-RISC, and the MINI. Additionally, PD patients received the UPDRS assessment. (3) The MINI was used by two staff psychiatrists (Dr. Y. Lee and Dr. YJ. Chiou) to reach a psychiatric diagnosis. (4) The HAMD-D was administered by Dr. Y. Lee and Dr. YJ. Chiou to evaluate depression severity. (5) Our trained research assistant collected the patients’ demographic and clinical data (including UPDRS), and the caregivers’ demographic data and clinical rating scales, including the FSS, BHS, CD-RISC. (6) Dr. Y. Lee and Dr. YJ. Chiou discussed the psychiatric diagnosis in the first three sessions of the research meeting to reach a psychiatric diagnostic consensus.
Statistical analyses
Descriptive and inferential statistics were analyzed using SPSS for Windows V. 16.0. The non-parametric test (Mann–Whitney U test) was suitably performed because the number of depressive patients and caregivers were far less than the number of non-depressive patients and caregivers. Descriptive statistics (chi-square and Mann–Whitney U tests) were used first to test the difference in demographic data and then to test the clinical characteristics between subjects with and without depressive disorder. To determine the impact of fatigue, resilience, and suicidal ideation on patients’ and caregivers’ depression, we demonstrated actor and partner effects by using the actor–partner interdependence model (APIM) with a distinguishable dyads regression model32. The APIM is a model that integrates a conceptual view of interdependence with the appropriate statistical techniques for measuring and testing dyadic relationships by a distinct regression model54. The APIM was assessed using structural equation modeling (SEM)54. The SEM statistical program was analyzed using SPSS Amos 24.0.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. We obtained informed consent in writing from all individuals with PD and their caregivers.
Author contributions
Y.L. participated in interpreting data, reviewing references, and drafting the manuscript. Y.-J.C., C.-F.H., Y.-Y.C., Y.-F.C. and T.-K.L. participated in data collection and patient recruitment. L.-J.W. participated in protocol development and revised the manuscript. All authors read and approved the final manuscript and contributed to the drafting and revising of the paper.
Funding
This study was supported by a Grant from the Ministry of Science and Technology, Taiwan (MOST 107-2314-B-182A-129 -MY2).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov. Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.Abbas MM, Xu Z, Tan LCS. Epidemiology of Parkinson's disease-east versus west. Mov. Disord. Clin. Pract. 2018;5:14–28. doi: 10.1002/mdc3.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol. 2018;17:559–568. doi: 10.1016/S1474-4422(18)30127-3. [DOI] [PubMed] [Google Scholar]
- 4.Müller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson's disease. Parkinsonism Relat. Disord. 2013;19:1027–1032. doi: 10.1016/j.parkreldis.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer RF. Non-motor symptoms in Parkinson's disease. Parkinsonism Relat. Disord. 2016;22(Suppl 1):S119–122. doi: 10.1016/j.parkreldis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S. Antidepressive treatments for Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2015;21:833–842. doi: 10.1016/j.parkreldis.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Timmer MHM, van Beek M, Bloem BR, Esselink RAJ. What a neurologist should know about depression in Parkinson's disease. Pract. Neurol. 2017;17:359–368. doi: 10.1136/practneurol-2017-001650. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Recognition and treatment of depression in Parkinson's disease. J. Geriatr. Psychiatry Neurol. 2003;16:178–183. doi: 10.1177/0891988703256053. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Martín P, et al. Caregiver burden in Parkinson's disease. Mov. Disord. 2007;22:924–931. doi: 10.1002/mds.21355. [DOI] [PubMed] [Google Scholar]
- 10.Grün D, Pieri V, Vaillant M, Diederich NJ. Contributory factors to caregiver burden in Parkinson disease. J. Am. Med. Dir. Assoc. 2016;17:626–632. doi: 10.1016/j.jamda.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin D, et al. Living and coping with Parkinson's disease: Perceptions of informal carers. Palliat. Med. 2011;25:177–182. doi: 10.1177/0269216310385604. [DOI] [PubMed] [Google Scholar]
- 12.D'Amelio M, et al. Predictors of caregiver burden in partners of patients with Parkinson's disease. Neurol. Sci. 2009;30:171–174. doi: 10.1007/s10072-009-0024-z. [DOI] [PubMed] [Google Scholar]
- 13.Carod-Artal FJ, Mesquita HM, Ziomkowski S, Martinez-Martin P. Burden and health-related quality of life among caregivers of Brazilian Parkinson's disease patients. Parkinsonism Relat. Disord. 2013;19:943–948. doi: 10.1016/j.parkreldis.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Meara J, Mitchelmore E, Hobson P. Use of the GDS-15 geriatric depression scale as a screening instrument for depressive symptomatology in patients with Parkinson's disease and their carers in the community. Age Ageing. 1999;28:35–38. doi: 10.1093/ageing/28.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, et al. Prevalence and associated factors of depressive disorder in caregivers of individuals with Parkinson disease. J. Geriatr. Psychiatry Neurol. 2020;2:2. doi: 10.1177/0891988720933359. [DOI] [PubMed] [Google Scholar]
- 16.Siciliano M, et al. Fatigue in Parkinson's disease: A systematic review and meta-analysis. Mov. Disord. 2018;33:1712–1723. doi: 10.1002/mds.27461. [DOI] [PubMed] [Google Scholar]
- 17.Herlofson K, Kluger BM. Fatigue in Parkinson's disease. J. Neurol. Sci. 2017;374:38–41. doi: 10.1016/j.jns.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 18.Robottom BJ, et al. What determines resilience in patients with Parkinson's disease? Parkinsonism Relat. Disord. 2012;18:174–177. doi: 10.1016/j.parkreldis.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Shamaskin-Garroway AM, Lageman SK, Rybarczyk B. The roles of resilience and nonmotor symptoms in adjustment to Parkinson's disease. J. Health Psychol. 2016;21:3004–3015. doi: 10.1177/1359105315590268. [DOI] [PubMed] [Google Scholar]
- 20.Kostić VS, et al. Suicide and suicidal ideation in Parkinson's disease. J. Neurol. Sci. 2010;289:40–43. doi: 10.1016/j.jns.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Kummer A, Cardoso F, Teixeira AL. Suicidal ideation in Parkinson's disease. CNS Spectr. 2009;14:431–436. doi: 10.1017/S109285290002040X. [DOI] [PubMed] [Google Scholar]
- 22.Shepard MD, et al. Suicide in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2019;90:822–829. doi: 10.1136/jnnp-2018-319815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee T, et al. Increased suicide risk and clinical correlates of suicide among patients with Parkinson's disease. Parkinsonism Relat. Disord. 2016;32:102–107. doi: 10.1016/j.parkreldis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Lökk J. Caregiver strain in Parkinson's disease and the impact of disease duration. Eur. J. Phys. Rehabil. Med. 2008;44:39–45. [PubMed] [Google Scholar]
- 25.Ertl MM, Trapp SK, González Arredondo S, Rodríguez Agudelo Y, Arango-Lasprilla JC. Perceived stress, resilience, and health-related quality of life among Parkinson's disease caregivers in Mexico. Health Soc. Care Commun. 2019;27:1303–1310. doi: 10.1111/hsc.12767. [DOI] [PubMed] [Google Scholar]
- 26.Tyler CM, et al. Structural equation modeling of parkinson's caregiver social support, resilience, and mental health: A strength-based perspective. Neurol. Res. Int. 2020;2020:7906547. doi: 10.1155/2020/7906547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen M, et al. Cause-specific mortality among spouses of Parkinson disease patients. Epidemiology. 2014;25:225–232. doi: 10.1097/EDE.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk JP, et al. Influence of disease severity on fatigue in patients with Parkinson's disease is mainly mediated by symptoms of depression. Eur. Neurol. 2013;70:201–209. doi: 10.1159/000351779. [DOI] [PubMed] [Google Scholar]
- 29.Berardelli I, et al. Suicide in Parkinson's disease: A systematic review. CNS Neurol. Disord. Drug Targets. 2019;18:466–477. doi: 10.2174/1871527318666190703093345. [DOI] [PubMed] [Google Scholar]
- 30.Kenny DA. Models of non-independence in dyadic research. J. Soc. Personal Relationships. 1996;13:279–294. doi: 10.1177/0265407596132007. [DOI] [Google Scholar]
- 31.Wan-Fei K, et al. Depression, anxiety and quality of life in stroke survivors and their family caregivers: A pilot study using an actor/partner interdependence model. Electron. Physician. 2017;9:4924–4933. doi: 10.19082/4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung ML, Moser DK, Lennie TA, Rayens MK. The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: Testing dyadic dynamics using Actor-Partner Interdependence Model. J. Psychosom. Res. 2009;67:29–35. doi: 10.1016/j.jpsychores.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlstedt M, Fereshtehnejad SM, Aarsland D, Lökk J. Determinants of dyadic relationship and its psychosocial impact in patients with Parkinson's disease and their spouses. Parkinsons Dis. 2017;2017:4697052. doi: 10.1155/2017/4697052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade R, Pachana NA, Dissanayaka N. Management of sleep disturbances in Parkinson's disease patients, carers and the patient and carer dyadic relationship: A scoping review. Clin. Gerontol. 2018;2:1–9. doi: 10.1080/07317115.2018.1539424. [DOI] [PubMed] [Google Scholar]
- 35.Hicks A, et al. The role of dispositional mindfulness in a stress-health pathway among Parkinson's disease patients and caregiving partners. Qual. Life Res. 2019;28:2705–2716. doi: 10.1007/s11136-019-02217-6. [DOI] [PubMed] [Google Scholar]
- 36.Starkstein SE, Brockman S. Management of depression in Parkinson's disease: A systematic review. Mov. Disord. Clin. Pract. 2017;4:470–477. doi: 10.1002/mdc3.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Affleck G, Tennen H. Construing benefits from adversity: Adaptational significance and dispositional underpinnings. J. Pers. 1996;64:899–922. doi: 10.1111/j.1467-6494.1996.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 38.Navarta-Sánchez MV, et al. Factors influencing psychosocial adjustment and quality of life in Parkinson patients and informal caregivers. Qual. Life Res. 2016;25:1959–1968. doi: 10.1007/s11136-015-1220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macchi ZA, et al. Patient and caregiver characteristics associated with caregiver burden in Parkinson's disease: A palliative care approach. Ann. Palliat. Med. 2020;9:S24–s33. doi: 10.21037/apm.2019.10.01. [DOI] [PubMed] [Google Scholar]
- 40.Prizer LP, et al. The presence of a caregiver is associated with patient outcomes in patients with Parkinson's disease and atypical parkinsonisms. Parkinsonism Relat. Disord. 2020;78:61–65. doi: 10.1016/j.parkreldis.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Fahn, S. Unified Parkinson's disease rating scale. Recent developments in Parkinson's disease volume II. Vol. 153 (Florham Park, 1987).
- 42.Yu R-L, et al. Cross-cultural differences of the non-motor symptoms studied by the traditional Chinese version of the international parkinson and movement disorder society-unified Parkinson's disease rating scale. Mov. Disord. Clin. Pract. 2017;4:68–77. doi: 10.1002/mdc3.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 44.Schneider RA. Chronic renal failure: Assessing the fatigue severity scale for use among caregivers. J. Clin. Nurs. 2004;13:219–225. doi: 10.1046/j.1365-2702.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang MY, Liu IC, Chiu CH, Tsai PS. Cultural adaptation and validation of the Chinese version of the fatigue severity scale in patients with major depressive disorder and nondepressive people. Qual. Life Res. 2016;45:89–99. doi: 10.1007/s11136-015-1056-x. [DOI] [PubMed] [Google Scholar]
- 46.Connor KM, Davidson JR. Development of a new resilience scale: The Connor–Davidson resilience scale (CD-RISC) Depress. Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Shi Z, Zhang Y, Zhang Z. Psychometric properties of the 10-item Connor–Davidson resilience scale in Chinese earthquake victims. Psychiatry Clin. Neurosci. 2010;64:499–504. doi: 10.1111/j.1440-1819.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. J. Consult. Clin. Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- 49.Kao YC, Liu YP, Lu CW. Beck Hopelessness Scale: Exploring its dimensionality in patients with schizophrenia. Psychiatr Q. 2012;83:241–255. doi: 10.1007/s11126-011-9196-9. [DOI] [PubMed] [Google Scholar]
- 50.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 51.Yang P, et al. Posttraumatic stress disorder in adolescents after Typhoon Morakot-associated mudslides. J. Anxiety Disord. 2011;25:362–368. doi: 10.1016/j.janxdis.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng YP, et al. Validity and reliability of the Chinese hamilton depression rating scale. Br. J. Psychiatry. 1988;152:660–664. doi: 10.1192/bjp.152.5.660. [DOI] [PubMed] [Google Scholar]
- 54.Cook WL, Kenny DA. The actor–partner interdependence model: A model of bidirectional effects in developmental studies. Int. J. Behav. Dev. 2005;29:101–109. doi: 10.1080/01650250444000405. [DOI] [Google Scholar]