Abstract
Recent evidence points to the relationship between lead toxicity and the function of the hypothalamic-pituitary-adrenal axis, which suggests that lead exposure could influence how an individual cope with stress. Here we test this hypothesis by investigating the behavioral effects of lead exposure in mice during the forced swimming test (FST), a parading in which animals are exposed to a stressful situation and environment. Swiss mice received either 180 ppm or 540 ppm of lead acetate (Pb) in their ad-lib water supply for 60–90 days, starting at postnatal day 30. Control (Ctrl) mice drank tap water. At the end of the exposure period, mice were submitted to a 5-min session of FST or to an open-field session of the same duration. Data from naïve animals showed that corticosterone levels were higher for animals tested in the FST compared to animals tested in the open-field. Blood-lead levels (BLL) in Pb-exposed mice ranged from 14.3 to 106.9 µg/dL. No differences were observed in spontaneous locomotion between Ctrl and Pb-exposed groups in the open-field. However, in the FST, Pb-treated mice displayed higher swimming activity than Ctrl ones and this effect was observed even for animals with BLL higher than 20 µg/dL. Furthermore, significant differences in brain glutathione levels, used as an indicator of led toxicity, were only observed for BLL higher than 40 µg/dL. Overall, these findings suggest that swimming activity in the FST is a good indicator of lead toxicity and confirm our prediction that lead toxicity influences behavioral responses associated to stress.
Keywords: Lead acetate, Locomotor activity, Turning activity, Stress, Glutathione, Animal model
Introduction
In spite of efforts to reduce environmental lead levels over the past decades, lead exposure still a major public health problem [1–4]. Lead is a potent neurotoxin [5–8] and, while its neurobehavioral effects are more pronounced when exposure occurs during prenatal development and childhood [7, 9], there is growing evidence suggesting that cumulative lead exposure later in life also has adverse effects on brain function [10–12]. The latter raises further concern when we turn our attention to occupational exposure, since both young and old adults are exposed to lead levels higher than those usually found in the environment [13–15]. Nonetheless, lead-neurotoxicity is difficult to determine since, lead has a short half-life in the bloodstream [16] and the neural substrates that underlie lead-related adverse impact on cognitive function are still poorly understood [17]. Interestingly, relative recent epidemiologic data indicates that stress can be a pivotal factor that exacerbate lead neurotoxicity [18–20], and thus an important covariate to be considered when assessing lead toxicity. However, a caveat of any epidemiological analysis concerns the number and type of covariates that have been employed and, for this reason, one cannot discard the possibility that factors other than lead toxicity and stress (such as genetic background, and socioeconomic status of the populations sampled) had influenced the outcomes of the aforementioned studies.
Relevant information about the effects of toxic compounds on brain function stem from behavioral studies using animal models. Behavioral toxicological screening is an important asset in determining the mechanisms of action of different chemical compounds [21]. Of particular interest are findings that suggest a strong link between stress and the neurobehavioral effects of lead exposure. Lead toxicity can increase corticosterone levels, which could modify brain catecholamines, and thus intensify the harmful impact of stressful situations on the function of the hypothalamic-pituitary-adrenal axis [22–25]. Given these relationships, it is reasonable to suppose that lead exposure could profoundly affect how one copes with a stressful situation. In this regard, the forced swimming test (FST) is an attractive behavioral paradigm to investigate this possibility. Originally developed to predict the efficacy of antidepressant drugs [26, 27], this test has also been proposed as a general form of response to inescapable stress [28–30]. When rodents are forced to swim in a cylinder filled with water from which they cannot escape, they will, after an initial brief period of vigorous activity, alternate between two very distinct behaviors: swimming and immobility [26, 27, 31–33]. Behavioral strategies that decrease energy expenditure, such as passive floating or slow swimming, are thought to reflect a better coping response to stress in the FST [34]. Thus, we hypothesize that lead-exposed animals would present increased swimming activity and reduced immobility in the FST. To examine this possibility, Swiss mice were subchronically exposed to lead acetate from adolescence to adulthood and then tested in the FST. For purposes of comparison, we also examined the effects of such exposure in the open-field test, a less stressful behavioral assay and also one of the most used for the detection of the neurotoxic effects of lead exposure in rodents [35–39]. Moreover, we also evaluated the effects of lead exposure on glutathione, a main antioxidant system in the brain [40–42] and used as a measure of lead-oxidative stress [43].
Material and methods
Animal treatment
All procedures were carried out in compliance with the Animal Care and Use Committee of the State University of Rio de Janeiro, in compliance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Swiss mice (n = 201, 106 males and 95 females, from 29 litters) were bred and maintained in a temperature-controlled room on a 12:12 h light/dark cycle (lights on: 2:00, lights off: 14:00). At weaning (postnatal day 30, PN30), animals from the same litter were separated by sex, housed in groups of 3–5 mice per cage, and assigned to the following experimental groups: Pb-treated (Pb180 and Pb540) and control (Ctrl). Lead acetate trihydrate (Sigma-Aldrich, Reag. Ph. Eur.) was added to the drinking water at two doses, 180 ppm (0.018%, 474 μM, Pb180 group) and 540 ppm (0.054%, 1,422 μM, Pb540 group), for at least 60 days (average exposure period = 73.6 ± 6.3 days, longest exposure period = 88 days). Control animals received lead-free drinking water for the same period. The concentrations of 180 and 540 ppm of lead were chosen in order to allow for a range of BBLs that: (1) was wide enough to adequately investigate a dose–response relationship and, dose–response relationship and, (2) was compatible to levels observed in studies of lead neurotoxicity in rodents [44, 45] as well as in studies dealing with occupational exposure in humans [46]. A third dosage of lead acetate of 90 ppm (0.009%, 237 μM, Pb90 group) was administered to an independent sample of animals for a similar time period (65.8 ± 4.7 days) as part of a follow-up experiment. Solutions were provided ad libitum and prepared freshly every week. Behavioral tests were conducted at the end of the period of exposure during the lights off phase (between 16:00 h and 17:30 h) In order to avoid the possibility that a previous experience in one behavioral test would influence the animal’s performance in a subsequent one, mice were randomly assigned to be either tested in the open field or in the FST. At the completion of behavioral testing, animals were euthanized, and blood was collected for later determination of lead concentrations.
Forced swimming test (FST)
Swimming activity and immobility time were measured in the FST as previously described [32, 47, 48]. Briefly, 46 Pb180 (22 males and 24 females), 34 Pb540 (21 males and 13 females), and 57 Ctrl animals (31 males and 26 females) were placed in the center of a plastic container (diameter = 21 cm, height = 23 cm) filled with water (depth = 16 cm) at about 25 ºC. The animal’s behavior was continuously recorded for 5 min by an overhead video camera and the container was cleaned and the water exchanged before testing another animal.
Swimming activity, expressed by the number of left (counterclockwise) and right (clockwise) 30º turns was measured by an observer blind to the animal’s treatment condition [32, 47, 48]. A transparent overlay with 30º axes was matched with the image of the circular container on the screen of the video monitor to aid with 30º turns quantification [48]. For each animal, the total swimming activity was considered as the sum of all consecutive 30º turns (to the left and right) during a testing session (5 min), whereas the time the animal remained floating with all limbs and tail motionless was deemed immobility time. All animals were submitted to a second and third forced swimming testing sessions in the following 2 days to evaluate session-to-session reproducibility.
Open field
Spontaneous locomotor activity was measured in an open-field arena as previously reported [47]. Briefly, 24 Pb180 (13 males and 11 females), 20 Pb540 (11 males and 9 females), and 20 Ctrl animals (8 males and 12 females) were tested in the open field. The setup consisted of a polypropylene box (37.6 × 30.4) surrounded by walls (17 cm), of which the floor was divided into 16 equal size rectangles (7.6 × 9.4 cm), 12 peripheral and 4 central. After placing a mouse inside the arena at one corner, its spontaneous locomotor activity was continuously recorded during 5 min with an overhead video camera. At the end of the testing session, the mouse was returned to its home cage, and the open-field arena was thoroughly cleaned before testing another animal.
Locomotor activity was quantified by the number of rectangles crossed: an animal had to place all its four legs on a given rectangle for a crossing to be counted. The total distance traveled (i.e. number of rectangles crossed) was used to quantify locomotor activity in the periphery (corresponding to the 12 rectangles adjacent to the walls) and in the central portion (corresponding to the 4 rectangles in the center of the arena) of the open-field. The behavior of animals was measured by an observer blind to treatment conditions.
Determination of blood lead levels (BLL)
After the completion of behavioral tests, animals were euthanized by cervical dislocation and blood samples were taken by heart puncture. Samples were kept chilled (− 20 °C) until measurements of BLL were performed (within 30 days of blood collection). Measurements were performed using an atomic absorption spectrometry graphite furnace (Perkin Elmer 5100), wavelength 283.3 nm, atomization at 1900 °C with a detection limit of 0.6 µg/dL.
Measurement of corticosterone
Serum corticosterone levels were evaluated in an independent group of age-matched adult male mice (n = 21). Thirty minutes after the end of a single FST or open-field testing session, animals were killed by decapitation (FST: n = 7; open-field: n = 7 from 6 litters) and approximately 0.5 ml of trunk blood was collected from each animal. Blood samples were also collected from non-manipulated (naïve) animals (n = 7 from 6 litters). Next, serum corticosterone levels were determined by radioimmunoassay as described previously [49]. Briefly, we used a specific commercial RIA kit (ICN Biomedicals Inc., Aurora, OH, USA) with an assay sensitivity of 50 ng/mL and intra assay variation coefficient of 7.1%. All samples were analyzed in duplicate.
Glutathione levels
Glutathione levels were evaluated in a separate sample of animals divided in three groups exposed to 90, 270 (0.027%, 711 μM) and 540 ppm of lead acetate trihydrate in the drinking water. Control animals received lead-free drinking water. After 60 days of lead exposure, animals were killed, brains were quickly removed from the skulls and kept on ice. Brain tissues were kept at − 20 ºC up to 2 days after had been processed and were preserved at − 80 ºC for up to 5 days before being processed.
Glutathione levels were measured using an enzymatic recycling procedure in which glutathione is sequentially oxidized by 5,5′-dithiobis- (2-nitrobenzoic acid, DTNB) and reduced by NADPH in the presence of the glutathione reductase. Then the extent of 2-nitro-5-thiobenzoic acid (TNB) formation is monitored at 412 nm and glutathione levels are evaluated by comparison to values from a standard curve. Briefly, three working solutions (0.3 mM NADPH, 6 mM DTNB, and ~ 50 units of glutathione reductase/ml) were prepared in stock buffer (125 mM Na-phosphate, 6.3 mM Na-EDTA) and the pH adjusted to 7.5. Brain tissues were deproteinized by vigorous (5 vol of 5% 5-sulfosalicylic acid to 1 vol brain tissue), followed by centrifugation at 10.000×g for 5 min. GSH and GSSG levels were determined by the glutathione reductase-DTNB recycling procedure by adding 10 µL of tissue sample to 2 µL of 2-vinylpyridine and 100µL of working solutions. The 2-vinylpyridine was used to mask the sulfhydryl group of GSH. Analyses were then carried out by comparisons to standard curves of GSH: 0.5, 1.0, 2.0 and 4.0 μM, respectively [50].
Statistical analysis
Comparisons involving BLL were carried out using univariate ANOVAs. Correlations between BLL, duration of exposure and behavioral variables were evaluated using the Pearson correlation coefficient (p < 0.05, two-tailed).
For all behavioral variables, univariate ANOVAs were carried out. Subjects were grouped by Treatment (Pb-treated X Ctrl) and Sex. The Fisher’s Least Significant Difference Test (FLSD) was used for the post-hoc analyses. Differences between experimental groups were also determined using Wilcoxon Mann–Whitney U and Fisher exact tests. All data is shown as mean and ± SEM (unless otherwise mentioned). Significance was assumed at the level of p < 0.05, two-tailed.
Results
First, to confirm that the FST is recorded in a stressful environment, we compared corticosterone plasma concentrations of naïve mice and animals tested in the FST and in the open field. As expected, corticosterone levels were significantly increased by the forced swimming experience compared to both naïve and animals tested in the open field (Fig. 1; ANOVA: F = 14.1, df = 2/14, p < 0.001). Of note, no difference in corticosterone concentrations were observed between animals tested in the open field and those that have not been manipulated (Fig. 1).
Fig. 1.
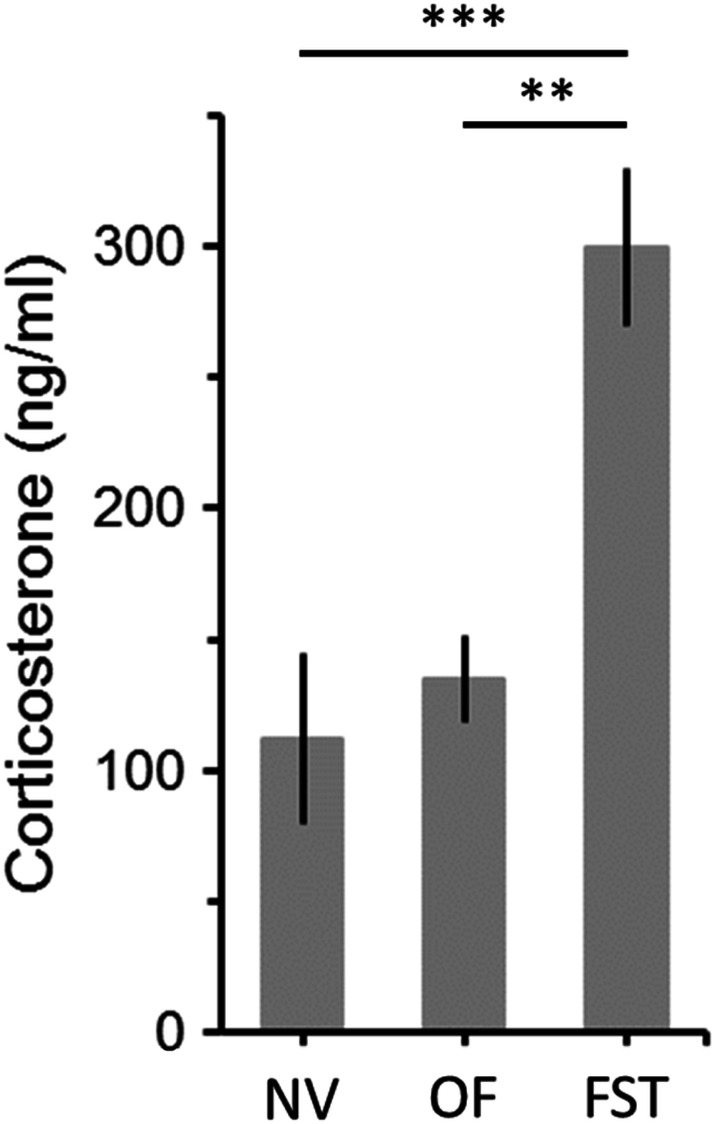
Effects of open-field (OF) and forced swimming test (FST) on plasma levels of corticosterone. Corticosterone was measured 4 min after the end of behavioral tests. Control animals were not subjected to any behavioral manipulations (naïve animals, NV). NV, n = 7 animals; OF, n = 7; FST, n = 7. Bars are means ± SEM. **p < 0.01, ***p < 0.001
Blood lead levels
In the control group, 68% of mice (n = 27) presented detectable BLL that ranged from 0.6 to 4.2 µg/dL (mean = 1.1 ± 0.2). In Pb-treated animals, while BLL displayed a wide range of concentrations. For mice treated with the 180 ppm solution, the BLL ranged from 14.3 to 62.7 µg/dL (mean = 35.1 ± 1.7) and for mice treated with the 540 ppm solution levels ranged from 52.0 to 106.9 µg/dL (mean = 75.4 ± 2.0). BLL of animals given the 540 ppm solution were significantly higher than those observed for mice given the 180 ppm one (F = 240.5, df = 1, p < 0.001). It is important to mention that we did not find a relationship between blood lead levels and time of exposure (180 ppm: Pearson's r = 0.14, P = 0.35; 540 ppm: Pearson's r = 0.25, p = 0.16).
Open-field
Confirming previous studies [51–54], Pb exposure during adulthood failed to show significant differences in spontaneous locomotor activity between controls and exposed animals in the open-field (Fig. 2a; ANOVA: F = 0.1, df = 2/63, p = 0,88). Moreover, no differences were observed regarding locomotor activity in the periphery and central portions of the arena (Table 1). Of note, the absence of significant treatment group differences was irrespective of BLL (total activity: Pearson's r = 0.07, p = 0.66; activity in the periphery: Pearson's r = 0.13, p = 0.40; activity in the central: Pearson's r = 0.12, p = 0.43).
Fig. 2.
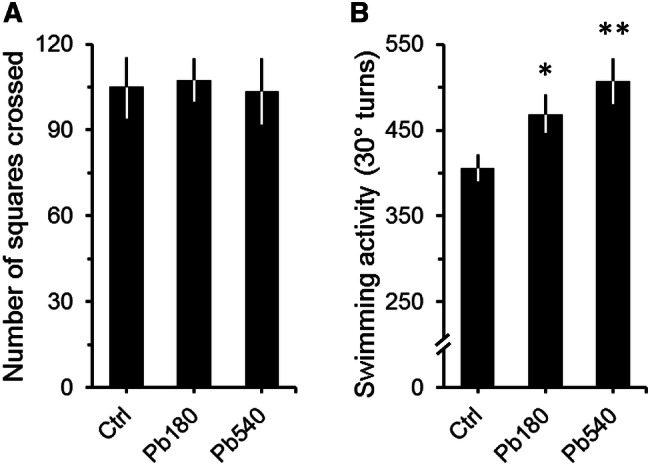
Effects of lead exposure on spontaneous locomotion (a) and swimming activity (b) of adult mice. a, b, Graphs depicting the spontaneous locomotor activity in the open-field (a) and the number of total 30° turns (turns to the left and right) in the FST (b) for controls (Ctrl) and animals that received either 180 ppm (Pb180) or 540 ppm (Pb540) of lead acetate in their ad-lib water supply. Lead exposure period was of at least 60 days (74 ± 6, max = 88 days). Ctrl group drank tap water. Note that while no significant differences were observed for locomotor activity (a), animals exposed to both doses of lead displayed significantly more 30° turns than controls (b). Ctrl, n = 57 animals from 15 litters; Pb180, n = 46 animals from 9 litters; Pb540, n = 34 from 6 litters. Bars are means ± SEM. *p < 0.05 and **p < 0.01 compared to Ctrl
Table 1.
Number of squares crossed in the open-field
| Group | Periphery | Center |
|---|---|---|
| Ctrl | 90.1 ± 8.1 | 14.9 ± 2.9 |
| Pb180 | 92.5 ± 6.0 | 15.0 ± 2.2 |
| Pb540 | 83.9 ± 9.0 | 19.7 ± 3.0 |
Values are means ± SEM
Forced swimming test
Contrary to what was observed in the open field, significant differences between Pb-exposed and control animals were found in the FST, where both 180 and 540 ppm exposed animals displayed on average more 30° turns than controls (Fig. 2b; ANOVA: F = 5.8, df = 2/134, p < 0.01). Interestingly, however, swimming activity did not differ between 180 and 540 ppm exposed groups (Fig. 2b). Moreover, these results were stable with subsequent testing sessions on different days in spite of the natural decline in overall natatory activity due to habituation to the test environment (Table 2; ANOVA: Second session, F = 3.8, df = 2/134, p < 0.05; Third session, F = 3.9, df = 2/134, p < 0.05). Of note, no differences in immobility times were observed between Pb-exposed and control animals (180 ppm, 31.5 ± 6.1 s; 540 ppm, 32.1 ± 8.4 s; Control, 40.4 ± 9.0 s; ANOVA: F = 0.3, df = 2/106, p = 0.74).
Table 2.
Number of total 30° turns in the FST
| Group | First session | Second session | Third session |
|---|---|---|---|
| Ctrl | 404.1 ± 19.1 | 308.3 ± 19.7 | 248.1 ± 17.1 |
| Pb180 | 467.7 ± 21.2* | 375.4 ± 21.6* | 316.1 ± 18.7** |
| Pb540 | 509.8 ± 25.6** | 383.1 ± 26.1* | 305.0 ± 22.7* |
Values are means ± SEM
*p < 0.05 and **p < 0.01 compared to Ctrl
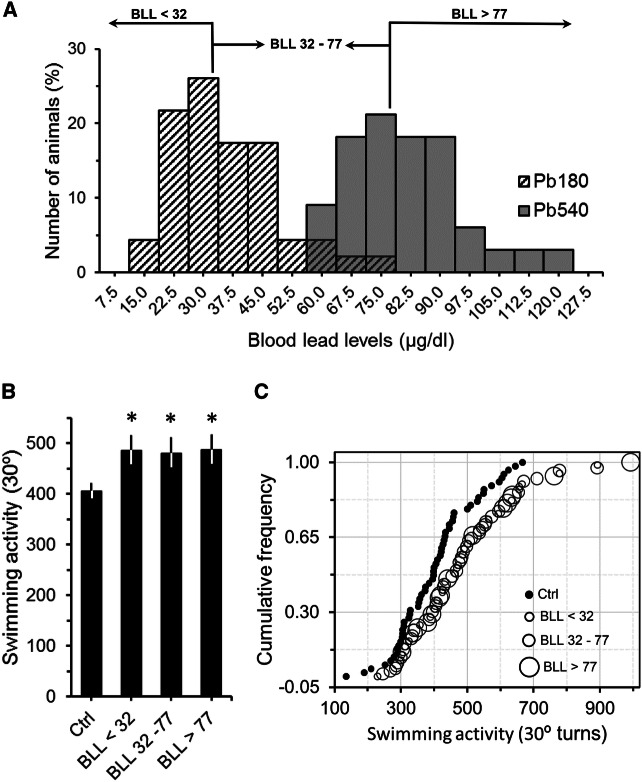
The lack of significant differences between the swimming activity of 180 and 540 ppm groups suggests that swimming activity is independent of the levels of Pb in the blood. In fact, swimming activity did not correlate with either BLL or with the exposure time (swimming activity vs BLL, Pearson's r = 0.07, p = 0.54; swimming activity vs time of exposure, Pearson's r = 0.01, p = 0.96). Furthermore, when we compared the swimming activity of animals with BLL lower than 32 µg/dL (i.e. values to the left of the peak of the 180 ppm distribution in Fig. 3a) to the swimming activity of animals with BLL between 32 and 77 µg/dL, and with BLL greater than 77 µg/dL (i.e. values between the peaks of the 180 and 540 ppm distributions, and values to the right of the peak of the 540 ppm distribution, respectively, Fig. 3a) we still did not find any significant differences (Fig. 3b). All exposed groups, even animals with BLL lower than 32 µg/dL, had swimming activity values significantly higher than controls (Fig. 3b; ANOVA: F = 4.9, df = 3/134, p < 0.01). Moreover, similar results were observed when we look at the cumulative frequency distribution of the data (Fig. 3c). Thus, based on these findings, it was not possible to determine at which BLL range animals do not show increased swimming activity. To explore this issue, we used an independent sample of adult mice that were exposed to 90 ppm of Pb for at least two months and then submitted to the FST. The BLL of these animals ranged from 4.4 to 16.6 µg/dL (mean = 9.6 ± 0.8). Figure 4 shows that although BLL were significantly higher than controls (Fig. 4a; ANOVA: F = 184.2, df = 1/35, p < 0.001), animals exposed to 90 ppm of Pb did not show increased swimming activity in the FST (Fig. 4b; ANOVA: F = 0.71, df = 1/36, p = 0.41). Therefore, our overall data indicate that adult animals with BLL higher than 20 µg/dL show increased swimming activity, which could be an indicative of lead neurotoxicity.
Fig. 3.
Sample stratification based on blood lead levels (BLL). a Histogram plotting BLL of animals that received 180 ppm (Pb180) or 540 ppm (Pb540) of lead acetate in their ad-lib water supply (same sample depicted in Fig. 2b). Note that BLL frequencies for both lead doses were consistent with a normal distribution. Cut points for low (< 32 µg/dL), middle (between 32 and 77 µg/dL) and high (> 77 µg/dL) BLL were based on the mean of each distribution (Pb180, mean = 35.1; Pb540, mean = 75.4). Vertical lines and horizontal arrows delineate data cut points and ranges, respectively. b Graph showing the averaged swimming activity in the FST as a function of sample stratification depicted in (a). Note that, regardless of BLL (low, middle or high), all lead exposed groups had an averaged swimming activity significantly higher than that observed for controls (Ctrl). Bars are means ± SEM. *p < 0.05 compared to Ctrl. c Cumulative frequency of animals plotted as a function of their total swimming activity for control (filled circles, Ctrl) and lead exposed animals (open circles). For lead exposed animals, symbol's size reflects BLL (low, middle or high). Note that the BLL distribution is skewed to the right relative to the Ctrl one and that BLL symbol sizes are scattered in no orderly manner (i.e. low to high levels or high to low), emphasizing an increase in swimming activity irrespective of BLL magnitude. Ctrl, n = 57 animals from 15 litters; Pb180, n = 46 animals from 9 litters; Pb540, n = 34 from 6 litters
Fig. 4.
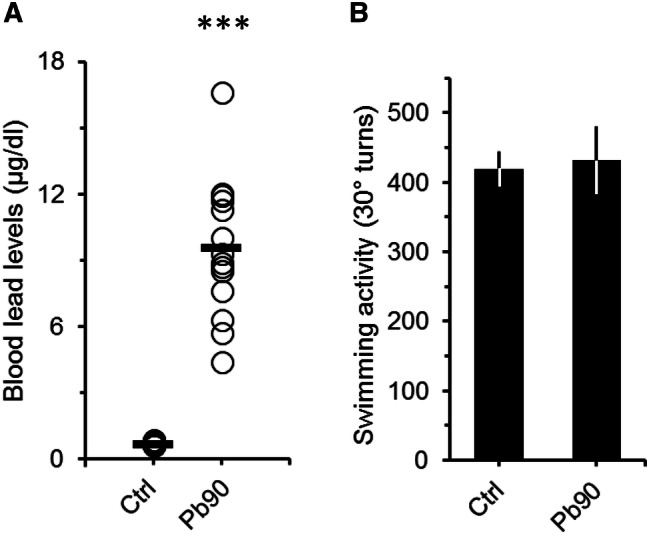
Blood lead levels (a) and swimming activity (b) of adult mice exposed to a lower dosage of lead. a Plot showing the blood lead levels (BLL) of controls (Ctrl) and mice that received 90 ppm (Pb90) of lead acetate in their ad-lib water supply. Lead exposure period was of at least 60 days (66 ± 5, max = 72). Controls drank tap water. Open circles represent BLL of a single animal. Black horizontal bars are the averaged BLL across animals. ***p < 0.001 compared to Ctrl. b Graph depicting the number of total 30° turns (turns to the left and right) in the FST for controls (Ctrl) and Pb90 animals. Note that the averaged swimming activity of the Pb90 animals was similar to that of the control group. Ctrl, n = 21 animals from 4 litters; Pb90, n = 17 animals from 3 litters. Bars are means ± SEM
Brain glutathione levels
Oxidative stress is a likely cause of lead-induced brain toxicity since the brain is particularly vulnerable to oxidative stress due to: (1) its high oxygen utilization, (2) weaker antioxidant capacity and (3) high content of oxidable polyunsaturated fatty acids [55, 56]. Thus, we next evaluated the effects of lead exposure on glutathione levels in an independent sample of adult Pb-exposed animals. Glutathione is one of the most important antioxidant systems, assisting in the detoxification and excretion of heavy metals. Interestingly, we only found significant differences in glutathione levels in animals that presented BLL higher than 40 µg/dL (Fig. 5; ANOVA: F = 9.4, df = 3/50, p < 0.001). In this regard, our behavioral data suggest that swimming activity is a very sensitive indicator of lead neurotoxicity.
Fig. 5.
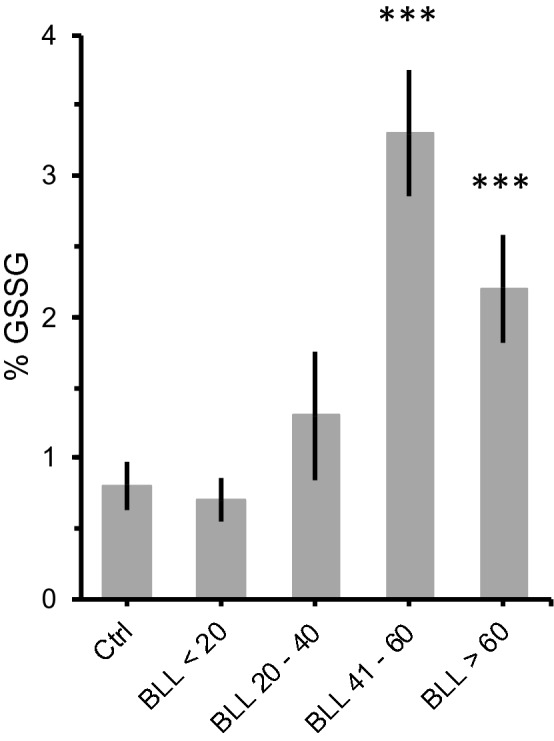
Effect of lead exposure on oxidative stress. Mean percentage of oxidized glutathione (% GSSG) in the brain of mice with different blood lead levels (BLL, in µg/mL). Animals were exposed to either 180 ppm (Pb180) or 540 ppm (Pb540) of lead acetate in their ad-lib water supply. Lead exposure period was of at least 60 days (68 ± 2, max = 73). Controls (Ctrl) drank tap water. Note that significant differences in % GSSG were only observed for BLL of 60 µg/dL or higher whereas an increase in swimming activity was evident for BLL lower than 32 µg/dL (Fig. 3). Ctrl, n = 18 animals from 4 litters; BLL < 20, n = 15 animals from 3 litters; BLL 20–40, n = 6 animals from 2 litters; BLL 41–60, n = 5 animals from 3 litters; BLL > 60, n = 7 animals from 2 litters. Bars are means ± SEM. ***p < 0.001 compared to Ctrl
Discussion
The current study was designed to evaluate the effects of lead exposure on the swimming activity and immobile behavior of adult mice in the FST. While no differences were found between Pb-treated and control groups in the open-field, marked differences were observed in the FST regarding swimming activity. The absence of differences between Pb-treated and Control groups is in agreement with a number of previous findings, where lead exposure was done either during adolescence or adulthood [51–54]. However, there is a vast literature showing that lead toxicity increases locomotor activity in the open-field of animals exposed during prenatal and lactation periods [36, 37, 39, 57]. The most likely explanation for such discrepancy is that lead neurotoxicity early during development is far more prominent since lead interferes with a series of neurodevelopmental events such as cell differentiation, synaptogenesis, myelination, programmed cell death, among others [7, 9].
Furthermore, regarding the observed lack of significant group differences with respect to spontaneous activity in the open-field, it is important to emphasize that we observed similar corticosterone levels between animals tested in the open-field and those that have not been manipulated. Moreover, significant differences, both behaviorally and related to corticosterone levels were only observed for mice tested in the FST. These findings support a link between lead toxicity and coping with stress. While in the open field, animals spend a great time in the corner of the testing arena [58] and frequently stop walking to perform behaviors such as grooming, rearing and sniffing, during a free-swimming session, mice spend most of the time swimming close to the wall of the testing arena attempting to escape from a frightening test situation [48]. In fact, when first placed in the aquatic arena, the animal’s behavior is typically characterized by vigorous swimming accompanied by frantic clawing at the walls of the plastic cylinder [32, 48]. As the test progress, animals display a progressive increase in the frequency and duration of episodes of immobile floating and a decrease of escape-directed behaviors [32, 34, 48, 59]. It has been proposed that the switch from active to passive coping strategies is an adaptive learned response which depends on the activation of the dopaminergic mesocorticolimbic system [34, 60]. Particularly, high levels of tonic dopamine activation support the execution of costly and risky defensive responses which characterize active coping strategies, whereas reduced levels of tonic dopamine block such responses [34, 60, 61]. Interestingly, Tye and collaborators (2013) have demonstrated that optogenetic phasic-tonic activation of ventral tegmental area dopaminergic neurons increase escape directed behavior (kicking activity) during the FST but not ambulation in open-field [61]. Therefore, one plausible explanation for the increased swimming activity observed in mice exposed to 180 and 540 ppm of lead is that it reflects a maladaptive coping strategy against an unescapable test situation caused by an overactive dopaminergic mesocorticolimbic system. This idea is in accordance with behavioral studies postulating that chronic lead exposure at low doses facilitates dopamine neurotransmission in the nucleus accumbens [62, 63]. Moreover, it is important to note that the responsiveness of mesolimbic-cortical dopaminergic circuitry during aversive situations depends on inputs from ventral hippocampus and amygdala [34]. These areas are targets for corticosteroid hormones released under stress [34, 64, 65]. Thus, natatory hyperactivity of lead-exposed animals in the FST may be also associated to toxic effects of lead on the function of the hypothalamic-pituitary-adrenal (HPA) axis [24, 66, 67]. Future studies are needed to examine the role of the mesolimbic system on lead toxicity stress-related coping strategies. Yet how lead toxicity could be affecting the HPA axis function? Lead exposure may modify stress responses by increasing HPA axis responsiveness [24, 68]. For instance, stress induced by cold temperatures leads to a prominent increase in corticosterone levels in rats chronically exposed to lead but not in control ones [24]. In humans, it was observed that the level of lead exposure during prenatal or early postnatal life is associated with significantly heightened salivary cortisol responses to acute stress [69].
Coping with stress in the FST has also been discussed in terms of immobile behavior [34, 70]. Based on our findings, one could expect that since lead-exposed animals displayed increased swimming activity, we should have also observed a decrease in immobility times for these animals. However, although immobility times were smaller in the mice exposed to 180 and 540 ppm of lead compared to the control group, this difference was slim and did not reach statistical significance. These results may be explained since average immobility times were only around 10% of the total time of the testing session. Moreover, previous findings from our group have demonstrated that there is not a direct relationship between 30° turn swimming activity and immobility times in the FST [31, 32].
Lastly, our analysis of glutathione (GSH) levels revealed that lead exposure during adulthood induces significant alterations in GSH metabolism. GSH is a coenzyme that can effectively neutralize oxidants, thus protecting cells against oxidative stress caused, for instance, by heavy metal exposure [55, 71]. Moreover, GSH has heightened functional importance in the central nervous system because, compared to other enzymes such as superoxide, catalase and glutathione peroxidase, its antioxidant activity is significantly greater in the brain than in other organs [72]. In response to high oxidative stress levels, GSH is oxidized to the dimeric glutathione peroxidase (GSSG), which, in turn, neutralizes hydrogen peroxide and other peroxides. The ratio of GSH/GSSG has been considered an effective indicator of lead toxicity [45]. At normal levels of oxidative stress, the ratio between GSSG and GSH is usually less than 1%, with no net loss of glutathione through oxidation. However, under excessive oxidative stress (i.e. lead poisoning) the activity of GSSG reductase is suppressed, leading to an accumulation of GSSG [50, 71]. In addition, lead binds to sulfhydryl groups of proteins, depleting the reserves of reduced GSH [73]. Interestingly, our results indicate a significant increase of %GSSG in lead exposed animals. However, such %GSSG raise was only observed at BLL above 40 μg/dL whereas our findings in the FST indicate that the BLL threshold to detect significant differences in swimming activity was between 20 and 35 µg/dL. This discrepancy suggests that oxidative stress is not the sole mechanism responsible for the increase in swimming activity promoted by lead. Accordingly, the neurotoxicity of lead has been associated with mechanisms such as the capability of lead to substitute for calcium and alter calcium homeostasis [7] and the inhibition of N-methyl-d-aspartate receptors [74]. Future studies are needed to systematically explore these possibilities in an effort to elucidate the underlying neurobehavioral mechanisms of lead poisoning in animal models of adult lead exposure.
Overall our results confirm the hypothesis that lead-exposed mice present increased swimming activity in the FST and suggests that cumulative lead exposure (from weaning to adulthood) may affect adaptative coping strategies to stressful situations. Moreover, the fact that significant differences in swimming activity between controls and lead-exposed animals were observed at relatively low BLL raises the concern that the effects of lead exposure on the dopaminergic and HPA systems may be taking place even at lower levels of exposure. With that in mind, the FST can be a valuable asset in studies investigating how lead exposure is associated to neurochemical changes due to stress, as well as functioning as a simple tool to verify lead poisoning in adult animal models using low doses of lead.
Acknowledgements
This work was supported by grants from: Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ-BRAZIL), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-BRAZIL) and SR2-UERJ. The authors are thankful to to Ulisses Risso for animal care.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
References
- 1.Benson SM, Talbott EO, Brink LAL, et al. Environmental lead and childhood blood lead levels in US children: NHANES, 1999–2006. Arch Environ Occup Heal. 2017;72:70–78. doi: 10.1080/19338244.2016.1157454. [DOI] [PubMed] [Google Scholar]
- 2.Bodeau-Livinec F, Glorennec P, Cot M, et al. Elevated blood lead levels in infants and mothers in benin and potential sources of exposure. Int J Environ Res Public Health. 2016;13:1–14. doi: 10.3390/ijerph13030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olympio KPK, Gonçalves CG, Salles FJ, et al. What are the blood lead levels of children living in Latin America and the Caribbean? Environ Int. 2017;101:46–58. doi: 10.1016/j.envint.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Pelc W, Pawlas N, Dobrakowski M, Kasperczyk S. Environmental and socioeconomic factors contributing to elevated blood lead levels in children from an industrial area of Upper Silesia. Environ Toxicol Chem. 2016;35:2597–2603. doi: 10.1002/etc.3429. [DOI] [PubMed] [Google Scholar]
- 5.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:0741–0749. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12:57–65. [PubMed] [Google Scholar]
- 7.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 8.Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Costa LG, Aschner M, Vitalone A, et al. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenga C, Gangemi S, Alibrandi A, et al. Relationship between lead exposure and mild cognitive impairment. J Prev Med Hyg. 2016;57:205–210. [PMC free article] [PubMed] [Google Scholar]
- 11.Murata K, Iwata T, Dakeishi M, Karita K. Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J Occup Health. 2009;51:1–12. doi: 10.1539/joh.k8003. [DOI] [PubMed] [Google Scholar]
- 12.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115:483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazi TG, Shah F, Afridi HI. Occupational and environmental lead exposure to adolescent workers in battery recycling workshops. Toxicol Ind Health. 2015;31:1288–1295. doi: 10.1177/0748233713485883. [DOI] [PubMed] [Google Scholar]
- 14.La-Llave-León O, Salas Pacheco JM, Estrada Martínez S, et al. The relationship between blood lead levels and occupational exposure in a pregnant population. BMC Public Health. 2016;16:1–9. doi: 10.1186/s12889-016-3902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laidlaw MAS, Filippelli G, Mielke H, et al. Lead exposure at firing ranges - a review. Environ Heal A Glob Access Sci Source. 2017;16:1–15. doi: 10.1186/s12940-017-0246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie LH, Wright RO, Bellinger DC, et al. Blood lead levels and cumulative blood lead index (CBLI) as predictors of late neurodevelopment in lead poisoned children. Biomarkers. 2011;16:517–524. doi: 10.3109/1354750X.2011.604133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santa Maria MP, Hill BD, Kline J. Lead (Pb) neurotoxicology and cognition. Appl Neuropsychol Child. 2018 doi: 10.1080/21622965.2018.1428803. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DC. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicology. 2008;29:828–832. doi: 10.1016/j.neuro.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healey N. Lead toxicity, vulnerable subpopulations and emergency preparedness. Radiat Prot Dosimetry. 2009;134:1–9. doi: 10.1093/rpd/ncp068. [DOI] [PubMed] [Google Scholar]
- 20.Peters JL, Kubzansky L, McNeely E, et al. Stress as a potential modifier of the impact of lead levels on blood pressure: the normative aging study. Environ Health Perspect. 2007;115:1154–1159. doi: 10.1289/ehp.10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice DC. Animal models of cognitive impairment produced by developmental lead exposure. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. Boca Raton: CRC Press/Taylor and Francis Group LLC.; 2006. [PubMed] [Google Scholar]
- 22.Cory-Slechta DA, Virgolini MB, Rossi-George A, et al. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 23.Glass TA, Bandeen-Roche K, McAtee M, et al. Neighborhood psychosocial hazards and the association of cumulative lead dose with cognitive function in older adults. Am J Epidemiol. 2009;169:683–692. doi: 10.1093/aje/kwn390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virgolini MB, Chen K, Weston DD, et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87:469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto BK, Kutscher CL. Drug and food-deprivation modulation of activity in rats given chronic dietary lead: significance of type of activity measure. Pharmacol Biochem Behav. 2002;15:505–512. doi: 10.1016/0091-3057(81)90285-9. [DOI] [PubMed] [Google Scholar]
- 26.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 28.Bulduk S, Canbeyli R. Effect of inescapable tones on behavioral despair in Wistar rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:471–475. doi: 10.1016/j.pnpbp.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Christianson JP, Drugan RC. Intermittent cold water swim stress increases immobility and interferes with escape performance in rat. Behav Brain Res. 2005;165:58–62. doi: 10.1016/j.bbr.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Drugan RC, Eren S, Hazi A, et al. Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol Biochem Behav. 2005;82:397–403. doi: 10.1016/j.pbb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Filgueiras CC, Abreu-Villaça Y, Krahe TE, Manhães AC. Unilateral hemispherectomy at adulthood asymmetrically affects immobile behavior of male Swiss mice. Behav Brain Res. 2006;172:33–38. doi: 10.1016/j.bbr.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Krahe TE, Filgueiras CC, Schmidt SL. Effects of rotational side preferences on immobile behavior of normal mice in the forced swimming test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2002;26:169–176. doi: 10.1016/S0278-5846(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 33.Thierry B, Steru L, Chermat R, Simon P. Searching-waiting strategy: a candidate for an evolutionary model of depression? Behav Neural Biol. 1984;41:180–189. doi: 10.1016/S0163-1047(84)90555-7. [DOI] [PubMed] [Google Scholar]
- 34.de Kloet ER, Molendijk ML. Coping with the forced swim stressor: Towards understanding an adaptive mechanism. Neural Plast. 2016;2016:1–13. doi: 10.1155/2016/6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leasure JL, Giddabasappa A, Chaney S, et al. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: Behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–495. doi: 10.1016/S0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 37.Silbergeld EK, Goldberg AM. Hyperactivity: a lead induced behavior disorder. Environ Health Perspect. 1974;7:227–232. doi: 10.1289/ehp.747227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szczerbak G, Nowak P, Kostrzewa RM, Brus R. Maternal lead exposure produces long-term enhancement of dopaminergic reactivity in rat offspring. Neurochem Res. 2007;32:1791–1798. doi: 10.1007/s11064-007-9306-0. [DOI] [PubMed] [Google Scholar]
- 39.Trombini TV, Pedroso CG, Ponce D, et al. Developmental lead exposure in rats: Is a behavioral sequel extended at F2 generation? Pharmacol Biochem Behav. 2001;68:743–751. doi: 10.1016/S0091-3057(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 40.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev. 1997;25:335–358. doi: 10.1016/S0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 41.Coyle J, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;80(262):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 42.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 43.Dobrakowski M, Pawlas N, Hudziec E, et al. Glutathione, glutathione-related enzymes, and oxidative stress in individuals with subacute occupational exposure to lead. Environ Toxicol Pharmacol. 2016;45:235–240. doi: 10.1016/j.etap.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Engstrom AK, Xia Z. Lead exposure in late adolescence through adulthood impairs short-term spatial memory and the neuronal differentiation of adult-born cells in C57BL/6 male mice. Neurosci Lett. 2017;661:108–113. doi: 10.1016/j.neulet.2017.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan Y, Zhao X, Yu J, et al. Lead-induced oxidative damage in rats/mice: a meta-analysis. J Trace Elem Med Biol. 2020;58:126443. doi: 10.1016/j.jtemb.2019.126443. [DOI] [PubMed] [Google Scholar]
- 46.Carocci A, Catalano A, Lauria G, et al. Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol. 2016;238:45–67. doi: 10.1007/398_2015_5003. [DOI] [PubMed] [Google Scholar]
- 47.Filgueiras CC, Ribeiro-Carvalho A, Nunes F, et al. Early ethanol exposure in mice increases laterality of rotational side preference in the free-swimming test. Pharmacol Biochem Behav. 2009;93:148–154. doi: 10.1016/j.pbb.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt SL, Filgueiras CC, Krahe TE. Effects of sex and laterality on the rotatory swimming behavior of normal mice. Physiol Behav. 1999;65:607–616. doi: 10.1016/S0031-9384(98)00184-X. [DOI] [PubMed] [Google Scholar]
- 49.Dutra-Tavares AC, Manhães AC, Silva JO, et al. Locomotor response to acute nicotine in adolescent mice is altered by maternal undernutrition during lactation. Int J Dev Neurosci. 2015;47:278–285. doi: 10.1016/j.ijdevneu.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/S0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 51.Correa M, Miquel M, Sanchis-Segura C, Aragon CMG. Effects of chronic lead administration on ethanol-induced locomotor and brain catalase activity. Alcohol. 1999;19:43–49. doi: 10.1016/S0741-8329(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 52.Nieto-Fernandez FE, Ruiz A, Ntukogu N, et al. Short term lead exposure induces a stress-like response in adult mice. Med Sci Monit. 2006;12:325–329. [PubMed] [Google Scholar]
- 53.Rice DC. PCBs and behavioral impairment: are there lessons we can learn from lead? Neurotoxicol Teratol. 1996;18:229–232. doi: 10.1016/S0892-0362(96)90003-3. [DOI] [PubMed] [Google Scholar]
- 54.Salinas JA, Huff NC. Lead and spatial vs. cued open field performance. Neurotoxicol Teratol. 2002;24:551–557. doi: 10.1016/S0892-0362(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 55.Abdulmajeed WI, Sulieman HB, Zubayr MO, et al. Honey prevents neurobehavioural deficit and oxidative stress induced by lead acetate exposure in male wistar rats- a preliminary study. Metab Brain Dis. 2015;31:37–44. doi: 10.1007/s11011-015-9733-6. [DOI] [PubMed] [Google Scholar]
- 56.Villeda-Hernández J, Barroso-Moguel R, Méndez-Armenta M, et al. Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Res Bull. 2001;55:247–251. doi: 10.1016/S0361-9230(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 57.Rodrigues ALS, Rocha JBT, Mello CF, Souza DO. Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacol Toxicol. 1996;79:150–156. doi: 10.1111/j.1600-0773.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 58.Ennaceur A, Michalikova S, Chazot PL. Do rats really express neophobia towards novel objects? Experimental evidence from exposure to novelty and to an object recognition task in an open space and an enclosed space. Behav Brain Res. 2009;197:417–434. doi: 10.1016/j.bbr.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Commissaris RL, Tavakoli-Nezhad M, Barron AJ, Pitts DK. Effects of chronic low-level oral lead exposure on prepulse inhibition of acoustic startle in the rat. Neurotoxicol Teratol. 2000;22:55–60. doi: 10.1016/S0892-0362(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 63.Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- 64.McKlveen JM, Myers B, Flak JN, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014;35:180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicology. 2006;27:11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 67.White LD, Cory-Slechta DA, Gilbert ME, et al. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Servatius RJ, Ottenweller JE, Bergen MT, et al. Persistent stress-induced sensitization of adrenocortical and startle responses. Physiol Behav. 1994;56:945–954. doi: 10.1016/0031-9384(94)90328-X. [DOI] [PubMed] [Google Scholar]
- 69.Gump BB, Stewart P, Reihman J, et al. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ Health Perspect. 2008;116:249–255. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anyan J, Amir S. Too depressed to swim or too afraid to stop? A reinterpretation of the forced swim test as a measure of anxiety-like behavior. Neuropsychopharmacology. 2018;43:931–933. doi: 10.1038/npp.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jozefczak M, Remans T, Vangronsveld J, Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012;13:3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ralf D. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 73.Hunaiti AA, Soud M. Effect of lead concentration on the level of glutathione, glutathione S-transferase, reductase and peroxidase in human blood. Sci Total Environ. 2000;248:45–50. doi: 10.1016/S0048-9697(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 74.Li XM, Gu Y, She JQ, et al. Lead inhibited N-methyl-d-aspartate receptor-independent long-term potentiation involved ryanodine-sensitive calcium stores in rat hippocampal area CA1. Neuroscience. 2006;139:463–473. doi: 10.1016/j.neuroscience.2005.12.033. [DOI] [PubMed] [Google Scholar]