Abstract
Glycosaminoglycans (GAGs) have been used to diminish the deleterious effects associated with aging by preventing the destruction of cartilage, bone, discs, and skin. The objective of this study was to evaluate the anti-aging effect of a newly prepared GAG derived from bumblebee (Bombus terrestris) queen (BTQG, 10 mg/kg). Gryllus bimaculatus (Gb, cricket) GAG (GbG, 10 mg/kg) or glucosamine sulfate (GS) was used as a positive control. N-glycans derived from BTQG contained hexose polymers including Hex4HexNAc3Pen1, Hex9, and Hex5HexNAc3dHex2 as the primary components. The GAGs were intraperitoneally administered to 14-month-old aged rats for 1 month. BTQG reduced the serum levels of free fatty acid, alkaline phosphatase (ALP), glutamate pyruvate transaminase (GPT), creatinine, and blood urea nitrogen (BUN), showing hepato-and renal-protective effects with anti-lipidemic activities comparable to GS. The changes of gene expression profile of liver tissue by cDNA microarray showed the simultaneous upregulation of 36 genes in the BTQG-treated rat group compared to the control group, including secretogranin II (Scg2), Activator (AP)-1-regulated protein-related reactive oxygen species (ROS) DNA damage repair, metallothionein 1a, and alpha-2 macroglobulin. The BTQG-treated group also showed 417 downregulated genes, including vimentin, moesin, and mitochondrial carbonic anhydrase. Insect glycosaminoglycan from the bumblebee (B. terrestris) queen may help decelerate the aging stage by ameliorating the aging effects on circulation, and liver and kidney function.
Keywords: Bumblebee (B. terrestris) queen glycosaminoglycan, Aged rat
Introduction
Aging is the natural progression of the body over time and is often associated with undesirable effects. Aging is associated with an increased prevalence of deteriorating diseases associated with an increased risk of cardiovascular disease and death [1]. Recently, it has been reported that glycosaminoglycan (GAG) heparin air sol could ameliorate pulmonary function in chronic obstructive pulmonary disease (COPD) patients in England [2]. Many insect GAGs have been found to produce nitric oxide in calf pulmonary artery endothelial cells and stimulate endothelial nitric oxide synthase [3].
Here, we showed that the intraperitoneal administration of natural insect GAGs to old rats exerted renal protective effects by decreasing the serum levels of blood urea nitrogen (BUN) and creatinine kinase. GAGs have been associated with age-related changes in matrix components by altering the structure of heparin sulfate on the surface of outgrowth endothelial cells. As an apicultural product, Bombus terrestris queen (BTQ) extract had the highest potential efficacy among all bumblebee extracts tested for treating inflammation in Sprague–Dawley (SD) rats [4]. It significantly reduced paw edema. We previously reported that the glycans from another bumblebee (B. ignitus) queen (longer-lived than other workers or males, so suitable for anti-aging) showed anti-cancer activity in in vitro and in vivo studies by strengthening the endocellular matrix [5]. Thus, we prepared GAG derived from a large number of BTQs or G. bimaculatus (cricket) to test and compare its anti-aging properties. Aging involves the progressive decline in the physiological capacity of an organism. It is manifested by accumulated alterations and destabilization across all systems. Aging is also associated with a differential gene expression pattern among metabolic and biosynthetic genes indicative of a decreased stress response. B. terrestris queen or cricket GAGs can be used to prevent kidney and liver diseases. They might also have applications in the field of tissue engineering as biomaterials and novel therapeutics.
GAGs from natural sources have been used as anti-aging agents in skin-based dermo-cosmetic treatments such as hyaluronic acid, hyaluronan, and glycosaminoglycan [6, 7]. As an apicultural product, buff-tailed BTQ has been produced by a rearing system through artificial pollination. Although some studies have isolated and characterized chitin from buff-tailed bumblebees (B. terrestris) [8], no animal studies have reported investigations using buff-tailed BTQ glycosaminoglycan (BTQG). Thus, we prepared GAGs derived from BTQs to test their anti-aging properties. Anti-aging effects are based on two mechanisms. One mechanism is through the removal of free radicals that cause damage to the aged cells of an organism. Another mechanism is by activating the cellular repair system with proper regulation of metabolic and biosynthetic genes [9]. Due to their biological activity, N-glycan of queen bumblebee origin and its fractions, including glycosaminoglycan, might have potential as cosmetics and functional food materials. In addition, they have been reported to possess antiphlogistic and arthritis- curing effects. Thus, queen bee origin GAG (including N-glycan) might have the potential for medicine development. Such applications can improve public health as well as the income of bee-keepers and bee-raising farmers. In this study, we reported that GAG from BTQ displayed anti-aging properties. Thus, it might have potential as an anti-aging agent. We also demonstrated the potential value of buff-tailed BTQG compared to field cricket Gb glycosaminoglycan in ameliorating the deleterious aspects of aging by measuring serum parameters and gene expression patterns of 14-month-aged rats administered with these insect GAGs for one month.
Materials and methods
Materials
Bombus terrestris bumblebee queens were reared and freeze-dried in the Department of Agricultural Biology, National Institute of Agricultural Sciences, Korea. G. bimaculatus was obtained from a cricket farm in Hwasung, Korea for use as a positive control material. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of insect glycosaminoglycan
Buff-tailed BTQG and GbG were purified using a previously reported method [5, 10], involving defatting with ethanol and acetone, protein enzymatic hydrolysis by treatment with alcalase (Sigma Aldrich, USA), protein precipitation with trichloroacetic acid (5%), impure materials cleansing with detergent such as cetylpyridinium chloride (5%), and dissolving of non-glycosaminoglycan with 2.5 M NaCl. GAGs were then acquired through cold ethanol precipitation, centrifugation, dialysis against water, and freeze-dry.
These crude GAGs were loaded onto a DEAE Sephadex A-25 gel chromatography column and a linear sodium chloride (NaCl) gradient from 0 to 2.5 M NaCl in phosphate buffer was applied to obtain pure GAG. To determine the purity, GAG and oligosaccharide mixtures digested by GAG enzymes (heparinase I, II, III, etc.) were fractionated by strong anion exchange (SAX) high-performance liquid chromatography, affording oligosaccharides. Liquid chromatography with a mass detector (LC–MS, 2020 and AXIMA Resonance, Shimadzu) was used to ascertain the purity of the GAGs for structural characterization.
N-glycan sequence analysis derived from BTQG
To evaluate the GAG structure by its N-glycan sequence, the DEAE Sephadex A-25 gel 0.5 M NaCl fraction of buff-tailed BTQG (BTQG 0.5 M) was further purified by enzymatic digestion (1000 units of PNGase F, New England Biolabs, MA, USA) to obtain low molecular weight N-glycan, followed by N-glycan enrichment via solid-phase (Graphitized carbon cartilage Grace davision discovery Sciences, IL, USA) extraction according to a previously published report [5, 11]. The N-glycan-derived BTQG was then chromatographed using 50% acetonitrile eluent and 2,5-dihydroxybenzoic acid as a matrix for mass spectrometry (MS) and MS/MS analysis using a matrix-assisted laser-ionization time-of-flight (MALDI-TOF) analyzer (AXIMA Resonance, Shimadzu) for detecting N-glycans from BTQG.
Neutral mono-sugar composition of BTQG by GC–MS analysis
To induce the trimethylsilylation of the samples, isolated BTQ and its N-glycan was hydrolyzed by 1 N HCl and then dried with N2 gas. The dried samples were added to pyridine and N, O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMS) (Sigma-Aldrich, USA), reacted for 30 min at 65 °C, and then injected onto the GC–MS. To confirm the target components, we also made a TMS mono- and di-carbohydrate standard mixture and blended it with a derivative sample of the TMS standard mixture. GC–MS analysis was performed using an Agilent 6890 Gas Chromatograph coupled to a 5973 N Mass-Selective Detector (Agilent, USA) [5].
Animal study and GAG treatment on aged rat
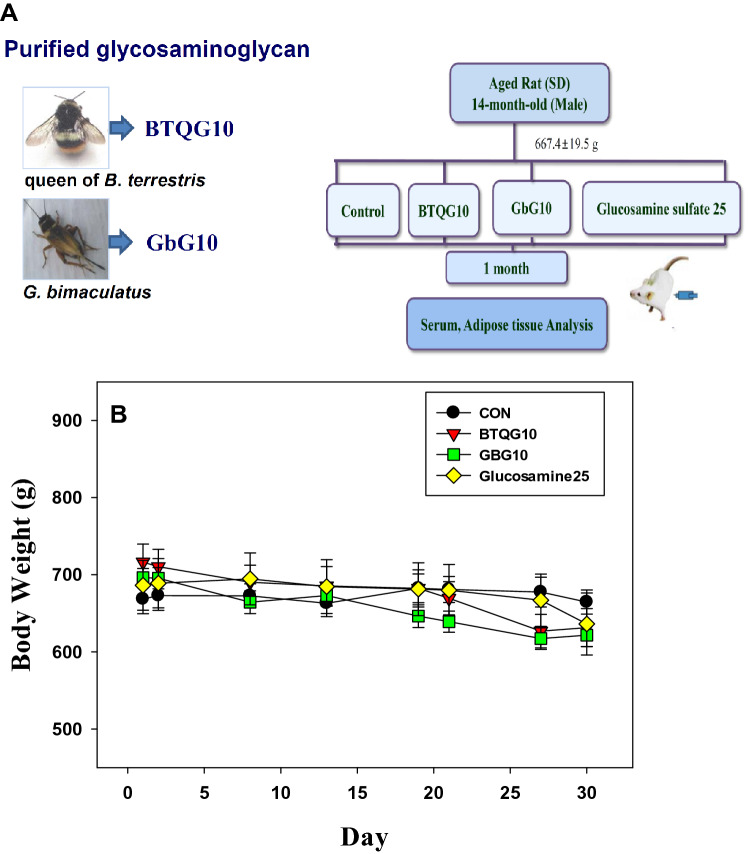
Sprague Dawley (SD) rats (male, 8-months-old) were supplied by Samtako Co. Ltd. (Osan, Korea). All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. All experiments were approved by the Laboratory Animal Ethical Committee of the National Institute of Agricultural Sciences, RDA, Korea (NAS201705), and followed the national guidelines for the care and use of animals. The rats were acclimated for six months under normal husbandry conditions and fed a normal diet (AIN-76A rodent diet, Research Diet, Inc., USA) and water ad libitum. At 14 months of age, the rats were segregated into four treatment groups of 12 rats each and distributed according to similar weights (667.4 ± 19.5 g). The treatments were given in phosphate-buffered saline (PBS) daily and administrated intraperitoneally.
The groups were control (CON), 10 mg/kg BTQG (BTQG10), 10 mg/kg GbG (GbG10), and 25 mg/kg glucosamine sulfate (GS25) (Whole Food Market, Austin, TX, USA). Each group was maintained on a normal diet (AIN-76A rodent diet, Research Diet) and treated for one month (Fig. 1a).
Fig. 1.
Animal study of aged rats treated with bumblebee queen- or cricket-glycosaminoglycan (BTQG or GbG) over a month. a Glycosaminoglycan of bumblebee queen- or cricket-glycosaminoglycan (BTQG or GbG) were purified from queen of B. terrestris or G. bimaculatus. Their animal scheme of aged rats continued for a one month with intraperitoneally sample or control treatment. b Glycosaminoglycan of bumblebee queen- or cricket-glycosaminoglycan (BTQG or GbG) were purified and their animal scheme of aged rats. BTQG10: bumblebee (queen of B. terrestris) glycosaminoglycan 10 mg/kg; GbG10: G. bimaculatus glycosaminoglycan 10 mg/kg; GS25: Glucosamine sulfate 25 mg/kg. b Effect of BTQG or GbG on body weight change in aged rat over one month. CON: PBS (as a vehicle) treated with murine normal diet. Each value represents mean ± SE. *Statistically significant from the control (p < 0.05) (N = 12)
Blood sampling and sero-biochemical assay
In the four groups [CON, BTQG10, GbG10, GS25 (n = 12)], blood (~ 5 ml) was collected from the posterior vena cava under light CO2 inhalation after one month of treatment and used for serum chemistry measurements. The parameters examined included phospholipid, hyaluronic acid, free fatty acid, albumin, alkaline phosphatase (ALP), glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), lactic dehydrogenase (LDH), creatinine phosphokinase (CK), glucose, total cholesterol triglyceride, HDL cholesterol, LDL cholesterol, creatinine, blood urea nitrogen (BUN), total protein, uric acid, and c-reactive protein (CRP). All parameters were evaluated using an autoanalyzer (Hitachi 7060 automatic clinical analyzer, Tokyo) in the Green Cross Lab, Korea.
Plasma assay for anti-coagulant activity detection
After one month of treatments, approximately 4 ml of anti-coagulated blood was collected from the posterior vena cava under light CO2 inhalation and centrifuged. The supernatant was used to determine the clotting time in the anticoagulant activity test. Activated partial thromboplastin time (aPTT), thrombin time, prothrombin time, and factor 1 (fibrinogen) as a fibrinolytic activity indicator were determined using the Hitachi 7060 automatic clinical analyzer according to the protocol of the Green Cross Laboratory.
D-dimer assay
To investigate these GAGs for clinical application as a fibrinolytic agent, the plasma samples (5 μl) from aged rat treated with GAG, were assayed using a TintElize® D-dimer (Biopool, Ventura, CA, USA) according to the manufacturer’s instructions [12].
Partial pharmacokinetic study
To identify the possible absorption route and the distribution of administered glycosaminoglycan (BTQ), a pharmacokinetic study of the sera and plasma of rats treated one month-intraperitoneally was performed by high-pressure anion-exchange chromatography (Dionex, Sunnyvale, CA, USA) with pulsed amperometric detection (HPAEC-PAD), using a Dionex CarboPac™ PA10 (4 × 250 mm) with a flow rate 1 ml/min.
RNA preparation and quantitative real-time PCR analysis
Total RNA was isolated from liver tissue from the aged rats using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized from 1 µg of total RNA using a high-capacity cDNA Reverse Transcription Kit (Amersham Biosciences Co., USA). Real-time polymerase chain reaction (RT-PCR) amplification was performed using Power SYBR Green Master Mix and a 7500 Real-Time PCR System (both from Applied Biosystems) according to the manufacturer’s instructions and a previously reported method [5]. The target mRNA levels were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control to determine the relative expression of target mRNA according to the cycling threshold method. All samples were analyzed in triplicate [13]. The primer sequences used for the amplification of genes involved in cell repair mechanisms and the GAPDH internal standard are listed in Table 1.
Table 1.
Sequences of PCR primers for amplification of genes involved in prevention of cardiovascular diseases related to aging using GAPDH as internal standard
| Gene name | Primer sequence | |
|---|---|---|
| ACE1 | Angiotensin converting enzyme 1 (Rat, ACE1) | 5′-CCACCGTTACCAGACAACTATCC-3' |
| 5′-GCGTATTCGTTCCACAACACCT-3’ | ||
| GSK-3b | Glycogen synthase kinase 3 beta (Rat, GSK-3b) | 5′-GGA TCT GCC ATC GAG ACA TT-3′ |
| 5′-GTG GCT CCA AAG ATC AGC TC-3′ | ||
| Heparanase | Heparanase (Rat) | 5′-CGGTTCTGACGGACTGCTT-3′ |
| 5′-AAAACCCATAGGAAAAGGCG-3′ | ||
| GPX1 | Glutathione peroxidase 1 (Rat, Gpx1) | 5′-TGAGAAGTGCGAGGTGAATG-3' |
| 5′-CGGGGACCAAATGATGTACT-3' | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 5′-GATTTGGCCGTATCGGAC-3' |
| 5′-GAAGACGCCAGTAGACTC-3' |
DNA microarray procedure
Microarray hybridization was performed on the rat liver samples [14]. Total RNA was isolated from liver tissue using a Qiagen RNeasy Midi Kit (Qiagen, Valencia, CA, USA). A FairPlay™ Microarray Labeling Kit (Stratagene, La Jolla, CA, USA) was used according to the manufacturer's instructions. The labeled DNA was loaded onto a microarray chip. A hybridization chamber was assembled with the Rat Genome 230 2.0 Array (Affymetrix Inc., Santa Clara, CA, USA) microarray chip and submerged in a water bath overnight at 60 °C. The microarray chip was washed in wash buffer, dried, and scanned with a BMS Array Scanner, Applied Precision Array WoRx eBiochip Reader (BioRad, Dallas, TX, USA).
Statistical analysis
The means and standard error of all parameters studied were determined for each group using the ANOVA test. A Student's t-test was carried out to determine the significant differences between the control and treated groups. The same treated groups were subjected to repeated measures ANOVA at different time points, followed by pairwise multiple comparisons (Tukey’s test).
Results
B. terrestris queen glycosaminoglycan (BTQG) preparation
The GAG salt fractions were dialyzed, freeze-dried and the glycosaminoglycan yield determined to be 3.5 g of BTQG per kg BTQ and 1.2 g of GbG per kg Gb.
Body weight and adipose fat changes
There were no significant differences in the mean body weight between the treatment groups (Fig. 1b, Tukey’s value p = 0.092). During the 1-month administration period, the body weights of the BTQG, and GbG-treated groups were comparable to those of the control. The mean weekly body weights are presented in Fig. 1b. Each glycosaminoglycan treatment caused a slight decrease in the body weight compared to the CON group between the first day (BTQG10, 107.14%; GbG10, 104.14%; and GS25, 102.64%) and last 30th day (BTQG10, 95.01%; GbG10, 93.54%; and GS25, 95.72%). The amount of adipose fat in the BTQG and GbG-treated groups was decreased. However, the decreases were not significantly different compared to the control group (data not shown).
Hematology and blood chemistry
Even though the normal physiological range of the blood values was based on a normal-aged rat experimental study, the plasma biochemical study results showed some dose-dependent changes in the anti-coagulant and anti-fibrinolytic parameters after one-month of treatment compared to the control group (Fig. 2a). There were comparable increases in Factor I (fibrinogen, mg/dL) in the treated groups compared to the control group (BTQG10, 132% increase; GbG10, 172.0% increase; GS25, 131.9% increase). There was a weak increase in prothrombin time in the GbG10-treated group (116.6% increase).
Fig. 2.
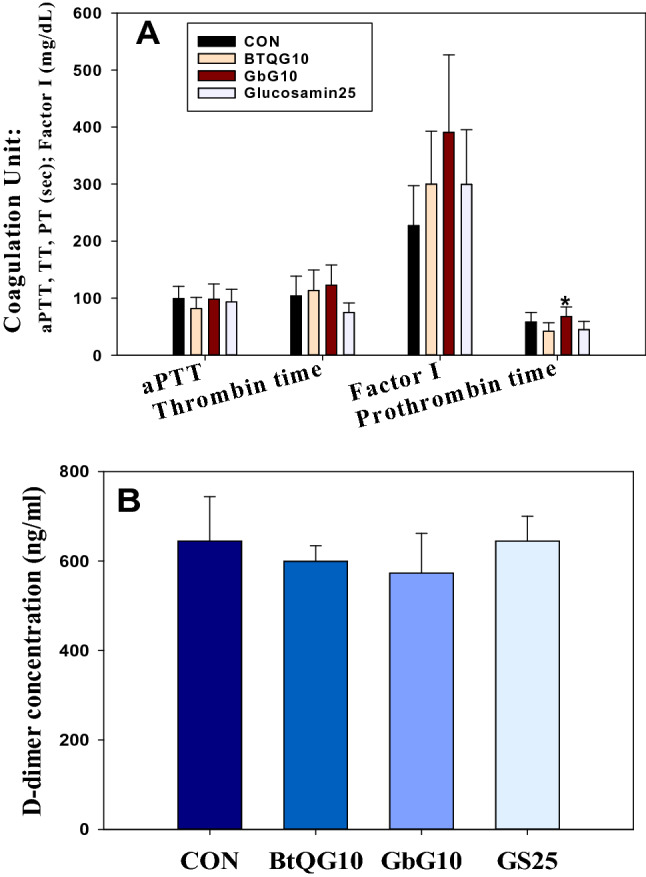
Anti-coagulation of effect bumblebee queen- or cricket-glycosaminoglycan. a Anticoagulant activity in plasma of aged rats treated with BtQG or GbG over a month. Each value represents mean ± SE. *Statistically significant from the control (p < 0.05) (N = 10). b D-dimer quantitation of 14-month old aged rat serum treated intraperitoneally with BTQG or GbG for one month. Each value represents mean ± SD. *Statistically significant from the control (p < 0.05) (N = 10)
Comparison of D-dimer concentration and anti-coagulation factor
D-dimer is a cross-linked fibrin degradation product that originates from the breakdown of a fibrin clot and is increasing in abnormal hemostatic state [12]. The D-dimer concentration in each GAG-treated aged rat plasma sample was somewhat lower than that in the control group (Fig. 2b).
Serological changes by buff-tailed bumblebee queen GAG one-month treatment
The mean levels of each serological biomarker are considered normal but can be used to assess the treatment effects to delay aging. Control and overused GbG oral dose (0, 40, 80, 160 mg/kg) toxicity serologic data of young same strain SD rat was referenced [15]. The mean serum glucose level in the BTQG10-treated group was lower than that in the control group of aged rats (388.44 mg/dL CON vs. 185.50 mg/dL BTQG10, 52.25% decrease; p < 0.05). It was also lower in the GbG10 group (248.09 mg/dL, 36.14% decrease; GbG10 vs. CON, p < 0.05) and the GS25 group (364.30 mg/dL, 6.22% decrease) (Fig. 3a).
Fig. 3.
Sero-biomarker study of aged rats treated with bumblebee queen- or cricket-glycosaminoglycan. a Antilipidemic effect on glucose, triglyceride and sero-cholesterol levels in aged rats treated with BTQG or GbG for one month. Each value represents mean ± SE. *Statistically significant from the control (p < 0.05) (N = 10). b Sero-biochemical decrease of albumin, creatinine, BUN, and total protein levels with nepro-protective effect in aged rat treated with BTQG or GbG for one month. Each value represents mean ± SE. *Statistically significant from the control (p < 0.05) (N = 10). c Hepatoprotective parameter, phospholipid, free fatty acid, ALP and ALT (sGPT) levels of in aged rat treated with BTQG or GbG for one month. Each value represents mean ± SE. *Statistically significant from the control (p < 0.05) (N = 10). d Heparin disaccharide plasma level of 14-month aged rat treated with BTQG, using HPAEC-PAD with Dionex CarboPac™ PA10 column (N = 10)
As the first anti-lipidemic parameter, the mean total cholesterol level was significantly decreased in aged rats after treatment (each group vs. CON (253.33 mg/dL), p < 0.05; BTQG10 (99.88 mg/dL) 60.58% decrease; GbG10 (113.00 mg/dL, 55.34% decrease; GS25 (197.40 mg/dL, 22.08% decrease).
As the second anti-lipidemic parameter, the triglyceride level (mg/dL) in each treated group was much lower than that (CON, 426.0 mg/dL) in the control group of aged rats. The difference of mean value was also significant [each group vs. CON, p < 0.05; BTQG10 (152.13 mg/dL), 64.3% decrease; GbG10 (200.55 mg/dL), 52.93% decrease; GS25 (239.4 mg/dL), 43.81% decrease]. As the third anti-lipidemic parameter, the LDL cholesterol (mg/dL) levels were decreased in the treated groups compared to the control group, 79.56 mg/dL (BTQG10, 31.75 mg/dL, 60.1% decrease; BTQG10 vs. CON, p < 0.05; GbG10, 34.09 mg/dL, 57.16% decrease; GbG10 vs. CON, p < 0.05; GS25, 65.80 mg/dL, 17.30% decrease) (Fig. 3a). Significant decreases in BUN levels (mg/dL) were also seen in all GAG-treated groups compared to the control CON, 23.67 mg/dL (each group vs. CON, p < 0.05; BTQG10, 14.61 mg/dL, 38.28% decrease; GbG10, 17.50 mg/dL, 26.07% decrease; GS25, 18.01 mg/dL, 23.92% decrease) (Fig. 3b).
In the sera from the BTQG10 and GbG10-treated groups, the mean phospholipid levels (mg/dL) were significantly lower than those in the sera of control, 383.67 mg/dL, aged rats after 1 month (BTQG10, 167.88 mg/dL, 56.25% decrease; BTQG10 vs. CON, p < 0.05; GbG10, 200.64 mg/dL, 47.71% decrease; GbG10 vs. CON, p < 0.05; GS25, 295.10 mg/dL, 23.09% decrease; CS25 vs. CON, p < 0.05). Free fatty acid levels (μEq/L) were also decreased in the treated groups (CON, 589.33 μEq/L: BTQG10, 436.25 μEq/L, 25.98% decrease; BTQG10 vs. CON, p < 0.05; GbG10, 388.00 μEq/L, 34.17% decrease; GbG10 vs. CON, p < 0.05; GS25, 486.70 μEq/L, 17.42% decrease). Also, the serum GPT (ALT) levels (IU/L) in the BTQG10 and GbG10-treated groups were significantly lower than those (CON, 97.11 IU/L) in the control group of aged rats (each group vs CON, p < 0.05; BTQG10, 24.25 IU/L, 75.03% decrease; GbG10, 36.36 IU/L, 62.56% decrease; GS25, 42.90 IU/L, 55.83% decrease) (Fig. 3c).
There were also analyzed all of tested sera parameter data using Tukey’s test of one-way RM ANOVA test. The differences in the mean values among the treatment groups, were statistically analyzed to be significant differences at followed p value: p ≤ 0.001: phospholipid, albumin, glucose, total cholesterol, LDL cholesterol, HDL cholesterol, total protein, inorganic phosphate; p ≤ 0.005: BUN, creatinine kinase (CK), triglyceride; p < 0.01: creatinine; p ≤ 0.05: ALT (sGPT), free fatty acid. There were no significant differences of mean values in ALP, AST (sGOT), LDH, uric acid, sodium, potassium, chloride, creatinine reactive protein (CRP) and calcium parameters by Tukey’s test.
Heparin disaccharide detection by partial pharmacokinetic study
In treated rat plasma and serum, heparin disaccharides including IV-a were detected and compared by calculating the area of the peak from the amperometric Au electrode detector (ED50A, Dionex) in a Bio-LC system (Fig. 3d). BTQG may be absorbed into the blood from the intestinal lumen in the abdominal cavity after an intraperitoneal injection as the heparin disaccharide form of BTQG produced by enzymatic digestion by endobacterium.
Gene expression by quantitative real-time PCR analysis
Some genes related to anti-aging activity and the mechanisms of action were elucidated by RT-PCR. The data are presented as PCR cycles (Ct value). The median CT values of four samples are presented. There was no difference between the treated groups and the control group (Ct value > 2.0) for the primers tested. The mean Ct value for heparanase in the GbG10 group showed about a two-fold decrease compared to the control. For glutathione peroxidase 1 (Gpx1), GbG10 treatment showed about a three-fold decrease. Angiotensin-converting enzyme (ACE) 1 was not increased in any group. For glycogen synthase kinase 3 beta (GSK-3b), samples from the BTQG10 showed about two-fold increases.
Rat gene array chip analysis
Microarray analysis using a rat 30 K cDNA clone array was performed to determine the gene-expression profiles in BTQG10, GbG10, and GS25-treated 14-month-old SD rat livers. This analysis might provide information on potential anti-aging markers. A total of 29,490 protein-coding transcripts could be analyzed by the Rat Gene ST 2.0 array chip. The control gene array chip used in this study was highly normalized, with about 0.98–1.02 ratios (within 2% standard error) of 5S-rRNA for ACE1, GSK-3beta, heparanase, GPX1, and GAPDH.
Gene expression change analysis by one-month GAG treatment
Total RNA was purified from the GAG-treated and control aged liver tissues and loaded onto gene array chips. Then, the DNA expression ratio was determined. The transcript levels of 30 K genes for young adult rats treated with cricket glycosaminoglycan (GbG) were previously reported [16, 17]. Compared to the control group, the BTQG10, GbG10, and GS25 groups exhibited the overexpression (more than twofold increases in the expression levels) of 266 (BTQG10), 338 (GbG10 most changed), and 248 (GS25) genes and the downregulation (more than a twofold decrease in expression level) of 614, 698 and 749 genes, respectively. Thirty-six commonly regulated genes were simultaneously upregulated in the three treated groups, whereas 417 genes were downregulated more than tenfold.
The upregulated or downregulated genes were related to cell apoptosis and cell signal transduction. Based on the predictive functions of these genes, about 370 genes were related to cell communication and about 200 genes were associated with transport. Eight genes were related to translation. Regarding the expression level of aging-related genes in the BTQG10, GbG10, and control group, 32 genes were upregulated simultaneously (Table 2) and 57 genes were downregulated (Table 3). Secretogranin II (Scg2) was upregulated 9.7–64-fold (Table 2). AP-1-regulated protein, which suppresses NO-induced apoptosis, was reported to perform important roles in repairing DNA damage by reactive oxygen species (ROS) [18]. In genes related to lifespan extension, the metallothionein 1a (Mt1a) gene was increased about 26–34-fold, metallothionein 1 M (Mt1m) was increased about 20–28-fold, metallothionein 1a was increased about 11–15-fold, and metallothionein 2A was increased about 11–14-fold in this study. Alpha-2 macroglobulin (A2m) is known to be a clue protein that can lengthen the lifespan of mice. A2m was increased by 24–58-fold in this study (Table 2).
Table 2.
Up-regulated genes in the liver tissues of aged rats treated with BTQG for one month
| Gene symbol | Gene description | Gene ontology category (molecular function term) | Pair mean ratio | |||
|---|---|---|---|---|---|---|
| BTQG10 | GbG10 | GS25 | ||||
| 1 | Scg2 | Secretogranin II | Cell communication: cytokine activity, apoptosis, DNA repair | 64.01 | 9.79 | 1.45 |
| 2 | Mt1a | Metallothionein 1a | Cell communication: metal binding | 26.26 | 34.89 | 11.85 |
| 3 | A2m | Alpha-2-macroglobulin | Protease binding, lengthen life span | 24.33 | 58.85 | 8.69 |
| 4 | Mt1m | Metallothionein 1 M | Cell communication, metal ion binding | 20.48 | 28.05 | 7.45 |
| 5 | Lcn2 | Lipocalin 2 | Apoptotic, cell death communication & transport: protease binding | 13.79 | 35.01 | 2.60 |
| 6 | S100a8 | S100 calcium binding protein A8 | Apoptotic process, adhesion, death | 12.07 | 5.13 | 0.89 |
| 7 | Mt1m | Metallothionein 1a | Cell communication, cell cycle, cell growth, transport | 11.87 | 5.59 | 6.72 |
| 8 | Mt2A | Metallothionein 2A | Cell communication: free radical scavenger | 11.43 | 4.08 | 6.27 |
| 9 | Ccnf | Cyclin F | Apoptotic, cell cycle, cell diversion | 6.59 | 10.79 | 1.24 |
| 10 | S100a9 | S100 calcium binding protein A9 | Apoptotic process, cell adhesion, cell communication: calcium binding | 6.20 | 3.50 | 0.70 |
| 11 | Mmp8 | Matrix metallopeptidase 8 | Metallopeptidase activity | 5.43 | 2.46 | 1.06 |
| 12 | Sult2a1 | Sulfotransferase family 2A | Cell communication: alcohol sulfotransferase activity | 5.27 | 3.90 | 12.17 |
| 13 | Wfdc21 | WAP 4-disulfide core domain 21 | Whey acidic protein intercellular signaling peptide | 5.17 | 11.69 | 32.48 |
| 14 | Cxcl1 | Chemokine (C-X-C motif) ligand 1 | Apoptotic process, cell adhesion, cell death: chemokine activity | 4.91 | 5.69 | 0.86 |
| 15 | Slc13a5 | Solute carrier family 13, member 5 | Transport: organic acid, sodium symporter activity | 4.61 | 6.09 | 1.25 |
| 16 | Marco | Macrophage receptor with collagenous structure | Transport: scavenger receptor activity | 4.57 | 2.25 | 2.23 |
| 17 | Abhd3 | Abhydrolase domain containing 3 | Hydrolase activity (3-oxo-5-alpha-steroid 4-dehydrogenase activity | 4.45 | 4.30 | 1.64 |
| 18 | Srd5a1 | Steroid-5-alpha-reductase | Behavior, cell communication: chemokine activity | 4.18 | 4.20 | 1.29 |
| 19 | Ubd | ubiquitin D | Proteasome binding | 4.04 | 2.82 | 1.49 |
| 20 | Slc13a3 | Solute carrier family 13, member 3 | Scavenger receptor, dicarboxylic acid transmembrane transporter activity | 3.97 | 4.33 | 2.23 |
| 21 | Abcg2 | ATP-binding cassette, subfamily G, member 2 | Transport: ATP binding | 3.95 | 3.97 | 1.64 |
| 22 | Spink3 | Serine peptidase inhibitor, Kazal type 3 | Serine type endopeptidase inhibitor activity | 3.74 | 8.06 | 1.78 |
| 23 | Osmr | oncostatin M receptor | Cell communication | 3.60 | 5.71 | 0.87 |
| 24 | Nqo1 | NAD(P)H dehydrogenase, quinone 1 | NAD(P)H dehydrogenase, quinone | 3.37 | 4.31 | 1.95 |
| 25 | Serpina7 | Serpin peptidase inhibitor, clade A, member 7 | Serine-type endopeptidase inhibitor activity | 3.38 | 3.34 | 1.11 |
PairMean ratio* means pair Mean ratio (test/control)
Table 3.
Down-regulated genes differentially expressed in the liver tissues of aged rats treated with BTQG for one month
| Gene symbol | Gene description | Gene ontology category (molecular function term) | Pair mean ratio | |||
|---|---|---|---|---|---|---|
| BTQG10 | GbG10 | GS25 | ||||
| 1 | Vim | vimentin | Growth: glycoprotein binding | 0.41 | 0.32 | 0.24 |
| 2 | Adam8 | ADAMmetallopeptidase domain 8 | Cell adhesion & communication | 0.39 | 0.37 | 0.61 |
| 3 | Emp1 | Epithelial membrane protein 1 | Growth: molecular function | 0.37 | 0.32 | 0.41 |
| 4 | Wbp5 | WW domain binding protein 5 | WW domain binding | 0.37 | 0.43 | 0.34 |
| 5 | Msn | Moesin | Cell adhesion: double-RNA binding | 0.36 | 0.26 | 0.15 |
| 6 | Gpx3 | Glutathione peroxidase 3 | Glutathione peroxidase activity | 0.35 | 0.33 | 0.23 |
| 7 | Bmpr2 | Bone morphogenetic protein receptor, type II | Cell communication: trans membrane receptor binding activity | 0.35 | 0.29 | 0.29 |
| 8 | Prss23 | Protease, serine, 23 | Serine endopeptidase activity | 0.35 | 0.27 | 0.28 |
| 9 | Car5b | Carbonic anhydrase 5b, mitochondrial | Carbonate dehydratase activity | 0.34 | 0.24 | 0.35 |
| 10 | Calcrl | Calcitonin receptor-like | Cell communication, transport: adrenomedullin receptor activity | 0.34 | 0.29 | 0.27 |
| 11 | Anxa1 | Annexin A1 | Cell adhesion, communication and cycle, transport: single-stranded DNA binding | 0.33 | 0.22 | 0.17 |
| 12 | Gpr116 | G protein-coupled receptor 116 | Cell communication: G protein-coupled receptor activity | 0.33 | 0.25 | 0.28 |
| 13 | Plat | Plasminogen activator, tissue | Cell communication: serine endopeptidase activity | 0.33 | 0.22 | 0.34 |
| 14 | Zdhhc2 | ZINC finger, DHHC-type containing 2 | Zinc ion binding | 0.33 | 0.29 | 0.32 |
| 15 | Dock9 | Dedicator of cytokinesis 9 | Cell communication: guanyl-nucleotide exchange factor activity | 0.32 | 0.25 | 0.38 |
| 16 | Cyth3 | Cytohesin 3 | Transport: ARF guanyl-nucleotide exchange factor activity | 0.31 | 0.26 | 0.24 |
| 17 | Efnb2 | Ephrin B2 | Cell adhesion & communication ephrin receptor binding | 0.31 | 0.29 | 0.25 |
| 18 | Mgp | Matrix Gla protein | Calcium ion binding | 0.31 | 0.37 | 0.24 |
| 19 | Oxct1 | 3-oxoacid CoA transferase 1 | 3-oxoacid CoA-transferase activity | 0.30 | 0.26 | 0.18 |
| 20 | Lyz2 | Lysozyme 2 | Lysozyme gene, antimicrobial | 0.29 | 0.17 | 0.05 |
| 21 | Tek | TEK tyrosine kinase, endothelial | Cell adhesion & communication protein tyrosine kinase activity | 0.29 | 0.23 | 0.23 |
| 22 | Anxa5 | Annexin A5 | Calcium binding, protein binding | 0.28 | 0.19 | 0.21 |
| 23 | Akr1cl | Aldo–keto reductase family 1, member C-like | Oxidoreductase activity | 0.27 | 0.19 | 0.18 |
| 24 | Gstm3 | Glutathione S-transferase mu 3 | Glutathione transferase activity | 0.26 | 0.13 | 0.61 |
| 25 | Naaa | N-acylethanolamine acid amidase | Transcription factor binding | 0.26 | 0.18 | 0.19 |
PairMean ratio* means pair Mean ratio (test/control)
Among the downregulated genes, some of them were related to rapid body reaction and ROS attack, including vimentin (VIM)-related wound healing; ADAM metallopeptidase, (Adam8) known to be a protein digestive enzyme; moesin (Msn), related to cytoskeletal reorganization; glutathione peroxidase (GPX3), known to cause cancer; mitochondrial carbonic anhydrase 5B (Car5B) that can induce oxidative damage; calcitonin receptor-like (Carcrl), related to repair; and annexin (ANXA1), soluble tissue plasminogen activator (Plat) (Table 3).
Characterization of buff-tailed bumblebee queen GAG
The monosaccharide composition, including the amino, acidic, and neutral sugars of BTQG were determined by GC–MS (Table 4). The primary amino monosaccharides of BTQG were present in the following order: D-glucosamine > N-acetyl-galactosamine > D-galactosamic acid > D-galacturonic acid > D-galactosamine > D-glucuronic acid. The main neutral monosaccharides found in BTQG were arabinose, rhamnose, and glucose, and the minor ones were galactose, xylose, and mannitol.
Table 4.
Acidic, amino, (A) and neutral (B) monosaccharide compositions of used BTQG (A)
| Acidic and amino sugar | BTQG (μg/mg) |
|---|---|
| D-glucuronic acid | 3.79 ± 0.24 |
| Glucosamine HCl | 44.06 ± 16.66 |
| Galactosamine HCl | 5.83 ± 3.14 |
| N-acetyl-glucosamine | 17.15 ± 4.48 |
| D-glucosaminic acid | 1.53 ± 1.21 |
| D- Galactosamic acid | 10.49 ± 4.39 |
| D-glucosamine 6- sulfate | 1.31 ± 0.02 |
| N-acetyl-D-galactosaminitol | 0.38 ± 0.09 |
| D-galacturonic acid | 6.68 ± 0.10 |
| Total monosugar sum | 91.21 |
| Neutral monosaccharide (hexose) | Content(ng/mg) |
|---|---|
| Glycosaminoglycan | |
| D (−) ribose | 0.93 ± 1.31 |
| L (+) rhamnose | 35.29 ± 4.14 |
| D (+) mannose | 0.32 ± 0.20 |
| D (+) xylose | 5.85 ± 5.30 |
| D (+) galactose | 13.64 ± 1.95 |
| L (+) arabinose | 73.43 ± 3.14 |
| D (−) fructose | 0.04 ± 0.05 |
| alpha-D ( +)-glucose | 30.95 ± 0.20 |
| beta-D ( +)-glucose | 0.38 ± 0.18 |
| Xylitol | 0.37 ± 0.01 |
| Mannitol | 2.20 ± 0.26 |
| Sorbitol | 0.11 ± 0.06 |
| Inositol | 0.12 ± 0.00 |
| Total content | 180.45 |
Each value represents mean ± SD. N = 3
Purified Bombus terrestris queen glycosaminoglycan was identified from the LC–MS chromatogram of the BTQG product digested by heparinase II (Fig. 4a). The N-glycans derived from BTQG included Hex7 and Hex8 (1377.5 m/z, Fig. 4b), Hex3HexNAc3dHex2, Hex4HexNAc3Pen1, Hex9, and Hex5HexNAc3dHex2 glycosylation structures as active ingredients (Fig. 4c). The neutral monosaccharides, hexose components of N-glycans from BTQG mainly were D (−) fructose 14.93%, D (−) ribose 13.75%, D (+) mannose 13.74% and D (+) galactose 13.70% by TMS GC–MS data base.
Fig. 4.
Identification of buff-tailed bumblebee queen GAG (BTQG) a LC–MS chromatogram of BTQG that digested by heparinase II. b N-glycan from BTQG using MALDI MS/MS TOF analyzer (with quadrupole ion trap) of the Hex8 at m/z 1337.5, c BTQG N-glycan chromatogram of MALDI (Matrix-assisted laser desorption/ionization) MS from m/z from 800 to 2200
Discussion
Aging is inevitable for living animals. Therefore, aging biomarkers in many model organisms ranging from worms such as Caenorhabditis elegans to rats, have been studied at the proteotoxicity level, especially, mitochondrial proteostasis in cellular metabolism and signaling [19, 20], mitochondrial-derived ROS levels [21], and others.
To extend lifespans and health spans, many trials such as treatment with arginine and anti-oxidant drugs have been conducted. The current anti-aging drug development trend is to repurpose gene-expression-based drugs to target aging [22].
GAG is a sulfated polysaccharide with a sulfate residue-oxidized polysaccharide. Typical GAGs include hyaluronic acid, chondroitin sulfate, dermatan sulfate, heparin, heparan sulfate, and keratin sulfate. Recently, since various biological activities of GAG and N-glycan have been identified, they have been used as medical biomaterials, cosmetics, and functional food materials. Hence, their value as restriction and functional molecules has increased.
Growth factor and cytokine generation can be controlled by GAG. It appears due to various diseases livestock disease and influenza virus from pig, cow and etc., with various international convention for the shark cartilage and shell the safety assurance as the raw material is difficult to acquire, whale, and the genus of tuna is the extermination prevention due to the reduction of the population and due to this, the annual production is restricted. It is the actual condition in which the transfer resource which is therefore new with the difficulty of this resource security is necessary.
For the safety assurance as raw material, thus, the party rank through MS and MS / MS the except impurity is separated using the porous carbon graphite cartridge with the queen bee origin N-glycan (N-glycan) the inventors selected the insect as the new GAG supply source and the protein moiety is in the alcohol extraction residue of the queen bee after manufacture the N-glycosidase F processing and the structure thereof were examined closely. In the present study, N-glycan was purified from Bombus terrestris queen bee glycosaminoglycan by ion-exchange chromatography using 0.5 M sodium chloride for elution. The product was characterized to have a linear structure consisting of a hexose polymer. The N-glycan contained Hex5HexNAc3dHex2, Hex7, Hex3HexNAc3dHex2, Hex4HexNAc3Pen1, and Hex9 glycosylation structures.
The BTQG-treated rat group showed upregulated genes compared to the control group, including secretogranin II (Scg2), activator protein (AP)-1-regulated protein related to ROS DNA damage repair, metallothionein 1a, metallothionein 1 M, and alpha-2 macroglobulin. The downregulated genes included vimentin, moesin, and mitochondrial carbonic anhydrase with anti-aging action.
As an anti-aging certifying study using 14-month old SD rats after treatment with B. terrestris queen glycosaminoglycan or cricket (G. bimaculatus) for one-month, the BTQG and GbG groups of rats not only showed dramatic, curative anti-lipidemic and kidney protective effects with consistent significance, but also anti-coagulant effects. The gene expression levels of 30 K cDNA showed that 370 genes involved in cell communication and 200 genes associated with transport were changed. The results of the microarray analysis of liver tissue from aged rats treated with BTQG or GbG for one-month revealed that 32 genes were commonly upregulated, whereas 57 genes were commonly downregulated by both BTQG and GbG (Fig. 5).
Fig. 5.
Proposed molecular or genetic mechanism of action of bumblebee (B. terrestris) queen glycosaminoglycan in aged rats
Of these commonly upregulated genes, secretogranin II (Scg2) is a suppressor protein related to nitric oxide-induced apoptosis [18]. Secretogranin-1 (chromogranin B, secretoneurin) can repair DNA damage as a pituitary neuroendocrine protein, strong angiogenic factor [23]. Metallothionein 1a, metallothionein 1 M, and metallothionein 2A have been reported to increase the lifespan of mice by increasing metallothionein (MT) gene expression.
MT can scavenge free radicals. It has anti-apoptotic properties in vitro [24, 25]. MT not only can suppress obesity but also can protect against diet-induced oxidative stress damage. It also plays a role in mediating in caloric restriction (CR) and the insulin/insulin-like signaling (IIS) pathway. Meanwhile, alpha-2 macroglobulin has been found to be a crucial protein that can extend the lifespan of aged rats. Thus, it can act as a positive marker concerning lifespan prolongation [26].
Of the commonly downregulated genes, vimentin (VIM) [27] is related to wound healing. ADAM metallopeptidase is a digestive enzyme protein [28]. Moesin is related to cytoskeletal reorganization. Glutathione peroxidase [29] can cause cancer. Carbonyl anhydrase [30] can induce oxidative damage. Calcitonin receptor-like protein is related to mitochondrial repair. Annexin [31] soluble plasminogen activator is a factor that can cause rapid body reactions and attack ROS.
BTQG treatment for one month reduced the serum levels of creatinine and BUN, exhibited anti-lipidemic effects with hepato- and renal-protective action, and maintained normal glucose levels in aged rats. BTQG and GbG had marked anti-inflammatory effects, decreased free fatty acid, ALP, and sGPT levels, and showed anticoagulant and antithrombotic effects, leading to factor 1 (fibrinogen) and thrombin time increases even though a weak decrease in prothrombin time and aPTT (partial thromboplastin time) was seen in BTQG-treated rat plasma. Such functional superiority of insect GAGs might be due to their structure, which is simpler than that of animal GAG, and insect feed diversity, such as pollen. Hence, glycosaminoglycans from insect such as bumblebee (B. terrestris) queen might help decelerate the aging process and ameliorate circulation, liver, and kidney function in humans as well as aged rats.
Acknowledgements
The authors acknowledge National Institute of Agricultural Sciences for financial (RDA, PJ011853) and technical supports. Technical support for analysis of N-glycan sequence data was obtained from Division of Mass Spectrometry Research of Korean Basic Science Institute.
Abbreviations
- aPTT
Activated partial thromboplastin time
- ALP
Alkaline phosphatase
- ALT(GPT)
Glutamate pyruvate transaminase
- AST(GOT)
Glutamate oxaloacetate transaminase
- BTQG10
B. terrestris Queen glycosaminoglycan 10 mg/kg
- BUN
Blood urea nitrogen
- CON
Control group
- CK
Creatinine phosphokinase
- CRP
C-reactive protein
- CS10
Chondroitin sulfate (10 mg/kg)
- GAG
Glycosaminoglycan
- GbG10
G. bimaculatus (A type of cricket) glycosaminoglycan 10 mg/kg
- GC–MS
Gas chromatography with mass detector
- HDL
High-density lipoprotein
- LDH
Lactate dehydrogenase
- LDL
Low-density lipoprotein
- PT
Prothrombin time
- T. Chol
Total cholesterol
- TG
Triglyceride
- TMS
Trimethylchlorosilane
Author contributions
MYA performed most of the experiments, prepared the manuscript: conceived of the study, participated in its design and coordination, collected and analyzed data, and prepared the manuscript. HJY reared and supplied the queen of the bumblebees. JSH participated in the genetic sequence alignment. JMJ carried out N-glycan sequence analysis. KKP participated in DNA microarray. All authors read and approved the final manuscript.
Funding
Financial support was obtained from Rural Development Administration Basic Research project (PJ011853 and PJ014198). The funding body allowed to the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
All data generated or analyzed during this study are indicated in this article.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
References
- 1.Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shute JK, Puxeddu E, Calzetta L. Therapeutic use of heparin and derivatives beyond anticoagulation in patients with bronchial asthma or COPD. Curr Opin Pharm. 2018;40:39–45. doi: 10.1016/j.coph.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Ahn MY, Kim SJ, Kim NJ, Hwang JS, Yun EY. Immune modulation of glycosaminoglycan derived from P. lewisi in TNF-α stimulated cells. Arch Pharm Res. 2015;38:1983–1991. doi: 10.1007/s12272-015-0616-5. [DOI] [PubMed] [Google Scholar]
- 4.Ahn MY, Han JW, Yoon HJ, Hwang JS. Anti-inflammatory effect of bumblebee alcohol extracts in CFA-induced rat edema. Toxicol Res. 2012;28:249–253. doi: 10.5487/TR.2012.28.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn MY, Kim BJ, Kim HJ, Jin JM, Yoon HJ, Hwang JS, Park KK. Anti-cancer effect of dung beetle glycosaminoglycans on melanoma. BMC Cancer. 2019;19:9. doi: 10.1186/s12885-018-5202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DF, Oh JH, Chung J. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83:174–181. doi: 10.1016/j.jdermsci.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Chang C, Yu C, Huang LLH. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell. 2017;16:451–460. doi: 10.1111/acel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majtán J, Bíliková K, Markovič O, Gróf J, Kogan G, Smiúth J. Isolation and characterization of chitin from bumblebee (Bombus terrestris) Int J Biol Macromol. 2007;40:237–241. doi: 10.1016/j.ijbiomac.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J Nutr. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Jo YY, Chang IM, Toida T, Park Y, Linhardt RJ. A new glycosaminoglycan from the giant African snail Achatina fulica. J Biol Chem. 1996;271:11750–11755. doi: 10.1074/jbc.271.20.11750. [DOI] [PubMed] [Google Scholar]
- 11.Magnelli PE, Bielik AM, Guthrie EP. Identification and characterization of protein glycosyation using specific endo- and exoglycosidases. J Vis Exp. 2011;58:e3749. doi: 10.3791/3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn MY, Hahn BS, Ryu KS, Kim JW, Kim YS. Purification and characterization of a serine protease with fibrinolytic activity from dung beetles, Catharsius molossus. Thromb Res. 2003;112:339–347. doi: 10.1016/j.thromres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Ahn MY, Kim BJ, Kim HJ, Hwang JS, Jung YS, Park KK. Anti-aging effect and gene expression profiling of dung beetle glycosaminoglycan in aged rats. Biomater Res. 2017;21:5. doi: 10.1186/s40824-017-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno T, Fukuda N, Nagase H, Tsunemi A, Tahira K, Matsumoto T, Hiraoka-Yamamoto J, Ikeda K, Mitsumata M, Sato Y, Soma M, Matsumoto K, Yamori Y. Atherogenic dyslipidemia and altered hepatic gene expression in SHRSP.Z-Leprfa/IzmDmer rats. Int J Mol Med. 2009;23:313–320. doi: 10.3892/ijmm_00000133. [DOI] [PubMed] [Google Scholar]
- 15.Ahn MY, Joo HJ, Kim JS, Yeon Y, Ryu HY, Choi BG, Song KS, Kim SH, Park MK, Jo YY. Toxicity assessment of Gryllus bimaculatus (a type of cricket) glycosaminoglycan. Toxicol Res. 2020 doi: 10.1007/s43188-020-00037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn MY, Kim BJ, Kim HJ, Yoon HJ, Jee SD, Hwang JS, Park KK. Anti-obesity effect of Bombus ignitus queen glycosaminoglycans in rats on a high-fat. Int J Mol Sci. 2017;18:681. doi: 10.3390/ijms18030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn MY, Hwang JS, Kim MJ, Park KK. Antilipidemic effects and gene expression profiling of the glycosaminoglycans from cricket in rats on a high fat diet. Arch Pharm Res. 2016;39(926):936. doi: 10.1007/s12272-016-0749-1. [DOI] [PubMed] [Google Scholar]
- 18.Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88:100. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moehle EA, Shen K, Dillin A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J Biol Chem. 2019;294:5396–5407. doi: 10.1074/jbc.TM117.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso AL, Fernandes A, Aquilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, Ortolano S, Pani G, Athanasopoulou S, Gonos ES, Schosserer M, Grillari J, Peterson P, Tuna BG, Dogan S, Meyer A. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Skulachev MV, Skulachev VP. New data on programmed aging—slow phenoptosis. Biochemistry (Moscow) 2014;79:977–993. doi: 10.1134/S0006297914100010. [DOI] [PubMed] [Google Scholar]
- 22.Dönertaş HM, Valenzuela MF, Partridge L. Gene expression-based drug repurposing to target aging. Aging Cell. 2018;2018:e12819. doi: 10.1111/acel.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakun M, Senatorski G, Rubel T, Lukasik A, Zielenkiewicz P, Dadlez M, Paczek L. Urine proteomes of healthy aging humans reveal extracellular matrix (ECM) alterations and immune system dysfunction. Age (Dordr) 2014;36:299–311. doi: 10.1007/s11357-013-9562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swindell WR. Metallothionein and the biology of aging. Ageing Res Rev. 2011;10:132–145. doi: 10.1016/j.arr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong W, Niu C, Lv L, Ni M, Ruan D, Chi L, Wang Y, Yu Q, Zhan K, Xuan Y, Wang Y, Tan Y, Wei T, Cai L, Jin L. Metallothionein prevents age-associated cardiomyopathy via inhibiting NF-κB pathway activation and associated nitrative damage to 2-OGD. Antioxid Redox Signal. 2016;25:936–952. doi: 10.1089/ars.2016.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thieme R, Kurz S, Kolb M, Debebe T, Holtze S, Morhart M, Huse K, Szafranski K, Platzer M, Hildebrandt TB, Birkenmeier G. Analysis of alpha-2 macroglobulin from the long-lived and cancer resistant naked mole-rat and human plasma. PLoS ONE. 2015;10:e0130470. doi: 10.1371/journal.pone.0130470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaki SM, Mohamed EA, Abde I, Fattah S, Abdullah H, Kaszubowska L. Age-associated functional morphology of thyroid and its impact on the expression of vimentin, cytokeratin and VEGF. The role of nigella in refinement. Folia Histochem Cytobiol. 2018;56:159–171. doi: 10.5603/FHC.a2018.0015. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Hong IA, Oh SH, Kwon YS, Cho SH, Lee KH. The effect of moesin overexpression on ageing of human dermal microvascular endothelial cells. Exp Dermatol. 2009;18:997–999. doi: 10.1111/j.1600-0625.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 29.Kong SZ, Li JC, Li SD, Liao MN, Li CP, Zheng PJ, Guo MH, Tan WX, Zheng ZH, Hu Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subcute aging in mice. Mar drugs. 2018;16:181. doi: 10.3390/md16060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbiscol E, Levine RL. Carbonic anhydrase III. Oxidative modification in vivo and loss of phosphatase activity during aging. J Biol Chem. 1995;270:14742–14747. doi: 10.1074/jbc.270.24.14742. [DOI] [PubMed] [Google Scholar]
- 31.Sheikh MH, Solito E. Annexin A1: uncovering the many talents of an old protein. Int J Mol Sci. 2018;19:1045. doi: 10.3390/ijms19041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are indicated in this article.