Abstract
To determine factors associated with delayed discharge of hospitalized patients with coronavirus disease (COVID-19). This retrospective cohort study included 47 patients with COVID-19 admitted to three hospitals in Quanzhou City, Fujian Province, China, between January 21, 2020 and March 6, 2020. Univariate and multivariate logistic regression analyses were conducted to identify factors associated with delayed discharge. The median length of hospital stay was 22 days. Patients in the delayed discharge group (length of hospital stay ≥ 21 days, n = 27) were more likely to have diarrhea, anorexia, decreased white blood cell counts, increased complement C3 and C-reactive protein levels, air bronchograms, undergo thymalfasin treatment, and take significantly longer to convert to a severe acute respiratory syndrome coronavirus (SARS-CoV-2) RNA-negative status than those in the control group (length of hospital stay, < 21 days; n = 20). In multivariate logistic regression analysis, the time to SARS-CoV-2 RNA-negative conversion (odds ratio [OR]: 1.48, 95% confidence interval [CI] 1.09–2.04, P = 0.01) and complement C3 levels (OR 1.14 95% CI 1.02–1.27, P = 0.03) were the only risk factors independently associated with delayed discharge from the hospital. Dynamic monitoring of complement C3 and SARS-CoV-2 RNA levels is useful for predicting delayed discharge of patients.
Subject terms: Diseases, Health care, Risk factors
Introduction
Since December 2019, a new form of coronavirus pneumonia, coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2), has caused a pandemic1–4. The common symptoms of COVID-19 include fever, lassitude, and dry cough. Similarly, some patients experience abdominal pain, diarrhea, nausea and vomiting, loss of appetite, and other gastrointestinal symptoms. A meta-analysis and systematic evaluation5 showed that 17.6% of patients with COVID-19 developed gastrointestinal symptoms, the most common of which were anorexia (26.8%), diarrhea (12.5%), nausea and vomiting (10.2%), and abdominal pain (9.2%). However, the timing and severity of gastrointestinal symptoms vary among different populations6. Moreover, the lymphocyte count is often reduced, and lung imaging usually shows ground-glass opacities in both lungs. The lesions are distributed mainly in the peripheral and subpleural regions of the lungs. Approximately 10% of those infected develop severe or critical disease, and case-fatality rates are high7–11. The large number of patients requiring hospitalization may exceed hospital capacity, and in many countries and regions, it is difficult to increase hospital capacity to meet the demand in a short period12,13. Therefore, increasing patient turnover and determining factors affecting the length of hospital stay may help relieve pressure on hospital admissions; however, there are no published studies on risk factors for delayed hospital discharge. Therefore, this study aimed to determine the clinical characteristics and duration of SARS-CoV-2 RNA shedding in patients confirmed to have COVID-19 and factors associated with delayed discharge from hospital.
Methods
Ethics declarations
This study was approved by the Ethics Committee of Fujian Provincial Center for Disease Control and Prevention (Min Ji Kong Lun Shen 2020; approval number: 001) and The First Hospital of Quanzhou Affiliated to Fujian Medical University (Quan Yi Lun 2020; approval number: 124). The requirement for written informed consent was not required for these two ethics committee owing to the retrospective nature of the study. Research was conducted in accordance with the relevant guidelines and regulations of the Chinese Health Commission on the prevention and control of COVID-19.
Patients and setting
We conducted a retrospective cohort study of the clinical characteristics of 47 patients with COVID-19 who were admitted to three designated hospitals in Quanzhou City, on the southeast coast of Fujian Province, China, between January 21, 2020 and March 6, 2020. Since the first COVID-19 case was confirmed on January 23, 2020, 22 cases of COVID-19 were diagnosed in just 1 week, and 47 cases were all confirmed within 3 weeks in Quanzhou. The criteria for all confirmed cases and their discharge conformed to the Diagnosis and Treatment Plan for Novel Coronavirus-Infected Pneumonia (6th Edition)14. The discharge criteria were: normal temperature for at least 3 days; significantly improved respiratory symptoms; pulmonary imaging showing significant improvement in acute exudative lesions; and nucleic acid test of two consecutive respiratory specimens, sampled at least 1 day apart, which was negative.
We classified 47 patients into the control group (length of hospital stay, < 21 days) and the delayed discharge group (length of hospital stay, ≥ 21 days). All patients underwent at least two SARS-CoV-2 nucleic acid tests. The specimens included throat swabs, sputum, nasopharyngeal swabs, alveolar lavage fluid, feces, anal swabs, and urine samples. The SARS-CoV-2 nucleic acid test results were judged based on real-time quantitative polymerase chain reaction. The time to SARS-CoV-2 nucleic acid-negative conversion refers to the time from the onset of symptoms to the date of the first negative nucleic acid test result of at least two consecutive negative test results. Negative results followed by a positive result were considered false-negative.
Data collection
Data on patient demographics, symptoms, signs, complications, laboratory test results, lung computed tomography findings, and clinical treatment were collected from the inpatient record system. Two independent reviewers extracted data and evaluated the suitability of the raw data. Before the final analysis, all differences were resolved through discussion.
Statistical analyses
All statistical analyses were conducted using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Figures were plotted using GraphPad Prism 8.1 (GraphPad Software Inc., San Diego, CA, USA). Continuous variables were compared using t-tests or Mann–Whitney U test and expressed as the median and (IQR). Categorical variables were compared using χ2 tests or Fisher’s exact test and expressed as frequencies and percentages. Variables showing significant differences were further analyzed using univariate and multivariate logistic regression to identify factors leading to delayed discharge of patients. P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
In total, 47 patients were enrolled in this study, including 27 in the delayed discharge group and 20 in the control group. The median length of hospital stay for all patients was 22 days. The median age of patients in the delayed discharge group (41 [range 31–54] years) was higher than that of patients in the control group (35 [range 31–45] years); however, the difference was not significant (P = 0.13). Table 1 shows the clinical characteristics according to group. No significant differences were observed between the groups in the prevalence of comorbidities, including hypertension, diabetes, cerebrovascular disease, malignant tumors, liver disease, and chronic respiratory disease, surgical history, and the incidence of fever, cough, lassitude, headache, hemoptysis, chest tightness, shortness of breath, rhinorrhea, dry throat, and nausea and vomiting. However, the number of patients with diarrhea and anorexia was significantly higher in the delayed discharge group than in the control group (both, P = 0.03). Similarly, no significant differences were observed between the groups in the time from symptom onset to admission, partial pressure of oxygen on admission, oxygenation index, mean arterial pressure, heart rate, and respiration.
Table 1.
Demographic and epidemiological characteristics of patients with COVID-19.
| All patients (n = 47) | Hospitalization days < 21 (n = 20) | Hospitalization days ≥ 21 (n = 27) | P value | |
|---|---|---|---|---|
| Age, median (IQR), years | 38 (31–50) | 35 (31–45) | 41 (31–54) | 0.13 |
| BMI, Median (IQR) (kg/m2) | 23.9 (20.7–26.4) (n = 39) | 23.4 (21.7–25.8) (n = 19) | 24.1 (20.1–28.3) (n = 20) | 0.77 |
| < 18.5 | 3 (7.69) | 2 (10.53) | 1 (5.00) | 0.79 |
| ≥ 23 | 23 (58.97) | 12 (63.16) | 11 (55.00) | 0.19 |
| Sex, male | 24 (51.06) | 8 (40.00) | 16 (59.26) | 0.19 |
| Exposure of seafood market in South China | 1 (2.13) | 1 (5.00) | 0 | 0.43 |
| Live in Wuhan ≥ 2 weeks | 34 (72.34) | 15 (75.00) | 19 (70.37) | 0.73 |
| Complications | ||||
| Hypertension | 10 (21.28) | 3 (15.00) | 7 (25.93) | 0.59 |
| Diabetes | 5 (10.64) | 3 (15.00) | 2 (7.41) | 0.72 |
| Cerebrovascular disease | 1 (2.13) | 0 | 1 (3.70) | > 0.99 |
| Malignant tumor | 1 (2.13) | 1 (5.00) | 0 | 0.43 |
| Chronic liver disease | 11 (23.40) | 6 (30.00) | 5 (18.52) | 0.57 |
| Respiratory disease | 4 (8.51) | 1 (5.00) | 3 (11.11) | 0.83 |
| Previous surgery | 9 (19.15) | 4 (20.00) | 5 (18.52) | > 0.99 |
| Signs and symptoms | ||||
| Fever | 39 (82.98) | 15 (75.00) | 24 (88.89) | 0.39 |
| Dry cough | 11 (23.40) | 3 (15.00) | 8 (29.63) | 0.41 |
| Expectoration | 26 (55.32) | 13 (65.00) | 13 (48.15) | 0.25 |
| Hemoptysis | 4 (8.51) | 1 (5.00) | 3 (11.11) | 0.83 |
| Fatigue | 19 (40.43) | 6 (30.00) | 13 (48.15) | 0.21 |
| Anorexia | 6 (12.77) | 0 | 6 (22.22) | 0.03 |
| Headache | 8 (17.02) | 4 (20.00) | 4 (14.81) | 0.94 |
| Diarrhea | 10 (21.28) | 1 (5.00) | 9 (33.33) | 0.03 |
| Pharyngalgia | 13 (27.66) | 6 (30.00) | 7 (25.93) | 0.76 |
| Shortness of breath | 9 (19.15) | 4 (20.00) | 5 (18.52) | > 0.99 |
| Chest tightness/pain | 11 (23.40) | 5 (25.00) | 6 (22.22) | > 0.99 |
| Stuffy and runny nose | 7 (14.89) | 3 (15.00) | 4 (14.81) | > 0.99 |
| Nausea and vomiting | 2 (4.26) | 0 | 2 (7.41) | 0.50 |
| Time from onset to admission (Quartile interval, day) | 3 (1–6) (n = 46) | 3 (2–6) | 2 (1–5) (n = 26) | 0.35 |
| Partial oxygen pressure (quartile interval, mmHg) | 90.7 (79.3–104) (n = 36) | 92.2 (80.1–106) (n = 15) | 89.1 (77.1–103) (n = 21) | 0.29 |
| Oxygenation index (quartile interval) | 431.5 (366.3–494.8) (n = 36) | 439 (382–505) (n = 15) | 424 (358–490) (n = 21) | 0.29 |
| Mean arterial pressure (quartile interval, mmHg) | 97 (87–104) (n = 46) | 93 (87–103) (n = 20) | 97 (89–102) (n = 26) | 0.28 |
| Heart rate (per minute) | 89 (85–96) | 89 (86–99) | 89 (84–92) | 0.21 |
| Respiratory rate (per minute) | 20 (20–21) | 20 (20–20) | 20 (20–22) | 0.65 |
Normal BMI (Asia standard): 18.5–22.9 kg/m2.
BMI body mass index, IQR interquartile range.
Laboratory tests
Table 2 shows the laboratory test results according to group. The proportion of patients with decreased white blood cell counts, C-reactive protein and complement C3 levels, and the time to SARS-CoV-2 nucleic acid-negative conversion was significantly higher in the delayed discharge group than in the control group (P = 0.03, 0.01, 0.04, and < 0.01, respectively). The most significant difference was observed in the time to SARS-CoV-2 nucleic acid-negative conversion (P < 0.01), with the shortest and longest time to negative conversion being 8 and 42 days, respectively (Fig. 1). No significant difference in lymphocyte counts, neutrophil counts, monocyte counts, neutrophil/lymphocyte ratios, procalcitonin levels, D-dimer levels, lactate dehydrogenase levels, hepatic function (total bilirubin, albumin, aspartate aminotransferase, and alanine aminotransferase levels), renal function (blood urea nitrogen and creatinine levels), cardiac enzymes (troponin levels and creatinine kinase-myocardial band), or humoral immunity (immunoglobulin A, immunoglobulin G, immunoglobulin M, and complement C3 levels) was observed between the two groups. Regarding lung computed tomography manifestations, no significant difference was observed between the groups in the presence of bilateral changes or ground-glass opacities; however, air bronchograms were significantly more common in the delayed discharge group than in the control group (18/27 [67%] vs. 4/20 [20%]; P = 0.004).
Table 2.
Laboratory findings of patients with COVID-19.
| All patients (n = 47) | Hospitalization days < 21 (n = 20) | Hospitalization days ≥ 21 (n = 27) | P value | |
|---|---|---|---|---|
| Laboratory findings | ||||
| Leucocytes (*10^9/L) | 5.47 (4.40–6.48) | 5.43 (4.40–6.35) | 5.75 (3.81–6.48) | 0.29 |
| < 3.5 (%) | 6 (12.77) | 0 | 6 (22.22) | 0.03 |
| Neutrophil (*10^9/L) | 3.23 (2.43–3.87) | 3.22 (2.46–3.81) | 3.41 (2.43–4.06) | 0.31 |
| Lymphocytes (*10^9/L) | 1.55 (1.10–1.94) | 1.56 (1.12–1.90) | 1.44 (1.03–1.88) | 0.57 |
| < 1.1 (%) | 12 (25.53) | 3 (15.00) | 9 (33.33) | 0.28 |
| Neutrophil-to-Lymphocyte ratio | 2.10 (1.56–2.92) | 1.83 (1.53–3.37) | 2.37 (1.63–2.83) | 0.78 |
| Monocytes (*10^9/L) | 0.48 (0.36–0.62) | 0.44 (0.34–0.54) | 0.48 (0.38–0.65) | 0.76 |
| Platelet (*10^9/L) | 228 (190–265) | 236 (203–262) | 212 (187–269) | 0.66 |
| Erythrocyte sedimentation rate (mm/h) | 17 (11–22) (n = 10) | 18 (16–19) (n = 2) | 17 (9–30) (n = 8) | > 0.99 |
| Prothrombin time (S) | 11.5 (11–11.8) (n = 43) | 11.4 (11–11.6) (n = 19) | 11.6 (11–11.8) (n = 24) | 0.71 |
| > 13 (%) | 2 (4.26) | 1 (5.00) | 1 (3.70) | > 0.99 |
| D-dimer (mg/L) | 0.32 (0.26–0.47) | 0.38 (0.28–0.52) | 0.29 (0.25–0.36) | 0.13 |
| Creatine kinase (U/L) | 69 (44–103) | 58 (42–93) | 72 (52–108) | 0.57 |
| Creatine kinase isoenzyme (U/L) | 14 (10–17) | 11 (8–15) | 15 (12–18) | 0.57 |
| Lactic dehydrogenase (U/L) | 172 (149–203) | 159 (144–188) | 176 (156–208) | 0.41 |
| Albumin (g/L) | 39.2 (36.3–42.1) | 38.1 (35.9–41.8) | 39.4 (37.1–42.5) | 0.68 |
| Albumin -to-Globulin ratio | 1.4 (1.3–1.5) | 1.3 (1.1–1.5) | 0.65 | |
| < 1.2 (%) | 12 (25.53) | 3 (15.00) | 9 (33.33) | 0.28 |
| Alanine transaminase (U/L) | 23 (14–34) | 24 (12–35) | 23 (15–28) | 0.63 |
| > 40 (%) | 8 (17.02) | 2 (10.00) | 6 (22.22) | 0.48 |
| Aspartate aminotransferase (U/L) | 24 (19–30) | 20 (16–26) | 27 (21–32) | 0.13 |
| > 35 (%) | 5 (10.64) | 1 (5.00) | 4 (14.81) | 0.55 |
| Total bilirubin (μmol/L) | 15.9 (11.3–23.9) | 17.8 (9–24.2) | 15.5 (11.7–21.0) | 0.78 |
| Blood urea nitrogen (mmol/L) | 3.56 (2.93–4.08) | 3.58 (2.88–4.04) | 3.56 (3.05–4.08) | 0.74 |
| Creatinine (μmol/L) | 64.6 (53.9–77.6) | 57 (51.1–70.9) | 68.7 (57.1–80.2) | 0.15 |
| Troponin I (ng/ml) | 0.002 (0.001–0.003) (n = 39) | 0.001 (0.001–0.003) (n = 15) | 0.003 (0.001–0.003) (n = 24) | 0.33 |
| Procalcitonin > 0.1 ng/ml (percentage, %) | 2 (4.88) (n = 41) | 2 (10.00) | 0 | 0.18 |
| C-reactive protein (mg/L) | 3.65 (0.51–14.4) | 0.52 (0.49–4.09) | 6.33 (3.42–18.9) | 0.01 |
| Complement C3 | 0.81 (0.74–0.95) (n = 45) | 0.78 (0.69–0.88) (n = 19) | 0.85 (0.75–1.08) (n = 26) | 0.04 |
| Complement C4 | 0.2 (0.15–0.3) (n = 45) | 0.18 (0.15–0.22) (n = 19) | 0.23 (0.18–0.36) (n = 26) | 0.09 |
| Negative conversion time of SARS-CoV-2 RNA after onset of symptoms | 24 (16–30) | 16 (14–21) | 30 (25–34) | < 0.001 |
| Positive for influenza A/B/RS virus IgM | 14 (29.79) | 4 (20.00) | 10 (37.04) | 0.35 |
| Imaging features | ||||
| Flaky/patchy shadows | 39 (82.98) | 14 (70.00) | 25 (92.59) | 0.10 |
| Ground glass shadow | 29 (61.70) | 13 (65.00) | 16 (59.26) | 0.69 |
| Broncho meteorology | 22 (46.81) | 4 (20.00) | 18 (66.67) | < 0.01 |
| Halo/anti-halo sign | 5 (10.64) | 1 (5.00) | 4 (14.81) | 0.55 |
| Consolidation | 12 (25.53) | 2 (10.00) | 10 (37.04) | 0.08 |
| Peripheral tape, subpleural | 30 (63.83) | 10 (50.00) | 20 (74.07) | 0.09 |
Figure 1.
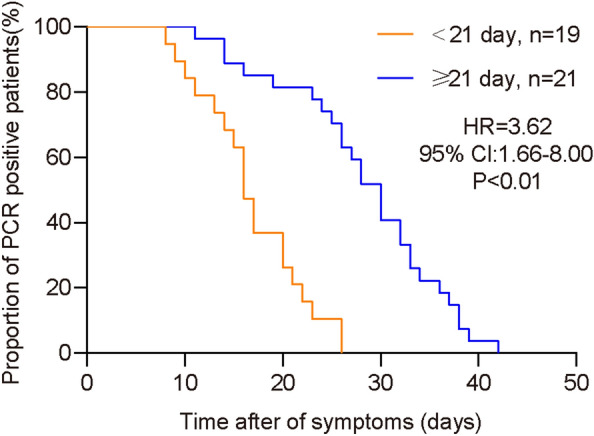
Time to severe acute respiratory syndrome coronavirus (SARS-CoV-2) nucleic acid-negative conversion by PCR analysis of upper respiratory tract samples. CI confidence interval, HR hazard ratio, PCR polymerase chain reaction.
Treatment
Table 3 shows the treatment provided according to group. The proportion of patients who were treated with traditional Chinese medicine and thymalfasin was significantly higher in the delayed discharge group than in the control group (both, P < 0.01). However, no significant difference was observed between the groups in the proportion of patients who received corticosteroids, antivirals, antibiotics, probiotics, acetylcysteine tablets, ambroxol/aminophylline, and supplemental oxygen or assisted ventilation.
Table 3.
Treatment of patients with COVID-19.
| All patients (n = 47) | Hospitalization days < 21(n = 20) | Hospitalization days ≥ 21(n = 27) | P value | |
|---|---|---|---|---|
| Antiviral therapy | 47 (100) | 20 (100) | 27 (100) | > 0.99 |
| Antibiotic | 16 (34.04) | 6 (30.00) | 10 (37.04) | 0.62 |
| Glucocorticoid | 13 (27.66) | 3 (15.00) | 12 (44.44) | 0.06 |
| Traditional Chinese medicine | 36 (76.60) | 9 (45.00) | 27 (100) | < 0.001 |
| Intestinal probiotics | 42 (89.36) | 19 (95.00) | 23 (85.19) | 0.55 |
| Acetylcysteine | 39 (82.98) | 14 (70.00) | 25 (92.59) | 0.10 |
| Ambroxol/theophylline | 8 (17.02) | 3 (15.00) | 5 (18.82) | > 0.99 |
| Thymalfasin | 19 (40.43) | 3 (15.00) | 16 (59.26) | < 0.01 |
| Oxygen uptake | 20 (42.55) | 7 (35.00) | 13 (48.15) | 0.37 |
| High flow oxygen therapy | 2 (4.26) | 1 (5.00) | 1 (3.70) | > 0.99 |
| Noninvasive ventilator | 1 (2.13) | 1 (5.00) | 0 | 0.43 |
| Invasive ventilation | 1 (2.13) | 0 | 1 (3.70) | > 0.99 |
| Ventilation treatment in prone position | 1 (2.13) | 0 | 1 (3.70) | > 0.99 |
Univariate and multivariate logistic regression analyses
Based on the above results, we performed univariate logistic regression analysis of nine factors, namely, diarrhea, anorexia, white blood cell counts, complement C3 levels, C-reactive protein levels, air bronchograms, the time to SARS-CoV-2 nucleic acid-negative conversion, treatment with traditional Chinese medicine, and thymalfasin treatment. Results of the logistic regression analyses are shown in Table 4. Diarrhea, thymalfasin treatment, complement C3 levels, the time to SARS-CoV-2 nucleic acid-negative conversion, and air bronchograms were associated with prolonged hospital stay. Multivariate logistic regression analysis showed that only complement C3 levels and the time to SARS-CoV-2 nucleic acid-negative conversion were independently associated with delayed discharge from hospital (Table 4). According to their regression coefficients, a binary logistic regression model was established as follows: Y = 0.13 × complement C3 + 0.39 × the time to nucleic acid-negative conversion − 16.94. Receiver operating characteristic curve analysis was used to evaluate the diagnostic value of this model. The area under the curve for predicting delayed discharge from hospital was 0.97 (95% confidence interval [CI] 0.93–1.00), which was significantly higher than that of complement C3 levels (0.64, 95% CI 0.49–0.80, P < 0.001) and the time to SARS-CoV-2 nucleic acid-negative conversion (0.87, 95% CI 0.76–0.97, P < 0.01). With a cut-off value of 0.52, the sensitivity, specificity, and positive and negative predictive values of this model were 89%, 95%, 95%, and 91%, respectively (Fig. 2).
Table 4.
Univariate and multivariate logistic regression analysis of factors associated with delayed discharge of patients with COVID-19.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Diarrhea | 0.11 (0.01–0.92) | 0.04 | ||
| Thymalfasin | 0.07 (0.01–0.34) | 0.001 | ||
| Complement C3 | 1.04 (1.00–1.08) | 0.04 | 1.14 (1.02–1.27) | 0.03 |
| Broncho meteorology | 8 (2.06–31.07) | 0.003 | ||
| Negative conversion time of SARS-CoV-2 RNA | 1.24 (1.10–1.39) | < 0.001 | 1.48 (1.09–2.03) | 0.01 |
CI confidence interval, OR odds ratio.
Figure 2.
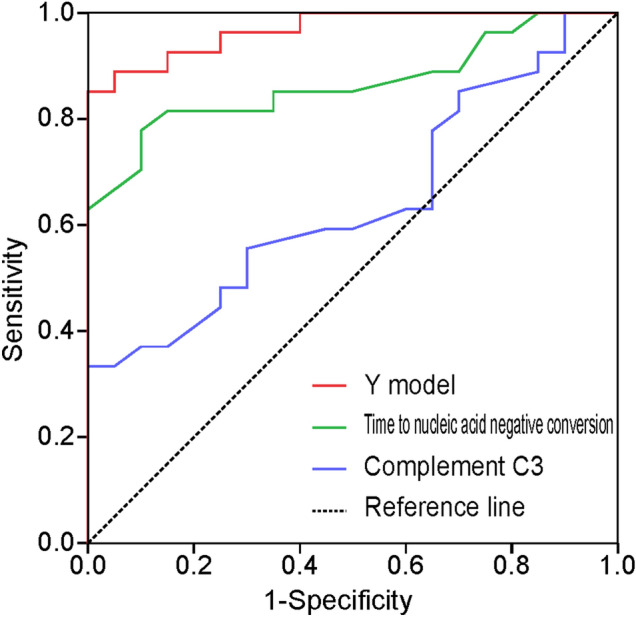
Receiver operating characteristic curves of the Y model, complement C3 levels, and time to nucleic acid-negative conversion for predicting delayed discharge from hospital.
Discussion
Previous research on COVID-19 has characterized the epidemiology, clinical features, and imaging findings of patients with COVID-19. However, information on risk factors for delayed discharge from hospital is limited. In this study, we identified risk factors associated with delayed discharge among 47 patients with COVID-19 who were admitted to three designated hospitals for COVID-19 in Quanzhou City, Fujian Province, China.
A previous study showed that the average length of hospital stay for patients with COVID-19 was 24.7 days15. Therefore, we selected 21 days (3 weeks) as the threshold for delayed discharge, which is easy to recall. In our study, the median (interquartile range [IQR]) length of hospital stay for patients with mild, moderate, and severe or critical disease was 20 (15–24), 22 (16–30), and 29 (22–35) days, respectively, showing a gradually increasing trend. Univariate and multivariate logistic regression analyses both showed that complement C3 levels and the time to SARS-CoV-2 nucleic acid-negative conversion were independent predictors of delayed discharge from hospital. This result is different from that reported in a previous study, which showed that patients with low arterial pressure of oxygen/fraction of inspired oxygen ratios, low lymphocyte counts, severe clinical presentations, and corticosteroid treatment are more likely to experience delayed clinical recovery16. This may be related to the relatively mild illness and small number of cases in our region. Moreover, we constructed a non-invasive Y model, including these variables, to predict delayed discharge from hospital. The sensitivity and specificity of this model were 89% and 95%, respectively.
Complement C3 levels were independently associated with delayed discharge from hospital. SARS-CoV-2 infection can activate innate and adaptive immune responses; however, uncontrolled innate inflammatory response and impaired adaptive immune response may lead to harmful local and systemic tissue injuries17. Complement C3, an immune inflammatory mediator, participates in the immune response of the body to eliminate viruses or bacteria. Increased complement C3 levels indicate that the body’s complement system has been activated, and there may be a strong inflammatory reaction. The stronger the inflammatory reaction, the greater the likelihood of severe disease and admission to an intensive care unit18. If a complement inhibitor is administered in the early stages of infection, the inflammatory injury can be alleviated, facilitating recovery17.
Negative SARS-CoV-2 nucleic acid test results indicate that the virus has been cleared, which is an important indicator of disease prognosis. Xiao et al.19 observed that the majority of patients who were positive for SARS-CoV-2 within 3 weeks after the onset of symptoms subsequently gradually became negative until they all tested negative at 6 weeks, suggesting SARS-CoV-2 viral replication has a relatively long period. In this study, the median time to SARS-CoV-2 nucleic acid-negative conversion was 24 days, consistent with the results of the study by Xiao et al.19; however, this was significantly longer than those obtained by Chen et al.20, which might be due to the longer time interval between SARS-CoV-2 nucleic acid tests in this study. In future diagnosis and treatment, in patients with improved clinical symptoms and imaging findings, the interval between SARS-CoV-2 nucleic acid tests should be reduced to shorten the hospital stay and reduce the financial burden of patients.
Although there have been no previous reports of diarrhea being associated with delayed discharge from hospital, a study reported a confirmed case with diarrhea as the first symptom, and patients with diarrhea had a higher probability of requiring ventilator support and being admitted to the intensive care unit21. In this study, only one critically ill patient required tracheotomy and ventilator support. Similarly, this patient had diarrhea, indicating that patients with COVID-19 with diarrhea need ventilator support and intensive care more often than those without diarrhea22. The presence of gastrointestinal symptoms is associated with a higher risk of hospitalization, which becomes more pronounced as the severity of the disease increases23,24. Furthermore, 90% (9/10) of patients with diarrhea in this study were in the delayed discharge group, implying that the length of hospital stay was longer for patients with diarrhea than for those without diarrhea; however, this finding requires further verification in multicenter studies with a larger sample size. Although the pathogenesis of gastrointestinal symptoms in patients with COVID-19 remains unclear, it may be related to the recruitment of inflammatory cells into the gastrointestinal tract, which releases inflammatory factors, and the expression of angiotensin-converting enzyme 2 in the gastrointestinal tract25. Similarly, studies have shown that air bronchograms are common during disease progression26 and are more common in fatal cases than in survivors, indicating its predictive value. Although univariate logistic regression analysis in this study equally showed that diarrhea and air bronchograms were associated with delayed discharge from hospital, the multivariate logistic regression analysis showed that they were not associated with delayed discharge. The lack of a statistically significant association may be attributable to the small sample size of this study.
Previous research reported that glucocorticoid use in patients with COVID-19 may lead to prolonged hospital stay27, and a study suggested that high-dose glucocorticoid use leads to an increased risk of mortality in patients with COVID-1928. In this study, although we treated patients on the short-term with low-dose glucocorticoid therapy, those who received glucocorticoid therapy tended to stay longer in hospital (P = 0.06). Therefore, the necessity of glucocorticoid use requires further investigation.
Other research has shown that male sex, delayed admission after the onset of symptoms, and invasive mechanical ventilation are factors associated with longer time to SARS-CoV-2 RNA-negative conversion29; however, they were not associated with delayed discharge in this study. A possible explanation is that SARS-CoV-2 RNA-negative conversion is only one of the discharge criteria, and temperature, clinical symptoms, and pulmonary imaging results were similarly considered.
This study had some limitations. First, the number of cases in this region is relatively small, and a larger sample size may be required for verification. Second, the antiviral therapy included lopinavir/ritonavir and interferon-α. Since nearly all patients were administered this antiviral therapy, we could not judge the effect of this treatment on delayed discharge. Third, the immune system is closely related to the severity of COVID-19 and clinical outcomes30,31, which may affect the length of hospital stay; however, we did not analysis this in our study.
In conclusion, high complement C3 levels and an extended time to SARS-CoV-2 RNA-negative conversion are risk factors for delayed discharge from hospital. Therefore, repeated and continuous monitoring of complement C3 levels and nucleic acid load are helpful for early assessment of discharge indications to increase the availability of beds and enable more patients to be treated in hospital.
Acknowledgements
We would like to thank the medical staff in the Department of Infectious Diseases and Department of Respiratory Diseases at The First Hospital of Quanzhou Affiliated to Fujian Medical University for the collection of clinical specimens.
Author contributions
PL, WC, ZS, XZ, and XY designed the study. PL, WC, and XY performed the analysis. HH, YL, MC, DL, and HC provided materials. PL, WC, and XY wrote the manuscript. WC, ZS, XZ, and XY critically reviewed the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81400625), the Natural Science Foundation of Fujian Province of China (2019J01593), the High-level Talent Innovation Project of Quanzhou (2018C067R), Young and Middle-aged Backbone Personnel Training Project of Fujian Health Commission (2020GGA076), and the Pilot Project of Fujian Provincial Department of Science and Technology (2020Y0005).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Peihuang Lin and Wenhuang Chen.
Contributor Information
Xibin Zhuang, Email: zxbqz@163.com.
Xueping Yu, Email: xupy15@fudan.edu.cn.
References
- 1.Li Q, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung K, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu J, Han B, Wang JJG. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S, et al. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020;80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litton E, et al. Surge capacity of intensive care units in case of acute increase in demand caused by COVID-19 in Australia. Med. J. Aust. 2020 doi: 10.5694/mja2.50596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoukat A, Wells CR, Langley JM, Singer BH, Galvani AP. Projecting demand for critical care beds during COVID-19 outbreaks in Canada. CMAJ. 2020 doi: 10.1503/cmaj.200457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Health Commission of the People's Republic of China. Diagnosis and treatment of COVID-19(trial version 6)[EB/OL] (2020-02-19) [2020-02-21]. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml.
- 15.Verity R, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Ardes D, et al. Duration of COVID-19: data from an Italian cohort and potential role for steroids. Microorganisms. 2020 doi: 10.3390/microorganisms8091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan Y, et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/s2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong P, et al. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct. Target Ther. 2020;5:256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan L, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive cross-sectional, multicenter study . Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz L, et al. Symptom profiles and risk factors for hospitalization in patients with SARS-CoV-2 and COVID-19: a large cohort from South America. Gastroenterology. 2020;159:1148–1150. doi: 10.1053/j.gastro.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trottein F, Sokol HJCR. Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep. 2020;32:107915. doi: 10.1016/j.celrep.2020.107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat R, et al. Chest imaging in patients hospitalized with COVID-19 infection—a case series. Curr. Probl. Diagn. Radiol. 2020 doi: 10.1067/j.cpradiol.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, et al. Risk factors associated with disease severity and length of hospital stay in COVID-19 patients. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu K, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu B, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


