Abstract
Hallucinations may arise from an imbalance between sensory and higher cognitive brain regions, reflected by alterations in functional connectivity. It is unknown whether hallucinations across the psychosis continuum exhibit similar alterations in functional connectivity, suggesting a common neural mechanism, or whether different mechanisms link to hallucinations across phenotypes. We acquired resting-state functional MRI scans of 483 participants, including 40 non-clinical individuals with hallucinations, 99 schizophrenia patients with hallucinations, 74 bipolar-I disorder patients with hallucinations, 42 bipolar-I disorder patients without hallucinations, and 228 healthy controls. The weighted connectivity matrices were compared using network-based statistics. Non-clinical individuals with hallucinations and schizophrenia patients with hallucinations exhibited increased connectivity, mainly among fronto-temporal and fronto-insula/cingulate areas compared to controls (P < 0.001 adjusted). Differential effects were observed for bipolar-I disorder patients with hallucinations versus controls, mainly characterized by decreased connectivity between fronto-temporal and fronto-striatal areas (P = 0.012 adjusted). No connectivity alterations were found between bipolar-I disorder patients without hallucinations and controls. Our results support the notion that hallucinations in non-clinical individuals and schizophrenia patients are related to altered interactions between sensory and higher-order cognitive brain regions. However, a different dysconnectivity pattern was observed for bipolar-I disorder patients with hallucinations, which implies a different neural mechanism across the psychosis continuum.
Subject terms: Neuroscience, Psychology
Introduction
Hallucinations are a hallmark feature of schizophrenia, but occur in a range of psychiatric, neurological and general medical conditions1, and even in a minority of healthy individuals2. Hallucinations, and other psychotic phenomena, can be understood to exist along a continuum, ranging from subclinical symptoms in healthy individuals on one end, to individuals with a severe psychotic illness on the other3. Patients with bipolar disorder who experience psychotic symptoms may hold a position in between both ends of the spectrum. However, it remains to be determined if hallucinations in non-clinical individuals, patients with schizophrenia, and patients with bipolar disorder share a common neural mechanism (and differ mainly in severity) or if hallucinations stem from different neural mechanisms across these three groups.
Functional magnetic resonance imaging (fMRI) has proven to be a powerful tool to investigate neural mechanisms associated with hallucinations. Studies have shown elevated levels of activity in sensory cortices during hallucinations in schizophrenia4,5, suggesting inadequate higher-order control over activity in these areas. Cognitive control is mediated by cognitive regions, including areas of the default-mode, central executive, and task-positive network6–9. Alterations in these networks have therefore been linked to hallucinations7. Furthermore, the insula and (para)hippocampal areas have been related to hallucinations9–12 suggesting the involvement of salience and memory processes. Taken together, hallucinations may arise from abnormal connectivity patterns among several areas in the functional connectome13–15.
The question arises if these findings in schizophrenia can be extended to hallucinations in other phenotypic groups. Hallucinations in healthy individuals and patients with bipolar disorder show partly overlapping phenomenological features with hallucinations in schizophrenia16–18, although differences in frequency, duration, and emotional content are consistently described16,19. In addition, neuropsychological studies indicate that cognitive control processes, including inhibition and executive functioning, are less altered in bipolar-I patients than in schizophrenia patients and may be used for compensatory strategies20–22.
This study sets out to compare functional connectome alterations between individuals with hallucinations across the psychosis continuum. To this end, we included patients with a schizophrenia spectrum disorder, bipolar-I disorder with and without a lifetime history of hallucinations, and healthy controls with and without hallucinations. Using a network-based approach, we examined whole-brain functional interactions between brain regions, allowing us to study whether similar neural mechanisms are present across diagnostic groups.
Based on the notion that hallucinations are related to dysconnectivity, we hypothesize that the functional connectome of all individuals with hallucinations will show widespread alterations, particularly in sensory areas including the auditory and visual cortex, limbic areas related to salience processing, and frontal areas related to cognitive control.
Materials and methods
Participants
This cross-sectional study included 116 patients with bipolar-I disorder, including 74 with a lifetime history of hallucinations (BD-H) and 42 without a lifetime history of psychotic experiences (BD), 99 patients with a schizophrenia spectrum disorder, all of whom endorsed lifetime hallucinations (SCZ-H), 40 non-clinical individuals with hallucinations (NC-H), and 228 healthy controls without any history of hallucinations (HC). Participants were recruited as part of five different studies that were all conducted at the University Medical Center Utrecht. See Supplementary Methods for more information on recruitment and characterization. The studies were approved by the Institutional Review Board of the University Medical Center Utrecht. All participants provided written informed consent. All methods and study procedures were conducted in accordance with the relevant guidelines and regulations. Presence of current or lifetime hallucinations and assessment of hallucination modality was established using the Comprehensive Assessment of Symptoms And History (CASH23). Hallucination severity was determined with the Positive and Negative Syndrome Scale (PANSS24) in patients with schizophrenia, and with the Psychotic Symptoms Rating Scales (PSYRATS25) in non-clinical individuals. Hallucination severity was not assessed in patients with bipolar-I disorder, as none of these participants experienced current hallucinations (see Table 1).
Table 1.
Participant characteristics on demographic and clinical variables (n = 483).
| SCZ-H (n = 99) | BD-H (n = 74) | BD (n = 42) | NC-H (n = 40) | HC (n = 228) | P value | |
|---|---|---|---|---|---|---|
| Age, mean (SD), years | 32.3 (10.9) | 46.2 (11.6) | 52.3 (11.9) | 43.1 (14.5) | 40.7 (14.6) | < 0.001* |
| Gender, m/f | 67/32 | 37/37 | 23/19 | 10/30 | 121/107 | < 0.001* |
| Handedness, R/L | 85/8 | 65/8 | 36/7 | 30/10 | 190/37 | 0.106 |
| Motion, mm | ||||||
| Relative mean displacement | 0.1 (0.04) | 0.1 (0.03) | 0.1 (0.03) | 0.08 (0.03) | 0.08 (0.03) | < 0.001* |
| Diagnosis, No. (%) | ||||||
| Schizophrenia | 59 (59.6) | |||||
| Schizoaffective disorder | 17 (17.2) | |||||
| Schizophreniform disorder | 1 (1.0) | |||||
| Schizotypal personality | 1 (1.0) | |||||
| Psychosis NOS | 20 (20.2) | |||||
| Bipolar-I disorder | 74 (100) | 74 (100) | ||||
| Medication, No. (%) | ||||||
| Antipsychotics | 83 (83.8) | 39 (52.7) | 9 (21.4) | 0 (0.0) | 0 (0.0) | |
| Lithium | 5 (5.1) | 42 (56.8) | 27 (64.3) | 0 (0.0) | 0 (0.0) | |
| Anti-depressants | 22 (22.2) | 16 (21.6) | 10 (23.8) | 2 (5.0) | 3 (1.3) | |
| Hallucinations lifetime, No. (%) | 99 (100) | 74 (100) | 0 (0.0) | 40 (100) | 0 (0.0) | |
| Auditory | 80 (80.8) | 39 (52.7) | 0 (0.0) | 40 (100) | 0 (0.0) | < 0.001* |
| Visual | 53 (53.5) | 51 (68.9) | 0 (0.0) | 33 (82.5) | 0 (0.0) | < 0.001* |
| Hallucinations current, No. (%) | 57 (65.5) | 0 (0.0) | 0 (0.0) | 40 (100) | 0 (0.0) | |
| Delusions lifetime, No. (%) | 72 (72.7) | 64 (86.5) | 0 (0.0) | 14 (35.0) | 1 (0.4) | |
Differences between continuous variables were tested with a one-way ANOVA, and differences in dichotomous variables with Chi-square tests. Significance was set at P < 0.05. Significant differences are indicated by an asterisk (*). Current hallucinations means experiences in the last month. The current hallucinations in the SCZ-H were based on the P3 > 2 PANSS, and in NC-H with the frequency item of the PSYRATS. Number of cases with missing data: Diagnosis n = 1 SCZ-H; Antipsychotics n = 10 HC; n = 5 SCZ-H; n = 3 BD-H; n = 1 BD. Lithium n = 10 HC; n = 6 SCZ-H. Antidepressants; n = 10 HC; n = 16 SCZ-H; Lifetime hallucinations n = 4 HC; Auditory n = 4 HC; n = 11 SCZ-H; n = 2 BD-H. Visual n = 5 HC; n = 15 SCZ-H. Olfactory n = 5 HC; n = 17 SCZ-H. Tactile n = 5 HC; n = 14 SCZ-H. Current hallucinations: SCZ-H = 23. Lifetime delusions; n = 5 HC; n = 3 NC-H; n = 21 SCZ-H.
NC-H non-clinical individuals with hallucinations, NOS not otherwise specified, BD-H bipolar-I disorder with lifetime history of hallucinations, BD bipolar-I disorder without lifetime history of hallucinations, SCZ-H schizophrenia spectrum disorder with hallucinations.
Data acquisition and preprocessing
See Supplementary Methods for a detailed description of acquisition parameters, preprocessing steps and motion correction.
Functional network construction
Average timeseries for each participant were extracted from N = 90 functional regions (nodes) of the automated anatomical labeling (AAL) atlas26 and N = 260 nodes of the Power atlas27 covering cortical and subcortical brain regions, but not the cerebellum. Wavelet decompositions to each of the regional time series were applied, extracting wavelet coefficients in scale 4 (0.05–0.10 Hz)28,29. Wavelet filtering was done by the maximal overlap discrete wavelet transform (MODWT) method using the WMSTA toolbox in Matlab (http://www.atmos.washington.edu/~wmtsa/). Functional connectivity was estimated between wavelet coefficients of any pair of regions i and j (edges) using wavelet coherence, done by the mscohere function in Matlab. See the Supplementary Material for more details on wavelet coherence.
Global connectome alterations
We analysed alterations in global network organization related to hallucinations. A detailed description of the graph measures can be found in the Supplemental Methods.
Network based statistics
A Network-Based Statistic (NBS) analysis30,31 was used to obtain information about the localization of connectivity alterations in the overall connectome. Interconnected components of altered connections were identified within the overall connectome using two-sided F-tests to explore increased and decreased levels of functional connectivity (instead of multiple one-sided t-tests). A Family Wise Error (FWE) adjusted P-value was calculated for each component using permutation testing (10,000 permutations) to determine the likelihood that a component of this size could arise by chance30. We compared all four participant groups (NC-H, SCZ-H, BD-H, BD) to controls (HC) in order to investigate if similar alterations are present in the hallucination groups, and if different alterations would be observed for the bipolar-I patients without hallucinations. We conducted these analyses for both the structural AAL-90 and functional Power-260 atlas, as there is some controversy regarding the use of a structural atlas in functional connectivity analyses.
Following Fornito et al.32, we organized all nodes of the AAL atlas into their corresponding lobes, and calculated the proportion of altered connections between each pair of lobes to pinpoint the lobes with the most alterations. In order to obtain more specific regional information on the most important nodes in the network, we ranked the nodes in the NBS networks on node degree per group, similar to Zalesky et al.34. In addition, a conjunction analysis was conducted to test for overlap of connections in each NBS network.
K-means clustering
To directly test for differences in dysconnectivity between the hallucination groups, we compared the three hallucination groups (NC-H, SCZ-H, and BD-H) in one NBS contrast. To help interpret the findings of this analysis, the connections in the resulting component were fed into a k-means clustering analysis (implemented in Matlab)33. This allowed us to cluster connections that behaved either similarly or different in terms of decreased or increased connectivity across the three hallucination groups (i.e. NC-H, SCZ-H, BD-H) (see Supplemental Material).
Symptom correlations
To test for associations with hallucination severity, PANSS item P3-Hallucinations was assessed for correlations with global network metrics using a non-parametric Spearman correlation within the schizophrenia group. Similarly, PSYRATS items frequency and severity of auditory hallucinations were explored for associations with network metrics in the non-clinical group. To correct for multiple testing, correlations are reported at P < 0.05 false discovery rate (FDR).
Hallucination modality
A significant difference was found in the reported hallucination modality across the three hallucination groups (χ2(4) = 366.0, P < 0.001 for auditory; χ2(4) = 265.3, P < 0.001 for visual, see Table 1). To explore effects of hallucination modality, we replicated the NBS analyses in a subgroup of participants only experiencing auditory hallucinations. We compared a subgroup of bipolar-I disorder patients who all only experienced auditory hallucinations (n = 39), to healthy controls (n = 228), as well as schizophrenia patients with only auditory hallucinations (n = 28) versus healthy controls (n = 228). Repeating our analyses in the non-clinical group with only auditory hallucinations was not feasible, as n = 7 participants experienced only auditory hallucinations.
Furthermore, we applied a random forest classification algorithm (part of the BrainWave software https://home.kpn.nl/stam7883/brainwave.html) to identify a set of connections in each NBS network that could differentiate between the experience of having either auditory or visual hallucinations within each group (i.e. NC-H, SCZ-H and BD-H). Each decision tree in the random forest is built using a bootstrap sample (thus with replacement), from the original data. Both bootstrap aggregating (i.e., bagging) and random feature selection avoid overfitting and reduce variance in the model, which results in uncorrelated trees34. Hence, the random forest classifier has the advantage that cross-validation is done internally, meaning that no separate test set of participants is needed to estimate the generalized error of the training set34.
The random forest parameters, mTry (i.e., the number of input variables chosen randomly at each split calculated by the square root of number of features) and nTree (i.e., the number of trees to grow for each forest) were set to 9 and 500, respectively.
In every classification, each feature is given a variable importance (VIMP) score between 0 and 1. Weighted accuracy, sensitivity and specificity were used to assess the random forest for its capability to distinguish between visual and auditory hallucinations. We computed weighted accuracies to correct for unequal group sizes35, see Supplementary Methods for more details on the random forest classifier.
Features fed into the classifier were selected based on their occurrence in the NBS network per group. The edges as presented in Supplementary Tables 13–15 were included for each of the groups. The number in front of each edge pair corresponds to the number of each edge pair in Supplementary Fig. 7. Two random forest parameters that need to be entered into the model were set at mTry = 9; and nTree = 500. mTry is the square root number of features, and the nTree is the number of trees to grow for each forest.
Note that in schizophrenia patients and non-clinical individuals, the condition ‘visual hallucinations’ means that participants experienced both auditory and visual hallucinations (the largest proportion of participants all endorsed auditory hallucinations, see Table 1). The condition ‘auditory hallucinations’ means that schizophrenia patients and non-clinical individuals experienced only auditory hallucinations. A clearer distinction between only auditory and only visual could be made in bipolar-I disorder patients. Please see Supplementary Methods for more information on cross validation of the random forest classifier.
Confounding effects
Due to differences in demographic variables and motion parameters, several validation analyses were conducted and reported in the Supplementary Methods and Results. A quality check of distance dependent effects of motion on edge strength per participant group can be found in Supplementary Fig. 1.
Results
Participant characteristics
A total of 483 participants were included in the analyses; see Table 1 for demographic and clinical characteristics, and Supplementary Tables 1, 2 for more information on exclusion of subjects (e.g. due to effects of motion). Schizophrenia patients were primarily characterized by lifetime auditory (80.8%) and visual (53.5%) hallucinations. Non-clinical individuals with hallucinations all experienced lifetime auditory hallucinations (100%) and the large majority (82.5%) also experienced visual hallucinations. Conversely, bipolar-I disorder patients mostly experienced visual hallucinations (68.9%), whereas about half (52.7%) experienced auditory hallucinations.
In line with previous studies, there was a higher proportion of males in the schizophrenia group, and a higher proportion of females in the non-clinical group (χ2(4) = 21.5, P < 0.001). A significant difference for age was found (F(4) = 21.7, P < 0.001), with the schizophrenia group being younger and bipolar-I disorder group being older than other three groups (post-hoc tests in Supplementary Tables 3, 4). As age and sex were not collinear, these variables were added as covariates in all analyses (see Supplementary Results). The groups also differed in motion parameters (F(4) = 20.3, P < 0.001), with patients with bipolar-I disorder and schizophrenia showing more movement than healthy controls and non-clinical individuals. Additional analyses revealed a minimal influence of motion on connectivity measures (see Supplementary Fig. 1).
Global connectome alterations
There were no significant differences in metrics of global network topology between the groups, see the Supplemental Results for more information.
Network based statistics
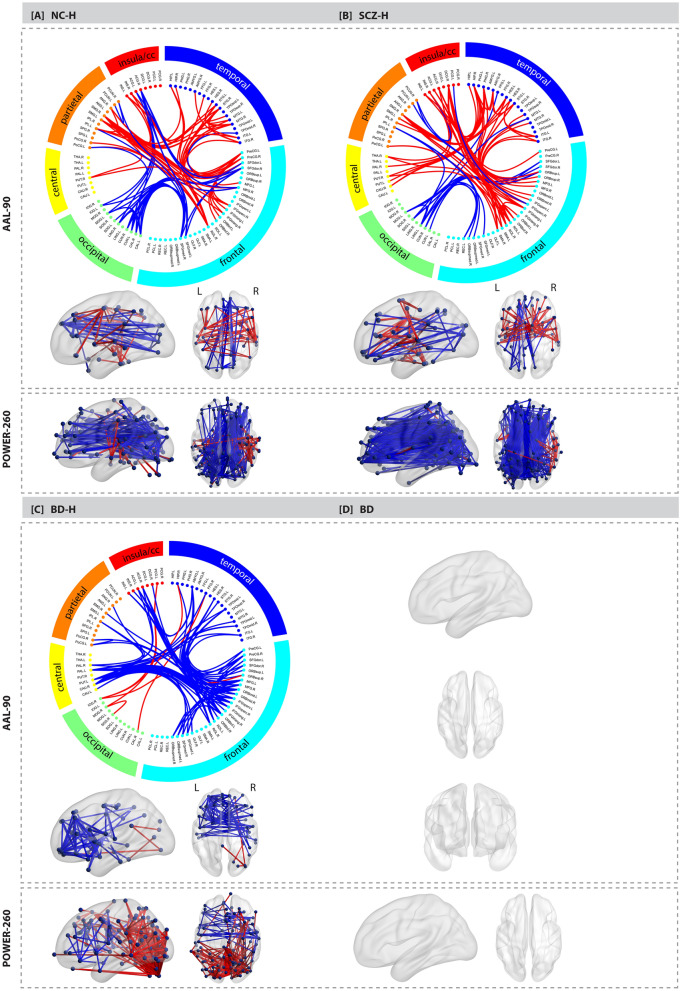
When separately comparing each hallucination group to controls, schizophrenia patients and non-clinical individuals with hallucinations exhibited a similar pattern of increased and decreased connectivity between a wide range of brain areas (P < 0.001 FWE corrected, for both; see Fig. 1A,B), whereas bipolar-I disorder patients with hallucinations revealed a markedly different pattern of mostly decreased connectivity (P = 0.012 FWE corrected; see Fig. 1C). Bipolar-I disorder patients without hallucinations did not exhibit significant alterations in connectivity relative to controls (P > 0.05, FWE corrected; see Fig. 1D). For a complete list of connections in each NBS network, see Supplementary Tables 6–8 for the AAL atlas and Supplementary Tables 10–12 for the Power atlas. These results were broadly replicated in matched subgroups for the AAL atlas, when age and sex were not included as covariates, see Supplementary Fig. 2.
Figure 1.
Functional connectome alterations in the non-clinical and clinical groups compared to healthy controls. The Network Based Statistics results of the AAL atlas are depicted in de upper grey panel and the results of the Power atlas in the lower grey panel: (a) non-clinical individuals with hallucinations (n = 40) vs healthy controls (n = 228); (b) schizophrenia patients with hallucinations (n = 99) vs healthy controls (n = 228); (c) bipolar-I disorder patients with lifetime history of hallucinations (n = 74) vs healthy controls (n = 228). (d) bipolar-I disorder patients without a lifetime history of psychosis (n = 42) did not differ from controls (n = 228). The edges are color-coded based on either an increase (red) or decrease (blue) in connectivity compared to controls. The circle plot depicts all 90 nodes of the AAL atlas clustered according to their cerebral lobe. Group differences were tested at 10,000 permutations P < 0.05 FWE corrected. Age and sex were included as covariates. The corresponding test-statistics, a list of altered connections (Supplementary Tables 6–8 for AAL atlas, Supplementary Tables 10–12 for Power atlas), and abbreviations for the AAL brain regions depicted in the circle plot (Supplementary Table 16) can be found in the Supplementary. BD bipolar-I disorder without lifetime history of hallucinations, BD-H bipolar-I disorder with lifetime history of hallucinations, CC cingulate cortex, NC-H non-clinical individuals with hallucinations, SCZ-H schizophrenia spectrum disorder with hallucinations.
Using the AAL atlas, the majority of connections comprising the NBS components in schizophrenia patients and non-clinical individuals were found between fronto-insula/cingulate regions, followed by fronto-temporal, temporo-temporal, fronto-occipital, and fronto-parietal alterations (see Fig. 2A,B). The NBS subnetwork in schizophrenia patients included more altered connections among fronto-insula/cingulate and temporal-central regions whereas altered fronto-occipital connections were more prevalent in non-clinical individuals. Similar alterations were found when using the Power atlas, including increased connectivity between the auditory, sensorimotor and cingulo-opercular control network (see Fig. 1 and Supplementary Fig. 3a,b for a circle plot of the results for the Power atlas). Decreased connectivity was found mainly between the visual, default mode, central executive and ventral attention networks in both groups.
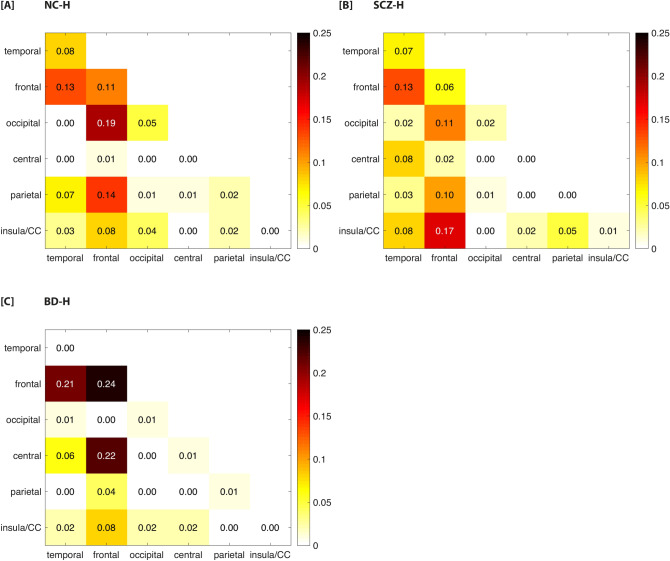
Figure 2.
Proportion of altered connections between each pair of lobes for the three hallucination groups relative to controls. The number of altered links between each pair of lobes was divided by the total number of altered pair-wise links based on the results of the AAL atlas. (a) non-clinical individuals with hallucinations (n = 40) vs healthy controls (n = 228); (b) schizophrenia patients with hallucinations (n = 99) vs healthy controls (n = 228); (c) bipolar-I disorder patients with lifetime history of hallucinations (n = 74) vs healthy controls (n = 228). Note that the hippocampus and amygdala are assigned to the temporal lobe. The putamen, pallidum and caudate are included in the central lobe. BD bipolar-I disorder without lifetime history of hallucinations, BD-H bipolar-I disorder with lifetime history of hallucinations, CC cingulate cortex, NC-H non-clinical individuals with hallucinations, SCZ-H schizophrenia spectrum disorder with hallucinations.
For bipolar-I disorder patients with hallucinations, the most pronounced alterations were found among fronto-temporal, fronto-central connections and between the bilateral frontal lobes when using the AAL atlas (see Fig. 2). These results were replicated using the Power atlas, reporting increased connectivity between the visual, default mode, subcortical and dorsal attention networks (see Fig. 1C and Supplementary Fig. 3C). Decreased connectivity was found between the auditory, sensorimotor, cingulo-opercular, salience and central-executive network.
In schizophrenia patients, the node with the highest number of altered connections in the NBS component was the left middle cingulate gyrus (see Table 2), whereas this was the right superior medial frontal gyrus for non-clinical individuals, and left amygdala for bipolar-I disorder patients with hallucinations.
Table 2.
Node degree of NBS network per group.
| NC-H (n = 40) | SCZ-H (n = 99) | BD-H (n = 74) | |||
|---|---|---|---|---|---|
| Node | Degree | Node | Degree | Node | Degree |
| Frontal_Sup_Medial_R | 12 | Cingulum_Mid_R | 17 | Frontal_Mid_Orb_L | 19 |
| Precentral_L | 12 | Hippocampus_R | 10 | Amygdala_L | 13 |
| Rolandic_Oper_R | 11 | Frontal_Sup_Orb_L | 9 | Caudate_R | 12 |
| SupraMarginal_R | 11 | Rolandic_Oper_R | 9 | Frontal_Mid_L | 12 |
| Temporal_Sup_R | 10 | Rolandic_Oper_L | 8 | Frontal_Inf_Orb_L | 10 |
| Frontal_Sup_R | 8 | SupraMarginal_R | 6 | Pallidum_L | 9 |
| Cingulum_Ant_R | 7 | Frontal_Inf_Oper_R | 5 | Putamen_L | 9 |
| Lingual_R | 7 | Heschl_R | 5 | Frontal_Inf_Oper_R | 6 |
| Calcarine_L | 6 | Lingual_L | 5 | Precentral_R | 6 |
| Cuneus_L | 6 | Occipital_Mid_L | 5 | Rolandic_Oper_R | 6 |
| Frontal_Mid_R | 5 | ParaHippocampal_R | 5 | Rolandic_Oper_L | 5 |
| Frontal_Sup_Medial_L | 5 | Temporal_Sup_L | 5 | Cingulum_Ant_L | 4 |
| Temporal_Mid_R | 5 | Amygdala_R | 4 | Cingulum_Ant_R | 4 |
| Cuneus_R | 4 | Cingulum_Ant_R | 4 | Frontal_Inf_Oper_L | 4 |
| Frontal_Sup_L | 4 | Frontal_Inf_Tri_L | 4 | Frontal_Mid_R | 4 |
| Insula_R | 4 | Cuneus_R | 3 | Temporal_Pole_Sup_L | 4 |
| Parietal_Inf_L | 4 | Heschl_L | 3 | Frontal_Inf_Tri_L | 3 |
| Precentral_R | 4 | Insula_L | 3 | Frontal_Med_Orb_R | 3 |
| Temporal_Sup_L | 4 | Insula_R | 3 | Frontal_Sup_Medial_L | 3 |
| Angular_R | 3 | Occipital_Sup_L | 3 | Heschl_R | 3 |
| Calcarine_R | 3 | Parietal_Inf_L | 3 | Occipital_Inf_R | 3 |
| Frontal_Inf_Oper_R | 3 | Supp_Motor_Area_R | 3 | Parietal_Inf_R | 3 |
| Insula_L | 3 | Temporal_Inf_L | 3 | Caudate_L | 2 |
| Occipital_Mid_L | 3 | Caudate_R | 2 | Cingulum_Post_L | 2 |
| SupraMarginal_L | 3 | Cingulum_Ant_L | 2 | Frontal_Med_Orb_L | 2 |
| Temporal_Inf_L | 3 | Frontal_Inf_Orb_R | 2 | Frontal_Mid_Orb_R | 2 |
| Temporal_Inf_R | 3 | Frontal_Sup_L | 2 | Frontal_Sup_L | 2 |
| Cingulum_Ant_L | 2 | Pallidum_R | 2 | Frontal_Sup_Medial_R | 2 |
| Frontal_Inf_Tri_R | 2 | Postcentral_L | 2 | Frontal_Sup_Orb_R | 2 |
| Hippocampus_L | 2 | Precuneus_R | 2 | Frontal_Sup_R | 2 |
| Occipital_Inf_L | 2 | Putamen_L | 2 | Fusiform_R | 2 |
| Postcentral_L | 2 | Putamen_R | 2 | Hippocampus_R | 2 |
| Precuneus_R | 2 | Rectus_L | 2 | Insula_L | 2 |
| Putamen_R | 2 | Supp_Motor_Area_L | 2 | Occipital_Mid_R | 2 |
| Temporal_Mid_L | 2 | SupraMarginal_L | 2 | Olfactory_R | 2 |
| Amygdala_R | 1 | Temporal_Inf_R | 2 | Pallidum_R | 2 |
| Frontal_Inf_Orb_L | 1 | Temporal_Mid_L | 2 | Postcentral_L | 2 |
| Frontal_Inf_Orb_R | 1 | Thalamus_R | 2 | Postcentral_R | 2 |
| Frontal_Inf_Tri_L | 1 | Cingulum_Post_L | 1 | Precentral_L | 2 |
| Frontal_Mid_L | 1 | Cuneus_L | 1 | Temporal_Inf_L | 2 |
| Fusiform_R | 1 | Frontal_Inf_Oper_L | 1 | Temporal_Pole_Mid_L | 2 |
| Heschl_R | 1 | Frontal_Inf_Orb_L | 1 | Angular_R | 1 |
| Hippocampus_R | 1 | Frontal_Med_Orb_L | 1 | Calcarine_R | 1 |
| Occipital_Sup_L | 1 | Frontal_Med_Orb_R | 1 | Cingulum_Mid_R | 1 |
| Postcentral_R | 1 | Frontal_Mid_L | 1 | Frontal_Inf_Orb_R | 1 |
| Precuneus_L | 1 | Frontal_Mid_Orb_R | 1 | Frontal_Inf_Tri_R | 1 |
| Temporal_Pole_Mid_R | 1 | Frontal_Mid_R | 1 | Frontal_Sup_Orb_L | 1 |
| Temporal_Pole_Sup_R | 1 | Frontal_Sup_Medial_L | 1 | Insula_R | 1 |
| Frontal_Sup_Orb_R | 1 | Occipital_Sup_R | 1 | ||
| Frontal_Sup_R | 1 | SupraMarginal_R | 1 | ||
| Fusiform_L | 1 | Temporal_Mid_R | 1 | ||
| Hippocampus_L | 1 | Temporal_Sup_L | 1 | ||
| Parietal_Inf_R | 1 | ||||
| Precentral_L | 1 | ||||
| Precuneus_L | 1 | ||||
| Temporal_Mid_R | 1 | ||||
| Temporal_Pole_Sup_R | 1 | ||||
NC-H non-clinical individuals with hallucinations, BD-H bipolar-I disorder with lifetime history of hallucinations, SCZ-H schizophrenia spectrum disorder with hallucinations.
The conjunction analysis revealed considerable variation across the exact connections comprising the respective NBS networks, see Supplementary Fig. 4 and Supplementary Table 5 for a list of overlapping connections.
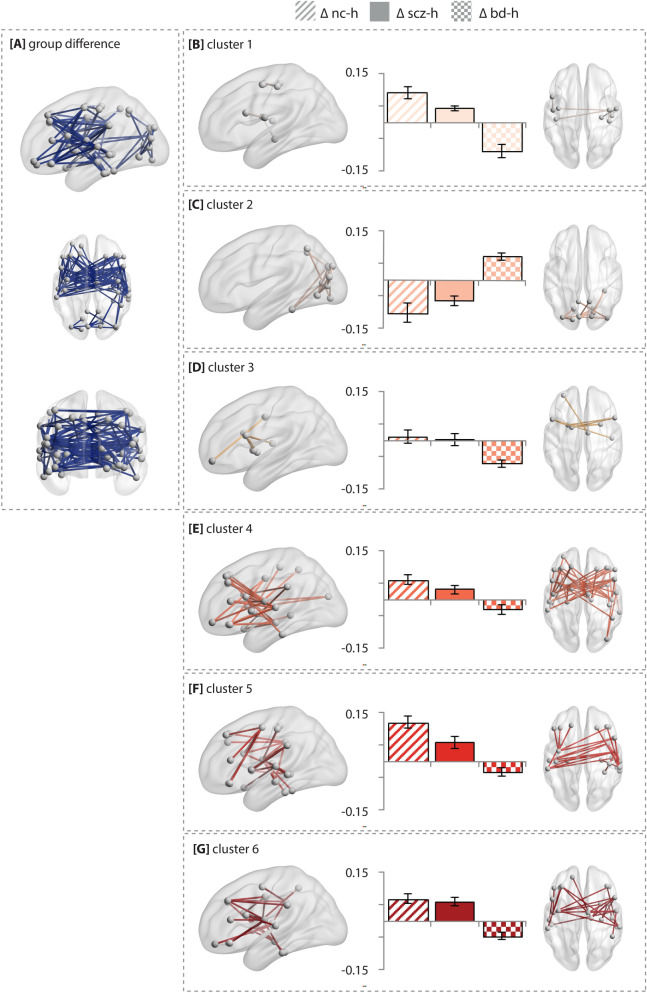
K-means clustering
A direct NBS comparison of the three hallucination groups yielded a subnetwork of connections that differed between non-clinical individuals, schizophrenia patients, and bipolar-I patients with hallucinations (P < 0.001; see Fig. 3A and Supplementary Table 9 for a list of connections). To help interpret the resulting subnetwork, a k-means clustering analysis was performed, which yielded six separate sets of connections across the hallucinating groups that behaved either similar or differently across groups (Fig. 3B–E). As presented in Fig. 3, a similar pattern of connectivity alterations was observed for schizophrenia patients and non-clinical individuals, including dysconnectivity among fronto-temporal, temporo-temporal, fronto-central and occipital areas (Fig. 3B–E). Bipolar-I disorder patients with hallucinations revealed an inversed pattern of dysconnectivity relative to schizophrenia and non-clinical individuals in terms of showing decreased versus increased connectivity and vice versa. See Supplementary Table 9 for a list of connections per cluster.
Figure 3.
Sets of connections clustered according to their behavior in clinical and non-clinical individuals with hallucinations. (A) The result of the overall group comparison between patients with schizophrenia (n = 99), patients with bipolar-I disorder with (n = 74) and non-clinical individuals with hallucinations (n = 40) using the AAL atlas. (B–E) the edges in the component that differed between the groups were clustered by a k-means clustering algorithm to elucidate differences across the groups with hallucinations. Error bars represent the standard deviation of connectivity strength within that particular cluster. The group differences were tested using an F-test at T = 8.0; P < 0.05 at 10,000 permutations. The test-statistics and AAL labels corresponding to the altered connections per cluster can be found in Supplementary Table 9. Age and sex were included as covariates. NC-H non-clinical individuals with hallucinations, BD-H bipolar-I disorder with lifetime history of hallucinations, SCZ-H schizophrenia spectrum disorder with hallucinations.
Symptom correlates
None of the clinical variables was associated with either the weighted global efficiency, weighted clustering coefficient, MST leaf fraction, MST diameter or edges within the NBS network in the schizophrenia patients with hallucinations, or non-clinical individuals (all P > 0.05, FDR corrected).
Hallucination modality
When replicating the NBS analysis in a subgroup of bipolar-I participants with only auditory hallucinations, we found similar alterations compared to the total sample of bipolar-I disorder patients with hallucinations, except that increased activity in the occipital cortex was not found in bipolar-I disorder patients with only auditory hallucinations (see Supplementary Fig. 6). When comparing schizophrenia patients with only auditory hallucinations (n = 28) versus healthy controls (n = 228), results were not significant at P > 0.05 FWE corrected.
Using the random forest machine learning algorithm, differentiating between auditory and visual hallucinations in non-clinical individuals was possible with a weighted accuracy of 77.5%, sensitivity of 0.0% and specificity of 93.9%, respectively. The connection between the left superior temporal gyrus and right rolandic operculum (no. 19) was the most important discriminating feature. In both schizophrenia patients and bipolar-I disorder patients, the weighted accuracy was at or below chance-level, meaning than differentiation was not possible (schizophrenia: weighed accuracy of 50.0%, sensitivity of 26.0% and specificity of 64.1%; bipolar-I disorder: weighted accuracy of 28.6%, sensitivity of 67.3% and specificity of 7.0%). See Supplementary Fig. 7 for an overview of the results per group.
Discussion
This study compared functional connectivity alterations related to hallucinations in a large sample of patients with schizophrenia, bipolar-I disorder, non-clinical individuals, and healthy controls. A range of connections was found to be altered in schizophrenia patients and non-clinical individuals with hallucinations. These alterations involved mainly increased connectivity between the insula, cingulate cortex, auditory, and language-related areas in non-clinical individuals and schizophrenia patients, compared to controls. The non-clinical individuals and schizophrenia patients exhibited remarkably similar disruptions of functional connectivity. In contrast, differential effects were observed for bipolar-I disorder patients with hallucinations versus controls, involving mainly decreased connectivity between fronto-temporal and fronto-striatal areas. Bipolar-I disorder patients without hallucinations did not show any connectivity alterations compared to controls. A direct group-wise comparison confirmed connectivity alterations that were similar in patients with schizophrenia and non-clinical individuals, but inversed (decreased versus increased connectivity) in bipolar-I disorder patients. Thus, contrary to our initial hypothesis, we did not find a similar pattern of functional connectivity alterations across the psychosis continuum. This implies that individuals with hallucinations on the psychosis continuum may not necessarily share a neural mechanism, despite overlapping phenomenological features.
Our results are in line with previous findings on schizophrenia and hallucinations, showing alterations in functional connectivity in areas related to sensory processing and cognitive control4,5,10,12,36–38. Previous studies also reported increased connectivity among frontal, anterior cingulate, and insular cortex and language-related areas in relation to hallucinatory experiences11,12.
Previous findings suggest similar functional connectivity alterations in healthy individuals with hallucinations as observed in schizophrenia with hallucinations38–40. Findings of altered structural connectivity in healthy individuals with hallucinations are also broadly consistent with alterations found in schizophrenia with hallucinations41,42. Our results confirm previous findings of similar brain alterations in healthy individuals with hallucinations and schizophrenia patients.
Previous studies have showed both similar6,43 and dissimilar44 connectome alterations in schizophrenia and psychotic bipolar disorder. Our findings could not confirm that bipolar-I patients with hallucinations showed similar connectivity alterations as schizophrenia patients and non-clinical individuals with hallucinations. However, decreased connectivity between frontal and temporal areas, as observed in our sample of bipolar patients with hallucinations, was previously reported in pediatric bipolar disorder patients and related to top-down control45. Decreased connectivity between fronto-striatal areas and fronto-temporal areas has also been related to mood dysregulation in bipolar disorder46,47.
Our findings should be interpreted in light of methodological considerations. Differences in hallucinatory modality across groups may be associated with the differential findings between schizophrenia patients and non-clinical individuals versus bipolar-I disorder patients with hallucinations. In the latter group, a larger proportion of patients experienced lifetime visual hallucinations (68.9%), with a smaller proportion (52.7%) reporting auditory hallucinations. Previous studies found modality-specific alterations in schizophrenia48,49. Indeed, we observed increased connectivity of the visual cortex in patients with bipolar-I disorder patients—but not after repeating the analysis in bipolar-I disorder patients with only auditory hallucinations—suggesting that the involvement of the visual cortex may be linked to the experience of visual hallucinations. To further explore this notion, we applied a random forest machine learning algorithm. Classification accuracies around chance level were found, meaning we did not find a set of connections able to discriminate between effects of auditory versus visual hallucinations. As the sensitivity was around 0% in non-clinical individuals, these results must be interpreted with caution. Because of using our dataset retrospectively, the dataset was not highly suitable to investigate effects of hallucination modality, as the majority of participants endorsed both auditory and visual hallucinations. Future studies could more thoroughly investigate this issue by recruiting patients who experience hallucinations in only one modality.
Furthermore, recent advances have indicated that structural atlases might not be best suited to investigate functional connectivity, we therefore replicated our results using the functional Power atlas. As the best method regarding the use of brain atlases is currently inconclusive, we included results of both atlases as this may benefit discussion in the field.
By taking advantage of a large cross-diagnostic sample, our findings add important new information on neural mechanism of hallucinations across the psychosis continuum. However, we were unable to include a group of schizophrenia patients without lifetime hallucinations, as most schizophrenia patients experience hallucinations in their lifetime. Furthermore, we included participants based on the criterion of having experienced lifetime hallucinations, regardless of current hallucination state. Thus, all participants share a general disposition to hallucinate, but there were differences between groups in current hallucination state. However, using resting state fMRI to study hallucinations is generally thought to be more sensitive to trait-associated connectivity alterations which are relatively stable over time50,51. This assertion is in line with our finding that current symptom severity was not significantly correlated with connectivity measures. Moreover, the average frequency of hallucinations in the non-clinical individuals was once per week (much less frequent than in the schizophrenia patients), but these individuals nonetheless exhibited a similar connectivity pattern as the schizophrenia patients. Together, this suggests that our resting state measurements reflect trait- rather than state-characteristics. Moreover, comparison of active hallucination state between schizophrenia and bipolar disorder may be biased by other confounding effects including mood state, as hallucinations in bipolar disorder occur mainly in manic or depressive episodes17.
Another limitations concerns the fact that the bipolar-I disorder patients in the current study were all in euthymic phase at time of scanning. Also, the group of bipolar-I disorder patients without hallucinations was smaller in size (n = 42) than the group of bipolar-I disorder patients with hallucinations (n = 74). Taken together, this could have contributed to the null-finding in bipolar-I patients without hallucinations, as larger sample sizes might be needed to pick up more subtle aberrations in this group.
Group-differences in medication use may also have influenced our results. Lithium is suggested to normalize brain function52–54, which may explain why bipolar-I disorder patients without lifetime hallucinations did not reveal connectivity differences compared to controls. However, lithium users were evenly balanced across both groups of bipolar-I disorder patients with and without hallucinations, and are thus unlikely to fully explain our results. Also, it is unlikely that our findings can be attributed to the use of antipsychotic medication, as the non-clinical individuals were free of medication use and nevertheless demonstrated similar alterations as the medicated patients with schizophrenia. Confounding factors such as drug-induced hallucinations in the non-clinical group are also unlikely, as none of the non-clinical participants had a positive urine test for illicit drugs55.
Potentially confounding effects of age, sex, and motion were tested in several ways, and were found to have minimal influence. Note that our analyses were conducted after stringent exclusion of participants with high motion. Afterward, the relative mean displacement ranged from 0.08 to 0.10 mm across groups, indicating high-quality data.
Conclusions
The findings of this study suggest that schizophrenia patients and non-clinical individuals with hallucinations exhibit similar alterations in functional connectivity. In contrast, a markedly different pattern of connectivity alterations was observed in bipolar-I patients with a lifetime history of hallucinations, encompassing different regions and involving mainly reductions in connectivity as opposed to increases. These findings suggest a similar neural mechanism for hallucinations in schizophrenia patients and non-clinical individuals, but a different neural mechanism in bipolar-I disorder. If our findings of a different neural mechanism for hallucinations in bipolar disorder can be replicated independently, this would warrant further investigation into whether hallucinations in bipolar-I disorder should be treated differently in clinical practice. Further elucidating the role of large-scale networks in the experience of hallucinations may enable tailored pharmaco-therapeutic interventions designed to restore the balance between these networks.
Supplementary Information
Acknowledgements
We would like to thank Prof. Dr. R.S. Kahn and Prof. Dr. R.A. Ophoff for their substantial contributions in obtaining funding and to the collection of the data. We would like to thank all participants for their participation, and all researchers for their efforts in making the BiG, TOPFIT, Spectrum, Simvastatin, and UH studies possible.
Funding
This work was supported by several grants. The BiG study was supported by the National Institute of Mental Health (R01 MH090553). The TOPFIT study was supported by the Dutch Diabetes Research Foundation (2007.00.040), Lilly Pharmaceuticals (Ho01-TOPFIT), Janssen Pharmaceuticals, and the Dutch Psychomotor Therapy Foundation. The Simvastatin study was supported by ZONMW TOP (40-00812-98-12154 and Stanley Foundation (12T-008). The Understanding Hallucinations study was supported by ZONMW TOP (40-00812-98-13009). The spectrum study was supported by NWO/ZonMW grant (Dutch Scientific Research Organization) Clinical Fellowship (40-00703-97-270) and NWO/ZonMW Innovation Impulse (VIDI) (017.106.301). The contribution of co-author KH was funded by an ERC Advanced Grant (693124) and a RCN grant (223273).
Data availability
The datasets generated during and/or analysed during the current study are not publicly available as this study was part of multiple larger studies of which not all data has yet been analysed and published. Data pertaining to this manuscript are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Supplementary Information published with this Article contained an error, where an old version of Figure S5 was used.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/14/2021
A Correction to this paper has been published: 10.1038/s41598-021-87849-w
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80657-8.
References
- 1.Sommer IE, Kahn RS. Psychosis susceptibility syndrome: An alternative name for schizophrenia. Lancet Psychiatry. 2014;1:111. doi: 10.1016/S2215-0366(14)70288-3. [DOI] [PubMed] [Google Scholar]
- 2.Sommer IE, Koops S, Blom JD. Comparison of auditory hallucinations across different disorders and syndromes. Neuropsychiatry. 2012;2:1–12. doi: 10.2217/npy.12.2. [DOI] [Google Scholar]
- 3.Van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: A psychosis continuum in the general population? Schizophr. Res. 2000;45:11–20. doi: 10.1016/S0920-9964(99)00224-8. [DOI] [PubMed] [Google Scholar]
- 4.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 5.Kompus K, Westerhausen R, Hugdahl K. The, “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: A meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Baker JT, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 2015;9:430. doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alderson-Day B, et al. Auditory Hallucinations and the brain's resting-state networks: Findings and methodological observations. Schizophr. Bull. 2016;42:1110–1123. doi: 10.1093/schbul/sbw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre S, et al. Network dynamics during the different stages of hallucinations in schizophrenia. Hum. Brain Mapp. 2016;37:2571–2586. doi: 10.1002/hbm.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diederen KM, et al. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- 11.Liemburg EJ, et al. Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophr. Res. 2012;135:15–22. doi: 10.1016/j.schres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chang X, et al. Resting-state functional connectivity in medication-naïve schizophrenia patients with and without auditory verbal hallucinations: A preliminary report. Schizophr. Res. 2017;188:75–81. doi: 10.1016/j.schres.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt A, et al. Approaching a network connectivity-driven classification of the psychosis continuum: A selective review and suggestions for future research. Front. Hum. Neurosci. 2015;8:1047. doi: 10.3389/fnhum.2014.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward ND, Cascio CJ. Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry. 2015;72:743–744. doi: 10.1001/jamapsychiatry.2015.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daalman K, et al. Auditory verbal hallucinations and cognitive functioning in healthy individuals. Schizophr. Res. 2011;132:203–207. doi: 10.1016/j.schres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Toh WL, Castle DJ, Thomas N, Badcock JC, Rossell SL. Auditory verbal hallucinations (AVHs) and related psychotic phenomena in mood disorders: analysis of the 2010 Survey of High Impact Psychosis (SHIP) data. Psychiatry Res. 2016;243:238–245. doi: 10.1016/j.psychres.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Waters F, Fernyhough C. Hallucinations: A systematic review of points of similarity and difference across diagnostic classes. Schizophr. Bull. 2017;43:32–43. doi: 10.1093/schbul/sbw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers AR, 3rd, Kelley MS, Corlett PR. Varieties of voice-hearing: Psychics and the psychosis continuum. Schizophr. Bull. 2017;43:84–98. doi: 10.1093/schbul/sbw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethridge LE, et al. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiarity and relation to generalized cognitive deficit. Schizophr. Res. 2014;159:491–498. doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badcock JC, Mahfouda S, Maybery MT. Hallucinations and inhibitory functioning in healthy young adults with high and low levels of hypomanic personality traits. Cogn. Neuropsychiatry. 2015;20:254–269. doi: 10.1080/13546805.2015.1021907. [DOI] [PubMed] [Google Scholar]
- 22.Thakkar KN, Schall JD, Logan GD, Park S. Cognitive control of gaze in bipolar disorder and schizophrenia. Psychiatry Res. 2015;225:254–262. doi: 10.1016/j.psychres.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25.Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: The psychotic symptom rating scales (PSYRATS) Psychol. Med. 1999;29:879–889. doi: 10.1017/S0033291799008661. [DOI] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Percival DB, Walden AT. Wavelet Methods for Time Series Analysis. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 29.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004;11:561–566. doi: 10.5194/npg-11-561-2004. [DOI] [Google Scholar]
- 30.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore E. Connectivity differences in brain networks. Neuroimage. 2012;60:1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- 32.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chyzhyk D, Graña M. Findings in resting-state fMRI by differences from K-means clustering. Stud. Health Technol. Inform. 2014;207:300–310. [PubMed] [Google Scholar]
- 34.Breiman L. University of California Random forest. Mach. Learn. 1999;45:1–35. [Google Scholar]
- 35.Chen, C., Liaw, A., Breiman, L. Using random forest to learn imbalanced data. Tech. Rep.666, Dept. Statistics, Univ. California, Berkeley, CA, (2004).
- 36.Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a cortocostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol. Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf ND, et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J. Psychiatry Neurosci. 2011;36:366–374. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lutterveld R, Diederen KM, Otte WM, Sommer IE. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum. Brain Mapp. 2014;35:1436–1445. doi: 10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diederen KM, et al. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 2013;43:1685–1696. doi: 10.1017/S0033291712002541. [DOI] [PubMed] [Google Scholar]
- 40.Sheffield JM, Kandala S, Burgess GC, Harms MP, Barch DM. Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:498–506. doi: 10.1016/j.bpsc.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drakesmith M, et al. Schizophrenia-like topological changes in the structural connectome of individuals with subclinical psychotic experiences. Hum. Brain Mapp. 2015;36:2629–2643. doi: 10.1002/hbm.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dellen E, et al. Structural brain network disturbances in the psychosis spectrum. Schizophr. Bull. 2016;42:782–789. doi: 10.1093/schbul/sbv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meda SA, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol. Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collin G, et al. Brain network analysis reveals affected connectome structure in bipolar I disorder. Hum. Brain Mapp. 2016;37:122–134. doi: 10.1002/hbm.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickstein DP, et al. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol. Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strakowski SM, et al. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am. J. Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 47.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 48.Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb. Cortex. 2013;23:1108–1117. doi: 10.1093/cercor/bhs082. [DOI] [PubMed] [Google Scholar]
- 49.Ford JM, et al. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr. Bull. 2015;41:223–232. doi: 10.1093/schbul/sbu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohlken MM, Hugdahl K, Sommer IE. Auditory verbal hallucinations: Neuroimaging and treatment. Psychol. Med. 2017;47:199–208. doi: 10.1017/S003329171600115X. [DOI] [PubMed] [Google Scholar]
- 51.Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: Brain networks under genetic control. Hum. Brain Mapp. 2009;30:1938–1946. doi: 10.1002/hbm.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh MK, Chang KD. The neural effects of psychotropic medications in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2012;21:753–771. doi: 10.1016/j.chc.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 54.Abramovic L, et al. The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. Eur. Neuropsychopharmacol. 2016;26:1741–1751. doi: 10.1016/j.euroneuro.2016.09.371. [DOI] [PubMed] [Google Scholar]
- 55.Sommer IE, et al. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr. Bull. 2010;36:633–641. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available as this study was part of multiple larger studies of which not all data has yet been analysed and published. Data pertaining to this manuscript are available from the corresponding author on reasonable request.