Abstract
Gastric cancer (GC) is a leading cause of cancer-induced mortality, with poor prognosis with metastasis. The mechanism of gastric carcinoma lymph node metastasis remains unknown due to traditional bulk-leveled approaches masking the roles of subpopulations. To answer questions concerning metastasis from the gastric carcinoma intratumoural perspective, we performed single-cell level analysis on three gastric cancer patients with primary cancer and paired metastatic lymph node cancer tissues using single-cell RNA-seq (scRNA-seq). The results showed distinct carcinoma profiles from each patient, and diverse microenvironmental subsets were shared across different patients. Clustering data showed significant intratumoural heterogeneity. The results also revealed a subgroup of cells bridging the metastatic group and primary group, implying the transition state of cancer during the metastatic process. In the present study, we obtained a more comprehensive picture of gastric cancer lymph node metastasis, and we discovered some GC lymph node metastasis marker genes (ERBB2, CLDN11 and CDK12), as well as potential gastric cancer evolution-driving genes (FOS and JUN), which provide a basis for the treatment of GC.
Subject terms: Cell biology, Cancer, Gastrointestinal cancer
Introduction
Globally, gastric cancer (GC) is the fifth most common and one of the leading cancers that cause mortality. In 2012, there were 952,000 cases diagnosed, resulting in an estimated 723,000 annual deaths1. Gastric cancer represents a poor prognosis, and the total 5-year survival is less than 30%, despite treatment with surgery2. Lymphatic metastasis was expected to be associated with poor outcomes, and in poorer stages, more distant lymph nodes can be revealed through histopathology finding3,4. More accurate diagnosis and precision therapy are the priorities of current clinical and fundamental research in gastric cancer. Based on distinct molecular subtypes in The Cancer Genome Atlas (TCGA) Network, gastric cancer is now regarded to have specific genomic abnormalities and targeted therapies5.
Biologists and clinicians are faced with many challenges, including gastric neoplasm metastasis. Genomic analysis revealed a series of significant actionable mutation loads or pathways in gastric cancer, such as PI3K/AKT/mTOR, CLD18, and HER2/EGFR, which are likely to induce primary gastric cancer to develop into metastases6,7. Recently, transcriptomic data have revealed that the RhoA pathway is involved in the invasion and migration of the ‘diffuse’ growth phenotype in gastric cancer8. Metastatic cascades have been depicted by several steps, including the dissemination of circulating cells, adhesion to blood vessel endothelial cells and proliferation9. However, the mechanism of gastric carcinoma lymph node metastasis remains unknown, partly because data from metastasis studies were generated with the bulk approach, which were likely to mask the roles of subpopulations. Therefore, heterogeneity caused by diverse tumour cell subsets and complex microenvironments has been a great challenge in diagnosis and treatment.
Single-cell RNA-seq (scRNA-seq) deciphers tissues into individual cells to distinguish neoplastic from nontumourous cells and to profile expression patterns to infer subclones10. ScRNA-seq can be used to analyse metastatic cancer cells whose bulk-level expression profiles are affected by metastasized local tissue11–13. In the study of GC scRNA-seq, Li et al. found that some specific marker genes, including SLC11A2, KLK7 and SULT2B1, were related to the development of early GC cells14. Single-cell gene expression studies revealed widespread changes in cell numbers, transcriptional status, and intercellular interactions in the GC tumour microenvironment15. ScRNA-seq can be employed in analysing different degrees of GC, which is a potential good predictor of GC prognosis16. These studies have paid attention to the tumour heterogeneity of GC but lack research on GC metastasis. To answer questions from the GC intratumoural perspective in metastasis, we performed single-cell level analysis on three GC patients with primary cancer and paired metastatic lymph node cancer tissue using the scRNA-seq approach.
Materials and methods
Experimental design
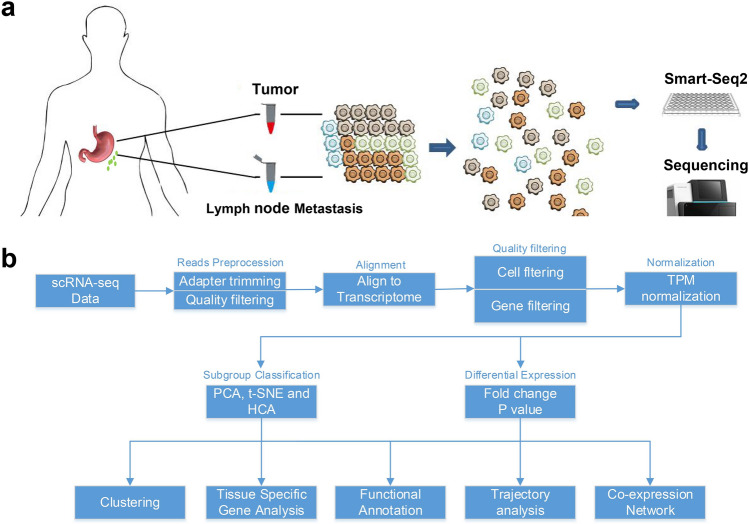
The experiment was performed by comparative sequencing analysis using scRNA-seq from the primary tumour tissue (TT) and paired lymph node (LN) metastasis tumour tissue in three gastric cancer patients. The clinical characteristics of each patient used in this study are shown in Table 1. Tumour tissues were obtained from Changhai Hospital affiliated to Second Military Medical University during operations. The study was approved by the Ethical Committee of Changhai Hospital (CHEC2016-157). Informed consent was written by each of the patients and their guardians, and all procedures were conducted per the Helsinki Ethical Principles for Medical Research. All libraries were prepared with the Smart-seq2 scRNA-seq protocol and sequenced on a HiSeq2500 instrument with 50 bp single-end sequencing mode (Fig. 1).
Table 1.
Clinical characteristics of each patient used in the scRNA-seq study and the cell number of each sample after quality control.
| Clinical characteristics | Patient | ||
|---|---|---|---|
| PT1 | PT2 | PT3 | |
| Numbers of analysis cells (TT/LN) | 19/4 | 27/13 | 19/12 |
| Age | 55 | 33 | 67 |
| Sex | Male | Male | Male |
| Histopathological diagnosis | Moderately differentiated adenocarcinoma | Moderatly low differentiation adenocarcinoma | Moderately low differentiation adenocarcinoma |
| Pathological stage | IIIA | IIA | IIIB |
| T | 1b | 2 | 3 |
| N | 2 | 0 | 1 |
| M | 0 | 0 | 0 |
| Site of origin | Gastric angle | Antrum | Cardia/GNor |
| Infiltration degree | Submucosal | Muscularis | Serosa |
| Tumour size (cm) | 2.5 × 4 × 0.4 | 2.6 × 2.1 × 0.5 | 5.5 × 5.5 × 0.6 |
| CEA (ng/mL) | 2.20 | 2.68 | 2.88 |
| CA724 (U/mL) | 6.53 | 1.64 | 1.65 |
| CA199 (U/mL) | < 2.20 | 14.93 | < 2 |
| HER2 IHC | 3 + | Negative | 1 + |
| HER2 FISH | Positive | Negative | Positive |
| HER2/CSP17 | > 2 | 1.07 | 2.51 |
| Ki67 | 80% | 80% | 80% |
| P53 | Negative | 10% | Negative |
PT patient, TT tumour tissue, LN lymph node, CEA carcinoembryonic antigen, CA carbohydrate antigen, CSP17 centromere-specific probe 17, HER2 human epidermalgrowth factor receptor-2, IHC immunohistochemistry, FISH fluorescence in situ hybridization.
Figure 1.
(a) Overview of the study design and sampling protocol. (b) Analysis pipeline in the current single-cell RNA-seq study.
Solid tumour decomposition and single cell isolation simulation
Biopsy or metastatic tumour were dissected and transferred to a 2 ml tube (Axygen, China), each containing 1 ml prewarmed M199 media (Thermo Fisher Scientific, USA), 2 mg/ml collagenase P (Roche, USA) and 10 U/µl DNase I (Roche, USA) as described by Tirosh et al.17. Tissues were digested for 60 min at 37 °C and then pipetted up and down every ten times every 10 min. The tissue suspensions were then filtered with a 70 µm nylon mesh (Thermo Fisher Scientific, USA) and centrifuged at 450g for 5 min. Pellets were resuspended for live cell staining using CFSE incubation for 5 min.
Single-cell whole-transcriptome library preparation and sequencing
Single cells from each tissue were manually picked under fluorescence microscopy (X71, Olympus, Japan) using a mouth pipette. Each of the harvested single cells was transferred into 2 µL of cell lysis buffer (CLB) in 0.2 mL PCR tubes. Libraries of isolated single cells were then prepared as per the Smart-seq2 protocol18 with modifications on reverse transcription and amplification cycles.
Oligo-dT primed RT (reverse transcription) was performed by Smartscribe (Takara, Japan) reverse transcriptase and locked TSO oligonucleotide (Exiqon, Danmark) upon single cells. Full-length cDNA amplification was conducted by PCR amplification for 22 cycles with Hifi HotStart ReadyMix reagent (KAPA Biosystems, USA) and purified by 0.6 × AMPure beads (BD, USA). Barcoded libraries were fragmented and segmented with a Library Prep kit (Nextera XT, Illumina, USA). Pooled libraries with unique N5–N7 barcodes were sequenced with a HiSeq 2500 sequencer (Illumina, USA) and a 50 SE read flow cell.
ScRNA-seq data analysis
Sequencing adapters and low-quality reads were first trimmed and removed using Trimmomatic19. Reads with a Phred score below 20 and trimmed sequence lengths less than 18 bp were discarded. The remaining high-quality reads were mapped to the human genome using the HiSat2 tool20 by using the human genome UCSC hg19 as a reference (ftp://genome-ftp.cse.ucsc.edu/goldenPath/hg19/chromosomes/) with a total of 22,335 genes. FeatureCounts software21 was used for the expression calculation of each gene, and raw count values of genes in each sample were obtained. A gene that was considered to be expressed in a sample had one more count in the sample. Read counts were normalized to TPM (transcript per million) values and then log2 transformed by using the “newSCESet” function of “scater” (https://github.com/davismcc/scater) package by R (https://www.r-project.org/).
Analyses, including principal component analysis (PCA), Pearson correlation, Student’s t-test and hierarchical clustering analysis (HCA), were performed using functions in R as follows: ‘prcomp’, ‘cor’, ‘t.test’ and ‘cluster’ in the ‘stats’ package and Heatmap in the ‘ComplexHeatmap’ package. The “ggplot” was used for the visualization of PCA.
Differentially expressed genes (DEGs) were calculated with fold-change and p-value between "treatment" and control groups. We set the fold change by a twofold cut-off, and FDR-adjusted p < 0.05 was regarded as the criterion for DEGs. This was carried out by using the “stat” package. Gene Ontology (GO) analysis results were obtained based on the Metascape (http://metascape.org)22.
Single-cell trajectory analysis. We used TSCAN, diffusion map, and monocle2 to perform pseudotime trajectory analysis for the evolution of gastric cancer cells. Cells were chosen based on Seurat cluster identification results.
Immunofluorescence
GC tumour tissues were embedded in paraffin and sliced by Servicebio (Shanghai, China). The antibodies were purchased from Abcam (Abcam, Cambridge, UK), including Anti-ERBB2 and Anti-Oligodendrocyte Specific Protein (CLDN11).
Results
Sequencing data processing and QC
After filtration with a per-gene average read > 1 across all samples, 94 out of 171 samples passed quality control (Table 1). A total of 7601 genes passed filtration and were adopted in further analysis. Each cell was sequenced with 20,000 ~ 200,000 uniquely mapped reads, which is sufficient to detect distinct subpopulation expression profiles17,23–25. Correlations between individual tumour cells from different samples showed a broad range of Pearson coefficients (r = − 0.1 ~ 0.98), implying prominent transcriptomic heterogeneity. However, despite the heterogeneity across the cells, most of the samples were clustered according to their tissue of origin (Figure S1).
Clustering of the primary tumour and metastatic tumour
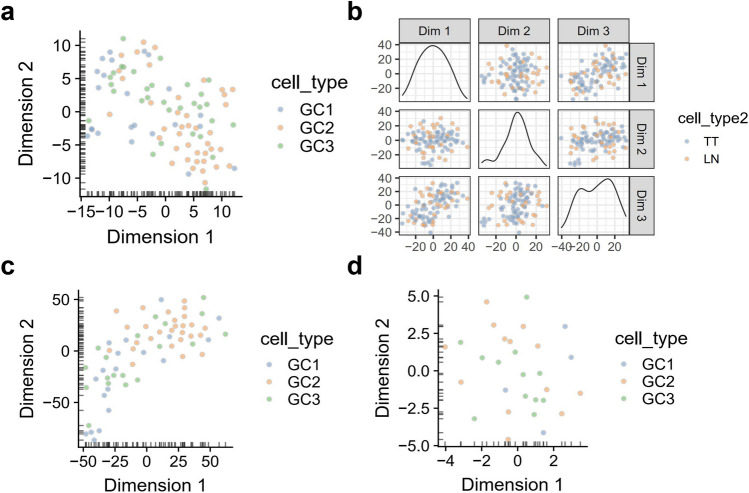
T-SNE was plotted to present the distribution of the single cells from the primary tumour and metastatic tumour in lower dimensions. Primary and metastatic tumour subgroups were partly merged (Fig. 2). Unsupervised T-SNE showed the separation of the primary tumour and metastatic tumour cell groups. In terms of tumour tissues, removal of nontumourous cells resulted in distinct patient-specific cancer heterogeneity. ScRNA-seq revealed specific carcinoma subpopulations and their characteristics from each patient. However, diverse microenvironmental populations were shared by the different patients, and nonmalignant cells did not show clustering of any specific subgroups.
Figure 2.
(a) T-SNE was plotted to present the distribution of the single cells from three patients in all primary tumour tissues. (b) Unsupervised T-SNE showing the separation of carcinoma cell groups. (c) In terms of gastric cancer tumour tissues, removal of noncarcinoma cells reveals intrinsic patient-specific tumour cell heterogeneity. (d) More randomly dispersed dots are shown in the metastatic tumour cells.
Intratumoural heterogeneity analysis
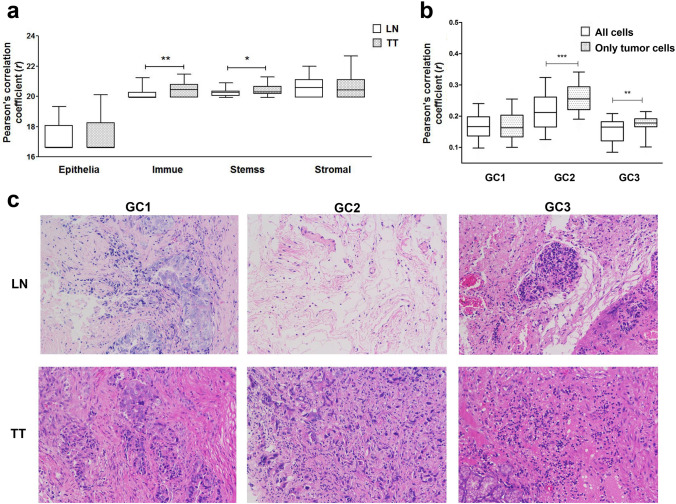
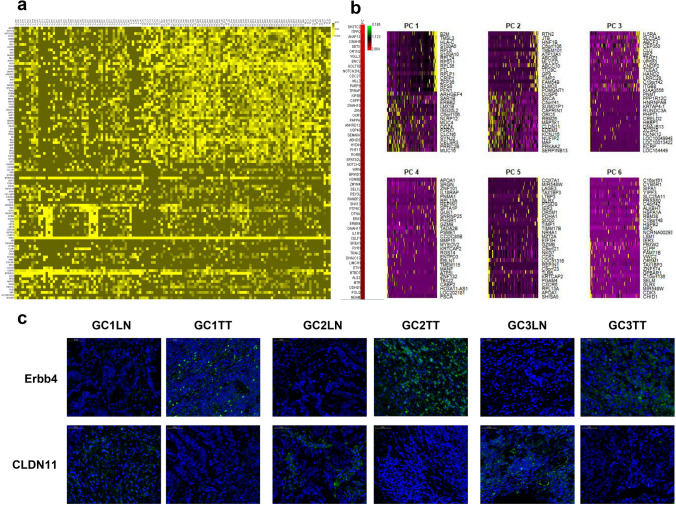
In terms of the intratumoural heterogeneity, the correlation analysis of single cells revealed heterogeneity within tumours across three patients (Fig. 3). Using bulk stemness, immune, stromal, and tumour scoring assessments, we found significant tumour and stromal scoring differences between primary tumour and metastatic tumour single cells, indicating compositional and functional changes in tumours. Population-wide comparison between TT and LN single cells revealed that NOTCH2, NOTCH2NL, KIF5B, and ERBB4 are highly expressed in primary cancer, while CDK12, ERBB2, and CLDN11 are overexpressed in metastatic cancer. The decomposition of four main principal components (PCs) in the datasets is shown in Fig. 4.
Figure 3.
Intratumoural heterogeneity analysis. Correlation analysis of single cells revealed heterogeneity within tumours across three patients. Robust bulk stemness, immune, stromal, and tumour scoring assessment between tumour and paratumour single cells.
Figure 4.
Tissue-specific markers. (a) Population-wide comparison between TT and LN single cells. Tissue-specific markers were calculated, and a heatmap was plotted using the top 100 highly expressed features based on previously defined clusters. NOTCH2, NOTCH2NL, KIF5B, and ERBB4 are highly expressed in primary cancer, while CDK12, ERBB2, and CLDN11 are overexpressed in metastatic cancer. Functional annotation revealed microtubule movement, and notch-based signalling was activated in the primary cells, indicating its metastatic propensity. (b) Decomposition of six main principal components (PCs) in the datasets. (c) IF of ERBB4 and CLDN11 in TT and LN.
ScRNA-seq analysis and trajectory analysis of cell clusters
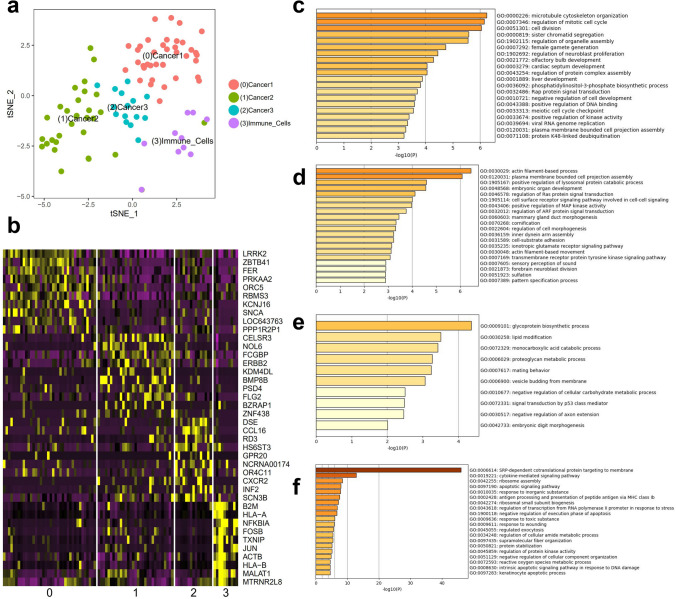
Seurat marker analysis revealed four main clusters in the overall single cells. Twelve significant principal components were extracted to identify four main clusters in the tumour tissues. The heatmap indicates markers highly expressed in each cluster. Functional annotations of each cluster are shown based on Seurat-calculated markers (Fig. 5).
Figure 5.
Seurat marker analysis revealed four main clusters in the overall single cells. Twelve significant principal components were extracted to identify four main clusters in the tumour tissues. (a) Four main clusters in the tumour tissues using TSNE. (b) Heatmap indicating markers highly expressed in each cluster. (c–f) Functional annotations of each cluster are shown based on Seurat-calculated markers.
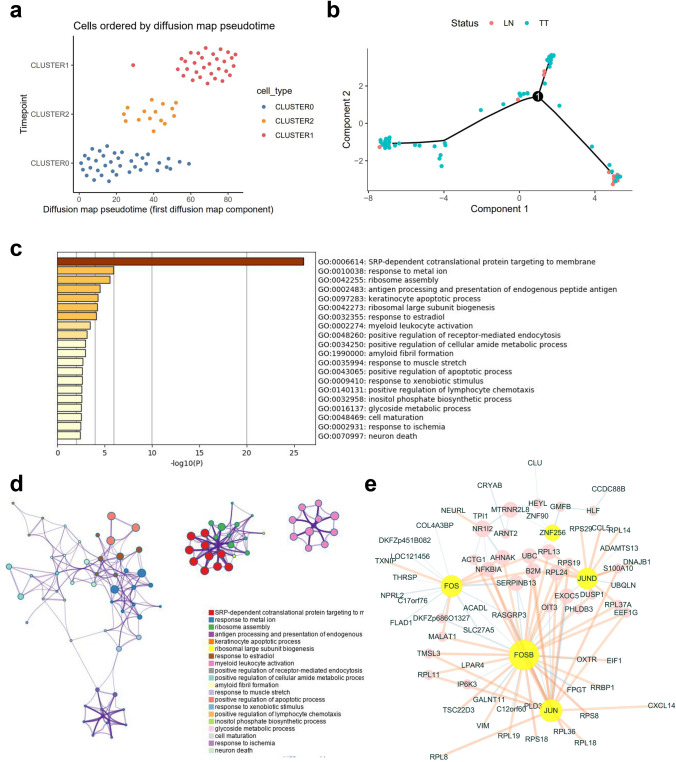
The pseudotime trajectory of GC clusters revealed a distinct pattern of postulated evolution state from Cluster0 > 2 > 1. The major genes (TOP1000) driving evolution were mainly involved in SRP-dependent cotranslational protein targeting to the membrane, response to the metal ion, and ribosome assembly. The kernel genes in evolution regulation include SERPINB13, NFKBIA, B2M, and RPL24. Transcription factors including FOS, FOSB, JUN, JUNB, and ZNF256 drive the regulatory networks (Fig. 6).
Figure 6.
Gastric-derived cell evolutionary trajectory. (a) The pseudotime trajectory of GC clusters revealed a distinct pattern of postulated evolution state from Cluster 0 > 2 > 1. (b) The evolutionary trajectory of TT and LN cells. (c,d) Evolution trajectory-based functional annotation (Top1000 gene). (e) Regulatory co-network of kernel genes in evolution regulation and a transcription factor-driven regulatory network.
Stem cell markers were applied to validate the multiple GC origin hypotheses. Based on canonical markers, we identified hepatocytes and macrophage cells using TSNE. Using TSCAN, we postulated a gastric-derived cell evolutionary trajectory. SLICER, TSCAN, and diffusion map pseudotime tools show the evolutionary trajectory of four tumour cell clusters (Figure S2).
Discussion
Tumour heterogeneity in gastric tumorigenesis and progression has recently attracted researchers’ attention. Based on some significant findings and theories of heterogeneity, target and immune therapies are in progress. However, there remains largely unknown heterogeneity in gastric cancer. By using single-cell isolation aided scRNA-seq, we could clearly identify the signatures of the primary tumour and metastatic tumour and reveal the role of heterogeneity, which causes metastasis in gastric cancer. Gastric carcinoma is common in lymph node metastasis, but the mechanism remains unknown. In our study, we revealed a subgroup of cells bridging the metastatic group and primary group, implying the transition state of cancer during the metastatic process by analysis of three patients’ transcriptomic data of single cells from primary gastric tumours and lymph node metastasis tumours.
Cancer heterogeneity has been shown to be a great challenge in cancer diagnosis and treatment26. Recently, scRNA-seq has been able to analyse abnormal cell-to-cell interactions, chemotherapy resistance, and immunosuppressive microenvironments from primary tissues or CTCs. For example, Chun et al. separated tumour cells and immune cells from primary breast cancer cells27. Patel et al. revealed unanticipated heterogeneity in primary glioblastoma, showing diverse regulatory signalling and therapy programmes28. Kim et al.’s scRNA-seq results combined both intratumoural SNV KRASG12D and expression heterogeneity of lung adenocarcinoma cells, deciphering subpopulations in anticancer drug responses29. In terms of the intratumoural cancer cell component. Our clustering data showed significant intratumoural heterogeneity.
Tissue-specific markers were calculated, and a heatmap was plotted using the top 50 highly expressed features based on six redefined clusters using cell markers. Tissue-specific markers were calculated, and a heatmap was plotted using the top 100 highly expressed features based on previously defined clusters. NOTCH2, NOTCH2NL, KIF5B, and ERBB4 are highly expressed in primary cancer, while ERBB2, CLDN11 and CDK12 are overexpressed in metastatic cancer. Previous studies suggested that the expression of Notch signalling pathway-associated proteins, such as Notch2, was significantly elevated in gastric cancer tissues compared to normal tissues30. In addition, evidence showed that higher KIF5B and ERBB4 promoted cancer cell proliferation31,32. Evidence suggests that CDK12, ERBB2, and CLDN11 play an associated metastatic role in cancer33–35. Several studies have shown that CLDN11 is related to tumour migration and metastasis35. Although the aetiologies of gastric cancer are partly clear and validated by experiments, the proposed “seed”-and- “soil” hypothesis has yet to be well explained36. Our findings implied that lymph node metastasis-prone subclones are more likely to share CLDN11, which is a member of the tight junction protein family that functions as a component of cell adhesion37. This explained cancer cell colonization in the lymph node after migrating from primary tissues. Future studies need to evaluate both transcriptomic and genetic alterations and even geographical information across different regions from primary metastatic tumours only to identify survival and evolution pressure in the cancer-related microenvironment, which promotes the identification of potential key drivers of gastric cancer.
TFs are proteins with special structures and functions that regulate gene expression. We noticed that several TF-regulating genes, including FOS and JUN, appeared to be particularly important during tumour evolution. JUN and FOS were determined to be critical genes related to GC38. ScRNA-seq reveals an expression pattern with high FOS and JUN at leukaemia evolution, which resolves following therapy but reoccurs following relapse and death39.
As this is a preliminary study, the limitation of our analysis is the small number of patient cases enrolled in this study. Nonetheless, we obtained a more comprehensive picture of gastric cancer lymph node metastasis at single-celled resolution, giving a new perspective on the biomarkers (ERBB2, CLDN11 and CDK12) involved in metastasis, pathways involved and driver genes (FOS and JUN) during the metastasis process, providing a basis for the treatment of GC.
Supplementary Information
Acknowledgements
This work was supported by the National Major Scientific and Technological Special Project for Significant New Drugs Development (2017ZX09304030).
Author contributions
B.W., X.B.Z. and S.B.Z. designed the work. B.W., S.B.Z., Y.Y.Z., K.C.X., and T.M.Z. joined the experiment. B.W., T.Q., J.L. and S.B.Z. analysed datasets. B.W., T.Q., J.L., S.B.Z. and X.B.Z. wrote this paper.
Data availability
Raw data of scRNA-seq were deposited in GEO (GSE158631).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sibo Zhu, Email: sibozhu@fudan.edu.cn.
Xianbao Zhan, Email: zhanxianbao@csco.org.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80881-2.
References
- 1.McGuire, S. World Cancer Report Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2014;7(418–419):2016. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurello P, et al. Classification of lymph node metastases from gastric cancer: Comparison between N-site and N-number systems. Our experience and review of the literature. Am. Surgeon. 2007;73:359–366. doi: 10.1177/000313480707300410. [DOI] [PubMed] [Google Scholar]
- 3.Grimes JA, et al. Agreement between cytology and histopathology for regional lymph node metastasis in dogs with melanocytic neoplasms. Vet. Pathol. 2017;54:579–587. doi: 10.1177/0300985817698209. [DOI] [PubMed] [Google Scholar]
- 4.Ehteshami Bejnordi B, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318:2199–2210. doi: 10.1001/jama.2017.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cislo M, et al. Distinct molecular subtypes of gastric cancer: From Lauren to molecular pathology. Oncotarget. 2018;9:19427–19442. doi: 10.18632/oncotarget.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brastianos PK, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon C, et al. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin. Cancer Res. 2016;22:971–983. doi: 10.1158/1078-0432.CCR-15-1356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Valastyan S, Weinberg RA. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller S, Diaz A. Single-cell mRNA sequencing in cancer research: Integrating the genomic fingerprint. Front. Genet. 2017;8:73. doi: 10.3389/fgene.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Neal JT, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(1972–1988):e1916. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bancells C, et al. Revisiting the initial steps of sexual development in the malaria parasite Plasmodium falciparum. Nat. Microbiol. 2019;4:144–154. doi: 10.1038/s41564-018-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, et al. Dissecting the single-cell transcriptome network underlying gastric premalignant lesions and early gastric cancer. Cell Rep. 2019;27:1934–1947. doi: 10.1016/j.celrep.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 15.Sathe A, et al. Single-cell genomic characterization reveals the cellular reprogramming of the gastric tumor microenvironment. Clin. Cancer Res. 2020;26:2640–2653. doi: 10.1158/1078-0432.CCR-19-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. 2020 doi: 10.1136/gutjnl-2019-320368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picelli S, et al. Full-length RNA-seq from single cells using smart-seq2. Nat. Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 19.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Smyth GK, Shi W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng GX, et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017;8:14049. doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, et al. Single-cell analyses of transcriptional heterogeneity in squamous cell carcinoma of urinary bladder. Oncotarget. 2016;7:66069–66076. doi: 10.18632/oncotarget.11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirosh I, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539:309–313. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung W, et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017;8:15081. doi: 10.1038/ncomms15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KT, et al. Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells. Genome Biol. 2015;16:127. doi: 10.1186/s13059-015-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du X, et al. Role of Notch signaling pathway in gastric cancer: a meta-analysis of the literature. World J. Gastroenterol. 2014;20:9191–9199. doi: 10.3748/wjg.v20.i27.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohno T, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat. Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Gong L, Qian Z, Song G, Liu J. ERBB4 promotes the proliferation of gastric cancer cells via the PI3K/Akt signaling pathway. Oncol. Rep. 2018;39:2892–2898. doi: 10.3892/or.2018.6343. [DOI] [PubMed] [Google Scholar]
- 33.Quigley DA, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;175:889. doi: 10.1016/j.cell.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, et al. Prognostic role of ERBB2, MET and VEGFA expression in metastatic colorectal cancer patients treated with anti-EGFR antibodies. Br. J. Cancer. 2016;114:1003–1011. doi: 10.1038/bjc.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, et al. Methylated claudin-11 associated with metastasis and poor survival of colorectal cancer. Oncotarget. 2017;8:96249–96262. doi: 10.18632/oncotarget.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidler IJ. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, et al. Systems proteomics view of the endogenous human Claudin protein family. J. Proteome Res. 2016;15:339–359. doi: 10.1021/acs.jproteome.5b00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansouri V, Rezaei Tavirani S, Zadeh-Esmaeel MM, Rostami-Nejad M, Rezaei-Tavirani M. Comparative study of gastric cancer and chronic gastritis via network analysis. Gastroenterol. Hepatol. Bed. Bench. 2018;11:343–351. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, et al. Evolution of multiple cell clones over a 29-year period of a CLL patient. Nat. Commun. 2016;7:13765. doi: 10.1038/ncomms13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data of scRNA-seq were deposited in GEO (GSE158631).