Abstract
Ellagitannins (esters composed of glucose and ellagic acid) are hydrolyzed to generate ellagic acid in gut followed by conversion of ellagic acid to urolithins such as urolithin A by intestinal bacteria. Since urolithins are absorbed by gut easier than ellagitannins and ellagic acid, and show various physiological activities (e.g. anti-cancer, anti-cardiovascular disease, anti-diabetes mellitus, anti-obesity and anti-Alzheimer disease activities), they are expected as excellent health-promoting phytochemicals. Here, using human monoblast U937 cells, we investigated the effect of ellagic acid and urolithin A on the superoxide anion (O2−)-generating system of phagocytes, which is consisted of five specific protein factors (membrane proteins: p22-phox and gp91-phox, cytosolic proteins: p40-phox, p47-phox and p67-phox). Twenty micromolar of urolithin A enhanced the all-trans retinoic acid (ATRA)-induced O2−-generating activity (to ~175%) while 20 μM ellagic acid inhibited the ATRA-induced O2−-generating activity (to ~70%). Semiquantitative RT-PCR showed that transcription level of gp91-phox was certainly decreased (to ~70%) in ATRA plus ellagic acid-treated cells, while that of gp91-phox was significantly increased (to ~160%) in ATRA plus urolithin A-treated cells. Chromatin immunoprecipitation assay suggested that urolithin A enhanced acetylations of Lys-9 residues of histone H3 within chromatin surrounding the promoter region of gp91-phox gene, but ellagic acid suppressed the acetylations. Immunoblotting also revealed that ATRA plus urolithin A-treatment up-regulated protein levels of p22-phox (to ~160%) and gp91-phox (to ~170%) although ATRA plus ellagic acid-treatment down-regulated protein levels of p22-phox (to ~70%) and gp91-phox (to ~60%). These results suggested that conversion of ellagic acid to urolithin A in gut may bring about reverse effects on the gp91-phox gene expression, resulting in opposite alterations in O2−-generating activity of intestinal macrophages.
Keywords: Ellagic acid, Urolithin A, gp91-phox, All-trans retinoic acid, Superoxide, U937
Abbreviations: ATRA, all-trans retinoic acid; ChIP, chromatin immunoprecipitation; H3K9, Lys-9 residues of histone H3; H3K14, Lys-14 residues of histone H3; O2−, superoxide anion; PMA, phorbol 12-myristate 13-acetate
Highlights
-
•
Ellagic acid down-regulated the ATRA-induced O2−-generating activity.
-
•
Ellagic acid significantly suppressed transcription of gp91-phox gene.
-
•
Urolithin An up-regulated the ATRA-induced O2−-generating activity.
-
•
Urolithin A significantly enhanced transcription of gp91-phox gene.
-
•
Production of urolithin A by gut bacteria may affect the intestinal macrophages.
1. Introduction
Ellagic acid (2,3,7,8-tetrahydroxy [1]-benzopyrano [5,4,3-cde] [1]benzopyran-5, 10-dione) exists in various vegetables and fruits (e.g. raspberry, strawberry, walnuts, pomegranate etc.) as ellagitannins that are esters composed of glucose and ellagic acid [1]. After hydrolysis reactions of ellagitannins, ellagic acid is generated. Furthermore, urolithins (dibenzo [b,d]pyran-6-one derivatives) such as urolithin A with anti-oxidant and anti-inflammatory effects are synthesized by the microbial conversion of ellagic acid and residual ellagitannins in human gut [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. Urolithins are absorbed by gut easier than ellagitannins and ellagic acid, and exert various physiological activities: anti-cancer, anti-cardiovascular disease, anti-diabetes mellitus, anti-obesity, anti-Alzheimer disease and so on [[2], [3], [4], [5], [6], [7], [8]]. Even at physiological concentration, urolithins can show effects on various chronic degenerative diseases [2]. Therefore, urolithins and their related compounds are expected as health-promoting phytochemicals. However, molecular mechanisms of physiological functions of urolithins remain unclear.
Here, we investigated the effect of ellagic acid and urolithin A on the superoxide anion (O2−)-generating system of phagocytes (e.g. neutrophil and macrophage) for killing the invading bacteria [10]. This system is consisted of five specific protein factors (membrane proteins p22-phox and gp91-phox, cytosolic proteins p40-phox, p47-phox and p67-phox), and small G-protein Rac [11]. Cytochrome b558, a heme-binding membrane protein, is a heterodimer protein formed by p22-phox and gp91-phox proteins, and mediates the final steps of electron transfer to molecular oxygen resulting in the O2− generation. In a response to infectious stimuli, phagocytes release O2− outside of the cells or inside of phagosomes where various stronger reactive oxygen species are formed from O2− step by step. These reactive oxygen species are used to kill the invading bacteria. U937, a human monoblastic leukemia cell line, has been used as an excellent model to study mechanisms of leukocyte differentiation [[12], [13], [14]]. Although U937 cells lack the O2−-generating activity, they are differentiated to macrophage-like cells and produce O2− by various differentiation-inducing agents such as interferon-γ and all-trans retinoic acid (ATRA) [[12], [13], [14]]. Of course, gene expressions of the five specific protein factors of the O2−-generating system are remarkably up-regulated by ATRA during differentiation [[15], [16], [17], [18]]. Moreover, various phytochemicals can affect gene expressions of the five specific protein factors of the O2−-generating system, resulting in alteration of its activity. For example, in recent year, we reported that curcumin [15], resveratrol [16], chalcone [17], hydroxychalcones [17] and sulforaphane [18] regulated the ATRA-induced O2−-generating activity via controlling gene expressions of these five specific protein factors in U937 cells. Our data in this study suggested that conversion of ellagic acid to urolithin A in gut may bring about reverse effects on the gp91-phox gene expression followed by opposite alterations in the O2−-generating activity of intestinal macrophages.
2. Materials and methods
2.1. Materials
Ellagic acid, PMSF (FUJIFILM Wako, Osaka, Japan), urolithin A (Cayman Chemical, Ann Arbor, MI, USA), phorbol 12-myristate 13-acetate (PMA), ATRA, luminol (Sigma, St Louis, MO, USA), plasmocin (InvivoGene, CA, USA) and Diogenes (National Diagnostics, Atlanta, GA, USA) were purchased. Monoclonal anti-gp91-phox antibody, monoclonal anti-p47-phox antibody (BD Biosciences, San Jose, CA, USA), monoclonal anti-p67-phox antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p40-phox antibody (GeneTex, Irvine, CA, USA), monoclonal anti-β-actin antibody, monoclonal anti-Na+/K+-ATPase antibody (Abcam, Cambridge, UK), and horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin (Promega, Madison, WI, USA) were obtained. Monoclonal anti-human p22-phox antibody (449) was kindly provided by Dr. Roos and Dr. Verhoeven (Sanquin Research, and Landsteiner Laboratory, Academic Medical Centre, University of Amsterdam, The Netherlands).
2.2. ATRA-induced monocytic differentiation of U937 cells
Human monoblastic leukemia cell line, U937 cells (RCB0435) were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan [16]. Cells were grown in RPMI-1640 culture medium containing 10% fetal bovine serum and 5 μg/mL plasmocin [[16], [17], [18]]. Cells (2.0 × 106) in 5 ml of the culture medium were incubated with 1 μM ATRA in the absence or presence (10 μM or 20 μM) of ellagic acid or urolithin A at 37 °C for 48 h.
2.3. Measurement of O2− generation
Measurement of O2− generation was carried out by Lumat3 LB9508 luminometer (Berthold Technologies, Bad Wildbad, Germany) using Diogenes-luminol chemiluminescence probes as described previously [[16], [17], [18]].
2.4. Semiquantitative RT-PCR
Cells (2.0 × 106) in 5 ml of the culture medium were incubated with 1 μM ATRA in the absence or presence of 20 μM ellagic acid or 20 μM urolithin A at 37 °C for 48 h. Semiquantitative RT-PCR was performed using specific sense and antisense primers of five human genes essential for the O2−-generation system (p22-phox, gp91-phox, p40-phox, p47-phox and p67-phox) as described previously [[14], [15], [16], [17], [18]]. Human GAPDH gene was used as internal controls. Semiquantitative RT-PCR data were obtained using a luminescent image analyzer STAGE-5100 (AMZ System Science, Osaka, Japan), analyzed by Quant-AMZ software (TotalLab., Newcastle upon Tyne, UK) as described [[16], [17], [18]].
2.5. Chromatin immunoprecipitation (ChIP) assay
Cells (2.0 × 106) in 5 ml of the culture medium were incubated with 1 μM ATRA in the absence or presence of 20 μM ellagic acid or 20 μM urolithin A at 37 °C for 48 h. ChIP assay was carried out using a ChIP assay kit (Merck, Darmstadt, Germany) according to the instruction manual as described previously [16,17]. To confirm the presence of human gp91-phox promoter sequences containing critical cis-element (and also Hox/Pbx consensus-like cis-element) and inverted PU.1 binding site [14,[19], [20], [21]], the immunoprecipitated DNA samples were analyzed by PCR technique. PCRs were performed at 96 °C for 20 s, 55 °C for 30 s and 72 °C for 30 s, for 36–40 cycles, using forward primer (5′-TCAGTTGACCAATGATTATTAGCCAATT-3′) and reverse primer (5′-CTATGCTTCTTCTTCCAATGACCAAAT-3′). PCRs were stopped before reaching the plateau. PCR products were analyzed using a luminescent image analyzer STAGE-5100 with Quant-AMZ software as described [16,17].
2.6. Immunoblotting
Immunoblotting was performed as described previously [[14], [15], [16], [17], [18]]. In brief, cells (2.0 × 106) in 5 ml of the culture medium were incubated with 1 μM ATRA in the absence or presence of 20 μM ellagic acid or 20 μM urolithin A at 37 °C for 48 h. Cells (5 × 106) were sonicated in 100 μl of 50 mM Tris-HCl buffer, pH 7.5, containing 0.25 M sucrose, 2 mM EDTA and 1 mM PMSF. Disrupted cells were divided into cytosolic fractions and membrane fractions by centrifugation. These protein samples were subjected to SDS-PAGE followed by immunoblotting. Data analyses were carried out using a luminescent image analyzer STAGE-5100. Human β-actin (for cytosolic fractions) and Na+/K+-ATPase (for membrane fractions) were used as controls [17,18].
2.7. Statistical analysis
Quantitative data are presented as averages of three separate experiments. Error bars indicate standard deviation. Statistical differences were calculated with Student's t-test.
3. Results and discussion
3.1. Urolithin A significantly enhances the ATRA-induced O2−-generating activity while ellagic acid inhibits the ATRA-induced O2−-generating activity
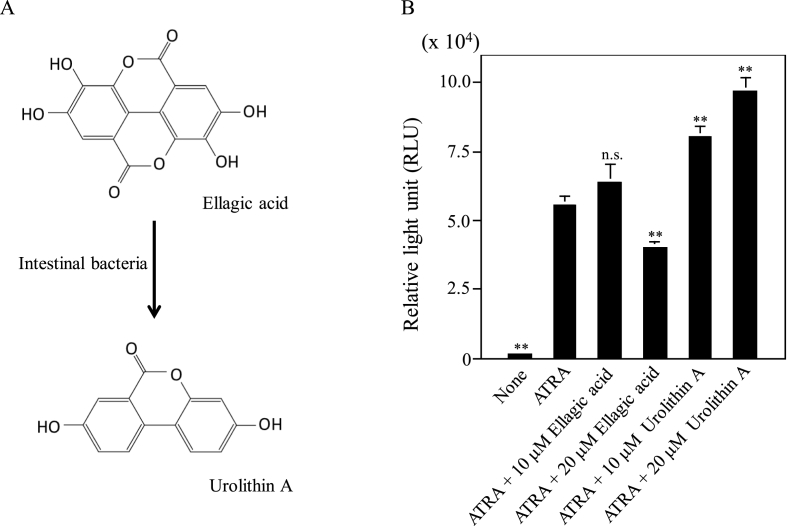
As well known phenomenon, ellagic acid is converted to urolithin A by intestinal bacteria and schematic is represented in Fig. 1A. To investigate the physiological functions of ellagic acid and urolithin A in monocytic differentiation, first, we examined the influences of both of them, on the ATRA-induced O2−-generating activity in U937 cells (Fig. 1B). As with our previous reports [[15], [16], [17], [18]], ATRA-treated U937 cells showed remarkable O2−-generating activity by PMA treatment while untreated U937 cells only generated a negligible level of O2− by PMA treatment. Although 10 μM of ellagic acid showed no effect on the ATRA-induced O2−-generating activity, 20 μM of ellagic acid significantly down-regulated the activity (to ~70%). In contrast, very interestingly, urolithin A could enhance the O2−-generating activity in a dose-dependent manner (10 μM: to ~150%, 20 μM: to ~175%). On the other hand, both ellagic acid and urolithin A showed little effect on the O2−-generating activity in the absence of ATRA (Supplementary Fig. 1). These results revealed that ellagic acid and urolithin A would play opposite roles in the regulation of the ATRA-induced O2−-generating activity in U937 cells.
Fig. 1.
Influences of ellagic acid and urolithin A on the ATRA-induced O2−-generating activity in U937 cells. (A) Conversion scheme of ellagic acid to urolithin A by intestinal bacteria. (B) Influences of ATRA plus ellagic acid or urolithin A on induction of the O2−-generating activity. O2− generation was determined after culture of the cells at 37 °C for 48 h in the absence (None) or presence of each agent (ATRA, ATRA plus ellagic acid or ATRA plus urolithin A) as in “Materials and methods.” Cells (1 × 106 cells/ml) were stimulated with 200 ng/ml PMA at 37 °C. PMA-induced chemiluminescences were measured at 10 min after stimulation. Data represent the averages of three separate experiments and error bars indicate standard deviation. **, P < 0.01 compared with the data of ATRA-treated cells; n. s., not significant.
3.2. Opposite effects of ellagic acid and urolithin A on transcription levels of the gp91-phox genes essential for the O2−-generating system during ATRA-induced monocytic differentiation
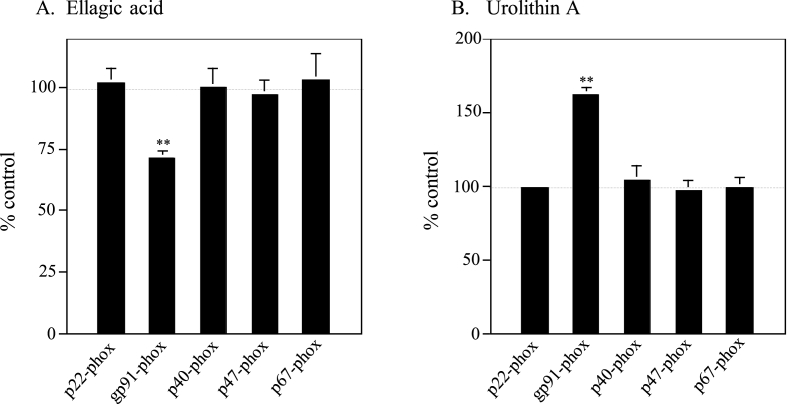
As shown in Fig. 1B, urolithin A significantly up-regulated the ATRA-induced O2−-generating activity while ellagic acid definitely down-regulated the activity in U937 cells. These findings suggested that ellagic acid and urolithin A may affect transcription of five genes of the essential components (p22-phox, gp91-phox, p40-phox, p47-phox and p67-phox) for the O2−-generation in the presence of ATRA. To confirm the effects of ellagic acid and urolithin A on transcription of these five genes in the presence of ATRA, we carried out semiquantitative RT-PCR and compared transcription levels of these five genes in ATRA plus ellagic acid or urolithin A-treated cells with those in ATRA-treated cells (Fig. 2). Total RNAs were prepared from ATRA-treated, ATRA plus ellagic acid-treated and ATRA plus urolithin A-treated U937 cells. Quantitative data of semiquantitative RT-PCR were indicated as percentages of control values obtained from ATRA-treated U937 cells. In ATRA plus ellagic acid-treated U937 cells, just transcription level of gp91-phox was certainly decreased (to ~70%) (Fig. 2A). In contrast, as expected, only transcription level of gp91-phox was significantly increased (to ~160%) in ATRA plus urolithin A-treated U937 cells (Fig. 2B). These results suggested that such reverse effects of ellagic acid and urolithin A on transcription of gp91-phox gene in the presence of ATRA resulted in their opposite influences on the ATRA-induced O2−-generating activity (see Fig. 1B).
Fig. 2.
Influences of co-treatment with ATRA and ellagic acid or urolithin A on transcription of the O2−-generating system-related genes. Total RNAs were extracted from ATRA-treated, ATRA plus ellagic acid-treated (A), and ATRA plus urolithin A-treated (B) U937 cells, and mRNA levels of p22-phox, gp91-phox, p40-phox, p47-phox and p67-phox were determined by semiquantitative RT-PCR as in “Materials and methods.” Data calibrated with the internal controls are indicated as percentages of control values (100%) obtained from ATRA-treated U937 cells and represent the averages of three separate experiments. Error bars indicate standard deviation. **, P < 0.01 compared with the data of ATRA-treated cells.
3.3. Urolithin A enhanced acetylations of Lys-9 residues of histone H3 within chromatin surrounding the promoter region of gp91-phox gene, but ellagic acid suppressed these acetylations
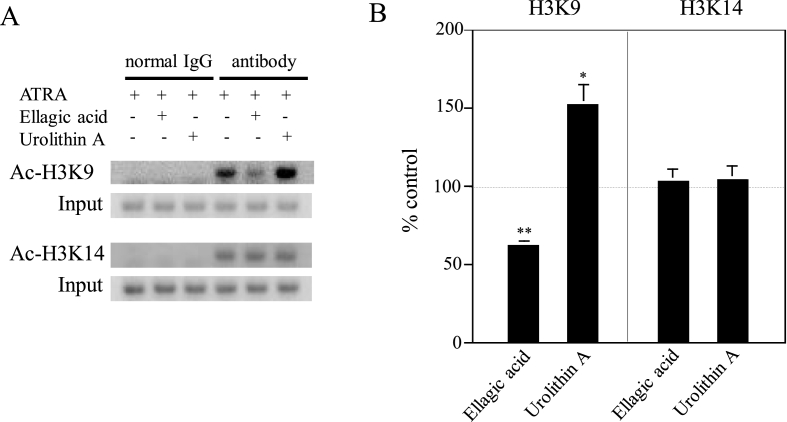
As mentioned above, our findings suggested that ellagic acid suppresses transcription of gp91-phox and urolithin A enhances it. To determine whether or not ellagic acid and urolithin A participate in acetylations of histone H3 within chromatin surrounding the promoter region of gp91-phox gene, we carried out ChIP assay using anti-acetylated Lys-9 and Lys-14 residues of histone H3 (H3K9 and H3K14) antibodies (Fig. 3). These two Lys residues of histone H3 are the typical acetylation sites that are involved in transcriptional activation [[22], [23], [24]]. As shown in our previous reports, ATRA remarkably accelerated acetylation levels of H3K9 residues within chromatin surrounding the promoter region of gp91-phox gene, but not H3K14 residues [16,17]. On the other hand, acetylation levels of H3K14 residues were enhanced by resveratrol and butein in the presence of ATRA [16,17]. In this study, we compared ATRA plus ellagic acid- or urolithin A-treated U937 cells with ATRA-treated U937 cells. Typical electrophoresis patterns are shown in Fig. 3A. As expected, ellagic acid caused remarkable down-regulation of acetylation levels of H3K9 residues within chromatin surrounding the promoter region of gp91-phox gene (to ~60% of control value obtained from ATRA-treated U937 cells) while urolithin A brought about significant up-regulation of them (to ~150% of control value obtained from ATRA-treated U937 cells) (Fig. 3B). In contrast, both ellagic acid and urolithin A did not affect acetylation levels of H3K14 residues within chromatin surrounding the promoter region of gp91-phox gene (Fig. 3B). These data revealed that ellagic acid down-regulates the ATRA-induced O2−-generating activity via suppressing acetylations of H3K9 residues within chromatin around the promoter regions of gp91-phox gene while urolithin An up-regulates the ATRA-induced O2−-generating activity through enhancing them. In other words, ellagic acid and urolithin A may regulate the ATRA-induced O2−-generating activity in opposite epigenetic manners. Further studies are required for exploring what kinds of histone acetylation-related enzymes participate in acetylations of H3K9 residues within chromatin around the promoter regions of gp91-phox gene, and how these two phytochemicals are involved in the histone acetylations.
Fig. 3.
Influences of co-treatment with ATRA and ellagic acid or urolithin A on acetylation levels of H3K9 and H3K14 residues within chromatin surrounding the promoter regions of gp91-phox gene. (A) Typical patterns of PCR. ChIP assay was performed as in “Materials and Methods”. PCR products were subjected to 2% agarose gel electrophoresis and analyzed using a luminescent image analyzer as described [16,17]. (B) Quantitative analysis. Cross-linked chromatins of ATRA-treated, ATRA plus ellagic acid-treated and ATRA plus urolithin A-treated U937 cells were co-precipitated by antibodies specific for acetylated H3K9 and H3K14 residues. PCRs were performed as in “Materials and methods.” Data are indicated as percentages of control values (100%) obtained from ATRA-treated U937 cells and represent the averages of three separate experiments. Error bars indicate standard deviation. *, P < 0.05; **, P < 0.01 compared with the data of ATRA-treated cells.
3.4. Opposite effects of ellagic acid and urolithin A on protein levels of cytochrome b558 composed of p22-phox and gp91-phox proteins during ATRA-induced monocytic differentiation
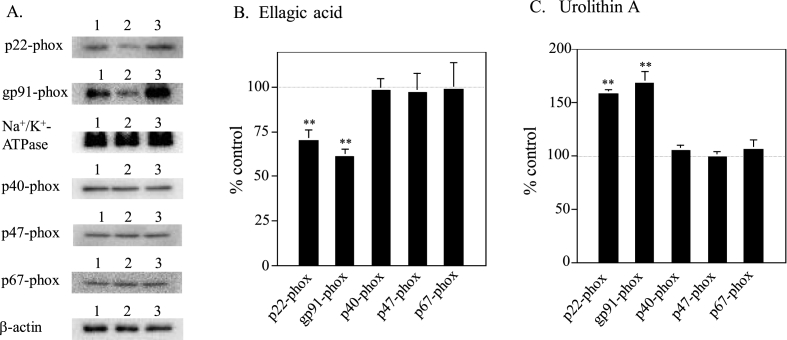
To investigate the effects of ellagic acid and urolithin A on amounts of five proteins (p22-phox, gp91-phox, p40-phox, p47-phox and p67-phox proteins) essential for the O2−-generation in phagocytes, immunoblotting was performed for these proteins using antibody specific for each protein. Quantitative data obtained from ATRA plus ellagic acid- or urolithin A-treated U937 cells were indicated as percentages of control values obtained from ATRA-treated U937 cells (Fig. 4). Both ellagic acid and urolithin A showed insignificant effect on protein level of three cytosolic factors (p40-phox, p47-phox and p67-phox proteins) as with amounts of their mRNA (see Fig. 2). On the other hand, ATRA plus ellagic acid-treatment down-regulated protein levels of p22-phox (to ~70%) and gp91-phox (to ~60%) (Fig. 4A). In contrast, as expected, ATRA plus urolithin A-treatment up-regulated protein levels of p22-phox (to ~160%) and gp91-phox (to ~170%) (Fig. 4B). Expression of gp91-phox protein limits the O2−-generating activity in U937 cells [13]. It is thought that the amount of p22-phox protein also was increased according to enhancement of gp91-phox protein since cytochrome b558 is a heterodimer protein composed of gp91-phox and p22-phox proteins. These findings suggested that protein levels of cytochrome b558 would reflect the changes of amount of gp91-phox mRNA caused by ellagic acid or urolithin A in the presence of ATRA, resulting in their opposite influences on the ATRA-induced O2−-generating activity (see Fig. 1B).
Fig. 4.
Influences of co-treatment with ATRA and ellagic acid or urolithin A on protein levels of the O2−-generating system-related factors. (A) Typical immunoblot profiles. Cytosolic (for p40-phox, p47-phox and p67-phox) and membrane (for p22-phox and gp91-phox) fractions were prepared from ATRA-treated (lane 1), ATRA plus ellagic acid-treated (lane 2) and ATRA plus urolithin A-treated (lane 3) U937 cells, and protein levels were determined by immunoblotting as in “Materials and methods.” Human β-actin (for cytosolic fractions) and Na+/K+-ATPase (for membrane fractions) were used as controls. Quantitative data of ATRA plus ellagic acid-treated (B) and ATRA plus urolithin A-treated (C) U937 cells are indicated as percentages of control values (100%) obtained from ATRA-treated U937 cells, and represent the averages of three separate experiments. Error bars indicate standard deviation. **, P < 0.01 compared with the data of ATRA-treated cells.
3.5. Concluding remarks
Both ellagic acid and urolithin A influenced protein level of only cytochrome b558 composed of p22-phox and gp91-phox among the four essential proteins (cytochrome b558, p40-phox, p47-phox and p67-phox) for the O2−-generating system. Although resveratrol enhanced the ATRA-induced O2−-generating activity via increase of cytochrome b558 protein in U937 cells, it also caused the accumulation of p47-phox protein [16]. The accumulation mechanisms of p47-phox protein caused by resveratrol are unresolved yet. In addition, both ellagic acid and urolithin A showed little cytotoxicity up to 20 μM (Supplementary Fig. 2). Therefore, ellagic acid and urolithin A may be used as modifiers having slight side effects for cytochrome b558 functions. Interferon-γ, one of trans activators for gp91-phox gene [14,21], improves the splicing efficiency of the gp91-phox gene primary transcripts in a particular group of X-linked chronic granulomatous disease patients [25,26]. For example, combination of interferon-γ and urolithin A may rescue a certain kind of X-linked chronic granulomatous disease patients with a mutation in gp91-phox gene from infectious diseases.
Moreover, in this study, we demonstrated using U937 cells that urolithin A epigenetically enhances the ATRA-induced O2−-generating activity via activating gp91-phox transcription while ellagic acid epigenetically suppresses the ATRA-induced O2−-generating activity via inhibiting gp91-phox transcription. As is well known, U937 cells have been used as an in vitro model for studying differentiation mechanisms and functions of macrophages [12]. Macrophages also exist in intestine and play important roles to maintain the intestinal homeostasis including intestinal immunity [27,28]. In addition, interestingly, urolithin A is generated from ellagic acid by intestinal bacteria [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. As is well known, a large number of bacteria live in human gut. It is believed that they act as key factors of health and disease through participating in various metabolic pathways in human gut [29]. Taken together, these findings showed the possibility that conversion of ellagic acid to urolithin A by intestinal bacteria may affect the intestinal homeostasis via controlling intestinal macrophage functions. Unfortunately, we have no data how much concentration of urolithin A affects human intestinal macrophages in human gut under physiological conditions. Although localization of urolithin A is especially difficult to be elucidated in human gut, these constraints will be resolved in the future.
Author statement
Hidehiko Kikuchi: Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Kaori Harata: Data curation, Writing – review & editing. Harishkumar Madhyastha: Writing – review & editing. Futoshi Kuribayashi: Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This work was supported in part by JSPS KAKENHI Grant Number 19K02329 (to H. K.) and 18K07804 (to F. K.).
Acknowledgements
We thank R. Madhyastha for proofreading of the manuscript. This work was supported in part by JSPS KAKENHI Grant Number 19K02329 (to H. K.) and 18K07804 (to F. K.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100891.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Cerda B., Periago P., Espin J.C., Tomas-Barberan F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Munoz C., Vaillant F. Metabolic fate of ellagitannins: implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014;54:1584–1598. doi: 10.1080/10408398.2011.644643. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Gonzalez C., Ciudad C.J., Noe V., Izquierdo-Pulido M. Health benefits of walnut polyphenols: an exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017;57:3373–3383. doi: 10.1080/10408398.2015.1126218. [DOI] [PubMed] [Google Scholar]
- 4.Burton-Freeman B.M., Sandhu A.K., Edirisinghe I. Red raspberries and their bioactive polyphenols: cardiometabolic and neuronal health links. Adv. Nutr. 2016;7:44–65. doi: 10.3945/an.115.009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomas-Barberan F.A., Gonzalez-Sarrias A., Garcia-Villalba R., Nunez-Sanchez M.A., Selma M.V., Garcia-Conesa M.T., Espin J.C. Urolithins, the rescue of "old" metabolites to understand a "new" concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- 6.Wu S., Tian L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum) Molecules. 2017;22:E1606. doi: 10.3390/molecules22101606. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muke G.E., Murray I.A., Espin J.C., Perdew G.H., Urolithin A is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Metabolites. 2018;8:E86. doi: 10.3390/metabo8040086. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey P. Gut microbiota in phytopharmacology: a comprehensive overview of concept, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019;147:104367. doi: 10.1016/j.phrs.2019.104367. [DOI] [PubMed] [Google Scholar]
- 9.Cortes-Martin A., Selma M.V., Tomas-Barberan F.A., Gonzalez-Sarrias A., Espin J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 2020;64 doi: 10.1002/mnfr.201900952. [DOI] [PubMed] [Google Scholar]
- 10.Morel F., Doussiere J., Vignais P.V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur. J. Biochem. 1991;201:523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- 11.Dagher M.C., Pick E. Opening the black box: lessons from cell-free systems on the phagocyte NADPH-oxidase. Biochimie. 2007;89:1123–1132. doi: 10.1016/j.biochi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL60 and U937 cell lines. J. Leukoc. Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi H., Fujinawa T., Kuribayashi F., Nakanishi A., Imajoh-Ohmi S., Goto M., Kanegasaki S. Induction of essential components of the superoxide generating system in human monoblastic leukemia U937 cells. J. Biochem. 1994;116:742–746. doi: 10.1093/oxfordjournals.jbchem.a124590. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi H., Kuribayashi F., Kiwaki N., Takami Y., Nakayama T. GCN5 regulates the superoxide-generating system in leukocytes via controlling gp91-phox gene expression. J. Immunol. 2011;186:3015–3022. doi: 10.4049/jimmunol.1000364. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi H., Kuribayashi F., Kiwaki N., Nakayama T. Curcumin dramatically enhances retinoic acid-induced superoxide generating activity via accumulation of p47-phox and p67-phox proteins in U937 cells. Biochem. Biophys. Res. Commun. 2010;395:61–65. doi: 10.1016/j.bbrc.2010.03.136. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi H., Mimuro H., Kuribayashi F. Resveratrol strongly enhances the retinoic acid-induced superoxide generating activity via up-regulation of gp91-phox gene expression in U937 cells. Biochem. Biophys. Res. Commun. 2018;495:1196–1200. doi: 10.1016/j.bbrc.2017.11.161. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi H., Mimuro H., Madhyastha H., Kuribayashi F. Chalcone skeleton promotes transcription of gp91-phox gene but inhibits expression of gp91-phox protein, and hydroxyl groups in hydroxychalcones participate in the stable expression of gp91-phox protein. Microbiol. Immunol. 2019;63:438–443. doi: 10.1111/1348-0421.12732. [DOI] [PubMed] [Google Scholar]
- 18.Akiyoshi S., Kikuchi H., Kuribayashi F., Madhyastha H., Minami H. Sulforaphane displays the growth inhibition, cytotoxicity and enhancement of retinoic acid-induced superoxide-generating activity in human monoblastic U937 cells. Fundam. Toxicol. Sci. 2019;8:319–325. [Google Scholar]
- 19.Suzuki S., Kumatori A., Haagen I.A., Fujii Y., Sadat M.A., Jun H.L., Tsuji Y., Roos D., Nakamura M. PU.1 as an essential activator for the expression of gp91phox gene in human peripheral neutrophils, monocytes, and B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6085–6090. doi: 10.1073/pnas.95.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bei L., Lu Y., Eklund E.A. HOXA9 activates transcription of the gene coding gp91Phox during myeloid differentiation. J. Biol. Chem. 2005;280:12359–12370. doi: 10.1074/jbc.M408138200. [DOI] [PubMed] [Google Scholar]
- 21.Kumatori A., Yang D., Suzuki S., Nakamura M. Cooperation of STAT-1 and IRF-1 in interferon-γ-induced transcription of the gp91phox gene. J. Biol. Chem. 2002;277:9103–9111. doi: 10.1074/jbc.M109803200. [DOI] [PubMed] [Google Scholar]
- 22.Allis C.D., Berger S.L., Cote J., Dent S., Jenuwien T., Kouzarides T., Pillus L., Reinberg D., Shi Y., Shiekhatter R., Shilatifard A., Workman J., Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi H., Kuribayashi F., Imajoh-Ohmi S., Nishitoh H., Takami Y., Nakayama T. GCN5 protects vertebrate cells against UV-irradiation via controlling gene expression of DNA polymerase η. J. Biol. Chem. 2012;287:39842–39849. doi: 10.1074/jbc.M112.406389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmodiya K., Krebs A.R., Oulad-Abdelghani M., Kimura H., Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genom. 2012;13:424. doi: 10.1186/1471-2164-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishibashi F., Mizukami T., Kanegasaki S., Motoda L., Kakinuma R., Endo F., Nunoi H. Improved superoxide-generating ability by interferon γ due to splicing pattern change of transcripts in neutrophils from patients with a splice site mutation in CYBB gene. Blood. 2001;98:436–441. doi: 10.1182/blood.v98.2.436. [DOI] [PubMed] [Google Scholar]
- 26.Errante P.R., Frazao J.B., Condino-Neto A. The use of interferon-gamma therapy in chronic granulomatous disease. Recent Pat. Anti-Infect. Drug Discov. 2008;3:225–230. doi: 10.2174/157489108786242378. [DOI] [PubMed] [Google Scholar]
- 27.Bain C.C., Schridde A. Origin, differentiation, and function of instetinal macrophages. Front. Immunol. 2018;9:2733. doi: 10.3389/fimmu.2018.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller P.A., Matheis F., Mucida D. Gut macrophages: key players in intestinal immunity and tissue physiology. Curr. Opin. Immunol. 2020;62:54–61. doi: 10.1016/j.coi.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cani P.D. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.