Abstract
Background
High-level disinfection protects tens-of-millions of patients from the transmission of viruses on reusable medical devices. The efficacy of high-level disinfectants for preventing human papillomavirus (HPV) transmission has been called into question by recent publications, which if true, would have significant public health implications.
Methods
Evaluation of the clinical relevance of these published findings required the development of novel methods to quantify and compare: (i) Infectious titres of lab-produced, clinically-sourced, and animal-derived papillomaviruses, (ii) The papillomavirus dose responses in the newly developed in vitro and in vivo models, and the kinetics of in vivo disease formation, and (iii) The efficacy of high-level disinfectants in inactivating papillomaviruses in these systems.
Findings
Clinical virus titres obtained from cervical lesions were comparable to those obtained from tissue (raft-culture) and in vivo models. A mouse tail infection model showed a clear dose-response for disease formation, that papillomaviruses remain stable and infective on fomite surfaces for at least 8 weeks without squames and up to a year with squames, and that there is a 10-fold drop in virus titre with transfer from a fomite surface to a new infection site. Disinfectants such as ortho-phthalaldehyde and hydrogen peroxide, but not ethanol, were highly effective at inactivating multiple HPV types in vitro and in vivo.
Interpretation
Together with comparable results presented in a companion manuscript from an independent laboratory, this work demonstrates that high-level disinfectants inactivate HPV and highlights the need for standardized and well-controlled methods to assess HPV transmission and disinfection.
Funding
Advanced Sterilization Products, UK-MRC (MR/S024409/1 and MC-PC-13050) and Addenbrookes Charitable Trust
Keywords: HPV, nosocomial transmission; Virus disinfection; Cervical cancer; Methodology for virus infection assay; High-level disinfectant
Research in Context.
Evidence before this study
Questions had been raised about the effectiveness of high-level disinfectants such as ortho-phthalaldehyde based on data with raft cultured HPV16 and 18 using in vitro infectivity assays. The work has not been reproduced by any other laboratories and there are multiple methodological and translational gaps that would need to be addressed to evaluate the reproducibility and clinical relevance of these data. The gaps include an understanding of:
-
•
Levels of HPV titres in clinically derived human lesions to know whether the levels being tested in model systems (e.g. raft tissue-derived and pseudovirus titres) were relevant to humans
-
•
Similarities and differences in clinically sourced virus titres across different HPV high risk (e.g. cervix) and low risk lesions (e.g. anogenital and laryngeal)
-
•
How to consistently and objectively evaluate the lowest infectious dose required to achieve infection (not captured by VGE/cell) and compare across viral preparations
-
•
Effect of squames on infectivity across in vitro and in vivo models
-
•
Differences in infectivity of raft tissue-derived, pseudovirus, and in vivo sources viruses
-
•
Viral titres that can be transferred from a lesion to a fomite surface
-
•
Effect of squames on viral survival over time on a fomite surface
-
•
Dose-response and kinetics of infection in an in vivo model and its relation to results in in vitro models
-
•
Impact of neutralizing agents on viral infectivity
-
•
Impact of virus isolation technique from raft culture on the infectivity readout
-
•
Impact of timing for readouts on infectivity in vitro
In order to evaluate the relevance of the clinical results it was necessary to develop novel translational methods to address the above gaps.
Added value of this study
The work presented in this publication together with work presented in the companion manuscript by the lab of Dr. Michelle Ozbun (https://doi.org/10.1016/j.ebiom.2020.103165) have addressed the gaps listed above. They have advanced our understanding of the in vitro to in vivo correlation for papillomavirus (PV) infectivity and have provided key insights on how to design infectivity and disinfection testing studies that have greater clinical relevance. The results have also demonstrated that high level disinfectants such as ortho-phthalaldehyde and hydrogen peroxide are effective at inactivating HPV on fomite surfaces that are representative of surfaces on medical devices. The publications that called this into question had methodologic issues related to their neutralizing agent that may have confounded their results.
Implication of all the available evidence
The data generated allow the healthcare community to have confidence in the use of disinfectants such as ortho-phthalaldehyde and hydrogen peroxide to disinfect medical devices and thereby protect patients against HPV transmission. The methods that have been developed, which combine quantification of viral load in patient lesions, with in vitro cell culture across multiple viral subtypes and in vivo testing with rigorous controls, provide a helpful framework for evaluating new therapeutic and disinfectant agents against other virus types.
Alt-text: Unlabelled box
1. Introduction
Human papillomaviruses (HPVs) cause a diverse range of skin lesions, from common or genital warts, to high-grade neoplasia. High-risk HPV-driven neoplasia can result in the development of several human cancers, of which cervical cancer is the most prevalent. Current HPV vaccines are not therapeutic [1], uptake has been variable (as low as 40% in the US and France) [2,3], and there remains no targeted antiviral therapy that can clear HPV-associated disease post-infection. High-risk HPVs are typically transmitted by close physical contact, but are stable in the environment and can be transmitted indirectly [4], either as free virions or as virus-laden epithelial cells (squames) from fomite surfaces such as medical devices [5,6]. This is true of transvaginal ultrasound probes, cryotherapy tips, thermal ablation probes and speculums, which if contaminated with high-risk HPV can facilitate virus transmission to the most vulnerable site of the body, the cervical transformation zone [7].
To date, there have been few studies addressing the susceptibility of HPV to clinical disinfectants, and as a consequence, the efficacy of disinfectants to inactivate HPV has been extrapolated from the use of surrogate non-enveloped viruses such as poliovirus [8], or through the use of surrogate papillomavirus particles that are produced in vitro (i.e. pseudoviruses and raft-cultured viruses). This is primarily due to the difficulties of producing high titres of infectious HPV particles in vitro, and the lack of a suitable assay to quantify infection, as HPVs do not cause cytopathic effects (CPE) or drive cell lysis in monolayer assays. The extent to which these assays and infection models mimic in vivo infection and the transmission via squames of clinically relevant HPV titres is uncertain [9,10].
Recently, there have been several publications from a single research group suggesting that certain high-level disinfectants may be ineffective in preventing HPV transmission from medical devices, based on an in vitro infectivity model using crude HPV preparations from organotypic (raft) culture [11,12]. Given these implications for public health, the objective of this work was to evaluate the clinical relevance and validity of these findings, which required the development and use of novel methods and models to quantify and compare: (i) Infectious titres of in vitro lab-produced, clinically-sourced (from patient cervical lesions), and animal-derived viruses, (ii) The dose-response and the kinetics of disease formation in these newly developed in vitro and in vivo models, and (iii) Efficacy of high-level disinfectants in inactivating PV in these systems. This work has provided key insights on the ‘viral load’ and potential infectious titre observed clinically, differences in infectivity between raft-derived viruses and in vivo derived viruses, the potential impact of disinfection neutralizing agents used as controls, and their interaction with the virus during infectivity assays, and the role of the exfoliating cells (or squames) in enhancing virus survival and in modulating transmission success.
2. Methods
2.1. Ethics
The collection of clinical material used in this study complied with the Helsinki Declaration of 1975, as revised in 1983, and was approved by an independent ethics committee (South Central Oxford B, 17/SC/0203) as per UK regulations.
2.2. Collection of patient HPV from cervical lesions
To evaluate the levels of HPV virions present in the surface layer of cervical lesions from patients with high-risk HPV (hrHPV) infection, a nitrocellulose membrane (254 mm diameter, GE Healthcare, Illinois, United States) was applied to the cervix for a contact time of 15 s, transferring surface cells and virus particles onto the membrane (patch sample). A total of 40 samples were collected from patients attending Cambridge University Hospitals between 2018 to 2019.
2.3. Typing and quantification of HPV on the membrane
Membranes were fixed with 4% paraformaldehyde, then permeabilised with 0.1% Triton X-100. Following Benzonase (Sigma-Aldrich, Missouri, United States) treatment at 37°C for 1 h (leaving only the potentially infectious encapsidated viral genome DNA), total DNA was extracted. Samples were typed and quantified using the high-risk Human Papillomavirus (HPV) Genotyping Real Time PCR kit (Shanghai ZJ Bio-Tech, Shanghai, China), using reference HPV genomes obtained from The International Human Papillomavirus Reference Centre as standards.
2.4. Production of papillomavirus
We employed three systems to produce/obtain infectious PV particles. Recombinant-based HPV16 pseudovirions (PsV) with reporter plasmid pCMV-Gluc2 (New England Biolabs, Massachusetts, United States) were produced using 293TT cells (RRID: CVCL_1D85) and p16sheLL (RRID: Addgene_37320) [13], [14], [15]. Organotypic raft culture-derived HPV18 was produced using NIKS cells (a spontaneously immortalised human keratinocyte cell line) and HPV18 genome [16], [17], [18]. Mus musculus PV type 1(MmuPV1) was extracted from mouse lesions [19]. Tissue homogenates and cell lysates were incubated at 37°C with Benzonase for 24 h. After a low-speed centrifugation step to remove cellular debris, the virus particles were pelleted by ultracentrifugation then resuspended with PBS with 10% FBS (cell-free virus) [20]. For MmuPV1 on fomites, virus with exfoliating cells on the lesion surface, was transferred onto the tip of a 3 mm diameter acrylonitrile butadiene styrene (ABS) stick (virus with exfoliating cells). Alternatively, 2 μL of cell-free virus (2 × 108 VGE) was placed onto the tip of the stick and left to dry for 1 h. The virus on fomite (ABS stick) was kept at room temperature (20–24°C) and 45–65% relative humidity (dark and no contact with circulating air).
2.5. Quantification of viral genome equivalents (VGE)
VGE represents the level of encapsidated viral DNA. Virus genome copy number was determined by SYBR Green qPCR (Thermo Fisher Scientific, Massachusetts, United States) using type-specific primers (Supplementary Table 1).
2.6. Antibodies
Antibodies used included rabbit anti-MmuPV1 L1 and E4 (developed in the lab, 1:200 for immunofluorescence), and mouse anti-HPV18, HPV-16 and MmuPV1 neutralising antibodies (which were a gift of Neil Christensen, Pennsylvania State University College of Medicine, 1:200 for neutralising assay) [21], [22], [23].
2.7. Virus infectious titre in vitro (quantification of virus transcripts)
The infectious titre of PV in vitro is described as the quantity of virus E1^E4 transcripts or Gaussia Luciferase (GLuc) reporter gene activity. HaCaT cells (RRID: CVCL_0038), which were cultured in Dulbecco Modified Eagle Medium (2 × 105cells/well in 6-well plates), were infected with the virus in the presence or absence of the neutralising antibody. After 24 h, the medium was exchanged, and the cells were incubated for a further 24 h before the cells or culture media were collected. The activity of GLuc in the media was measured using the Gaussia Luciferase Assay Kit (New England Biolabs). Following total RNA extraction and first-strand cDNA synthesis, virus E1^E4 was also quantified by SYBR Green qPCR using type-specific primers (Supplementary Table 1).
2.8. Infectious foci assay
Viral transcripts in individually infected HaCaT cells were detected and visualised using RNAscope in situ hybridization (Advanced Cell Diagnostics, Minnesota, United States) following the manufacturer's instruction. The probe used for MmPV1 RNA detection was MusPV-E6-E7 (Cat No. 409771), and the probe used for HPV18 RNA was HPV-HR18 (Cat No. 312591). The proportion of virus RNA-positive cells was quantified and the multiplicity of infection (MOI) was determined by inverse Poisson distribution[24].
2.9. Quantification of MmuPV infectivity in vivo
All animal procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and in compliance with the ARRIVE guidelines. The protocols were approved by Animal Welfare and Ethical Review Body (AWERB) of the University of Cambridge and the Home Office (the project licence number: 70/8113).
Tails of athymic nude mice (Hsd:Athymic Nude-Foxn1nu, female, 6–8 weeks of age, after a week of acclimatisation; ENVIGO, Indianapolis, United States) were inoculated with different doses (in 2 μL volume) of cell-free MmuPV1 following a 3 mm long scarification of the epidermis (up to 3 inoculation sites per a mouse tail), then observed at weekly intervals for 16 weeks for the appearance of palpable/visible lesions. The sample size (the number of inoculation sites of each group were between 10 and 33 (specified in Figures). The percent of infected sites with lesions was plotted as a reverse Kaplan-Meier curve. The statistical significance is shown using the log-rank test. The sample size was determined using the outcomes shown in Fig. 3a. In this study, in vivo experiments were conducted by two persons but not randomised. Potential confounders such as the order of treatments and measurements, or animal/cage location were not controlled. There were no exclusions of animals during the experiments.
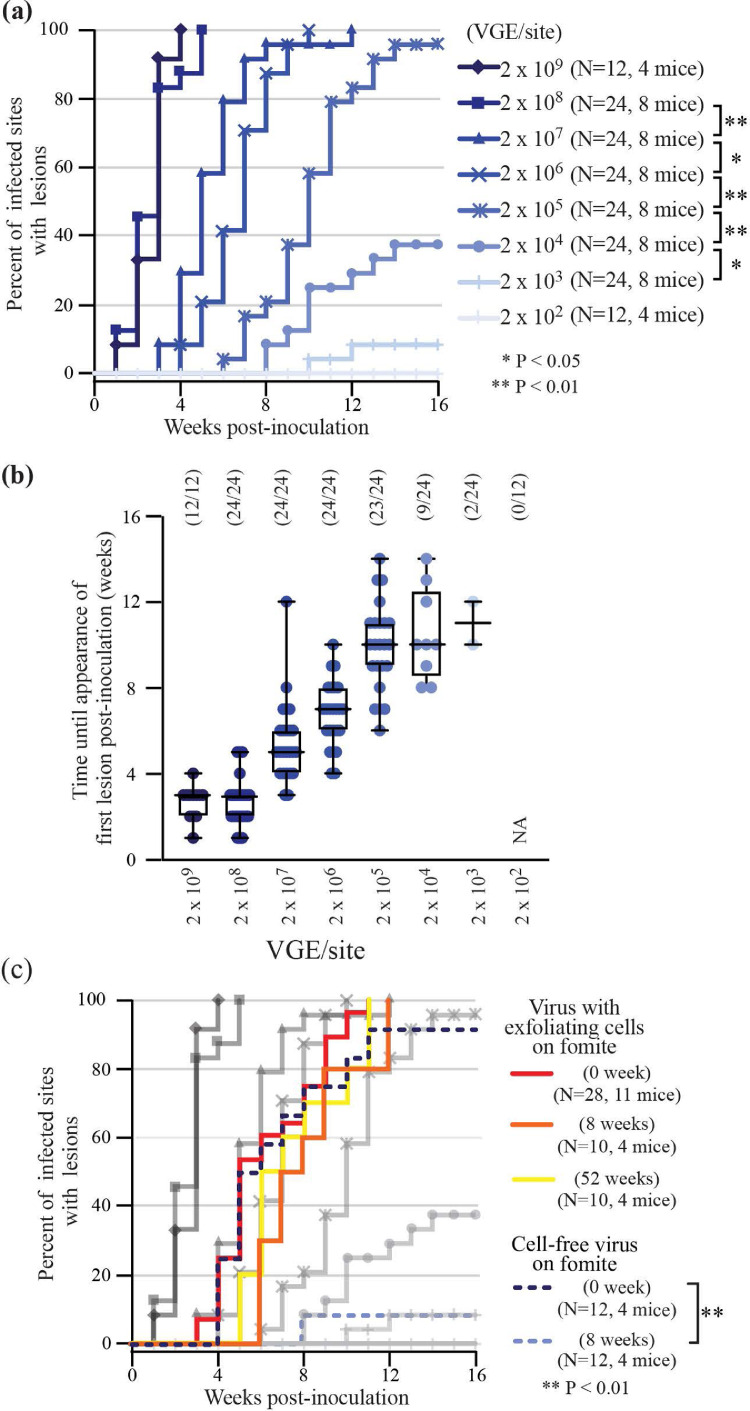
Fig. 3.
Quantification of virus infectivity in vivo using MmuPV1 model
(a) Proportion of lesions formed at infected sites over a 16-week post-inoculation observation period with differing virus doses. * indicates p < 0.05, **indicates p < 0.01 (the log-rank test). The numbers of sites of inoculation (N) and mice in each group are shown. (b) Number of weeks until appearance of first lesion post-inoculation at differing virus doses are shown as Mean and Range. The numbers of sites of inoculation and lesion formed are shown. (c) Proportion of lesions formed at infected sites over a 16-week post-inoculation observation period for either virus with exfoliating cells on fomite (kept for 0, 8 and 52 weeks) or cell-free virus on fomite (kept for 0 and 8 weeks). Fig. 3a results are shown for comparison, in grey. **indicates p < 0.01 (the log-rank test). The numbers of sites of inoculation (N) and mice in each group are shown.
2.10. Disinfection assay
2 μL of virus sample, which contains 10% of FBS as an organic load (soil), was incubated with 8 μL of each disinfectant (0.55% ortho-phthalaldehyde (OPA, Advanced Sterilization Products, California, United States), 30% hydrogen peroxide (Sigma-Aldrich), 1000/5000 ppm hypochlorite (Advanced Sterilization Products) or 70% ethanol) for 12 minutes at room temperature (<25°C). 80% of each disinfectant as working concentration was chosen in light of the European Standard for the quantitative suspension test for the evaluation of virucidal activity [8]. The contact time (12 min) was chosen as specified by the manufacturer of OPA for comparison. Hypochlorite and hydrogen peroxide were subsequently neutralised by adding 90 μL 1% cysteine/peptone (for hypochlorite) and 200 units/mL catalase (for hydrogen peroxide) in DMEM supplemented with 10% FBS for 1 h. OPA was neutralised by adding 10 μL 10% glycine or lysine for 10 min, then incubated for 50 min in 80 μL DMEM + 10% FBS. Next, 100 μL of disinfectant- or neutralised disinfectant-treated virus was added to 2 mL culture medium for infection. For these in vivo model, 2 μL of disinfectant- or neutralised disinfectant-treated virus was used for inoculation. For virus on fomites, the tip of the fomite (ABS stick) was incubated with 1 mL of each disinfectant (80% as working concentration) for 12 min at room temperature (<25°C), then incubated with 1 mL of neutralised disinfectant for 10 min, twice. Mice were then inoculated with the treated virus. Virus incubated with neutralised disinfectant was used as control.
2.11. Evaluation of neutralised OPA and virus charge
For neutralised OPA, 2 μL neutralised OPA (glycine- or lysine-HCl) was diluted with 1 mL 20 mM HEPES (pH 7.4), then passed through a Sartobind S or Q pico ion exchange membrane (Sartorius, Göttingen, Germany) following equilibration with 20 mM HEPES. The flow-through was collected, and OPA-glycine and OPA-lysine were quantified via spectrometer by measuring absorbance at 220 nm. This measurement was subtracted from the input, and the result was given as the amount of neutralised OPA sequestered to the membrane. For virus, 2 μL virus sample with/without 98 μL neutralised OPA was diluted with 1 mL 20 mM HEPES, then passed through an ion-exchange membrane following equilibration. The membrane was washed twice with 1 mL 20 mM HEPES. Virus sequestered to the membrane was eluted with 1 mL 20 mM HEPES/0.5 M NaCl. The buffer was exchanged to PBS using a Zeba Spin Desalting Column, 40K MWCO (Thermo Fisher Scientific), and the sample was subject to subsequent analyses. The pI and charge of neutralised OPA was estimated using SPARC online calculator [25].
2.12. Statistics
Data are given as means ± standard deviation (SD). For comparison of two groups (Fig. 1b), Mann Whitney test was used (two-tailed). Percent of infected sites with lesions was plotted as a reverse Kaplan-Meier curve (Fig. 3 and 7). For comparison of two groups, the log-rank test was used.
Fig. 1.
Variation in human papillomavirus VGE titres at the surface of the cervix
(a) Exfoliating cells from 40 patients were collected from the surface of the cervix using a nitrocellulose patch (see Methods). (b) After Benzonase digestion to remove non-encapsidated viral DNA, quantitative HPV typing was carried to determine virus type and titre (VGE/mm2; Y-axis). For each patient, associated disease was classified either as LSIL (blue), HSIL (red), or as encompassing both HSIL and LSIL together (yellow). p = 0.058, LSIL versus HSIL (Mann Whitney test). (c) Detail of HPV types and VGE abundance in LSIL and HSIL are shown. Virus titre in the exfoliating cervical cells of the cervix of 40 different patients are shown as VGE/mm2. HPV types detected at the lesion surface are listed, with the most prominent HPV type in each case highlighted in red and virus titres.
Fig. 7.
Evaluation of efficacy of disinfectants using MmuPV1 in vivo infection assay
Proportion of lesions formed at infected sites over a 16-week observation period following inoculation with cell-free MmuPV1 previously incubated with (a) disinfectant or (b) neutralised disinfectant, or MmuPV11 with exfoliating cells on fomite previously incubated with (c) disinfectant or (d) neutralised disinfectant. The numbers of sites of inoculation (N) and mice in each group are shown.
2.13. Role of Funding Source
This work is supported by Advanced Sterilization Products (ASP) and UK-MRC (MR/S024409/1 and MC-PC-13050), Addenbrookes Charitable Trust. ASP had no role in study design, data collection and data analysis but provided input on interpretation and proofreading of the manuscript (Jeremy Yarwood, Marc Rogers, Ankur Sharma, Gary Eichenbaum are listed as authors). UK-MRC and Addenbrookes Charitable Trust have no role in study design and data collection, data analysis, interpretation or writing of the manuscript. Addenbrookes Charitable Trust funded Aslam Shiraz PhD programme. Advanced Sterilization Products funded part of Aslam Shiraz PhD programme and paid for the work done in this study including the in vivo work. UK Medical Research Council (sponsor reference MC-PC-13050 & MR/S024409/1) funded Prof. John Doorbar's lab and Taylor Saunders-Woods PhD programme.
3. Results
3.1. Quantitation of viral genome equivalents in the exfoliating cell layers of the cervix from patients with HPV
A ‘patch-sampling’ approach was used to collect exfoliating cervical cells from women attending for routine colposcopy (Fig. 1a), and the titre of viral genome equivalents (VGE) per mm2 of surface epithelium was quantified (from 40 patients (one patch per patient)), Figure 1c. VGE is a measure of encapsidated viral genomes or ‘virus particles', and is distinct from the more commonly used term ‘viral load’, which counter-intuitively is a measure of HPV DNA abundance, irrespective of whether genomes are packaged, present as free episomes, or integrated into the host cell chromosome. Most patients were positive for multiple HPV types using the VGEVGE assay. Among these patients, a single HPV type (shown in red in Fig. 1c) was found to contribute most (>90%) to the overall titre. In individuals where only a single HPV type was detected, HPV16 was present in 81.2% (9 of 11 cases) of individuals, albeit at low titres (<1 × 104 VGE/mm2). Amongst those with the most productive low-grade squamous intraepithelial lesion (LSIL) (i.e., those with titres >1 × 107 VGE/mm2, Fig. 1c shadowed in grey), HPV18 was the predominant type (37.5%, 3 of 8 cases). Regions of high-grade neoplasia were often accompanied by low-grade disease (33% of high-grade squamous intraepithelial lesion (HSIL) cases also contained LSIL), which is reflected in the wide range of titres in the samples (1.27 × 106 VGE/mm2 to 1.45 VGE/mm2 in HSIL alone; 9.72 × 108 VGE/mm2 to 0.339 VGE/mm2 in LSIL alone).
3.2. Measuring HPV infectious titre using ‘transcript quantitation’ and ‘infectious centre’ assays
For these experiments, high-risk HPV virions at clinically relevant titres were prepared from the differentiated layers of organotypic raft cultures. Although several high-risk HPV types can be propagated in raft culture[16], HPV18 was used as it was the predominant type found in productive LSILs (>1 × 107 VGE/mm2, Fig. 1c). Parallel infectivity studies were carried out using high-risk HPV PsV and MmuPV1.
Viral E1^E4 transcript abundance for HPV18 and MmuPV1, and reporter gene activity for PsV, were in all cases proportional to the infecting virus (VGE) dose in HaCaT cells (Figs. 2a and 2b). The approach had high detection sensitivity and could reveal the extent of infection at VGE titres as low as 1.0 × 10−2/cell, as well as showing a wide dynamic range (>5 log10), a characteristic that is required for the evaluation of inhibitory agents targeting the infection process. For both HPV18 and MmuPV1, the pre-incubation of virions with anti-L1 neutralising antibodies abolished detectable infection (Figs. 2a and 2b)b. The infectious centre assay was more laborious and less sensitive, with a detection threshold of 50 VGE/cell. Human papillomavirus infection of monolayer keratinocytes is not associated with cytopathic effect (CPE) and is not productive, which limits infectious centres to the initially infected cell and its immediate daughter cells (Figs. 2cc and 2d). Both HPV18 and MmuPV1 were, however, found to have similar particle/infectivity ratios in this assay, with around one infectious event per 450 VGE (Figs. 2cc and 2d).
Fig. 2.
Quantification of virus infectious titre in vitro
(a, b) Measurement of E1^E4 viral gene transcripts or Gaussia luciferase reporter gene activity in HaCaT cells infected with differing doses of HPV18 (red), MmuPV1 (green) and PsV (black). Infectious titre shown with 5-6 log10 dynamic range. Neutralising assay compares virus titre following incubation with the neutralising antibody or isotypic control. AU, arbitrary unit; NT, not tested; ND, not detected. Data is obtained with biological triplicates and shown as Mean and SD. (c, d) RNAscope® visualisation of E6 and E7 viral gene transcripts in HaCaT cells infected with HPV18 (top) or MmuPV1 (bottom); virus titre shown as VGE/cell; proportion of virus-RNA positive cells (cell boundary in red) given as %.; boxed regions enlarged in (d). Scale bar; 100 μm
3.3. The kinetics of papillomavirus disease formation in vivo; correlation with in vitro assays
To establish the in vivo relevance of the cell-based assays and patient-derived titres, PV viral load, fomite transmission and infectivity was evaluated using the MmuPV1 mouse model. After tail scarification and inoculation with a range of MmuPV1 titres reflecting those seen in clinical lesions (see Fig. 1 and Supplementary Figure 1), athymic nude mice were monitored weekly for the appearance of palpable/visible lesions. The disease was apparent between weeks one and fourteen post-infection, with the timing of lesion appearance being directly proportional to the inoculation dose (Figs. 3a and 3b) as demonstrated in the in vitro assays (Fig. 2). The limit of in vivo detection was 2.0 × 103 VGE, with the infectious disease model having a dynamic range of approximately 6 log10 of virus dose. This data indicates that the rate of in vivo lesion formation is influenced by the virus titre, and when considered alongside the in vitro data, suggest that this reflects the number of initiating infection events.
3.4. Role of exfoliating cells in modulating the papillomavirus in vivo infectivity
During natural transmission, papillomaviruses are shed and transmitted within exfoliating cells or squames [26], and although the transmission vehicle is thought to modify virus survival and infection kinetics, this remains to be addressed in relation to disease development. To examine this, virus collected on fomites from in vivo infected MmuPV1 lesions was quantified using the VGE assay, followed by an assessment of virus transfer-efficiency to uninfected skin. Fomites that had direct lesional contact showed a median virus titre of 5.2 × 106 VGE/mm2, which is towards the upper end of what was seen for HPV at the cervical surface (Fig. 1). One log10 reduction in VGE titre (median 5.1 × 105/mm2) occurred following fomite-mediated transfer to new sites of infection (Supplementary Figure 2), with the infectivity of ‘virus with exfoliated cells’ being similar to that seen with ‘cell-free’ virus at titres of 2.0 × 106 VGE to 2.0 × 107 VGE per site (Fig. 3c). As papillomaviruses are thought to be relatively stable in the environment [4], next we examined the role of the exfoliated cell in modulating virus desiccation sensitivity. Although infectious immediately after deposition on fomites, cell-free virus lost viability in the in vivo assays upon storage, with a >3 log10 reduction in titre after 8 weeks at room temperature (Fig. 3c). In contrast, virus collected with exfoliating cells showed minimal loss of infectious viability over this period, and surprisingly showed no significant drop in infectivity even after 52 weeks of desiccation at room temperature (Fig. 3c). These results suggest that papillomaviruses are extremely stable when shed from lesional surfaces, and that exfoliating cells facilitate papillomavirus survival in the environment. When taken together, this data also suggests that the risk of in vivo disease formation may be extrapolated from in vitro test results (Fig. 4).
Fig. 4.
Viral titration assay results provide a scale to interpret each read-out of virus titration in the context of VGE, infectious titre and actual capability of in vivo lesion formation.
3.5. In vitro efficacy of disinfectants in controlling papillomavirus transmission
In in vitro assays, both HPV18 and MmuPV1 were found to be similarly susceptible to both aldehyde-based cross-linking agents and oxidizing agents (OPA, hydrogen peroxide and hypochlorite). These are the two most widely used disinfectant classes, and in both cases, the observed >4-log10 reduction in infectivity (Fig. 5a) exceeds current EU and FDA guidelines regarding the suitability of disinfectants for use in high-level disinfection [8]. In agreement with previous results, ethanol showed no ability to abrogate papillomavirus infectivity. Similar results were obtained with the pseudoviruses, except in the case of ethanol which conferred a one log10 infectivity reduction (Fig. 5b).
Fig. 5.
Evaluation of disinfectant efficacy using in vitro infection assay
(a, b) Measurement of viral infectivity (E1^E4 viral gene transcripts or reporter gene activity shown as Mean and SD) of HPV18, MmuPV1 and PsV in HaCaT cells following incubation with viruses treated with disinfectants or their neutralised equivalent (except 70% ethanol). AU, arbitrary unit; ND, not detected. Data were obtained with biological triplicates and shown as Mean and SD.
An important finding in the disinfection testing was that OPA that had been neutralised with glycine (subsequently referred to as OPA-glycine, see Fig. 6), which is the standard negative control for evaluating OPA efficacy, was also able to inhibit papillomavirus infectivity in the in vitro assay (Fig. 6a), whereas OPA neutralised with lysine (subsequently referred to as OPA-lysine) showed no inhibitory activity (Figs. 5 and 6a). OPA-glycine and OPA-lysine differ in overall charge at neutral pH (Fig. 6b), raising the hypothesis that a transient electrostatic association between OPA-glycine and positively charged PV particles may underlie the observed infectivity reduction, as reported for other negatively charged molecules, such as carrageenan [15]. In support of this hypothesis, neat OPA-lysine, which has a slight positive charge, and the negatively charged neat OPA-glycine were differentially sequestered during anion-exchange chromatography (Fig. 6c), while papillomavirus particles were only sequestered during cation-exchange chromatography, with 500 mM NaCl being required for elution (Fig. 6d). OPA-glycine, but not OPA-lysine, effectively blocked virus binding to the cation-exchange column, which supports the hypothesis that negatively charged molecules such as OPA-glycine modify overall papillomavirus charge electrostatically (Figs. 6d and 6e). These results may explain the comparable effects of OPA and neutralised OPA (OPA-glycine) on in vitro HPV infectivity reported recently [11,12], highlighting a need for including appropriate neutralised negative controls that do not bind to the virus when evaluating disinfectants.
Fig. 6.
OPA-glycine disrupts the electrostatic charge of virus particles and inhibits infection
(a) Virus infectivity (E1^E4 viral gene transcripts or reporter gene activity) of HPV18, MmuPV1 and PsV in HaCaT cells following incubation with OPA-glycine and OPA-lysine. ND, not detected. (b) Schematic representation of the reaction of OPA with glycine and lysine. Estimated pI and charge at pH 7.4 are shown. (c) Relative amount of cation-exchange (cation) or anion-exchange (anion) membrane-sequestered OPA-glycine and OPA-lysine during ion-exchange chromatography. AU, arbitrary unit. (d) Measurement of infectious dose? of membrane-sequestered PsV, and PsV plus OPA-glycine or OPA-lysine. (e) Relative VGE of membrane-sequestered HPV18 and MmuPV1 in the presence of OPA-glycine or OPA-lysine. All data were shown as Mean and SD and obtained with biological triplicates.
3.6. In vivo efficacy of disinfectants in controlling papillomavirus transmission
In order to evaluate the efficacy of disinfectants on physiologically relevant in vivo transmission, extracted MmuPV1 was incubated with OPA or hydrogen peroxide, both of which achieved a >4-log10 reduction in infectious titre (Fig. 7a), with no lesion development, even at 16 weeks post-infection. Hypochlorite showed at least a 3-log10 reduction but failed to achieve complete inhibition, with efficacy improving at higher concentration (5,000 ppm). 70% ethanol did not inhibit disease formation in vivo (Fig. 7a), similar to the in vitro results shown in Fig. 5. Next, we evaluated the efficacy of disinfectants against ‘virus in the presence of exfoliated cells’ on fomites. While OPA, hydrogen peroxide and hypochlorite all showed at least a 3-log10 reduction in infectious titre, complete inhibition was never seen, with 1000 ppm hypochlorite showing the lowest efficacy. Similarly, 70% ethanol showed no reduction in virus infectivity (Fig. 7c). In contrast to what was seen with OPA-glycine, the neutralised disinfectants had either little or no effect on disease formation in vivo (Figs. 7b and d), which most likely reflects the extended kinetics of the in vivo infection process (days rather than hours), and the presence of tissue proteins at the wound site, which may competitively dissociate molecules such as OPA-glycine that bind electrostatically to the virion surface. These results, using an in vivo transmission model with ‘naturally produced’ papillomavirus, support our in vitro studies showing that the OPA, hypochlorite and hydrogen peroxide all show sufficient effectiveness for PV disinfection, while 70% ethanol was found to be ineffective. Furthermore, whether the virions were cell-free or present with exfoliating cells had a significant impact on susceptibility to disinfection, suggesting that being shed within an anuclear squame confers an important survival advantage on the virus, protecting it against both desiccation and disinfection. The study highlights the need to use physiologically relevant models for the evaluation of virus control measures and emphasises the importance of combining disinfection with other decontamination methods such as physical cleaning.
4. Discussion
4.1. Characterization of HPV titres and types in clinical lesions
Several reports have highlighted a risk of nosocomial high-risk HPV transmission during cervical examination and treatment [5,6], however, there has been no quantitative assessment of infectious virus titres at the cervix of patients with HPV, or the risk of disease transmission through fomite contact. To examine this, we quantified for the first time the level of virus production from the lesion surface of patients with cervical lesions (Fig. 1), and have used this information to cover a clinically relevant range of virus doses in our experiments (Fig. 4). There was significant variation in viral titres recovered on the patch across lesions (ranging from 0.34 VGE/mm2 to 9.72 × 108 VGE/mm2) with a trend for lower viral titres in individuals presenting with HSIL, which typically represent abortive or non-productive infections. LSIL, and heterogeneous lesions where HSIL was accompanied by LSIL, generally showed higher overall VGE titres at their surface. Interestingly, a subset of LSIL showed only very low virus titres, which is compatible with previous reports suggesting that a proportion of lesions in this category may be non-productive [27], or lack a HPV causal association (See Fig. 1c) [28].
4.2. In vitro to in vivo correlation of papillomavirus disease formation
Prior to this work, the extent to which in vitro infectivity assays predict the risk of virus transmission in vivo was not known. Similarly, an infectious dose has not yet been linked to the risk of disease formation for any papillomavirus, and indeed there are limited opportunities to gather such in vivo data, other than from mice. Because of this, and because MmuPV1 and HPV show similar in vitro infectivity profiles, we sought to relate virus dose (VGE and infectious) to the kinetics of in vivo disease development following MmuPV1 infection. We have adapted recombinant PsV technologies and organotypic raft culture systems and used these alongside a MmuPV1 in vivo infection model to obtain high concentrations of infectious papillomavirus particles (Figs. 2 and 3). Laboratory-based in vitro and in vivo systems were used to quantify virus titre, and to provide a comprehensive evaluation of papillomavirus infectivity and susceptibility to disinfectants and their subsequent ability to drive in vivo disease formation (Fig. 4). On the basis of the data presented herein, in which an in vitro to in vivo correlation has been established for disinfection, it appears that the two infectivity assays are sufficient to provide an in vitro standard for evaluating the efficacy of agents such as disinfectants that may be used to control the spread of infection. The infectious centre assay identifies the number of infected cells and infectious virus particles, and provides insights into the rate of new lesion formation, whereas the transcript quantitation assay provides a high throughput method to evaluate the relative extent of viral transcription within a group of cells exposed to different conditions.
The development of the MmuPV1 model allows additional in vivo validation using native virus extracted after productive infection of mice. The preparation of high titre virus stocks from the MmuPV1 model is more straightforward than from the HPV18/16 organotypic raft system, and as both MmuPV1 and HPV18 showed similar behaviour in in vitro assays, it appears that MmuPV1 can be used as a convenient surrogate to study HPV transmission and infection. Such in vivo infection assays can be modified to mimic the natural transmission and infection route, including the ‘worst-case scenario’, where direct PV inoculation occurs at the scarification site in an immunocompromised host.
4.3. Role of exfoliating squames in papillomavirus transmission and disease formation
The results obtained in the mouse model highlight the importance of evaluating the role of squames in viral disinfectant susceptibility and transmission (Figs. 3 and 7). During natural transmission, the virus is not shed as free virions, but rather is released from the surface of the skin in exfoliating cells or squames [26]. Importantly, virus collected along with exfoliating cells showed greater resistance to environmental stresses, such as desiccation and oxidation, and was found to persist for at least a year with only a minimal drop in infectivity. Importantly for this study, virus released in exfoliating cells showed a significant increase in disinfectant resistance when compared to laboratory purified virions and PsV, which should be addressed in future studies of papillomavirus transmission and disinfection resilience. Among the disinfectants we tested here, OPA, and comparably hydrogen peroxide, showed the highest efficacy for virus inactivation in exfoliating cells (Fig. 7). In this context, it is worth noting that cells in the upper epithelial layers, where virus assembly occurs, contain very high levels of the viral E4 protein, which assembles into amyloid-like fibres and is thought to contribute to virus release, transmission, survival, and most probably also disinfectant resistance [10]. The currently accepted protocols, which use cell-free virus in the presence of simple organic load or ‘soil’ to evaluate the viricidal activity of chemical disinfectants [8,11,12], do not fully evaluate the role of the abundant E4 protein and the contents of exfoliating cells, which will include components of the cornified envelope along with some structural keratins and filaggrin.
4.4. Efficacy of high-level disinfectants across model systems
In contrast to the previously published results by Meyers et al. [11,12], our results clearly show that papillomaviruses are susceptible to both conventional aldehyde-, chlorine- and oxidant-based disinfectants, but are resistant to alcohol-based disinfection, although PsVs show partial susceptibility to the latter. This difference is thought to be due to the difference in maturation of the virus particle, and specifically the extent of cross-linking between L1 viral capsid proteins, which is lower in recombinant PsVs and quasivirions when compared to raft-derived or native virions [29,30]. This work focused solely on the effectiveness of disinfectants alone, particularly in comparison to OPA, whereas protection and disinfection in clinical practice involves the use of barriers to infection, such as disposable covers for transvaginal probes, along with mechanical cleaning systems. Given the high degree of HPV inactivation following incubation with disinfectants alone, and the additional physical cleaning steps that go into high-level disinfection[31], the results demonstrate that there is likely to be a minimal risk of HPV transmission following high-level disinfection, which should be addressed in future studies.
4.5. Importance of the choice of model systems and appropriate controls
We have also shown that PV particles are positively charged at physiological pH, and that OPA-glycine, but not OPA-lysine, can affect the charge of the virus particle. The charge of the virus is, in general, considered to play an important role in the virus-host cell interaction prior to infection. For example, cationic polymers are typically used to increase the efficiency of retrovirus infection in vitro, and are considered to create an electrostatic environment that promotes the optimum interaction between negatively charged retrovirus and heparan sulfate proteoglycans (HSPGs) [32]. The binding to HSPGs is also considered an important step in HPV infection [33]. Taking these points together, we hypothesise that negatively charged OPA-glycine neutralises the positive charge of the virus particle and inhibits PV binding to the host cells in our in vitro infection model. Negatively charged carrageenan, indeed, is known to inhibit PV infection [15]. Glycine is used as a general OPA neutraliser, and was chosen in the previously published reports by Meyers et al.[11,12]. In our in vitro and in vivo infection model systems however, OPA-glycine showed distinctive effects on papillomavirus infectivity, probably reflecting the different kinetics of the infection process between in vitro and in vivo infection models (Figs. 6a and 7). Taking these results and the work presented in the companion manuscript by the lab of Dr Michelle Ozbun together (https://doi.org/10.1016/j.ebiom.2020.103165) [34], it is clear that the proper evaluation of virus infectivity and the efficacy of disinfectants requires a clear understanding of the characteristics of the infection models (in vitro and in vivo), and the source of virus (cell-free virus, virus with exfoliating cells and/or clinically sourced virus), along with the appropriate use of validated controls (e.g. for virus titre, contact time, concertation of disinfectant and the choice of neutralisers).
5. Conclusion
The results herein can provide confidence to patients and the healthcare community that there is a minimal risk for transmission of HPV from medical devices following high-level disinfection with aldehyde-based disinfectants. The methods that have been developed, which combine quantification of viral load in patient lesions, assessment of viral-titre response for infection in cell culture, and dynamics of disease formation in vivo, provide a helpful framework for evaluating the dynamics of viral transmission, susceptibility for disinfection and their implications for clinical transmission.
6. Contributors
Dr. Nagayasu Egawa and Dr. Aslam Shiraz are joint first authors. They performed the experiments including study design, data collection, analysis, interpretation and writing. Dr. Robin Crawford aided in data collection from patients. Dr. Taylor Saunders-Wood assisted in generating some of the in vivo data. Dr. Jeremy Yarwood, Dr. Marc Rogers, Dr. Ankur Sharma & Dr. Gary Eichenbaum assisted with interpretation of the data and proofreading. Prof. John Doorbar was involved in all aspects of the study including writing and manuscript preparation. All authors read and approved the final version of the manuscript.
Declaration of interests
Dr Egawa, Dr. Shiraz and Prof. Doorbar report grants from Janssen Pharmaceuticals/ Advanced Sterilization Products (ASP), a manufacturer and distributor of OPA disinfectant, during the conduct of the study. Dr Yarwood is an employee of ASP. Dr Rogers is an employee of ASP. Dr Sharma reports personal fees from ASP outside the submitted work. Dr Eichenbaum was (and still is) an employee and shareholder of Johnson & Johnson (J&J) and funding for the work came from ASP, which was a subsidiary of J&J until it was sold to Fortive in 2019. Dr Saunders-Wood and Dr Crawford have nothing to disclose.
Data sharing statement
All data generated or analysed during this study are included in this article. Anti-HPV18, HPV-16 and MmuPV1 neutralising antibodies can be requested from Dr. Neil Christensen, Pennsylvania State University College of Medicine. MmuPV1 virions, anti-MmuPV1 antibodies (L1 and E4), and virus particles can be provided upon availability by written request to the corresponding author. All other materials are commercially available or have been described in our previous manuscripts.
Acknowledgments
We are extremely grateful for the support offered by Mr Peter Baldwin (Consultant Gynae-Oncologist) for his unwavering support at Cambridge University Hospitals (CUH). The CUH Colposcopy team (Joyce Eletu-Odibo, Kate Malliou & Gemma Ferguson) and Research team (Cherry Publico-Sanchez & Rutendo Nyagumbo). This work is supported by Advanced Sterilization Products, UK-MRC (MR/S024409/1 and MC-PC-13050) and Addenbrookes Charitable Trust.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103177.
Appendix. Supplementary materials
Supplementary Figure 1: (a) A representative image showing the appearance of early MmuPV1 lesions at 2-week post-inoculation of 2.0 × 108 VGE/site. Before the appearance of a palpable lesion (+), a change in skin colour was usually seen (-/+). (b) Histological images of an early lesion; H&E staining (HE), E6E7 viral transcripts (E6E7) and viral E4 and L1 proteins (E4 and L1). Scale bar; 100 μm
Supplementary Figure 2: (a) Exfoliating cells were collected from the surface of productive mouse papillomas using a plastic stick (fomite) made of acrylonitrile butadiene styrene, prior to inoculation onto the tail of another mouse after scarification. (b) After Benzonase digestion to remove non-encapsidated viral DNA, MmuPV1 on the fomite and at the site of inoculation was quantified (VGE/mm2; Y-axis).
References
- 1.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines–immune responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen-Huu NH, Thilly N, Derrough T, Sdona E, Claudot F, Pulcini C. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.11.081. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman RK, Raviotta JM, Nowalk MP, Moehling KK, Reis EC, Humiston SG. Using the 4 Pillars Practice Transformation Program to increase adolescent human papillomavirus, meningococcal, tetanus-diphtheria-pertussis and influenza vaccination. Vaccine. 2017;35(45):6180–6186. doi: 10.1016/j.vaccine.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden RB, Lowy DR, Schiller JT. Papillomavirus is resistant to desiccation. J Infect Dis. 1997;176(4):1076–1079. doi: 10.1086/516515. [DOI] [PubMed] [Google Scholar]
- 5.Storment JM, Monga M, Blanco JD. Ineffectiveness of latex condoms in preventing contamination of the transvaginal ultrasound transducer head. South Med J. 1997;90(2):206–208. doi: 10.1097/00007611-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Rooks VJ, Yancey MK, Elg SA, Brueske L. Comparison of probe sheaths for endovaginal sonography. Obstet Gynecol. 1996;87(1):27–29. doi: 10.1016/0029-7844(95)00336-3. [DOI] [PubMed] [Google Scholar]
- 7.Egawa N, Egawa K, Griffin H, Doorbar J. Human Papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7(7):3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Publication BS. BS EN 14476:2013+A2:2019, Chemical disinfectants and antiseptics. quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements (Phase 2/Step 1) BSI Standards Publication. 2019 [Google Scholar]
- 9.Bryan JT, Brown DR. Association of the human papillomavirus type 11 E1E4 protein with cornified cell envelopes derived from infected genital epithelium. Virology. 2000;277(2):262–269. doi: 10.1006/viro.2000.0599. [DOI] [PubMed] [Google Scholar]
- 10.Doorbar J. The E4 protein; structure, function and patterns of expression. Virology. 2013;445(1-2):80–98. doi: 10.1016/j.virol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Ryndock E, Robison R, Meyers C. Susceptibility of HPV16 and 18 to high level disinfectants indicated for semi-critical ultrasound probes. J Med Virol. 2016;88(6):1076–1080. doi: 10.1002/jmv.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers J, Ryndock E, Conway MJ, Meyers C, Robison R. Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants. J Antimicrob Chemother. 2014;69(6):1546–1550. doi: 10.1093/jac/dku006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardone G, Moyer AL, Cheng N, Thompson CD, Dvoretzky I, Lowy DR. Maturation of the human papillomavirus 16 capsid. mBio. 2014;5(4):e01104–e01114. doi: 10.1128/mBio.01104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78(2):751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2(7):e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa N, Wang Q, Griffin HM, Murakami I, Jackson D, Mahmood R. HPV16 and 18 genome amplification show different E4-dependence, with 16E4 enhancing E1 nuclear accumulation and replicative efficiency via its cell cycle arrest and kinase activation functions. PLoS Pathog. 2017;13(3) doi: 10.1371/journal.ppat.1006282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores ER, Allen-Hoffmann BL, Lee D, Sattler CA, Lambert PF. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology. 1999;262(2):344–354. doi: 10.1006/viro.1999.9868. [DOI] [PubMed] [Google Scholar]
- 18.Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O'Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114(3):444–455. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 19.Cladel NM, Budgeon LR, Balogh KK, Cooper TK, Hu J, Christensen ND. A novel pre-clinical murine model to study the life cycle and progression of cervical and anal papillomavirus infections. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozbun MA, Patterson NA. Using organotypic (raft) epithelial tissue cultures for the biosynthesis and isolation of infectious human papillomaviruses. Curr Protoc Microbiol. 2014;34:1–8. doi: 10.1002/9780471729259.mc14b03s34. 14B 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozbun MA. Infectious human papillomavirus type 31b: purification and infection of an immortalized human keratinocyte cell line. J. Gen. Virol. 2002;83(Pt 11):2753–2763. doi: 10.1099/0022-1317-83-11-2753. [DOI] [PubMed] [Google Scholar]
- 22.Christensen ND, Dillner J, Eklund C, Carter JJ, Wipf GC, Reed CA. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology. 1996;223(1):174–184. doi: 10.1006/viro.1996.0466. [DOI] [PubMed] [Google Scholar]
- 23.Cladel NM, Jiang P, Li JJ, Peng X, Cooper TK, Majerciak V. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg Microbes Infect. 2019;8(1):1108–1121. doi: 10.1080/22221751.2019.1637072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figliozzi RW, Chen F, Chi A, Hsia SC. Using the inverse Poisson distribution to calculate multiplicity of infection and viral replication by a high-throughput fluorescent imaging system. Virol Sin. 2016;31(2):180–183. doi: 10.1007/s12250-015-3662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilal SH, Karickhoff SW, Carreira LA. A rigorous test for SPARC's chemical reactivity models: estimation of more than 4300 Ionization pKas. Quant Struct-Act Relat. 1995;14:348–355. [Google Scholar]
- 26.Bryan JT, Brown DR. Transmission of human papillomavirus type 11 infection by desquamated cornified cells. Virology. 2001;281(1):35–42. doi: 10.1006/viro.2000.0777. [DOI] [PubMed] [Google Scholar]
- 27.Griffin H, Wu Z, Marnane R, Dewar V, Molijn A, Quint W. E4 antibodies facilitate detection and type-assignment of active HPV infection in cervical disease. PLoS One. 2012;7(12):e49974. doi: 10.1371/journal.pone.0049974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin H, Soneji Y, Van Baars R, Arora R, Jenkins D, van de Sandt M. Stratification of HPV-induced cervical pathology using the virally encoded molecular marker E4 in combination with p16 or MCM. Mod Pathol. 2015;28(7):977–993. doi: 10.1038/modpathol.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway MJ, Cruz L, Alam S, Christensen ND, Meyers C. Differentiation-dependent interpentameric disulfide bond stabilizes native human papillomavirus type 16. PLoS One. 2011;6(7):e22427. doi: 10.1371/journal.pone.0022427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapp M, Fligge C, Petzak I, Harris JR, Streeck RE. Papillomavirus assembly requires trimerization of the major capsid protein by disulfides between two highly conserved cysteines. J Virol. 1998;72(7):6186–6189. doi: 10.1128/jvi.72.7.6186-6189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabler IM, Lazarovitch T, Haifler M, Lang E, Shapira G, Zelig S. Sterility of reusable transrectal ultrasound transducer assemblies for prostate biopsy reprocessed according to food and drug administration guidelines–bacteriologic outcomes in a clinical setup. Urology. 2011;77(1):17–19. doi: 10.1016/j.urology.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 32.Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys J. 2004;86(2):1234–1242. doi: 10.1016/S0006-3495(04)74197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozbun MA. Extracellular events impacting human papillomavirus infections: Epithelial wounding to cell signaling involved in virus entry. Papillomavirus Res. 2019;7:188–192. doi: 10.1016/j.pvr.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozbun M, Bondu V, Patterson N, Sterk R, Waxman A, Bennett E. Infectious Titres of Human Papillomaviruses (HPVs) in patient lesions, methodological considerations in evaluating HPV infectivity and implications for the efficacy of high-level disinfectants. E Bio Med. 2020 doi: 10.1016/j.ebiom.2020.103165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: (a) A representative image showing the appearance of early MmuPV1 lesions at 2-week post-inoculation of 2.0 × 108 VGE/site. Before the appearance of a palpable lesion (+), a change in skin colour was usually seen (-/+). (b) Histological images of an early lesion; H&E staining (HE), E6E7 viral transcripts (E6E7) and viral E4 and L1 proteins (E4 and L1). Scale bar; 100 μm
Supplementary Figure 2: (a) Exfoliating cells were collected from the surface of productive mouse papillomas using a plastic stick (fomite) made of acrylonitrile butadiene styrene, prior to inoculation onto the tail of another mouse after scarification. (b) After Benzonase digestion to remove non-encapsidated viral DNA, MmuPV1 on the fomite and at the site of inoculation was quantified (VGE/mm2; Y-axis).