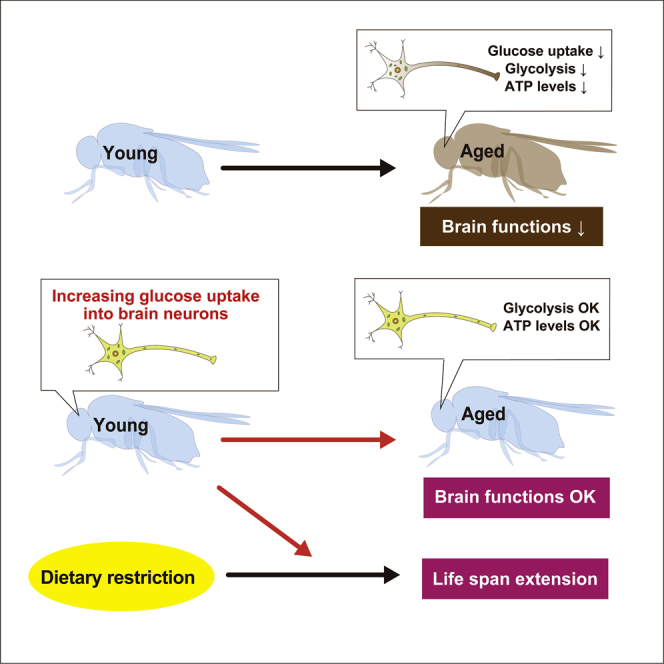
Summary
Brain neurons play a central role in organismal aging, but there is conflicting evidence about the role of neuronal glucose availability because glucose uptake and metabolism are associated with both aging and extended life span. Here, we analyzed metabolic changes in the brain neurons of Drosophila during aging. Using a genetically encoded fluorescent adenosine triphosphate (ATP) biosensor, we found decreased ATP concentration in the neuronal somata of aged flies, correlated with decreased glucose content, expression of glucose transporter and glycolytic enzymes and mitochondrial quality. The age-associated reduction in ATP concentration did not occur in brain neurons with suppressed glycolysis or enhanced glucose uptake, suggesting these pathways contribute to ATP reductions. Despite age-associated mitochondrial damage, increasing glucose uptake maintained ATP levels, suppressed locomotor deficits, and extended the life span. Increasing neuronal glucose uptake during dietary restriction resulted in the longest life spans, suggesting an additive effect of enhancing glucose availability during a bioenergetic challenge on aging.
Subject areas: Biological Sciences, Cellular Neuroscience, Endocrinology, Neuroscience, Physiology
Graphical Abstract
For a Figure360 author presentation of this figure, see https://doi.org/10.1016/j.isci.2020.101979
Highlights
-
•
Imaging of Drosophila brain reveals aged neurons suffer from energy deficits
-
•
Increased neuronal glucose uptake attenuates age-dependent declines in ATP
-
•
Increased glucose uptake is beneficial despite age-dependent mitochondrial damage
-
•
Increased neuronal glucose uptake and dietary restriction further extend life span
Biological Sciences; Cellular Neuroscience; Endocrinology; Neuroscience; Physiology
Introduction
Aging is associated with progressive declines in physiological integrity and functions alongside increases in vulnerability and the risk of developing a number of diseases (Kirkwood and Austad, 2000). The nervous system regulates motor and sensory functions and orchestrates metabolic control and stress responses in peripheral tissues via neuroendocrine signals (Alcedo and Kenyon, 2004; Burkewitz et al., 2015; Cohen et al., 2009; Libert et al., 2007; Satoh and Imai, 2014; Ulgherait et al., 2014; Zhang et al., 2018). Thus, age-associated changes in neurons are likely to play a critical role in organismal aging.
The brain requires a large amount of energy: it consumes about 20% of the oxygen and 25% of the glucose in the human body (Belanger et al., 2011; Mink et al., 1981; Raichle et al., 1970). Most of the adenosine triphosphate (ATP) required to support neuronal function is supplied by the metabolism of glucose through glycolysis and mitochondrial oxidative phosphorylation (Belanger et al., 2011). However, aging induces changes in both glucose availability and energy production, including declines in glucose uptake, electron transport chain activity, and aerobic glycolysis in the brain (Goyal et al., 2017; Hoyer, 2000). This implies that strategies aimed at increasing glucose metabolism in neurons may protect against organismal aging.
By contrast, dietary restriction (DR) and a reduction in insulin signaling have been demonstrated to have anti-aging effects (Guarente, 2008). DR causes circulating glucose concentrations to fall (Guarente, 2008). Thus, the pro-aging effects of reductions in brain glucose metabolism and the anti-aging effects of reducing insulin-stimulated glucose uptake are apparently contradictory. To resolve this discrepancy, it is necessary to elucidate how aging affects glucose metabolism in brain neurons and how such age-related changes in neurons interact with the anti-aging effects of DR.
In the present study, we show that aged brain neurons suffer from ATP deficits. Using a genetically encoded fluorescent ATP biosensor, we show that ATP concentrations decrease in the somas of brain neurons during aging due to a reduction in glycolysis or glucose availability. Increased neuronal glucose uptake by expression of a glucose transporter hGlut3 is sufficient to ameliorate age-dependent declines in ATP via glycolysis. Of particular interest, we demonstrate that increasing glucose uptake into neurons significantly extends the organismal life span under DR conditions, suggesting that increasing neuronal glucose metabolism optimizes the anti-aging effects of energetic challenges.
Results
An ATP biosensor (ATeam) identifies local changes in ATP levels in Drosophila brain neurons
Neurons are highly polarized structurally and functionally and require tight local management of ATP production (Rangaraju et al., 2014). To determine the local ATP concentration in fly brain neurons, we used the fluorescence resonance energy transfer (FRET)-based ATP biosensor, ATeam (Tsuyama et al., 2013). ATeam detects changes in ATP concentrations in the range of 0.5–4 mM at 25°C (Tsuyama et al., 2013, 2017), which is appropriate for the physiological ATP concentrations in neurons (Erecinska and Silver, 1989; Pathak et al., 2015; Rangaraju et al., 2014). The concentrations of intracellular ATP are much higher than the expression levels of ATeam; therefore, the FRET signal derived from ATeam is not likely to be affected by its intracellular distribution (Tsuyama et al., 2013, 2017).
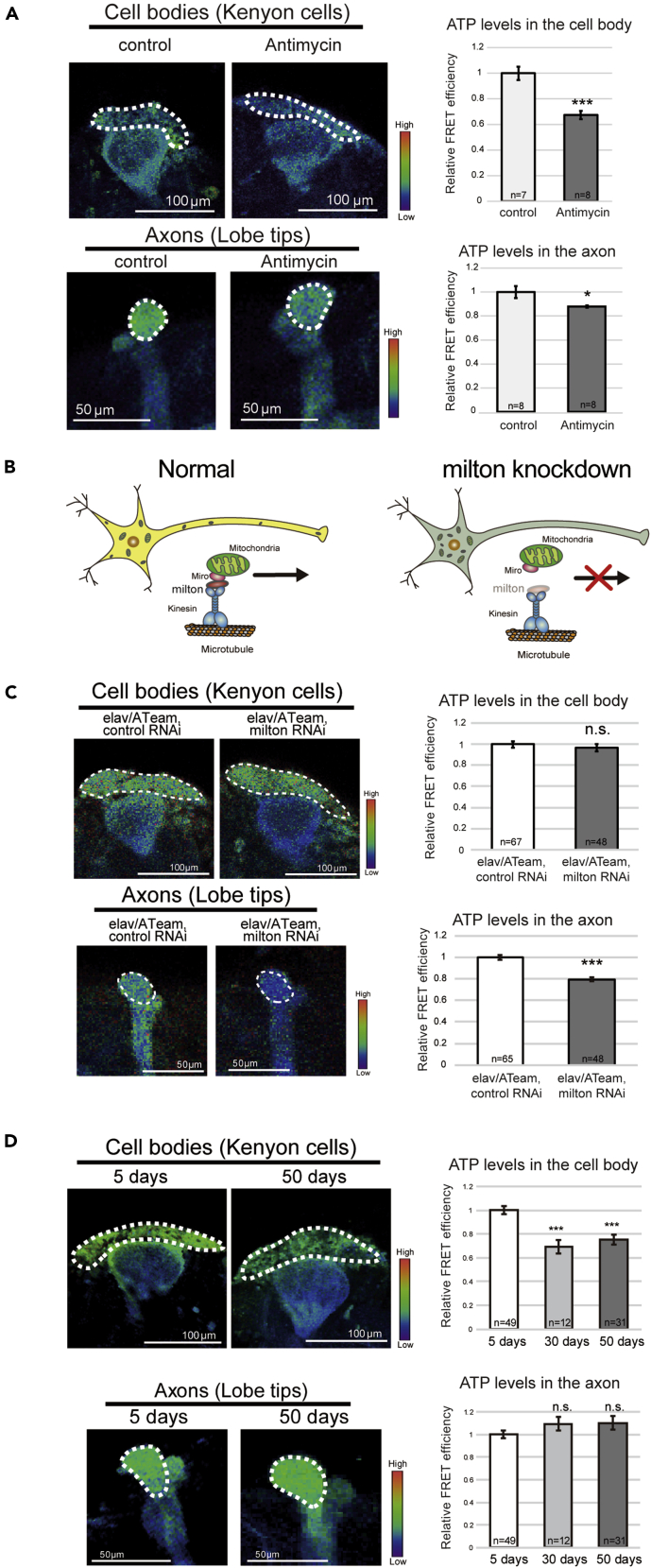
It has been reported that the ATeam signal decreases in response to the inhibition of cellular respiration in the neurons of Drosophila larvae (Tsuyama et al., 2013, 2017). To confirm that ATeam is capable of detecting changes in ATP concentration in adult fly brains, we used the Gal4-UAS system to express ATeam in neurons under the control of a pan-neuronal driver elav-GAL4 and inhibited respiratory complex III by treatment with antimycin (AM). We focused on the mushroom body, where axons, dendrites, and cell bodies are easily identifiable as lobes, calyxes, and Kenyon cells, respectively. AM treatment significantly reduced the FRET signal from ATeam in both the cell bodies and axons (Figure 1A), which indicates that ATeam can identify reductions in ATP concentrations in fly brain neurons.
Figure 1.
ATP concentration decreases in the cell body but not in the axon during aging
(A) A reduction in ATP concentration, caused by the inhibition of mitochondrial respiration by antimycin in adult fly brain neurons, was identified using ATeam. ATeam expression was driven by the pan-neuronal driver elav-GAL4. Quantification of the FRET signal (right panels) is shown (mean ± standard error [SE], n = 7–8; ∗p < 0.05, ∗∗∗p < 0.001; Student's t-test).
(B) Schematic representation of mitochondrial transport. The knockdown of milton, an adapter protein for mitochondrial transport, depleted mitochondria in the axon.
(C) Neuronal knockdown of milton reduced ATP concentration in Kenyon cells and lobe tips. Flies carrying the driver with control RNAi (elav/ATeam, control RNAi) were used as controls. Data are mean ± SE, n = 48–65; not significant (n.s.), p > 0.05, ∗∗∗p < 0.001; Student's t-test).
(D) The FRET efficiency of ATeam in Kenyon cells (A) and lobe tips (B) of the mushroom body of 5-, 30-, and 50-day-old flies (n = 12–49; n.s., p > 0.05, ∗∗∗p < 0.001; one-way analysis of variance (ANOVA), followed by Tukey's HSD multiple comparisons test). ATeam expression was driven in neurons by the pan-neuronal driver Elav-GAL4.
Next, we determined whether ATeam could identify local changes in ATP concentration. To reduce ATP concentration in the axons only, axonal mitochondria were genetically depleted by knocking down milton, an adapter protein for the kinesin motor that is essential for mitochondrial anterograde transport (Figure 1B) (Stowers et al., 2002). Previously, RNAi-mediated neuronal knockdown of milton was reported to effectively deplete mitochondria in axons (Iijima-Ando et al., 2012). Likewise, we found the FRET signal from ATeam decreased in axons but not in the cell bodies of milton-deficient fly brain neurons (Figure 1C). These results indicate that ATeam is capable of identifying local changes in ATP concentration in adult brain neurons.
ATP concentration decreases in the cell body but not in the axon of brain neurons during aging
To determine whether ATP concentration changes in brain neurons in an age-related manner, we compared the ATP concentrations in the mushroom body structure of young (5-day-old), middle-aged (30-day-old), and old (50-day-old) flies. We found that the ATP concentration in the cell bodies in the mushroom body structure of middle-aged (30-day-old) and old (50-day-old) flies was ~70% of that in young flies (5-day-old) (Figure 1D). By contrast, there were no significant changes in axonal ATP concentrations during aging (Figure 1D). ATeam detects ATP levels as cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) signal ratio, suggesting that the reduction in ATeam signal was not due to the loss of neurons. To confirm this, we carried out immunostaining of the neuronal marker elav and ultrastructural analyses of mushroom body region with electron microscopy. There were no vacuoles or signs of neuronal loss (Figure S1), indicating that the brains of 50-day-old flies were structurally intact, as previously reported (He and Jasper, 2014; Tonoki and Davis, 2015; White et al., 2010). Taken together, these results show that the ATP concentration, specifically in the cell bodies of brain neurons, decreases during aging.
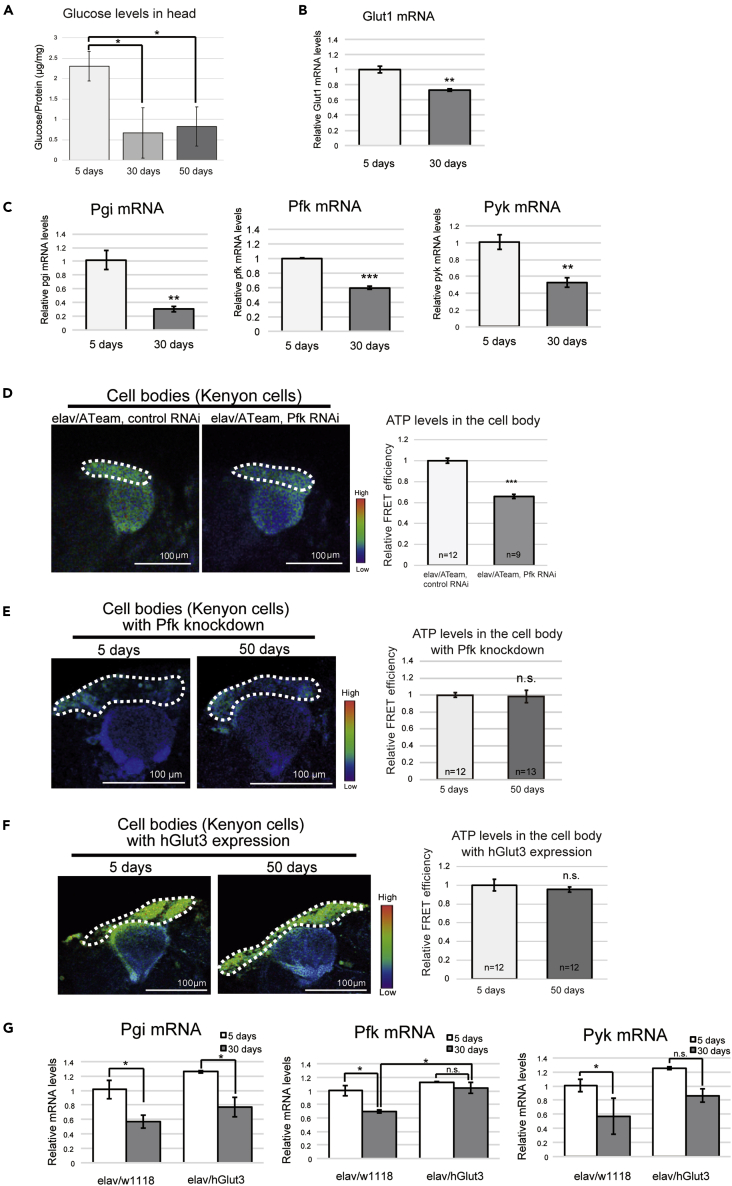
Decreases in glycolytic gene expression contribute to the age-related reduction in ATP
Most of the ATP produced in the brain is supplied by the metabolism of glucose (Belanger et al., 2011). To gain insight into the underlying reduced ATP concentrations, we measured glucose availability in the brain decreases during aging in Drosophila. We found that the glucose concentration in head extracts was significantly lower in aged flies (30- and 50-day-old) than in young (5-day-old) flies (Figure 2A). Glucose uptake by brain neurons is mostly constitutive because the glucose transporter isoforms expressed in neurons in the central nervous system (CNS), Glut1 and Glut3 in mammals and Glut1 in Drosophila, are constitutively localized to the plasma membrane and insensitive to insulin (Chintapalli et al., 2007; Flier et al., 1987). We measured dGlut1 mRNA expression during aging using real-time quantitative PCR (qRT-PCR), which revealed that its expression was slightly but significantly lower in the heads of aged than in those of young flies (Figure 2B).
Figure 2.
Neuronal expression of the glucose transporter hGlut3 attenuates the age-related reduction in ATP concentration in brain neuron and glycolytic gene expression
(A) Glucose concentration in head lysates decreased during aging. The glucose concentrations in 5-, 30- and 50-day-old fly heads, normalized to protein concentration, are shown (mean ± SE, n = 6; ∗p < 0.05; one-way ANOVA, followed by Tukey's HSD multiple comparisons test).
(B) dGlut1 mRNA expression decreased in fly heads during aging. qRT-PCR was performed using RNA extracted from the heads of 5- and 30-day-old flies (mean ± SE, n = 3; ∗∗p < 0.01; Student's t-test).
(C) The expression of glycolytic genes decreased in the fly brain during aging. The mRNA expression of genes encoding glycolytic enzymes in the heads of 5- and 30-day-old flies was quantified using qRT-PCR (mean ± SE, n = 3; ∗∗p < 0.01, ∗∗∗p < 0.001; Student's t-test).
(D) Neuronal knockdown of Pfk, which encodes a rate-limiting glycolytic enzyme, reduced ATP concentration in 3-day-old fly neurons (mean ± SE, n = 9–12; ∗∗∗p < 0.001; Student's t-test).
(E) The age-related reduction in ATP concentration in neuronal cell bodies was not identified in Pfk-deficient neurons. The expression of ATeam and Pfk RNAi was driven by the pan-neuronal driver elav-GAL4. The 5-day-old and 50-day-old flies were subjected to imaging analyzes at the same time. The experiments were repeated with timely independent cohorts of flies obtained from different crosses to analyze more than 12 brains for each age. Data are mean ± SE; n.s., p > 0.05, ∗p < 0.05; Student's t-test.
(F and G) Neuronal expression of hGlut3 ameliorates the age-related reduction in ATP concentration and glycolytic gene expression and the decline in physical function. (F) ATP concentration did not decrease with aging in neurons overexpressing hGlut3. Expression of ATeam and hGlut3 was driven by the pan-neuronal driver elav-GAL4. The 5-day-old and 50-day-old flies were subjected to imaging analyzes at the same time. The experiments were repeated with timely independent cohorts of flies obtained from different crosses to analyze more than 12 brains for each age. (mean ± SE; n.s., p > 0.05; Student's t-test). (G) Neuronal expression of hGlut3 attenuated the age-associated decline in glycolytic gene expression. The mRNA expression of genes encoding key glycolytic enzymes was quantified using qRT-PCR in the heads of 5- and 30-day-old flies. Flies carrying the driver alone were used as controls. Data are mean ± SE, n = 3; n.s., p > 0.05, ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA, followed by Tukey's HSD multiple comparisons test.
In human brains, the rate of glycolysis changes during aging (Goyal et al., 2017). To determine the contribution of changes in glycolytic rate to the age-related reduction in ATP in the cell bodies of Drosophila brain neurons, we measured the expression of genes encoding the key glycolytic enzymes in young and aged fly heads including glucose-6-phosphate isomerase (Pgi), phosphofructokinase (Pfk), and pyruvate kinase (Pyk). qRT-PCR analysis revealed that the mRNA expression of Pgi, Pfk, and Pyk was significantly lower in aged flies than in young flies (Figure 2C). To evaluate the contribution of glycolysis to ATP production in brain neurons, we knocked down Pfk, which encodes phosphofructokinase, a rate-limiting enzyme in glycolysis. Neuronal knockdown of Pfk reduced ATP concentration by up to 65% in 3-day-old flies (Figure 2D), indicating that glycolysis plays a critical role in ATP production in brain neurons. To determine whether the decline in glycolytic activity contributes to the age-related decrease in ATP concentration, we measured the ATeam signal in Pfk-deficient young and aged brain neurons. The neurons of 5-day-old flies with Pfk knockdown showed a reduced ATP concentration, with no further reduction in 50-day-old flies (Figure 2E). This result suggests that a lower rate of glycolysis and/or events upstream of glycolysis are responsible for the age-related reduction in ATP in cell bodies of brain neurons.
Neuronal expression of the glucose transporter hGlut3 attenuates the age-related reduction in ATP concentration in brain neurons and glycolytic gene expression
We next asked whether increasing glucose uptake could ameliorate the age-related reduction in ATP in the brain neurons. Expression of hGlut3 in Drosophila cells has been reported to increase glucose uptake (Besson et al., 2015). We found that neuronal expression of hGlut3 ameliorated the age-related reduction in ATP in the neuronal cell bodies (Figure 2F). By contrast, neuronal expression of hGlut3 did not increase the ATP concentration in young flies (Figure S2). These results suggest that glucose availability is the rate-limiting factor specifically in the brain neurons of old flies and that overexpression of glucose transporter can increase ATP production in the neurons of aged flies.
We also examined whether glucose transporter overexpression attenuates the age-related changes in glycolytic activity. We found that neuronal expression of hGlut3 suppressed the age-related decrease in the mRNA expression of the glycolytic gene, Pfk (Figure 2G). Taken together, these results suggest that neuronal overexpression of hGlut3 ameliorates age-related declines in ATP concentration by increasing glucose metabolism in aged fly neurons.
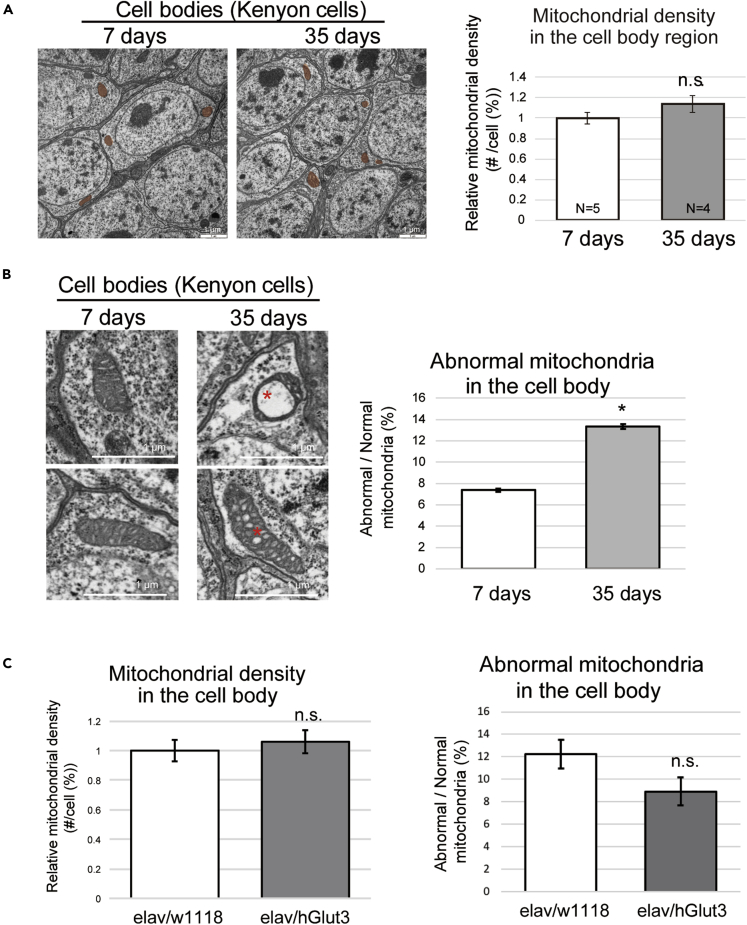
The age-related increase in abnormal mitochondria is not reversed by overexpression of glucose transporter in neurons
Since mitochondrial dysfunction is implicated in aged brains, we examined whether the reduced number and increased damage to mitochondrial was associated with an age-related reduction in ATP levels in brain neurons. We first measured the mitochondrial density in the brain neurons of young and aged flies by transmission electron microscopy. In the cell body, where ATP concentrations decrease (Figure 1D), the number of mitochondria did not change significantly during aging (Figure 3A). Thus, the age-related reduction in ATP in the cell bodies of neurons was not the result of a decrease in the number of mitochondria.
Figure 3.
Age-related changes in the number and quality of mitochondria in brain neurons
(A) The number of mitochondria in the neuronal cell body did not significantly change during aging. More than four brain hemispheres from flies of each age were analyzed using transmission electron microscopy (TEM), and the numbers of mitochondria in more than 30 cells in each hemisphere were counted. Representative images are shown, with the mitochondria highlighted in orange.
(B) The ratio of the number of abnormal mitochondria to the total number of mitochondria increased in the cell body during aging. More than four brain hemispheres from flies of each age were examined using TEM, and more than 30 cells were analyzed in each hemisphere. The number of mitochondria containing vacuoles or disorganized cristae (asterisks) was counted. (mean ± SE, n = 4–5; n.s., p > 0.05, ∗p < 0.05; Student's t-test).
(C) Neuronal overexpression of hGlut3 did not affect the age-associated changes in mitochondria in the brain. The mitochondrial density (left) and the ratio of the number of abnormal mitochondria to the total number of mitochondria (right) in neurons expressing hGlut3 in the Kenyon cell region of the brains were analyzed under TEM 50 days after eclosion (mean ± SE, n = 4–6; n.s., p > 0.05; Student's t-test).
Next, we analyzed the quality of mitochondria in the brain neurons of young and aged flies. Dysfunctional mitochondria are recognized by the abnormal structure of their cristae (Brandt et al., 2017; Daum et al., 2013). Ultrastructural analyses of the mitochondria revealed that the number of abnormal mitochondria in cell bodies slightly but significantly increased during aging (7% in 7-day-old flies and 13% in 35-day-old flies, Figure 3B). By contrast, in the axon, where ATP concentration does not decrease (Figure 1D), the number of mitochondria decreased slightly, but the number of abnormal mitochondria did not significantly change during aging (Figure S3), which might be because of the presence of a robust quality control system in this location (Lin et al., 2017). These results indicate that a slight but significant increase in the number of damaged mitochondria occurs alongside a decrease in ATP concentration in the cell bodies of neurons during aging.
Interestingly, while neuronal overexpression of hGlut3 ameliorated the age-related reduction in ATP in the neuronal cell bodies (Figure 2F), it neither increased the number of total mitochondria nor reduced the ratio of abnormal mitochondria (Figure 3C). These results suggest that increasing glucose uptake is sufficient to ameliorate energetic deficits in aged brain neurons.
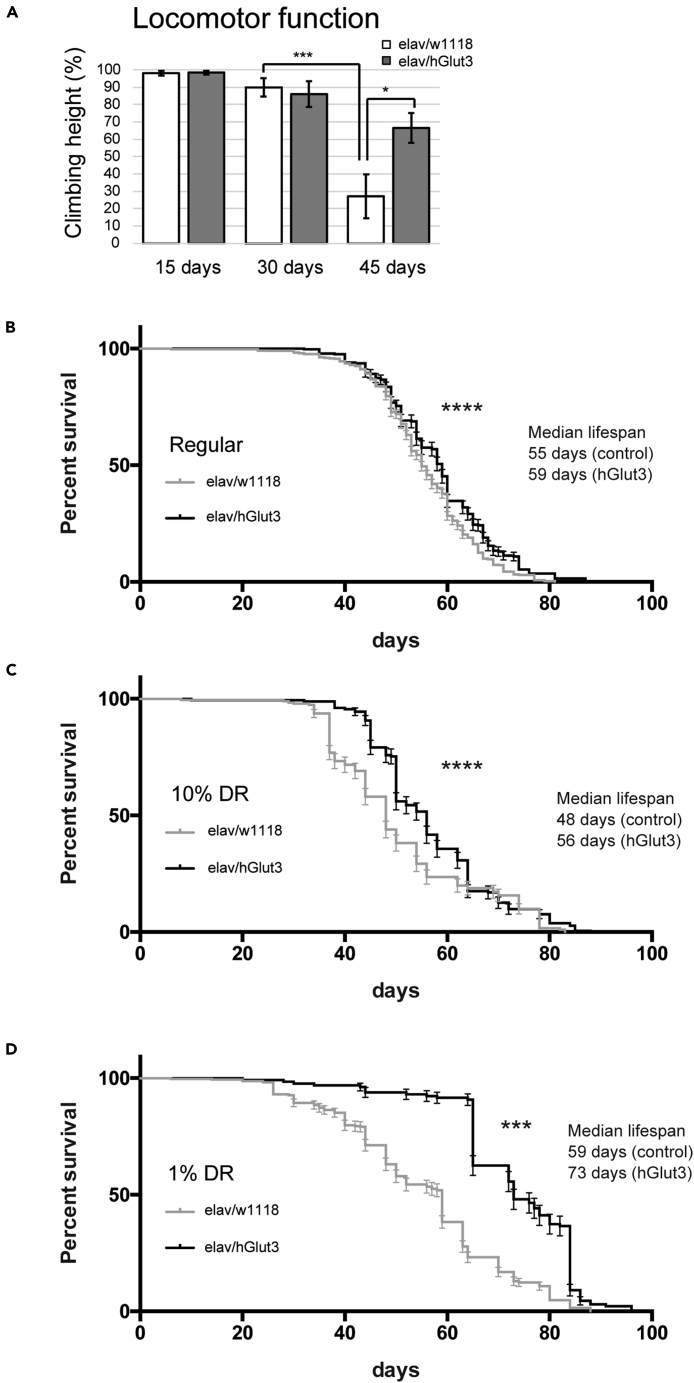
Increasing neuronal glucose uptake ameliorates declines in physical performance in aged flies and has an additive effect with DR in extending life span
Finally, we examined whether the increase in glucose uptake into neurons promoted the health and life span of flies. We first analyzed the effects of neuronal overexpression of hGlut3 on the age-related decline in physical function using a climbing assay, a robust behavioral assay taking advantage of the innate negative geotaxis behavior. In wild-type flies, climbing ability declined by 45 days of age, whereas flies with neuronal expression of hGlut3 performed significantly better at this age (Figure 4A), indicating that the increase in glucose uptake into neurons promoted health span in flies. We also found that flies with neuronal expression of hGlut3 lived slightly but significantly longer than control flies (Figure 4B).
Figure 4.
Increasing neuronal glucose uptake attenuates declines in physical performance in aged flies and has an additive effect with dietary restriction (DR) in extending life span
(A) Neuronal expression of hGlut3 ameliorated the age-associated neuronal dysfunction. Flies were tapped to the bottom of the vial, and the distance that they then climbed in 30 s was measured (mean ± SE, n = 12–39; ∗p < 0.05, ∗∗∗p < 0.001; Student's t-test). Flies carrying the driver alone were used as controls.
(B–D) Neuronal expression of hGlut3 and DR synergistically extended life span. Flies were raised on regular cornmeal food and maintained on regular cornmeal food (B) or two types of DR diet (C and D) (C: food without cornmeal and containing 10% (w/v) of yeast and glucose (10%), D: food without cornmeal and containing 1% (w/v) of yeast and glucose (1%) after eclosion. Error bars represent 95% confidence intervals. Data are expressed as mean ± SE, n = 131-601, ∗∗∗; p < 0.001, ∗∗∗∗; p < 0.0001, log-rank test.
DR has been reported to extend the life span in most species, including Drosophila (Bauer et al., 2005). Since DR causes circulating glucose concentrations to fall (Guarente, 2008), the anti-aging effects of DR and increasing in glucose uptake into neurons might be independent and additive. To determine the relationship between neuronal uptake of glucose and DR in aging, we analyzed the effects of neuronal expression of hGlut3 on life span under various dietary conditions. Flies were raised on regular cornmeal food, and after eclosion, they were fed regular food, DR food (10%) (10% yeast, 10% glucose, no cornmeal), or DR food (1%) (1% yeast, 1% glucose, no cornmeal). Flies on DR food showed reduced levels of glucose in the head and body with greater effects seen with 1% food than 10% food (Figure S4A). The life span was significantly extended on 1% food (Figure S4B), and life span extension by neuronal expression of hGlut3 was more prominent under DR conditions. While the median life span on regular food was 55 days in control and 59 days in hGlut3-expressing flies maintained on regular food (Figure 4B), on 1% food, the median life span was 59 days in control and 73 days in hGlut3-expressing flies (Figure 4D). Maintaining flies on 10% food did not affect the life span of control flies, but it extended the life span of hGlut3-expressing flies (median life span 48 days in control and 56 days in hGlut3-expressing flies (Figure 4C)).
We also analyzed glucose levels after hGlut3 expression in the aged flies under normal and DR conditions. We found that hGlut3 expression in neurons did not restore glucose levels in aged flies under normal or DR conditions (Figure S5). This result suggests that life span extension caused by neuronal expression of hGlut3 is not due to gross changes in total levels of glucose in the brain but is associated with efficient uptake of glucose and its utilization in neurons. When glucose availability is limited under DR conditions, efficiency of glucose utilization would have more impact than under normal conditions. Enhancement of glucose uptake by overexpression of hGlut3 may, therefore, be prominent under DR conditions. Taken together, these results suggest that increased glucose uptake by neurons enhances the anti-aging effects of DR at the organismal level.
Discussion
In the present study, we showed that aged brain neurons suffer from energy deficits, along with decreases in glucose concentration, the expression levels of glucose transporter and glycolytic enzymes (Figures 1 and 2), and mitochondrial quality (Figure 3). Increasing glucose uptake by neurons was sufficient to attenuate energetic deficits (Figure 2) despite persistent mitochondrial damage (Figure 3) and suppressed age-dependent locomotor decline (Figure 4). Interestingly, the effects of increasing glucose uptake on life span extension were more prominent under DR (Figures 4B–4D), suggesting that increasing neuronal uptake of glucose in concert with bioenergetic challenges could benefit health and life span in flies.
During aging, the ATP supply to brain neurons decreases because of global reductions in glucose metabolism, including in oxygen consumption, glycolysis, and mitochondrial respiratory capacity (Conley et al., 2000; Goyal et al., 2017; Jang et al., 2018; Kety, 1956; Martin et al., 1991). Our results suggest that glucose uptake was a limiting factor for ATP supply in aged brain neurons. An increase in glucose uptake attenuated not only the reduction in neuronal ATP concentration during aging but also the age-related reduction in the expression of glycolytic enzymes (Figure 2). In mammalian cells, it has been reported that an elevated concentration of intracellular glucose activates hypoxia-inducible factor-1α (Guo et al., 2008), which promotes the expression of glycolytic enzymes (Lum et al., 2007). An increase in glucose uptake did not suppress the age-related reduction in mitochondrial quality (Figure 3), suggesting that enhanced glucose supply can produce sufficient ATP despite mitochondrial damage to produce sufficient ATP in vivo.
DR is known to extend life span in many species, but it may reduce glucose supply to the brain and worsen energy deficits in neurons. We used DR conditions that are similar to Bauer et al. (Bauer et al., 2005), under which both the levels of sugar and yeast are reduced. Unlike Bauer et al., we saw an initial increase in mortality followed by a prolongation of life span (Figure S4), although the extension of life span was less than that observed in previous works (Bauer et al., 2005; Bross et al., 2005). This difference may be due to different laboratory media used to maintain stocks. The food used in our facility is relatively rich in yeast and cornmeal (4% yeast, 10% glucose, 9% cornmeal) compared with that used by Bauer et al. (2% yeast, 10% sucrose, 5% cornmeal); this difference may have affected the response to DR in the studied offspring.
Our results suggest that increased glucose uptake by neurons further improves the anti-aging effects of DR, presumably by counteracting neuronal energy deficits under DR conditions (Figure 4). Lower extracellular glucose caused by DR may recapitulate some of the conditions observed in the aged brain, in which lower cerebral blood flow limits glucose supply (Goyal et al., 2017; Hoyer, 2000; Noda et al., 2002). In Drosophila, life span extension by DR is mostly modulated by dietary yeast (Grandison et al., 2009; Lee et al., 2008; Mair et al., 2005): DR in Drosophila research is often performed simply by reducing the amount of yeast. In this study, both sugar and yeast were reduced in the DR condition, which caused a reduction in overall glucose availability (Figure S4). One might suspect that a reduction in sugar would have a negative effect on survival and hGlut3 overexpression simply restored glucose availability. However, the glucose content in the head was not restored by neuronal expression of hGlut3 (Figure S5), suggesting this was not the case. Instead, our results suggest that enhancing neuronal glucose uptake had more impact under conditions in which glucose availability is limited. In support of this, a previous study that did not use DR showed no effect of neuronal expression of dGlut1 or hGlut3 on life span (Besson et al., 2015; Niccoli et al., 2016).
The anti-aging effects of DR are reported to be mediated by a reduction in the intake of protein or amino acids (Mirzaei et al., 2014). Amino acid restriction affects life span through effects on amino acid sensing pathways, such as mechanistic target of rapamycin complex 1 and the integrated stress response (Mirzaei et al., 2014). The additive effects of DR and neuronal expression of hGlut3 on life span may be explained by the respective peripheral activation of anti-aging pathways by DR and increased neuronal glucose uptake and metabolism. Thus, a strategy aimed at both increasing glucose uptake by neurons and restricting amino acid intake may be effective at reducing the effects of aging.
In summary, we have shown that glucose uptake by brain neurons is a critical regulator of organismal and brain aging. Further elucidation of the cross-talk among nutrient signaling pathways in the central nervous system and peripheral tissues during aging will lead to the identification of novel strategies for the extension of the healthy life span of an organism.
Limitations of the study
We demonstrated that increased glucose uptake in neurons further extends life span under a DR condition. We used a DR protocol with a reduction in total calories. Since it has been reported that specific nutrients and their relative ratios play roles in their effects on life span extension (Mirzaei et al., 2014), it would be interesting to test the effects of neuronal expression of hGlut3 under other dietary conditions, such as limiting specific nutrients. In this study, we expressed hGlut3 in all neurons using a pan-neuronal elav-Gal4 driver and did not analyze the effect of increasing glucose uptake in specific types of neurons, glial cells, or in the other non-neuronal tissues on life span. Such experiments will elucidate cell type- and tissue-specific roles of glucose metabolism in aging and life span. We showed that increasing neuronal glucose uptake counteracts their age-dependent reduction in pfk expression, while the mechanism by which increasing intracellular glucose affects glycolytic gene expression remains unknown. Our analysis on the age-dependent change in mitochondrial quality was limited to the ultrastructural analysis, and mitochondrial functions were not analyzed. We hope to address these issues in the future for better understanding of the multifactorial processes of aging.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kanae Ando (k_ando@tmu.ac.jp).
Material availability
This study did not generate new materials.
Data and code availability
This study did not generate data sets or analyze codes.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgment
The authors thank Dr. Hiromi Imamura from the Graduate School of Biostudies, Kyoto University, for the ATP biosensor transgenic fly line and discussions. The authors thank Dr. Marie Thérèse Besson, Aix-Marseille Université, for UAS-hGlut3 fly line. The authors are grateful to Dr. Shin-ichi Hisanaga from the Department of Biological Sciences, Tokyo Metropolitan University, for critical comments. The authors thank Tetsuya Miyashita for technical support. This work was supported by a research award from the Japan Foundation for Aging and Health (to K.A) and a Grant-in-Aid for Scientific Research on Challenging Research (Exploratory) [JSPS KAKENHI Grant number 19K21593] (to K.A.), NIG-JOINT (71A2018, 25A2019) (to K.A.), and [JSPS Grant-in-Aid for JSPS Research Fellow 18J21936] (to M.O.), and the Research Funding for Longevity Science 19-7 from the National Center for Geriatrics and Gerontology, Japan (to K.M.I.).
Author contribution
M.O., K.M.I., and K.A. designed the experiments, interpreted the data, and wrote the manuscript; M.O., A.A., T.S., and E.S. conducted the experiments; and M.O., E.S., and K.A. analyzed the data. All authors read and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2020.101979.
Supplemental information
References
- Alcedo J., Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- Bauer J.H., Poon P.C., Glatt-Deeley H., Abrams J.M., Helfand S.L. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr. Biol. 2005;15:2063–2068. doi: 10.1016/j.cub.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Besson M.T., Alegria K., Garrido-Gerter P., Barros L.F., Lievens J.C. Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD Drosophila model. PLoS One. 2015;10:e0118765. doi: 10.1371/journal.pone.0118765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt T., Mourier A., Tain L.S., Partridge L., Larsson N.G., Kuhlbrandt W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife. 2017;6:e24662. doi: 10.7554/eLife.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross T.G., Rogina B., Helfand S.L. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell. 2005;4:309–317. doi: 10.1111/j.1474-9726.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Burkewitz K., Morantte I., Weir H.J.M., Yeo R., Zhang Y., Huynh F.K., Ilkayeva O.R., Hirschey M.D., Grant A.R., Mair W.B. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell. 2015;160:842–855. doi: 10.1016/j.cell.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V.R., Wang J., Dow J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Cohen D.E., Supinski A.M., Bonkowski M.S., Donmez G., Guarente L.P. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley K.E., Jubrias S.A., Esselman P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum B., Walter A., Horst A., Osiewacz H.D., Kuhlbrandt W. Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc. Natl. Acad. Sci. U S A. 2013;110:15301–15306. doi: 10.1073/pnas.1305462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M., Silver I.A. ATP and brain function. J. Cereb. Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Flier J.S., Mueckler M.M., Usher P., Lodish H.F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Goyal M.S., Vlassenko A.G., Blazey T.M., Su Y., Couture L.E., Durbin T.J., Bateman R.J., Benzinger T.L., Morris J.C., Raichle M.E. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26:353–360.e3. doi: 10.1016/j.cmet.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison R.C., Piper M.D., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Bragina O., Xu Y., Cao Z., Chen H., Zhou B., Morgan M., Lin Y., Jiang B.H., Liu K.J. Glucose up-regulates HIF-1 alpha expression in primary cortical neurons in response to hypoxia through maintaining cellular redox status. J. Neurochem. 2008;105:1849–1860. doi: 10.1111/j.1471-4159.2008.05287.x. [DOI] [PubMed] [Google Scholar]
- He Y., Jasper H. Studying aging in Drosophila. Methods. 2014;68:129–133. doi: 10.1016/j.ymeth.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update. Exp. Gerontol. 2000;35:1363–1372. doi: 10.1016/s0531-5565(00)00156-x. [DOI] [PubMed] [Google Scholar]
- Iijima-Ando K., Sekiya M., Maruko-Otake A., Ohtake Y., Suzuki E., Lu B., Iijima K.M. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1. PLoS Genet. 2012;8:e1002918. doi: 10.1371/journal.pgen.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.Y., Blum A., Liu J., Finkel T. The role of mitochondria in aging. J. Clin. Invest. 2018;128:3662–3670. doi: 10.1172/JCI120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S.S. Human cerebral blood flow and oxygen consumption as related to aging. J. Chronic Dis. 1956;3:478–486. doi: 10.1016/0021-9681(56)90146-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B., Austad S.N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W., Taylor P.W., Soran N., Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S., Zwiener J., Chu X., Vanvoorhies W., Roman G., Pletcher S.D. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lin M.Y., Cheng X.T., Tammineni P., Xie Y., Zhou B., Cai Q., Sheng Z.H. Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron. 2017;94:595–610 e596. doi: 10.1016/j.neuron.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J.J., Bui T., Gruber M., Gordan J.D., DeBerardinis R.J., Covello K.L., Simon M.C., Thompson C.B. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W., Piper M.D., Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A.J., Friston K.J., Colebatch J.G., Frackowiak R.S. Decreases in regional cerebral blood flow with normal aging. J. Cereb. Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- Mink J.W., Blumenschine R.J., Adams D.B. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- Mirzaei H., Suarez J.A., Longo V.D. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 2014;25:558–566. doi: 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T., Cabecinha M., Tillmann A., Kerr F., Wong C.T., Cardenes D., Vincent A.J., Bettedi L., Li L., Gronke S. Increased glucose transport into neurons rescues abeta toxicity in Drosophila. Curr. Biol. 2016;26:2291–2300. doi: 10.1016/j.cub.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A., Ohba H., Kakiuchi T., Futatsubashi M., Tsukada H., Nishimura S. Age-related changes in cerebral blood flow and glucose metabolism in conscious rhesus monkeys. Brain Res. 2002;936:76–81. doi: 10.1016/s0006-8993(02)02558-1. [DOI] [PubMed] [Google Scholar]
- Pathak D., Shields L.Y., Mendelsohn B.A., Haddad D., Lin W., Gerencser A.A., Kim H., Brand M.D., Edwards R.H., Nakamura K. The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem. 2015;290:22325–22336. doi: 10.1074/jbc.M115.656405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Posner J.B., Plum F. Cerebral blood flow during and after hyperventilation. Arch. Neurol. 1970;23:394–403. doi: 10.1001/archneur.1970.00480290014002. [DOI] [PubMed] [Google Scholar]
- Rangaraju V., Calloway N., Ryan T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A., Imai S. Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat. Commun. 2014;5:4211. doi: 10.1038/ncomms5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R.S., Megeath L.J., Gorska-Andrzejak J., Meinertzhagen I.A., Schwarz T.L. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Tonoki A., Davis R.L. Aging impairs protein-synthesis-dependent long-term memory in Drosophila. J. Neurosci. 2015;35:1173–1180. doi: 10.1523/JNEUROSCI.0978-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyama T., Kishikawa J., Han Y.W., Harada Y., Tsubouchi A., Noji H., Kakizuka A., Yokoyama K., Uemura T., Imamura H. In vivo fluorescent adenosine 5'-triphosphate (ATP) imaging of Drosophila melanogaster and Caenorhabditis elegans by using a genetically encoded fluorescent ATP biosensor optimized for low temperatures. Anal. Chem. 2013;85:7889–7896. doi: 10.1021/ac4015325. [DOI] [PubMed] [Google Scholar]
- Tsuyama T., Tsubouchi A., Usui T., Imamura H., Uemura T. Mitochondrial dysfunction induces dendritic loss via eIF2alpha phosphorylation. J. Cell Biol. 2017;216:815–834. doi: 10.1083/jcb.201604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M., Rana A., Rera M., Graniel J., Walker D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K.E., Humphrey D.M., Hirth F. The dopaminergic system in the aging brain of Drosophila. Front. Neurosci. 2010;4:205. doi: 10.3389/fnins.2010.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Gong J., Zhang W., Xiao R., Liu J., Xu X.Z.S. Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev. 2018;32:258–270. doi: 10.1101/gad.309625.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate data sets or analyze codes.