Abstract
Over the last years, the potential use of Black Soldier Fly meal (BSF) as a new and sustainable aquafeed ingredient has been largely explored in several fish species. However, only fragmentary information is available about the use of BSF meal-based diets in sturgeon nutrition. In consideration of a circular economy concept and a more sustainable aquaculture development, the present research represents the first comprehensive multidisciplinary study on the physiological effects of a BSF diet during sturgeon culture in an aquaponic system. Siberian sturgeon (Acipenser baerii) juveniles were fed over a 60-days feeding trial on a control diet (Hi0) and a diet containing 50% of full-fat BSF meal respect to fish meal (Hi50). Physiological responses of fish were investigated using several analytical approaches, such as gas chromatography-mass spectrometry, histology, Fourier Transformed Infrared Spectroscopy (FTIR), microbiome sequencing and Real-time PCR. While aquaponic systems performed optimally during the trial, Hi50 group fish showed lower diet acceptance that resulted in growth and survival reduction, a decrease in hepatic lipids and glycogen content (FTIR), a higher hepatic hsp70.1 gene expression and a worsening in gut histological morphometric parameters. The low feed acceptance showed by Hi50 group sturgeon highlighted the necessity to improve the palatability of BSF-based diet designed for sturgeon culture.
Subject terms: Metabolism, Reverse transcription polymerase chain reaction, Animal physiology
Introduction
Siberian sturgeon (Acipenser baerii) is one of the most valuable species in aquaculture, due to the production of caviar and high-quality meat for human consumption. Restocking programs are also of great interest for this species since it is included in the list of endangered wild populations1. Compared to other sturgeon species, Acipenser baerii shows rapid growth rate, resistance to pathogens, a relatively short reproductive cycle (7–8 years) and can be reared using a wide range of diets and environmental conditions2,3. Owing to its features and high commercial value, intensive aquaculture of Siberian sturgeon is presently carried out in different parts of the world and, in recent years, several studies regarding farming-related biology4 and nutritional requirements1,5 have been performed. To ensure proper growth and constant productivity, aquafeeds commonly used in sturgeon’s rearing are mainly represented by high-energy diets largely based on fish meal (FM) and fish oil (FO) to meet proper protein and n3 highly unsaturated fatty acids requirement6,7. However, for a more sustainable aquaculture development, the use of FM and FO should be limited for both ecological and economic reasons8. For this reason, the discovery of novel nutritious and more sustainable ingredients for aquafeeds formulation is crucial9.
Over the last years, several ingredients have been evaluated as FM substitutes in sturgeon aquaculture, ranging from vegetable ones like soybean meal5, rice concentrate10, sesame oil cake, corn gluten11, and spirulina microalgae12 to animal ones such as poultry by-products7. Nowadays, with the goal of a further reduction of aquaculture’s environmental footprint, insect species like the Black Soldier Fly (Hermetia illucens; BSF) represent very promising candidates as FM alternatives13. The great interest in the BSF meal as aquafeed ingredient is due to their eco-friendly rearing in terms of land use, water consumption, CO2 emissions and on high feed conversion efficiency (BSF larvae are able to grow on low value organic by-products converting them into valuable biomass)14,15. Furthermore, insects like Diptera and Coleoptera are part of the natural diet of Siberian sturgeon16. It is well known that these insects possess bioactive compounds like chitin, which at certain concentrations are able to boost the fish immune system and promote gut microbiota diversification17,18. From the nutritional point of view, BSF larvae have a suitable protein content and the amino acid composition is similar to that of FM19. However, BSF meal fatty acid profile has also some disadvantages, such as a high content of saturated fatty acids (SFA) and an extremely low content in polyunsaturated (PUFA) ones20. PUFA are particularly important for fish since deficiencies in these compounds may cause a general deterioration of fish health, poor growth, low feed efficiency and often high mortality21–23. Previous studies demonstrated that a proper PUFA dietary content is essential to sustain both larval and adult Siberian sturgeon growth and welfare24,25; these compounds play a pivotal role in sturgeon’s fillet and caviar quality26.
Some recent studies tested different defatted BSF meal inclusion levels in aquafeed formulation for several fish species, but results on fish physiological responses are still controversial27–30. This topic, however, has scarcely been investigated in sturgeon aquaculture and most of the results are limited to zootechnical analyses31–33. Nowadays, several laboratory approaches (histology, molecular biology, gas chromatography and infra-red spectroscopy) are available to evaluate fish welfare and quality and represent valid tools to assess the inclusion of new ingredients, like insect meal, in aquafeed production23,34–36. In addition, the use of full-fat BSF meal is preferable to the highly defatted in order to reduce manufacturing costs37,38. This aspect has been recently addressed by Truzzi et al.39. These authors developed an enrichment procedure to increase insects’ PUFA content that allowed to include up to 50% of BSF prepupae meal compared to FM in zebrafish diet without impairing fish growth and welfare38. Because of this positive result, this same enriched full-fat BSF dietary inclusion percentage was chosen for the present study, expecting to obtain more promising results respect to Caimi et al.32 that evidenced a significant reduction of feed consumption and growth performance in Siberian sturgeon juveniles fed on a diet in which FM was 50% replaced with highly defatted BSF larvae meal.
In the present study, Siberian sturgeon juveniles were fed over a 60-days feeding trial on a control diet (based on FM and FO; Hi0) and a diet containing 50% of enriched BSF meal (according to Truzzi et al.)39 respect to FM (Hi50). Results obtained on zootechnical performances, fillet fatty acid composition, liver and gut integrity, expression of genes involved in fish growth, stress and immune response and gut microbiome represent the first multidisciplinary investigation on the physiological effects of BSF-based diets in sturgeon juveniles. Furthermore, this is the first feeding trial using insect-based diets performed in an aquaponics system. This green technology combines aquaculture (production of fish) with horticulture (vegetables production) saving energy, water and nutrients40, representing an important step for the development of a sustainable aquaculture in a future zero-waste generation41,42.
Results
Water chemistry
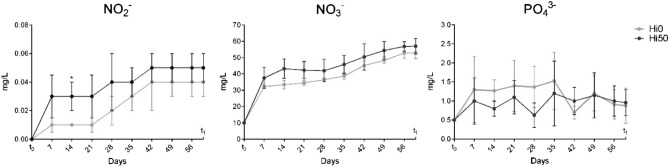
Nitrite (NO2-), nitrate (NO3−) and phosphate (PO43−) weekly trends are shown in Fig. 1. No significant differences were detected between the two experimental groups. Ammonia values were lower than 0.05 mg/L for both Hi0 and Hi50 at each sampling time.
Figure 1.
Trend of aquaponic system water parameters measured during the trial. Values are shown as mean (knots) ± SD (n = 3).
Sturgeon survival and growth
Siberian sturgeon survival was significantly (p < 0.0001) lower in Hi50 (80 ± 4%) compared to Hi0 (97 ± 3%). Considering the specific growth rate (% weight gain day−1), Hi50 (1.8 ± 0.9%) was significantly (p < 0.0001) lower than Hi0 (2.9 ± 0.8%).
Fatty acids composition
Diets
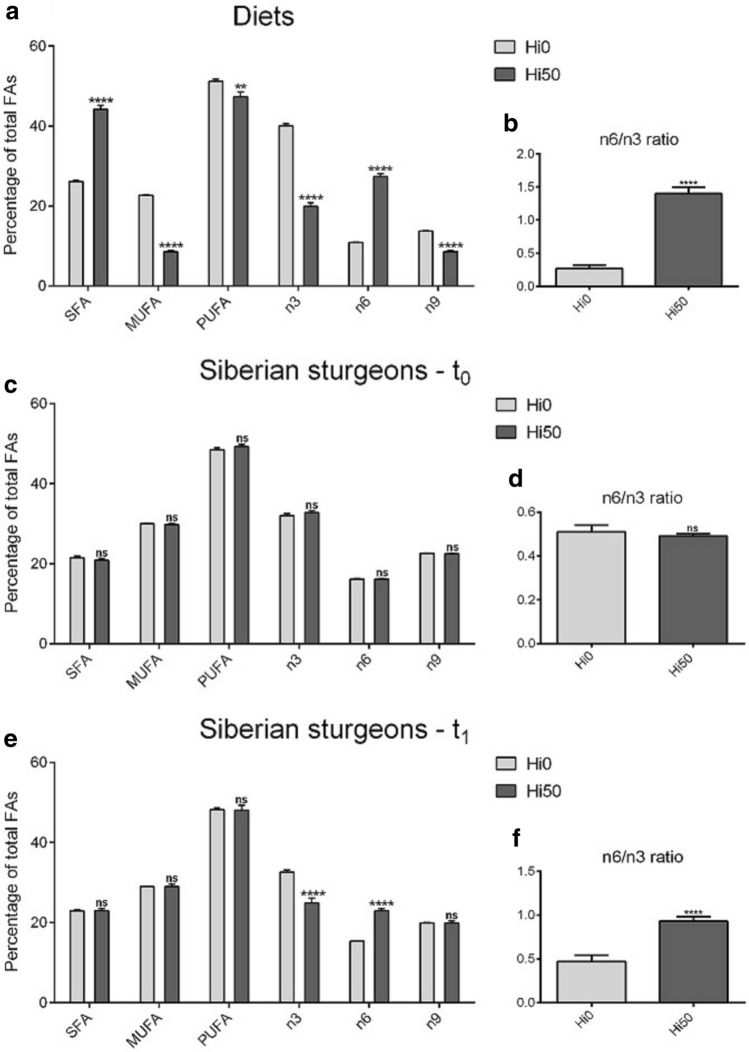
Figure 2a shows the percentages of fatty acids (FA) classes of the two experimental diets. The Hi50 diet resulted in a significantly (p < 0.0001) higher percentage of SFA (44.2 ± 1.0%) and significantly lower MUFA (8.6 ± 0.3%; p < 0.0001) and PUFA (47.3 ± 1.2; p < 0.01) compared to Hi0 diet (26.1 ± 0.3, 22.7 ± 0.1 and 51.2 ± 0.5% for SFA, MUFA and PUFA, respectively). In addition, the inclusion of BSF full-fat prepupae meal in the diet resulted in a significant (p < 0.0001) decrease of n3 (40.1 ± 0.5 and 19.9 ± 1.0% for Hi0 and Hi50, respectively) and n9 (13.7 ± 0.1 and 8.6 ± 0.3% for Hi0 and Hi50, respectively) percentages and a significant (p < 0.0001) increase in n6 percentage (10.9 ± 0.1 and 27.4 ± 0.7% for Hi0 and Hi50, respectively). Therefore, the n6/n3 ratio showed significant differences (p < 0.0001) between experimental diets (0.27 ± 0.05 and 1.40 ± 0.10 for Hi0 and Hi50, respectively; Fig. 2b).
Figure 2.
Percentage of SFA, MUFA and PUFA (as % of total FA) and omega 3 (n3), omega 6 (n6) and omega 9 (n9) FA contribution (%) to lipid profile. (a,b) Experimental diets including 0 and 50% of BSF meal respect to FM (Hi0 and Hi50); (c,d) Siberian sturgeon fillets at t0; (e,f) Fillets of Siberian sturgeon fed on the different diets at t1. Significant differences between Hi0 and Hi50, compared within the same FA class, are indicated as follows: ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Values are shown as mean ± SD (n = 3 for experimental diets; n = 9 for sturgeon at both t0 and t1).
Considering the specific FA composition (Table 1), the Hi50 diet was characterized by a significantly higher percentages of lauric (12:0), stearic (18:0), 7-hexadecenoic (16:1n9), linoleic (18:2n6) and α-linolenic (18:3n3) acids compared to the Hi0 diet. In the Hi0 diet palmitoleic (16:1n7), oleic (18:1n9), eicosapentaenoic (EPA, 20:5n3) and docosahexaenoic (DHA, 22:6n3) acids were significantly more abundant. Finally, the DHA/EPA ratio was significantly (p < 0.05) higher in the Hi50 diet compared to the Hi0 diet.
Table 1.
Fatty acid composition (as % of total FA) of experimental diets and Siberian sturgeon at t0 and t1.
| Diets | Sturgeon | |||||
|---|---|---|---|---|---|---|
| Hi0 | Hi50 | t0 | t1 | |||
| Hi0 | Hi50 | Hi0 | Hi50 | |||
| 10:0 | 0.020 ± 0.001 | 0.570 ± 0.010**** | 0.005 ± 0.001 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 |
| 12:0 | 0.18 ± 0.01 | 8.1 ± 0.7**** | 0.15 ± 0.04 | 0.12 ± 0.03 | 0.10 ± 0.03 | 2.35 ± 0.60**** |
| 13:0 | 0.06 ± 0.001 | n.d | 0.032 ± 0.001 | 0.029 ± 0.001 | 0.030 ± 0.002 | 0.044 ± 0.005 |
| 14:0 | 5.67 ± 0.06 | 4.6 ± 0.3** | 2.80 ± 0.09 | 2.67 ± 0.04** | 3.81 ± 0.12 | 3.73 ± 0.21ns |
| 14:1n5 | 0.53 ± 0.01 | n.d | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.41 ± 0.01 | 0.82 ± 0.07**** |
| 15:0 | 0.61 ± 0.01 | 0.58 ± 0.03 ns | 0.61 ± 0.01 | 0.59 ± 0.01*** | 0.60 ± 0.01 | 0.57 ± 0.01**** |
| 15:1n5 | n.d | n.d | n.d | n.d | n.d | n.d |
| 16:0 | 14.5 ± 0.3 | 17.1 ± 0.6** | 13.9 ± 0.1 | 13.7 ± 0.1*** | 14.7 ± 0.2 | 12.3 ± 0.1**** |
| 16:1n9 | 0.36 ± 0.01 | 6.3 ± 0.3**** | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.74 ± 0.06** |
| 16:1n7 | 6.25 ± 0.04 | n.d | 4.37 ± 0.01 | 4.29 ± 0.01**** | 5.81 ± 0.11 | 5.60 ± 0.42ns |
| 16:2n7 | 0.28 ± 0.01 | n.d | 0.30 ± 0.02 | 0.28 ± 0.01 | 0.13 ± 0.02 | 0.21 ± 0.01 |
| 17:0 | 0.53 ± 0.09 | n.d | 0.48 ± 0.09 | 0.55 ± 0.08 | 0.50 ± 0.01 | 0.41 ± 0.06 |
| 17:1n7 | n.d | n.d | n.d | n.d | n.d | n.d |
| 18:0 | 3.87 ± 0.01 | 13.1 ± 1.8*** | 3.21 ± 0.02 | 3.00 ± 0.02**** | 2.95 ± 0.39 | 3.44 ± 0.16** |
| 18:1n9 | 9.94 ± 0.06 | 2.30 ± 0.20**** | 17.7 ± 0.1 | 17.6 ± 0.1* | 16.5 ± 0.3 | 17.1 ± 0.2*** |
| 18:1n7 | 2.26 ± 0.01 | n.d | 2.66 ± 0.01 | 2.66 ± 0.02ns | 2.91 ± 0.04 | 2.61 ± 0.17**** |
| 18:2n6 | 9.06 ± 0.03 | 27.4 ± 2.3*** | 12.9 ± 0.1 | 12.9 ± 0.1ns | 12.5 ± 0.3 | 17.5 ± 1.3**** |
| 18:3n6 | n.d | n.d | n.d | n.d | n.d | n.d |
| 18:3n3 | 1.91 ± 0.06 | 3.4 ± 0.3** | 2.51 ± 0.01 | 2.51 ± 0.01ns | 2.03 ± 0.12 | 1.86 ± 0.06** |
| 20:0 | 0.36 ± 0.01 | n.d | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.19 ± 0.01 | 0.12 ± 0.02 |
| 20:1n9 | 1.83 ± 0.03 | n.d | 3.17 ± 0.04 | 3.19 ± 0.01ns | 2.25 ± 0.12 | 1.80 ± 0.27*** |
| 20:2n6 | 0.33 ± 0.03 | n.d | 1.26 ± 0.05 | 1.24 ± 0.03ns | 1.10 ± 0.02 | 1.93 ± 0.07**** |
| 20:3n6 | 0.26 ± 0.02 | n.d | 0.57 ± 0.08 | 0.53 ± 0.02 ns | 0.53 ± 0.01 | 1.26 ± 0.07**** |
| 21:0 | n.d | n.d | n.d | n.d | n.d | n.d |
| 20:4n6 | 1.23 ± 0.01 | n.d | 1.48 ± 0.02 | 1.49 ± 0.02ns | 1.27 ± 0.04 | 2.27 ± 0.16**** |
| 20:3n3 | 0.17 ± 0.01 | n.d | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.30 ± 0.02 | 0.30 ± 0.04 |
| 20:5n3 | 17.3 ± 0.2 | 4.8 ± 0.4**** | 6.15 ± 0.04 | 6.26 ± 0.03**** | 9.29 ± 0.18 | 4.51 ± 0.05**** |
| 22:0 | 0.24 ± 0.01 | n.d | 0.081 ± 0.001 | 0.077 ± 0.003 | 0.063 ± 0.003 | 0.039 ± 0.009 |
| 22:1n9 | 1.53 ± 0.03 | n.d | 1.19 ± 0.05 | 1.19 ± 0.03ns | 0.62 ± 0.06 | 0.25 ± 0.14**** |
| 23:0 | n.d | n.d | n.d | n.d | n.d | n.d |
| 24:0 | n.d | n.d | n.d | n.d | n.d | n.d |
| 22:6n3 | 20.7 ± 0.3 | 11.7 ± 1.5*** | 22.9 ± 0.1 | 23.6 ± 0.2**** | 21.0 ± 0.3 | 18.2 ± 1.7*** |
| 24:1n9 | n.d | n.d | n.d | n.d | n.d | n.d |
| DHA/EPA | 1.19 ± 0.03 | 2.5 ± 0.5* | 3.7 ± 0.1 | 3.8 ± 0.1* | 2.26 ± 0.06 | 4.0 ± 0.3**** |
Experimental diets included 0 and 50% of BSF meal respect to FM (Hi0 and Hi50). Siberian sturgeon fed diets including 0 and 50% of BSF meal (Hi0 and Hi50). Significant differences between Hi0 and Hi50 within rows and separately from diets, t0 and t1 are indicated as follows: ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Values are shown as mean ± SD (n = 3 for experimental diets; n = 9 for sturgeon at both t0 and t1). Statistical analysis was performed only for FAs > 0.5% (FA with a percentage < 0.5% were excluded from any statistical analyses because their concentrations were close to the limit of detection).
Sturgeon
As shown in Fig. 2c,d, no significant differences were observed between the experimental groups at t0 in terms of either FA composition (SFA: 21.5 ± 0.4 and 20.9 ± 0.4%; MUFA: 30.0 ± 0.1 and 29.8 ± 0.2%; PUFA: 48.5 ± 0.5 and 49.3 ± 0.5%; n3: 32.0 ± 0.5 and 32.8 ± 0.5%; n6: 16.2 ± 0.1 and 16.2 ± 0.1%; n9: 22.6 ± 0.1 and 22.5 ± 0.1% for Hi0 and Hi50, respectively) or n6/n3 ratio (0.51 ± 0.03 and 0.49 ± 0.01 for Hi0 and Hi50, respectively). In terms of the specific composition, slightly significant differences were detected for some fatty acids due to physiological differences among fish (for specific details, see Table 1).
Considering FA content of Siberian sturgeon fillets at t1 (Fig. 2e), no significant differences were detected between the experimental groups in terms of SFA (22.9 ± 0.3 and 23.0 ± 0.5% for Hi0 and Hi50 respectively), MUFA (29.0 ± 0.1 and 29.0 ± 0.6% for Hi0 and Hi50, respectively), PUFA (48.2 ± 0.5 and 48.1 ± 1.2% for Hi0 and Hi50, respectively) and n9 (19.9 ± 0.1 and 19.9 ± 0.5% for Hi0 and Hi50, respectively) content. However, the Hi50 group was characterized by a significantly (p < 0.0001) lower n3 percentage (24.9 ± 1.2%) and a significantly (p < 0.0001) higher n6 percentage (23.0 ± 0.5%) than Hi0 (32.6 ± 0.5 and 15.4 ± 0.1% for n3 and n6, respectively). Consequently, the n6/n3 ratio (Fig. 2f) was significantly (p < 0.0001) higher in Hi50 (0.93 ± 0.05) compared to Hi0 (0.47 ± 0.07).
In terms of specific FA composition at t1 (Table 1), Hi50 showed significantly higher percentages of lauric (12:0), stearic (18:0), oleic (18:1n9), linoleic (18:2n6) and dihomo-γ-linolenic (20:3n6) acids than Hi0. Conversely, significantly higher percentages of α-linolenic (18:3n3), eicosapentaenoic (20:5n3) and docosahexaenoic (22:6n3) acids were detected in Hi0 respect to Hi50. The DHA/EPA ratio was significantly (p < 0.0001) higher in Hi50 compared to Hi0.
Histology
Histological analyses at t0 were performed in order to evaluate liver and small intestine histological integrity at the beginning of the experiment. Sturgeons exhibited a homogeneous hepatic parenchyma with hepatocytes characterized by a moderate degree of intra-cytoplasmic lipid deposition (Supplementary Fig. S1a,b). The percentage of fat fraction (PFF) in the liver parenchyma did not show significant differences between Hi0 (49.2 ± 7.6%) and Hi50 (51.5 ± 9.4%) groups at t0. Histology of the small intestine (Supplementary Fig. S1c,d) evidenced a regular morphology of mucosal folds, with finger-shaped folds formed by a mono-stratified epithelial layer of enterocytes intercalated with goblet cells, followed by a thin submucosal layer surrounded by the outer muscular layer.
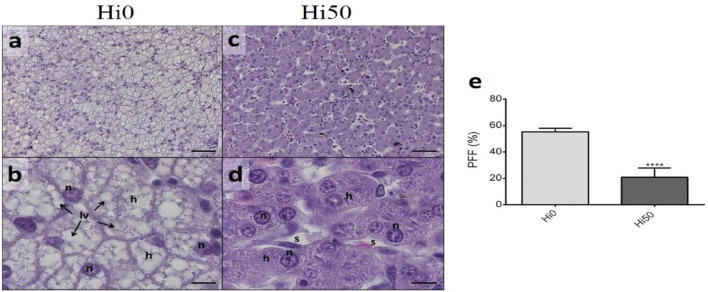
Liver analysis at t1 showed a considerable difference in parenchyma lipid accumulation between Hi0 and Hi50. Specifically, swollen hepatocytes filled of fat with limited cytoplasm were observed in livers of Hi0 sturgeons (Fig. 3a,b), while fat deposition was significantly reduced in the Hi50 group (Fig. 3c,d). PFF analysis at t1 confirmed this result highlighting significant differences in lipid deposition (p < 0.001) between Hi0 (55.3 ± 2.7%) and Hi50 (20.9 ± 7.0%) (Fig. 3e).
Figure 3.
Example of liver histomorphology and percentage of fat fraction (PFF) in hepatic tissue of Siberian sturgeon at t1. (a,b) Hi0; (c,d) Hi50; (e) PFF. Histology scale bars: (a,c) 50 μm; (b,d) 10 μm. Letters: h hepatocyte, n nucleus, lv lipid vesicles, s hepatic sinusoids. For PFF, values are shown as percentage mean ± SD (n = 15). Significant differences between Hi0 and Hi50 are indicated as follows: ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Sturgeon fed diets including 0 and 50 of BSF meal (Hi0 and Hi50, respectively).
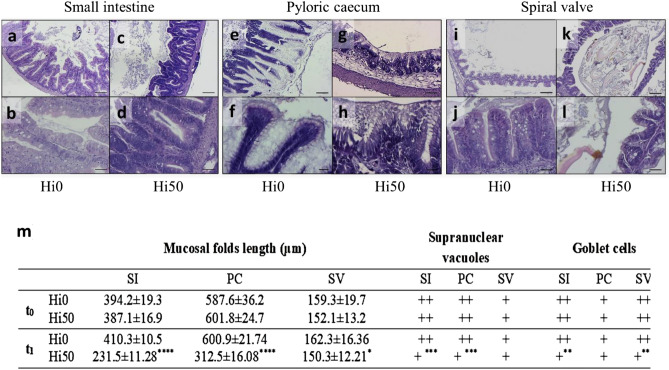
Representative histological images of small intestine (SI), pyloric caecum (PC) and spiral valve (SV) are shown in Fig. 4. In the morphometric analysis of these gut tracts (Fig. 4m), no significant differences were detected between the groups at t0, while mucosal folds atrophy, with a significant reduction of folds length, was observed at t1 in Hi50 SI (Fig. 5c,d; p < 0.0001), PC (Fig. 4g,h, p < 0.0001) and SV (Fig. 4k,l, p < 0.05) compared to the Hi0 group (Fig. 4a,b,e,f,i,j for SI, PC and SV, respectively). In addition, a significant (p < 0.0001) reduction of supranuclear vacuoles in SI and PC and a significant (p < 0.01) reduction in the relative abundance of goblet cells in SI and SV were observed in Hi50 compared to Hi0.
Figure 4.
Siberian sturgeon’ small intestine (SI), pyloric caeca (PC) and spiral valve (SV) histology at t1 and histological indexes (mucosal folds length, supranuclear vacuoles abundance and goblet cells number) calculated in these gut tracts. (a–d) small intestine; (e–h) pyloric caecum; (i–l) spiral valve. Histology scale bars: (a,c,e,g) 100 μm; (b,d,f,h,j,l) 50 μm; (i,k) 200 μm. For histological indexes (m), values are showed as mean ± SD (n = 15). Scores: supranuclear vacuoles + scattered, ++ abundant; goblet cells + 0 to 4 per villus, ++ more than 4 per villus. Significant differences, calculated within the same sampling time, between Hi0 and Hi50 are indicated as follows: ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Sturgeon fed diets including 0 and 50 of BSF meal (Hi0 and Hi50, respectively).
Figure 5.
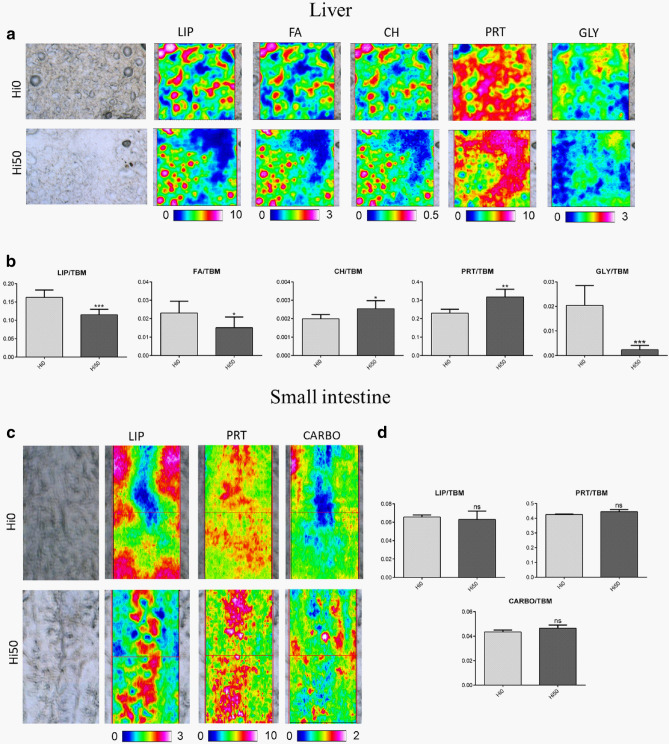
Spectral analysis of liver and small intestine samples of Siberian sturgeon collected at t1. (a) Microphotographs of liver samples and topographical distribution of: lipids (LIP maps), fatty acids (FA maps), unsaturated alkyl chains (CH maps), proteins (PRT maps), and glycogen (GLY maps) (map size 164 × 164 µm2). (b) Statistical analysis of liver biochemical composition: LIP/TBM (relative amount of total lipids), FA/TBM (relative amount of fatty acids), CH/TBM (degree of unsaturation in lipid alkyl chains), PRT/TBM (relative amount of total proteins) and GLY/TBM (relative amounts of glycogen). (c) Microphotographs of small intestine samples and topographical distribution of: lipids (LIP maps), proteins (PRT maps) and carbohydrates (CARBO maps) (map size 328 × 164 µm2). (d) Statistical analysis of small intestine biochemical composition: LIP/TBM (relative amount of total lipids), PRT/TBM (relative amount of total proteins) and CARBO/TBM (relative amount of carbohydrates). Significant differences between Hi0 and Hi50 are indicated as follows: ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Data are reported as mean ± SD (n = 6). Sturgeons fed diets including 0 and 50 of BSF meal (Hi0 and Hi50, respectively).
FTIR analysis
The spectral analysis of liver samples collected from Siberian sturgeons at t1 showed differences in the biochemical composition between Hi0 and Hi50. The IR maps (Fig. 5a), as well as the statistical analysis of specific band area ratios (Fig. 5b), showed a significant decrease of total lipids (LIP maps; LIP/TBM, p < 0.001), fatty acids (FA maps; FA/TBM, p < 0.05) and glycogen (GLY maps; GLY/TBM, p < 0.01) in Hi50 compared to Hi0. An increase in unsaturated lipids (CH maps; CH/TBM, p < 0.05) and proteins (PRT maps; PRT/TBM, p < 0.01) in Hi50 was also observed.
For the small intestine samples, both the IR maps (LIP, PRT and CARBO maps; Fig. 5c) and the statistical analysis of specific band area ratios (LIP/TBM, PRT/TBM and CARBO/TBM; Fig. 5d) did not show significant modifications between Hi0 and Hi50.
Sturgeon gut microbiome
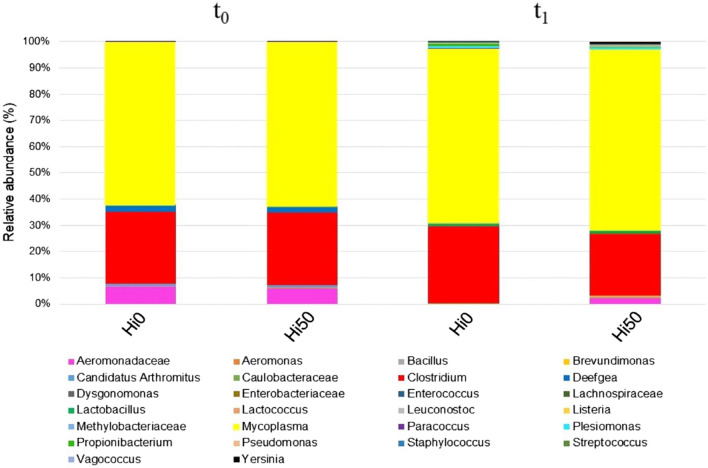
No significant differences were observed in the alpha diversity values (Shannon, Chao1 and number of OTUs) between Hi0 and Hi50 at both t0 and t1. However, a higher number of bacterial groups at genus or family level were identified in Hi50 (22 groups) compared to Hi0 (17 groups) samples at t1. Relative abundances of bacterial taxa were examined to determine the effect of the diet on gut microbiota composition. The average values (Fig. 6) of the biological replicates at both sampling times were found to be very similar. The taxonomic analysis showed the dominance (> 58%) of Mycoplasma in all samples analysed, followed by Clostridium, with relative abundances comprised between 22.64% (Hi50, t1) and 28.27% (Hi0, t1). Aeromonadacean bacteria were found in gut samples from both Hi0 and Hi50 at t0 (about 6%), and exclusively in Hi50 sampled at t1 with the relative abundance of 2.08%. Bacteria of the genus Deefgea were present in both experimental groups exclusively at t0 with relative abundance of about 2%. Additional bacteria were detected sporadically in some samples, with a relative abundance < 1%. Lactobacillus, Paracoccus, Propionibacterium and Streptococcus were identified solely at t1 in both experimental groups, while Listeria was found only in Hi50.
Figure 6.
Relative abundances (%) of bacterial community in Siberian sturgeon gut samples at both t0 and t1 as identified by MiSeq Illumina. Sturgeon fed diets including 0 and 50 of BSF meal (Hi0 and Hi50, respectively).
Real-time PCR results
Real-time PCR analyses were performed on liver samples in order to test the expression of genes involved in fish growth (igf1) and stress response (hsp70.1). Gene expression of tnfa was investigated in intestine samples.
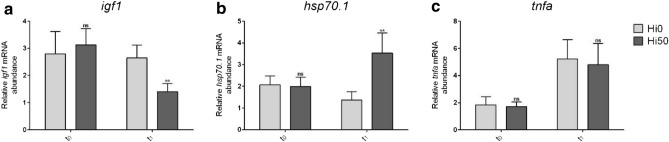
As shown in Fig. 7, the expression of the genes analysed did not show significant differences between the experimental groups at t0. At t1, results evidenced a significant (p < 0.01) downregulation for igf1 (Fig. 7a) and a significant (p > 0.01) upregulation for hsp70.1 (Fig. 7b) in Hi50 compared to Hi0. For tnfa (Fig. 7c), no significant differences in gene expression were detected between the experimental groups.
Figure 7.
Relative mRNA abundance of genes analysed at t0 and t1 in Siberian sturgeon. (a) igf1 and (b) hsp70.1 were analysed in liver samples; (c) tnfa was analysed in intestine samples. Fish fed diets including 0 and 50 of BSF meal (Hi0 and Hi50, respectively). Significant differences between Hi0 and Hi50, compared within the same sampling time, are indicated as follows: ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. Values are shown as mean ± SD (n = 5).
Discussion
The inclusion of BSF meal in aquafeed, as well as the physiological responses of fish, have been recently investigated in several important commercial species like Atlantic salmon (Salmo salar), rainbow trout (Oncorhinchus mykiss) and European seabass (Dicentrarchus labrax)28,30,43.
Information available in this field for the Siberian sturgeon is still fragmentary, in fact completely lacking if related to aquaponic systems. Previous studies, based on a limited number of laboratory approaches, highlighted that a defatted BSF prepupae meal dietary inclusion level higher than 25% impaired fish growth and welfare31–33. Based on these results and previous studies which demonstrated the possibility to use higher full-fat dietary BSF meal inclusion levels23,38 after an enrichment procedure of the insect biomass39, the present study aimed to: (i) test, for the first time, a 50% enriched full-fat BSF prepupae meal inclusion level in a practical diet for juvenile sturgeons in an aquaponic system; (ii) apply a set of laboratory techniques to examine comprehensively the physiological responses of the fish.
The results showed that the inclusion of a 50% enriched full-fat BSF prepupae meal negatively affects fish growth and survival. These conclusions are supported by the expression of the growth markers analysed and agree with previous studies reporting that inclusion of BSF meal levels higher than 40% often impairs fish growth and welfare32,36. A possible explanation of these differences between the two experimental groups can be related to the FA composition of the diets. Şener et al.44 reported high EPA and DHA levels in Russian sturgeon fed on diets rich in linoleic acid. In agreement with this study, our FA analyses showed the ability of sturgeon to convert linoleic acid (18:2n6) and α-linolenic acid (18:3n3) to EPA and DHA by desaturation and elongation enzymatic pathways45,46. Since biochemical conversions require expenditure of energy by the fish, they can explain the observed growth delay in Hi50 compared to Hi047.
It should be pointed out, however, that a lower diet acceptance was observed in the Hi50 group compared to Hi0 and the laboratory analysis performed suggest that the fish entered a fasting condition. Fasting is normally characterized by a growth reduction due mainly to a decrease in IGFs production by hepatocytes48. In turn, these changes induce hepatic lipolysis to provide peripheral tissue with free FA as energy source48,49. A similar scenario was observed in the present study: Hi50 growth reduction was coupled with a lower igf1 gene expression and a scarce lipid accumulation in the hepatic parenchyma. These results are also supported by PFF calculation and FTIR analysis which showed an overall decrease in both total lipids (LIP/TBM) and fatty acids (FA/TBM) in the Hi50 liver samples compared to Hi0. Furthermore, a severe reduction of hepatic glycogen, which represents the first energy reserve mobilized to face food restrictions50,51, was detected by FTIR in Hi50 compared to Hi0. Furné et al.52 demonstrated that the Adriatic sturgeon (Acipenser naccarii) responded to fasting with a precocious mobilization of hepatic glycogen and a high hepatic lipid-degradation capacity. Accordingly, our results represent a strong evidence that fish entered a fasting period. In addition, since malnutrition or fasting are nowadays considered stressors22,53,54, this stressful situation is fully supported by the higher hepatic hsp70.1 gene expression detected in Hi50 compared to Hi0.
Histological analyses of intestinal tracts are also useful to provide evidence of fasting status in fish. Fasting is usually associated to a reduction in mucosal fold number and height, reduction in supra-nuclear lipid droplets and reduction in goblet cells numbers55,56. Accordingly, our Hi50 group showed atrophy of mucosal folds and a dramatic decrease of enterocyte vacuolization and goblet cells number compared to Hi0. In a study performed by Caimi et al.33 on Siberian sturgeon over a 118-days period, lower (37.5%) levels of defatted BSF meal dietary inclusion did not show these negative effects on spiral valve and liver histology. On the other hand, in a 60-days feeding trial, a 15% dietary inclusion of full-fat BSF prepupae caused a thinning of the mucosa and a parallel thickening of the muscular layer in the proximal intestine while not affecting villus height31. Overall, our analyses of the intestine histology and tnfa gene expression did not reveal any sign of inflammation in either of the experimental groups. Similar results were reported in other studies30,36,57 that evidenced a positive role of BSF meal dietary inclusion on fish gut welfare. BSF meal contains lauric acid and chitin, that possess anti-inflammatory and immune-boosting properties58. BSF meal is also known to rise biodiversity in the fish microbiome, necessary to improve fish health, metabolism, nutrition and immunity17,59. Our Hi50 diet contained higher percentages of lauric acid and chitin and supported a higher number of bacterial groups (at genus or family level) in the fish gut compared to Hi0, possibly explaining the absence of intestinal inflammatory events. The dominance of Mycoplasma, followed by Clostridium, was observed in all samples analysed, regardless of the diet. An increased relative abundance of Mycoplasma was detected by Rimoldi et al.59 in the autochthonous gut microbiota of rainbow trout fed with BSF-based diets. This author attributed the beneficial action on host health to the production of antibacterial compounds, such as lactic and acetic acid.
In conclusion, the present study demonstrated the feasibility of aquaponic systems for sturgeon culture and for testing new aquafeed ingredients like insect meal. However, the general low feed acceptance showed by the Hi50 group fish compared to Hi0 indicates the need of further studies aimed at improving the palatability of BSF-based diets intended for sturgeon culture.
Methods
All methods were carried out in accordance with relevant guidelines and regulations.
Ethics
All zootechnical trials were conducted in agreement with the Italian legislation on experimental animals and were approved by the Ethics Committee of the Università Politecnica delle Marche (Aut. No. 01/2019).
Insects rearing and fish diet production
For details on insects rearing and fish diet production, see Supplementary Information section.
Fish, aquaponic system and experimental design
The 60-days feeding trial was conducted at the aquaponics facility “Cooperativa Agricola Tanto Sole” (Treia, Macerata, Italy). Juvenile Siberian sturgeons, purchased from Azienda Agricola Pisani Dossi s.s., Cisliano, MI, Italy), were acclimated for 1 week in a single 500 L tank equipped with mechanical, biological and UV filtration (Panaque, Viterbo, Italy). Tank temperature was 18 ± 0.5 °C; ammonia (NH3) and nitrite (NO2−) were < 0.05 mg/L and nitrate (NO3−) 10 mg/L, according to sturgeon rearing requirements60. At the end of the acclimation period, fish were randomly allocated into six Media Based Aquaponic Systems (80 specimens per tank). Each aquaponic system consisted of a 1.56 m2 hydroponic unit for plants cultivation and a 600 L fish tank, for a total volume of 720 L of water.
Fish unit
The six systems were maintained at constant temperature (18.0 ± 0.5 °C) by chillers TK500 (Teco, Ravenna, Italy). Evaporated water was replaced on request and the systems were subjected to a natural photoperiod (11L/13D). Water samples were collected weekly in order to test ammonia (NH3), nitrite (NO2−), nitrate (NO3−) and phosphate (PO43−) using Hanna reagents and a HI83399 spectrophotometer (Hanna instruments, Villafranca Padovana, Italy).
Hydroponic unit
Each hydroponic unit was filled with expanded clay with biological and mechanical filtration function40, necessary to guarantee a physical support for plant growth. Specifically, in each hydroponic unit, 16 lettuce (Lactuca sativa; initial weight: 2.95 ± 0.5 g) and 3 celery (Apium graveolens; initial weight: 20.8 ± 5.0 g) seedlings were planted two days before introduction of the fish (density = 12 plants/m2). Recirculating water flow from the fish tank to the hydroponic unit was regulated by a 1900 L/h pump (Eheim GmbH & Co, Deizisau, Germany) completing 3 water renewals per hour. Specifically, water was pumped from the fish tank to the hydroponic unit, and then returned to the fish unit through a siphon. The siphon was equipped with further synthetic foam for extra mechanical filtration (foam was cleaned once per week).
Feeding trial
At the beginning of the experiment (t0), the six aquaponic systems were randomly assigned to the experimental groups (Hi0, Hi50) according to an experimental design with triplicate tanks per dietary treatment. Feeding trial duration was 60 days, in which sturgeons almost triplicated their weight and were fed as follows: fish fed on the 0% of BSF meal diet (Hi0 group); fish fed on the diet including 50% of BSF full-fat prepupae meal (Hi50 group). Feed particle were 0.5–1 mm in size. Sturgeons were fed three times a day the experimental diets (3% body weight daily). At the beginning (t0) and at the end of the feeding trial (t1), after a 10-h fasting period, the required fish were sampled, euthanized with a lethal dose of MS222 (0.3 g/L; Merck KGaA, Darmstadt, Germany) and properly stored for further analyses.
Biometry
For growth measurements, 60 sturgeons per dietary group (n = 3) at both t0 and t1 were randomly collected from the different tanks. Wet weight was measured with an OHAUS Explorer (OHAUS Europe GmbH, Greifensee, Switzerland) analytical balance (precision 0.1 mg). The specific growth rate (SGR) was calculated as follows: SGR% = [(lnWf – lnWi)/t) × 100, where Wf is the wet weight determined at t1, Wi, the wet weight determined at t0, and t, the number of days (60). During the trial, dead fish were removed and recorded to estimate the final survival rate.
Fatty acid composition
Lipid content and fatty acid composition of experimental diets (n = 3) and fish fillet (9 sturgeon per dietary group; n = 3) were determined after sample homogenization (homogenizer MZ 4110, DCG, Eltronic, Monza, Italy) and freeze-drying (Edwards EF4, Crawley, Sussex, England). Lipid extraction was carried out on lyophilized powders with the Folch method (1957)61 for diets and with Microwave-Assisted Extraction (MAE) method for fish62. All lipid extracts were evaporated under laminar flow inert gas (N2) until constant weight to determine lipid content; then, they were re-suspended in 0.5 mL of n-epthane for fatty acid analysis. Fatty acid methyl esters (FAMEs) were prepared according to Canonico et al.63 using methyl ester of nonadecanoic acid (19:0; Dr. Ehrenstorfer GmbH, Augsburg, Germany) as internal standard. A gas-chromatographic (GC) system (Agilent-6890, Milano, Italy) coupled with a Mass Selective Detector (MS) (Agilent-5973N quadrupole, Milano, Italy) was used to determine FAMEs. A CPS ANALITICA CC-wax-MS (30 m × 0.25 mm ID, 0.25 μm film thickness) capillary column was used to separate FAMEs. Instrumental conditions were set up according to Truzzi et al.64. Analyses were carried out on three aliquots per sample. For each aliquot, at least three runs were performed on the GC–MS.
Histology
Liver, small intestine and spiral valve from 15 different sturgeons per dietary group (n = 3) were randomly collected at both t0 and t1 and processed according to Piccinetti et al.65. For details, see Supplementary Information section. In order to evaluate the percentage of fat fraction in the liver (PFF), three sections per fish (15 fish per dietary group; n = 3), at 100 µm intervals, were acquired and analysed by mean of the ImageJ software setting an homogeneous threshold value according to Zarantoniello et al.38. Non evaluable areas on sections, such as blood vessels and bile ducts, were not considered. Results were reported as mean ± SD of the area occupied by fat on the total hepatic parenchyma analysed on the section. A semi-quantitative evaluation was performed on small intestine, pyloric caecum and spiral valve morphology based on mucosal folds height, supranuclear vacuolization of enterocytes and abundance of goblet cells as previously described in Urán et al.66. Specifically, for the morphometric evaluation of mucosal folds height, ten transversal sections per fish (15 fish per dietary group) of small intestine, pyloric caecum and spiral valve, at 200 μm intervals, were analysed as described in Cardinaletti et al.28. All the undamaged and non-oblique folds were measured (at least 150 measurements per fish) using ZEN 2.3 software (Carl Zeiss Microscopy GmbH) and measurements were reported as height mean ± SD (µm)28. For the semi-quantitative analysis of supranuclear vacuoles and goblet cells, 3 whole intestine circular transversal sections per fish (15 fish per dietary group), at 200 μm intervals, were analysed. The sections were analysed by experienced staff in two independent blinded evaluations and an arbitrary unit was assigned as described in Panettieri et al.67. Scores were assigned as follows: supranuclear vacuoles + = scattered, + + = abundant; goblet cells + = 0/4 per villus, + + > 4 per villus.
FTIR measurements
Samples of liver and small intestine collected at t1 from 6 different sturgeons per dietary group (n = 3), were quickly dissected and immediately frozen at − 80 °C. Samples were then prepared for infrared spectroscopy (IR) measurements68 as reported in Supplementary Information section.
Sturgeon gut microbiome
RNA extraction and cDNA synthesis
Gut samples from Hi0 and Hi50 groups were collected at t0 and t1. Specifically, 9 different sturgeons per dietary group (n = 3) were collected and processed as previously described by Zarantoniello et al.38. The obtained cell pellets were covered with RNA later Stabilization Solution (Ambion, Foster City, CA, USA) and stored at − 80 °C until the extraction of total microbial RNA performed by Quick-RNA Miniprep kit (Zymo Research, CA, USA). The quantity and purity of the extracted RNA were checked using a Nanodrop ND 1000 (Thermo Fisher Scientific). Moreover, the absence of residual DNA contamination was checked by PCR as described by Garofalo et al.69. Each sample RNA (10 μL) was reverse-transcribed in cDNA using oligo (dT) and random hexamer primers from SensiFAST cDNA Synthesis Kit for RT-qPCR (Bioline, London, UK).
16S rRNA gene amplicon target sequencing
The portion of 16S rRNA gene (V3–V4 region) from each sample cDNA was amplified by PCR as previously described by Klindworth et al.70. The PCR products were further processed and sequenced by MiSeq Illumina instrument (Illumina, San Diego, California, USA) following the procedure detailed by Osimani et al.18.
Molecular analyses
RNA extraction, cDNA synthesis and real-time PCR
Total RNA extractions from liver and small intestine samples from 15 sturgeons per dietary group (n = 3) at both t0 and t1 were performed using RNAzol RT reagent (R4533, Merck KGaA) according to Olivotto et al.71 and Vargas-Abùndez et al.72. For details on methods and primers sequences (reported in Supplementary Table S2), see Supplementary Information section.
Statistical analysis
All data (except for microbiome) were analysed by t-test, with diet as the explanatory variable and presented as mean ± SD. The statistical software package Prism5 (GraphPad Software) was used. Significant differences between Hi0 and Hi50 were indicated as follows: ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001. For microbiome bioinformatics analyses, raw reads were first merged with the FLASH software and analysed with the QIIME 1.9.0 software73; the detailed pipeline was described by Ferrocino et al.74. OTUs clustering was obtained at 97% of similarity and centroids sequencing were mapped against the Greengenes 16S rRNA gene database. OTU tables generated by QIIME were rarefied at the lowest number of reads and showed the highest reached taxonomic resolution. The vegan package of R was used for the alpha diversity calculation.
Supplementary Information
Acknowledgements
Authors would like to thank Alghitaly for providing the microalgae and Prof. F. Rindi for the English revision. This study was funded by Ricerca Scientifica 2017 Cariverona to I.O., NUTRIFISH Project N°. 2017.0571.
Author contributions
I.O., M.Z., B.R., V.Nozzi, E.G. and V.M. wrote the manuscript. I.O., M.Z., B.R., G.G., V.Nozzi, L.F. and S.R. planned the experimental design, performed fish trials, biometric, molecular, histological and statistical analyses. C.T. and F.G. performed the fatty acid analyses. F.T. and G.C. produced the experimental diets. E.G., G.G., V.Notarstefano performed spectroscopic analyses. P.R. and N.I. cultured the insects. A.O. and V.M. performed microbiome analyses. All authors revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80379-x.
References
- 1.Babaei S, Abedian-Kenari A, Hedayati M, Yazdani-Sadati MA. Growth response, body composition, plasma metabolites, digestive and antioxidant enzymes activities of Siberian sturgeon (Acipenser baerii, Brandt, 1869) fed different dietary protein and carbohydrate: Lipid ratio. Aquac. Res. 2017;48:2642–2654. doi: 10.1111/are.13096. [DOI] [Google Scholar]
- 2.Bronzi P, Rosenthal H, Gessner J. Global sturgeon aquaculture production: An overview. J. Appl. Ichthyol. 2011;27:169–175. doi: 10.1111/j.1439-0426.2011.01757.x. [DOI] [Google Scholar]
- 3.Ashouri G, et al. Compensatory growth, plasma hormones and metabolites in juvenile Siberian sturgeon (Acipenser baerii, Brandt 1869) subjected to fasting and re-feeding. Aquac. Nutr. 2020;26:400–409. doi: 10.1111/anu.13002. [DOI] [Google Scholar]
- 4.Williot P, Nonnotte G, Chebanov M. The Siberian sturgeon (Acipenser baerii, Brandt, 1869) The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) New York: Springer; 2018. [Google Scholar]
- 5.Liu H, et al. Nutrients apparent digestibility coefficients of selected protein sources for juvenile Siberian sturgeon (Acipenser baerii Brandt), compared by two chromic oxide analyses methods. Aquac. Nutr. 2009;15:650–656. doi: 10.1111/j.1365-2095.2008.00634.x. [DOI] [Google Scholar]
- 6.Luo L, et al. Effects of early long-chain n-3HUFA programming on growth, antioxidant response and lipid metabolism of Siberian sturgeon (Acipenser baerii Brandt) Aquaculture. 2019;509:96–103. doi: 10.1016/j.aquaculture.2019.05.032. [DOI] [Google Scholar]
- 7.Zhu H, et al. Replacement of fish meal with blend of rendered animal protein in diets for Siberian sturgeon (Acipenser baerii Brandt), results in performance equal to fish meal fed fish. Aquac. Nutr. 2011;17:e389–e395. doi: 10.1111/j.1365-2095.2010.00773.x. [DOI] [Google Scholar]
- 8.Tacon AGJ, Metian M. Feed matters: Satisfying the feed demand of aquaculture. Rev. Fish. Sci. Aquac. 2015;23:1–10. doi: 10.1080/23308249.2014.987209. [DOI] [Google Scholar]
- 9.Sarker PK, et al. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile nile tilapia (Oreochromis niloticus) PLoS ONE. 2018;13(7):e0201315. doi: 10.1371/journal.pone.0201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicuro B, Piccinno M, Daprà F, Gai F, Vilella S. Utilization of rice protein concentrate in siberian sturgeon (Acipenser baerii Brandt) nutrition. Turkish J. Fish. Aquat. Sci. 2015;15:313–319. [Google Scholar]
- 11.Jahanbakhshi A, Imanpoor MR, Taghizadeh V, Shabani A. Hematological and serum biochemical indices changes induced by replacing fish meal with plant protein (sesame oil cake and corn gluten) in the Great sturgeon (Huso huso) Comp. Clin. Path. 2013;22:1087–1092. doi: 10.1007/s00580-012-1532-4. [DOI] [Google Scholar]
- 12.Palmegiano GB, et al. Effects of Spirulina and plant oil on the growth and lipid traits of white sturgeon (Acipenser transmontanus) fingerlings. Aquac. Res. 2008;39:587–595. doi: 10.1111/j.1365-2109.2008.01914.x. [DOI] [Google Scholar]
- 13.Cammack JA, Tomberlin JK. The impact of diet protein and carbohydrate on select life-history traits of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae) Insects. 2017;8:56. doi: 10.3390/insects8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spranghers T, et al. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017;97(8):2594–2600. doi: 10.1002/jsfa.8081. [DOI] [PubMed] [Google Scholar]
- 15.Meneguz M, et al. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018;98(15):5776–5784. doi: 10.1002/jsfa.9127. [DOI] [PubMed] [Google Scholar]
- 16.Pyka J, Kolman R. Feeding intensity and growth of Siberian sturgeon Acipenser baerii Brandt in pond cultivation. Arch. Pol. Fish. 2003;11(2):287–294. [Google Scholar]
- 17.Terova G, et al. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019;29:465–486. doi: 10.1007/s11160-019-09558-y. [DOI] [Google Scholar]
- 18.Osimani A, et al. Hermetia illucens in diets for zebrafish (Danio rerio): A study of bacterial diversity by using PCR-DGGE and metagenomic sequencing. PLoS ONE. 2019;14(12):e0225956. doi: 10.1371/journal.pone.0225956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belghit I, et al. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar) Aquaculture. 2019;503:609–619. doi: 10.1016/j.aquaculture.2018.12.032. [DOI] [Google Scholar]
- 20.Ewald N, et al. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manage. 2020;102:40–47. doi: 10.1016/j.wasman.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Tocher DR. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010;41:717–732. doi: 10.1111/j.1365-2109.2008.02150.x. [DOI] [Google Scholar]
- 22.Olivotto I, et al. Live prey enrichment, with particular emphasis on HUFAs, as limiting factor in false percula clownfish (Amphiprion ocellaris, Pomacentridae) larval development and metamorphosis: Molecular and biochemical implications. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011;159(3):207–218. doi: 10.1016/j.cbpa.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Zarantoniello M, et al. Zebrafish (Danio rerio) physiological and behavioural responses to insect-based diets: A multidisciplinary approach. Sci. Rep. 2020;10:10648. doi: 10.1038/s41598-020-67740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo L, et al. The impact of dietary DHA/EPA ratio on spawning performance, egg and offspring quality in Siberian sturgeon (Acipenser baeri) Aquaculture. 2015;437:140–145. doi: 10.1016/j.aquaculture.2014.11.036. [DOI] [Google Scholar]
- 25.Luo L, et al. n-3 Long-chain polyunsaturated fatty acids improve the sperm, egg, and offspring quality of Siberian sturgeon (Acipenser baerii) Aquaculture. 2017;473:266–271. doi: 10.1016/j.aquaculture.2017.02.021. [DOI] [Google Scholar]
- 26.Vaccaro AM, Buffa G, Messina CM, Santulli A, Mazzola A. Fatty acid composition of a cultured sturgeon hybrid (Acipenser naccarii x A. baerii) Food Chem. 2005;93:627–631. doi: 10.1016/j.foodchem.2004.09.042. [DOI] [Google Scholar]
- 27.Elia AC, et al. Influence of Hermetia illucens meal dietary inclusion on the histological traits, gut mucin composition and the oxidative stress biomarkers in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2018;496:50–57. doi: 10.1016/j.aquaculture.2018.07.009. [DOI] [Google Scholar]
- 28.Cardinaletti G, et al. Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss) Animals. 2019;9(5):251. doi: 10.3390/ani9050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawole FJ, et al. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture. 2020;518:734849. doi: 10.1016/j.aquaculture.2019.734849. [DOI] [Google Scholar]
- 30.Li Y, et al. Total replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal does not compromise the gut health of Atlantic salmon (Salmo salar) Aquaculture. 2020;520:734967. doi: 10.1016/j.aquaculture.2020.734967. [DOI] [Google Scholar]
- 31.Józefiak A, Nogales-Mérida S, Rawski M, Kierończyk B, Mazurkiewicz J. Effects of insect diets on the gastrointestinal tract health and growth performance of Siberian sturgeon (Acipenser baerii Brandt, 1869) BMC Vet. Res. 2019;15:1–11. doi: 10.1186/s12917-019-2070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caimi C, et al. First insights on black soldier fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture. 2020;515:734539. doi: 10.1016/j.aquaculture.2019.734539. [DOI] [Google Scholar]
- 33.Caimi C, et al. Could dietary black soldier fly meal inclusion affect the liver and intestinal histological traits and the oxidative stress biomarkers of Siberian sturgeon (Acipenser baerii) juveniles? Animals. 2020;10(1):155. doi: 10.3390/ani10010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgini E, et al. New insights on the macromolecular building of rainbow trout (O. mykiss) intestine: FTIR Imaging and histological correlative study. Aquaculture. 2018;497:1–9. doi: 10.1016/j.aquaculture.2018.07.032. [DOI] [Google Scholar]
- 35.Vargas A, et al. Rearing zebrafish on black soldier fly (Hermetia illucens): Biometric, histological, spectroscopic, biochemical, and molecular implications. Zebrafish. 2018;15:404–419. doi: 10.1089/zeb.2017.1559. [DOI] [PubMed] [Google Scholar]
- 36.Zarantoniello M, et al. A six-months study on black soldier fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019;9:8598. doi: 10.1038/s41598-019-45172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, et al. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I) Renew. Energy. 2017;111:749–756. doi: 10.1016/j.renene.2017.04.063. [DOI] [Google Scholar]
- 38.Zarantoniello M, et al. Black Soldier Fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture. 2020;518:734659. doi: 10.1016/j.aquaculture.2019.734659. [DOI] [Google Scholar]
- 39.Truzzi C, et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020;259:114309. doi: 10.1016/j.anifeedsci.2019.114309. [DOI] [Google Scholar]
- 40.Nozzi V, Graber A, Schmautz Z, Mathis A, Junge R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy. 2018;8:27. doi: 10.3390/agronomy8030027. [DOI] [Google Scholar]
- 41.Robaina L, Pirhonen J, Mente E, Sánchez J, Goosen N. Fish diets in aquaponics. In: Goddek S, Joyce A, Kotzen B, Burnell GM, editors. Aquaponics Food Production Systems. Cham: Springer; 2019. pp. 333–352. [Google Scholar]
- 42.Kledal PR, Thorarinsdottir R. Aquaponics: A commercial niche for sustainable modern aquaculture. In: Hai FI, Visvanathan C, Boopathy R, editors. Sustainable Aquaculture. Cham: Springer; 2018. pp. 173–190. [Google Scholar]
- 43.Magalhães R, et al. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax) Aquaculture. 2017;476:79–85. doi: 10.1016/j.aquaculture.2017.04.021. [DOI] [Google Scholar]
- 44.Şener E, Yildiz M, Savaş E. Effects of dietary lipids on growth and fatty acid composition in Russian sturgeon (Acipenser gueldenstaedtii) juveniles. Turk. J. Vet. Anim. Sci. 2005;29(5):1101–1107. [Google Scholar]
- 45.Gawlicka A, Herold MA, Barrows FT, de la Noue J, Hung SSO. Effects of dietary lipids on growth, fatty acid composition, intestinal absorption and hepatic storage in white sturgeon (Acipenser transmontanus R.) larvae. J. Appl. Ichthyol. 2002;18:673–681. doi: 10.1046/j.1439-0426.2002.00371.x. [DOI] [Google Scholar]
- 46.Imanpoor MR, Asghari M, Asadi R. Requirements for n-3 highly unsaturated fatty acids in feeding juvenile Iranian sturgeon (Acipenser persicus) and its effects on growth, carcass quality, and fatty acid composition. Aquac. Int. 2011;19:1035–1046. doi: 10.1007/s10499-011-9420-5. [DOI] [Google Scholar]
- 47.Bell MV, Tocher DR. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new directions. In: Arts MT, Brett MT, Kainz M, editors. Lipids in Aquatic Ecosystems. New York: Springer; 2009. pp. 211–236. [Google Scholar]
- 48.Bertucci JI, et al. Nutrient regulation of endocrine factors influencing feeding and growth in fish. Front. Endocrinol. 2019 doi: 10.3389/fendo.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Sánchez J, Le Bail PY. Growth hormone axis as marker of nutritional status and growth performance in fish. Aquaculture. 1999;177(1–4):117–128. doi: 10.1016/S0044-8486(99)00073-3. [DOI] [Google Scholar]
- 50.Metón I, Fernández F, Baanante IV. Short- and long-term effects of refeeding on key enzyme activities in glycolysis-gluconeogenesis in the liver of gilthead seabream (Sparus aurata) Aquaculture. 2003;225(1–4):99–107. doi: 10.1016/S0044-8486(03)00281-3. [DOI] [Google Scholar]
- 51.Piccinetti CC, et al. The effects of starving and feeding on Dover sole (Solea solea, Soleidae, Linnaeus, 1758) stress response and early larval development. Aquac. Res. 2015;46(10):2512–2526. doi: 10.1111/are.12410. [DOI] [Google Scholar]
- 52.Furné M, et al. The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012;182:63–76. doi: 10.1007/s00360-011-0596-9. [DOI] [PubMed] [Google Scholar]
- 53.Piccinetti CC, et al. Growth and stress factors in ballan wrasse (Labrus bergylta) larval development. Aquac. Res. 2017;48:2567–2580. doi: 10.1111/are.13093. [DOI] [Google Scholar]
- 54.Piccinetti CC, et al. Malnutrition may affect common sole (Solea solea L.) growth, pigmentation and stress response: Molecular, biochemical and histological implications. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;161(4):361–371. doi: 10.1016/j.cbpa.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Zeng LQ, et al. The effects of starvation on digestive tract function and structure in juvenile southern catfish (Silurus meridionalis Chen) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;162:200–211. doi: 10.1016/j.cbpa.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 56.Zaldúa N, Naya DE. Digestive flexibility during fasting in fish: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014;169:7–14. doi: 10.1016/j.cbpa.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, et al. Gut health and vaccination response in pre-smolt Atlantic salmon (Salmo salar) fed black soldier fly (Hermetia illucens) larvae meal. Fish Shellfish Immunol. 2019;86:1106–1113. doi: 10.1016/j.fsi.2018.12.057. [DOI] [PubMed] [Google Scholar]
- 58.Sogari G, Amato M, Biasato I, Chiesa S, Gasco L. The potential role of insects as feed: A multi-perspective review. Animals. 2019;9:1–15. doi: 10.3390/ani9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rimoldi S, Gini E, Iannini F, Gasco L, Terova G. The effects of dietary insect meal from Hermetia illucens prepupae on autochthonous gut microbiota of rainbow trout (Oncorhynchus mykiss) Animals. 2019;9(4):143. doi: 10.3390/ani9040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gisbert E, Williot P. Advances in the larval rearing of Siberian sturgeon. J. Fish Biol. 2002;60(5):1071–1092. doi: 10.1111/j.1095-8649.2002.tb01705.x. [DOI] [Google Scholar]
- 61.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 62.Truzzi C, Illuminati S, Annibaldi A, Antonucci M, Scarponi G. Quantification of fatty acids in the muscle of Antarctic fish Trematomus bernacchii by gas chromatography-mass spectrometry: Optimization of the analytical methodology. Chemosphere. 2017;173:116–123. doi: 10.1016/j.chemosphere.2016.12.140. [DOI] [PubMed] [Google Scholar]
- 63.Canonico L, et al. Conversion of raw glycerol to microbial lipids by new Metschnikowia and Yarrowia lipolytica strains. Ann. Microbiol. 2016;66:1409–1418. doi: 10.1007/s13213-016-1228-0. [DOI] [Google Scholar]
- 64.Truzzi C, Illuminati S, Antonucci M, Scarponi G, Annibaldi A. Heat shock influences the fatty acid composition of the muscle of the Antarctic fish Trematomus bernacchii. Mar. Environ. Res. 2018;139:112–128. doi: 10.1016/j.marenvres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 65.Piccinetti CC, et al. Transfer of silica-coated magnetic (Fe3O4) nanoparticles through food: A molecular and morphological study in zebrafish. Zebrafish. 2014;11(6):567–579. doi: 10.1089/zeb.2014.1037. [DOI] [PubMed] [Google Scholar]
- 66.Urán PA, et al. Time-related changes of the intestinal morphology of Atlantic salmon, Salmo salar L., at two different soybean meal inclusion levels. J. Fish Dis. 2009;32:733–744. doi: 10.1111/j.1365-2761.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- 67.Panettieri V, et al. Honey bee pollen in meagre (Argyrosomus regius) juvenile diets: Effects on growth, diet digestibility, intestinal traits, and biochemical markers related to health and stress. Animals. 2020;10:231. doi: 10.3390/ani10020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Notarstefano V, et al. Investigation of human pancreatic cancer tissues by Fourier transform infrared hyperspectral imaging. J. Biophotonics. 2020;13:e201960071. doi: 10.1002/jbio.201960071. [DOI] [PubMed] [Google Scholar]
- 69.Garofalo C, et al. Study of the bacterial diversity of foods: PCR-DGGE versus LH-PCR. Int. J. Food Microbiol. 2017;242:24–36. doi: 10.1016/j.ijfoodmicro.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olivotto I, et al. Cloning and expression of high choriolytic enzyme, a component of the hatching enzyme system, during embryonic development of the marine ornamental fish Chrysiptera parasema. Mar. Biol. 2004;145:1235–1241. doi: 10.1007/s00227-004-1404-9. [DOI] [Google Scholar]
- 72.Vargas-Abúndez AJ, et al. Insect meal based diets for clownfish: Biometric, histological, spectroscopic, biochemical and molecular implications. Aquaculture. 2019;498:1–11. doi: 10.1016/j.aquaculture.2018.08.018. [DOI] [Google Scholar]
- 73.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrocino I, et al. RNA-based amplicon sequencing reveals microbiota development during ripening of artisanal versus industrial Lard d’Arnad. Appl. Environ. Microbiol. 2017;83(16):e00983. doi: 10.1128/AEM.00983-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.