Abstract
We describe the case of a 33-year-old woman with recurrent granulomatous mastitis associated with Corynebacterium kroppenstedtii. This organism has been increasingly associated with granulomatous mastitis, specifically the cystic neutrophilic histopathologic variant, although currently there is a paucity both of reported cases and genomic sequence data. We highlight the challenges in the diagnosis and treatment of this entity, in particular focusing on the various methods of microbiologic identification, including MALDI-TOF, 16 s rRNA PCR and whole-genome sequencing.
Keywords: Corynebacterium, Whole-genome sequencing
Case presentation
A 33-year-old woman was referred to the Infectious Diseases clinic with a mass in her left breast. She was pregnant at the time of assessment at 21 weeks and 6 days gestational age (gravida 3, para 1). She had a 2-year-old son and stopped breastfeeding 11 months prior to conception of her current pregnancy. Her past medical history was otherwise significant for a previous cholecystectomy. The patient developed progressive pain and edema along the medial aspect of her left breast 13 days prior to assessment. There were no skin changes or nipple discharge. She presented to the emergency department of our hospital 2 days later and was prescribed cephalexin for presumed bacterial mastitis. She was referred for breast imaging and underwent an ultrasound of her left breast 2 days later. This demonstrated a lobulated, hypoechoic lesion with peripheral vascularity, measuring 7.7 × 3.4 cm in the largest dimension. There was overlying skin thickening and subcutaneous edema, with no abnormal axillary lymphadenopathy. The radiographic appearance was concerning for a breast abscess. Ultrasound-guided biopsy and aspirate of this lesion was performed, with 6 specimens obtained for evaluation.
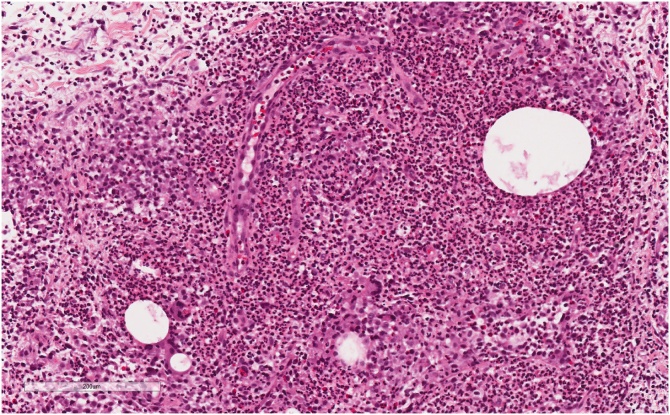
Histopathologic examination demonstrated extensive mixed chronic inflammatory infiltrates, including lymphocytes, plasma cells and neutrophils, centred around breast lobules (Fig. 1). The neutrophils were arranged around microcystic lipid spaces, some of which contained gram positive bacterial organisms. The overall appearance was felt to be in keeping with cystic neutrophilic granulomatous mastitis. Gram stain demonstrated few polymorphonuclear cells with no bacteria. Specimens were planted on Columbia agar with 5% sheep blood, chocolate agar and MacConkey agar and incubated at 37 °C, with no growth at 48 h. 16S rRNA PCR was subsequently performed on a tissue specimen, and Sanger sequencing identified Corynebacterium kroppenstedtii.
Fig. 1.
Hematoxylin and esosin stain of cystic neutrophilic granulomatous mastitis. Breast parenchyma with granulomatous inflammation and cystic spaces rimmed by neutrophils. Gram-positive bacteria were identified in the cystic spaces.
The patient was seen in the Infectious Diseases clinic after these investigations. She had significant clinical improvement with 2 weeks of cephalexin. The patient had minimal pain at her left breast and was systemically well. Physical examination demonstrated a residual nodule in her left breast, with no overlying inflammatory features. She completed a 4-week course of cephalexin, and her disease remained quiescent throughout her pregnancy. Treatment with doxycycline or trimethoprim-sulfamethoxazole, which have more typically been used to treat breast infections associated with Corynebacterium, was considered, but their use was precluded by teratogenicity.
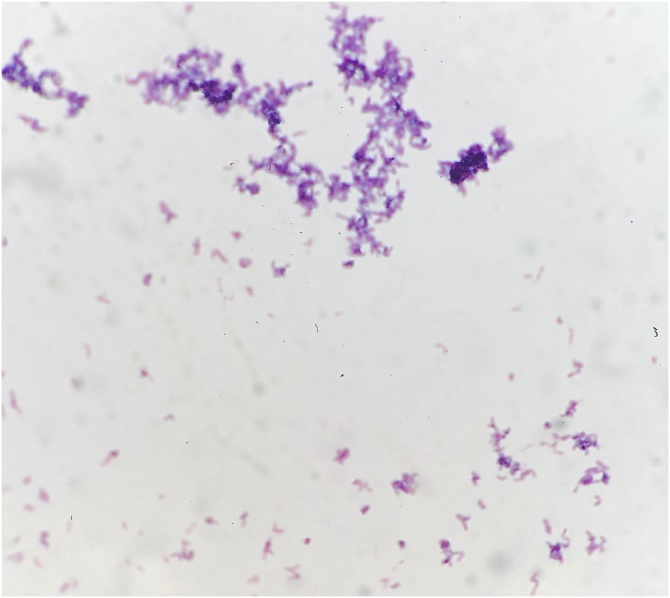
Unfortunately, the patient was seen 3 months after delivery with worsening pain and drainage from her left breast. Ultrasound-guided aspiration was repeated, with demonstration of a complex fluid collection measuring 4.0 × 2.3 cm in the largest dimension. Gram stain demonstrated few polymorphonuclear cells with no bacteria. Given the prior identification of C. kroppenstedtii by 16S rRNA PCR, the aspirate was planted on sheep blood agar enriched with 0.1 % Tween 80, in addition to standard media, and incubated at 37 °C. Small colonies were seen on the Tween 80-enriched sheep blood agar at 24 h and appeared as non-branching gram-positive bacilli on gram stain (Fig. 2). The colonies were identified as C. kroppenstedtii by matrix-associated laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Antibiotic sensitivity testing demonstrated susceptibility to cefotaxime, ciprofloxacin, doxycycline, erythromycin, gentamicin, linezolid, tetracycline and vancomycin based on Clinical and Laboratory Standards Institute breakpoints. The patient completed another 4 weeks of cephalexin, with good response. Repeat ultrasound at end-of-therapy demonstrated interval reduction in the size of the collection, and she has remained well at her most recent assessment, with no significant pain or drainage from her left breast.
Fig. 2.
Gram stain of C. kroppenstedtii. A single colony was selected from blood agar media and bacteria were Gram positive and displayed rod like morphology suggestive of Corynebacterium.
Additionally, we characterized the isolate using whole-genome sequencing. To facilitate this, DNA was extracted from a 48 -h sheep blood agar culture using MasterPure Complete DNA and RNA Purification Kit (Lucigen, Middleton, USA) as per the manufacturer’s protocol. Extracted DNA was quantified using Qubit 1X dsDNA HS Kit (Thermo Fisher Scientific, Waltham, USA) and used as input for library preparation with Nextera DNA Flex Library Prep Kit (Illumina, San Diego, USA) according to the manufacturer’s instructions. Normalized libraries were sequenced on a MiniSeq (Illumina, San Diego, USA) using a mid-output 2 × 150 bp paired-end run that resulted in 633 642 raw reads, respectively. Genome assembly and analysis was performed using the Shovill pipeline on the Galaxy platform [1]. Globally, the draft genome was 2,511,697 bp in length with GC content 56.8 %.
Discussion
Granulomatous mastitis is an uncommon inflammatory disease of the breast occurring in women of reproductive age, within a few years of giving birth. Patients typically present with a unilateral, firm and mildly tender breast mass, often resembling a malignant lesion [2]. There may be features of local inflammation, including erythema and sinus tract formation [3].
Granulomatous mastitis is largely considered an idiopathic condition, with various possible inflammatory and infectious disease associations including amyloidosis, sarcoidosis, hyperprolactinemia, granulomatosis with polyangiitis, tuberculosis and fungal infection [2]. However, cystic neutrophilic granulomatous mastitis, a specific subtype of granulomatous mastitis, has been increasingly associated with C. kroppenstedtii. Histopathologically, cystic neutrophilic granulomatous mastitis is characterized by lipogranulomas, comprised of lipid vacuoles bordered by neutrophils and an outer layer of macrophages, with surrounding mixed inflammatory infiltrate of lymphocytes, neutrophils and giant cells [4]. The vacuoles may contain gram positive bacilli, as seen in our case [4].
Corynebacterium are catalase-positive, non-sporulating, gram positive bacilli, of which more than 90 species have been identified. Non-diphtheroid species are ubiquitous; they colonize mucocutaneous surfaces and frequently contaminate clinical specimens. However, they are capable of invasive infection in appropriate hosts, such as patients who are immunocompromised or have prosthetic devices.
C. kroppenstedtii was first isolated in 1998 from the sputum sample of a woman with pneumonia [5]. The relationship with granulomatous mastitis was described in 2002 in a retrospective study of 24 women with histopathologically-confirmed disease and isolation of Corynebacterium species, of which the majority were C. kroppenstedtii [6]. Since then, the association has been documented in multiple case reports and case series [2,[7], [8], [9], [10], [11], [12], [13]]. C. kroppenstedtii is a lipophilic organism lacking mycolic acids in its cell membrane, conferring the propensity to infect lipid-rich breast tissue [7]. This property also explains the organism’s fastidious nature, and growth on regular media can be challenging, as seen in our case [14]. The addition of a lipid component, such as Tween 80, to sheep blood agar may enhance growth [14]. Microbiologic identification can be performed with MALDI-TOF mass spectrometry and 16S rRNA PCR [14].
The management of cystic neutrophilic granulomatous mastitis associated with C. kroppenstedtii can be challenging. The diagnosis is often delayed given the non-specific symptomatology and imaging findings, need for biopsy for histopathologic evaluation, and difficulty of isolating C. kroppenstedtii by routine culture methods. Lipid components enhance the usually fastidious growth of C. kroppenstedtii, but are generally not added without strong clinical or microbiologic suspicion. No guidelines exist for the treatment of granulomatous mastitis by C. kroppenstedtii, though typically involves prolonged courses of antibiotics for several weeks. The antibiotic susceptibilities of C. kroppenstedtii have been rigorously investigated. A study of 11 breast tissue and aspirate specimens that grew C. kroppenstedtii in culture found susceptibility to rifampin, tetracycline, trimethoprim-sulfamethoxazole, linezolid and vancomycin; resistance to beta-lactams was observed, though this may have been biased by treatment of beta-lactam-sensitive strains [15]. Beta-lactams, fluoroquinolones, clindamycin, aminoglycosides and macrolides have also been used successfully [8]. Given the lipophilic nature of C. kroppenstedtii and its tropism for breast tissue, lipophilic antibiotics, such as doxycycline and trimethoprim-sulfamethoxazole, may be more effective due to greater penetration into lipid-rich environments [15]. Surgery should be considered and was previously thought to be integral to management, but recent evidence suggests procedures outside of drainage of fluid collections are not indicated, and may be associated with increased recurrence and scarring [16,17]. Interestingly, cases of idiopathic granulomatous mastitis, where C. kroppenstedtii has not been identified though is not necessarily absent, are widely treated with corticosteroids [18], and often resolve after diagnosis without medical or surgical intervention. Whether cystic neutrophilic granulomatous mastitis associated with C. kroppenstedtii is phenotypically unique from classic idiopathic granulomatous mastitis, apart from its known histopathologic distinctions, and responds differently to standard treatment requires further elucidation.
The natural history of granulomatous mastitis is characterized by long duration to resolution, despite adequate antibiotic and surgical management. A study of 145 episodes found an average time to resolution of 5 months, with a range of up to 20 months [16]. As in our patient, recurrent disease is also common, and several previous published cases have involved multiple antibiotic courses and surgical procedures [7,8,13].
Conclusion
Our case highlights the importance of considering C. kroppenstedtii in patients with granulomatous mastitis. Growth on conventional media is challenging due to the organism’s fastidious nature; recognition of this disease-bacteria association allows for adjustments to culture methods that will enhance growth. Our case also demonstrates the utility of whole genome sequencing as an adjunctive tool for microbiologic identification.
Declaration of Competing Interest
No COI
Sources of funding for your research
None
Consent
AC; Informed consent obtained in writing
SM, FL, PA; NA
CT, RK; Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
KT; Consent has been obtained
Authors contribution
AC; Formal Analysis Review and Editing
SM; Sequencing analysis infrastructure and technical support
CT; data collection, writing
FL; Data collection, manuscript review
KT; software, analysis
PA; Library construction and sequencing
RK; data collection, data analysis, project supervision and conception, editing and reviewing
CRediT authorship contribution statement
Charlie Tan: Investigation, Formal analysis, Writing - original draft. Fang-I Lu: Investigation, Writing - review & editing. Patryk Aftanas: Data curation, Formal analysis. Kara Tsang: Software, Formal analysis. Samira Mubareka: Resources, Writing - review & editing. Adrienne Chan: Formal analysis, Writing - review & editing. Robert Kozak: Investigation, Formal analysis, Writing - original draft, Writing - review & editing, Supervision.
Acknowledgements
The authors thank Nancy McKinnon, Lacey Kucma and Amber Linkenheld-Struk for their technical assistance.
References
- 1.Jalili V., Afgan E., Gu Q., Clements D., Blankenberg D., Goecks J. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020;48(W1):W395–W402. doi: 10.1093/nar/gkaa434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor G.B., Paviour S.D., Musaad S., Jones W.O., Holland D.J. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109–119. [PubMed] [Google Scholar]
- 3.Eaton E.F., Mathews R.E., Lane P.S., Paddock C.S., Rodriguez J.M., Taylor B.B. A 9-Point risk assessment for patients who inject drugs and require intravenous antibiotics: focusing inpatient resources on patients at greatest risk of ongoing drug use. Clin Infect Dis. 2019;68(6):1041–1043. doi: 10.1093/cid/ciy722. [DOI] [PubMed] [Google Scholar]
- 4.Wu J.M., Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73(8):445–453. doi: 10.1136/jclinpath-2019-206180. [DOI] [PubMed] [Google Scholar]
- 5.Collins M.D., Falsen E., Akervall E., Sjoden B., Alvarez A. Corynebacterium kroppenstedtii sp. nov., a novel corynebacterium that does not contain mycolic acids. Int J Syst Bacteriol. 1998;48(Pt 4):1449–1454. doi: 10.1099/00207713-48-4-1449. [DOI] [PubMed] [Google Scholar]
- 6.Paviour S., Musaad S., Roberts S., Taylor G., Taylor S., Shore K. Corynebacterium species isolated from patients with mastitis. Clin Infect Dis. 2002;35(11):1434–1440. doi: 10.1086/344463. [DOI] [PubMed] [Google Scholar]
- 7.Johnson M.G., Leal S., Plongla R., Leone P.A., Gilligan P.H. The brief case: recurrent granulomatous mastitis due to Corynebacterium kroppenstedtii. J Clin Microbiol. 2016;54(8):1938–1941. doi: 10.1128/JCM.03131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraiya N., Corpuz M. Corynebacterium kroppenstedtii: a challenging culprit in breast abscesses and granulomatous mastitis. Curr Opin Obstet Gynecol. 2019;31(5):325–332. doi: 10.1097/GCO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 9.Gautham I., Radford D.M., Kovacs C.S., Calhoun B.C., Procop G.W., Shepardson L.B. Cystic neutrophilic granulomatous mastitis: the Cleveland Clinic experience with diagnosis and management. Breast J. 2019;25(1):80–85. doi: 10.1111/tbj.13160. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone K.J., Robson J., Cherian S.G., Wan Sai Cheong J., Kerr K., Bligh J.F. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology. 2017;49(4):405–412. doi: 10.1016/j.pathol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Co M., Cheng V.C.C., Wei J., Wong S.C.Y., Chan S.M.S., Shek T. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–747. doi: 10.1016/j.pathol.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Kutsuna S., Mezaki K., Nagamatsu M., Kunimatsu J., Yamamoto K., Fujiya Y. Two cases of granulomatous mastitis caused by Corynebacterium kroppenstedtii infection in Nulliparous Young Women with hyperprolactinemia. Intern Med. 2015;54(14):1815–1818. doi: 10.2169/internalmedicine.54.4254. [DOI] [PubMed] [Google Scholar]
- 13.Le Fleche-Mateos A., Berthet N., Lomprez F., Arnoux Y., Le Guern A.S., Leclercq I. Recurrent breast abscesses due to Corynebacterium kroppenstedtii, a human pathogen uncommon in caucasian women. Case Rep Infect Dis. 2012;2012 doi: 10.1155/2012/120968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauch A., Fernandez-Natal I., Soriano F. A microbiological and clinical review on Corynebacterium kroppenstedtii. Int J Infect Dis. 2016;48:33–39. doi: 10.1016/j.ijid.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Dobinson H.C., Anderson T.P., Chambers S.T., Doogue M.P., Seaward L., Werno A.M. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species. J Clin Microbiol. 2015;53(9):2895–2899. doi: 10.1128/JCM.00760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis J., Cocco D., Matz S., Hsu C.H., Brown M.J., Lee J. Re-evaluating if observation continues to be the best management of idiopathic granulomatous mastitis. Surgery. 2019;166(6):1176–1180. doi: 10.1016/j.surg.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Shin Y.D., Park S.S., Song Y.J., Son S.M., Choi Y.J. Is surgical excision necessary for the treatment of Granulomatous lobular mastitis? BMC Womens Health. 2017;17(1):49. doi: 10.1186/s12905-017-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey T.S., Mackinnon J.C., Bressler L., Millar A., Marcus E.E., Ganschow P.S. Idiopathic granulomatous mastitis--a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20(3):258–266. doi: 10.1111/tbj.12263. [DOI] [PubMed] [Google Scholar]