Abstract
Background
Fibroblast growth factor 21 (FGF21) has become a promising therapeutic target for metabolic diseases such as type 2 diabetes (T2D), obesity and non-alcoholic steatohepatitis. However, the clinical application of natural FGF21 molecule is limited because of its instability in vitro and short half-life in vivo. To improve FGF21’s therapeutic property, we screened high receptor binding FGF21 analogs and made FGF21-Fc-GLP-1 dual-targeted constructs to investigate their activity in a number of experiments .
Methods
Utilizing phage display high-throughput screening we identified mutations that could improve β-Klotho binding property of FGF21. IgG4 Fc was fused to FGF21 variants to extend the in vivo half-life. We further explored the potential synergistic actions of FGF21 with the incretin glucagon-like peptide-1 (GLP-1) by generating GLP-1-Fc-FGF21 dual agonists.
Findings
Two Fc-FGF21 variants showed enhanced β-Klotho binding affinity in vitro as well as improved glucose lowering effect in vivo. One of the dual agonists, GLP-1-Fc-FGF21 D1, provided potent and sustained glucose lowering effect in diabetic mice models. It also demonstrated superior weight loss effect to GLP-1 or FGF21 alone. Moreover, GLP-1-Fc-FGF21 D1 exhibited strong anti-NASH effect in the high-fat diet-induced ob/ob model as it improved liver function, serum and hepatic lipid profile and reduced NAFLD activity score with an efficacy superior to either FGF21 or GLP-1 analogs alone.
Interpretation
This novel GLP-1/FGF21 dual agonist is worth clinical development for the treatment of T2D, obesity and NASH.
Funding
HEC Pharm R&D Co., Ltd, National natural science fund of China.
Keywords: Diabetes, NASH, FGF21, GLP-1, Fusion protein
Research in context.
Evidence before this study
Glucagon-like peptide-1 (GLP-1)’s effects on blood glucose and body weight have been well established with several GLP-1 analogs approved for type 2 diabetes (T2D) and one analog Liraglutide approved for obesity treatment. The multifaceted functions of GLP-1 have made it a popular anchor target for multi-targeted drug design aiming for agents with better efficacies on metabolic diseases. Fibroblast growth factor 21 (FGF21) is a promising molecule for the treatment of diabetes and other metabolic diseases. However, several FGF21 analogs that are currently under clinical development showed limited efficacy on glycemic control and body weight management although better efficacy on non-alcoholic steatohepatitis. The complemental functions of GLP-1 and FGF21 make it interesting to test whether a combination of these two hormones could provide superior efficacies in treating metabolic diseases. Indeed, a very recent study reported the design and construction of a GLP-1-FGF21 fusion protein connected by an elastin-like polypeptide linker. The resulted dual agonist showed potent glucose and body weight lowering efficacy.
Added value of this study
In this study we optimized an FGF21 protein and presented a novel long-acting GLP-1/FGF21 dual-targeted fusion protein. This dual agonist can potently activate both GLP-1 and FGF21 receptors and in different mice models, the dual agonist shows potent anti-diabetic and anti-obesity effects. More importantly, we show that the dual agonist has superior anti-NASH efficacies in mice disease models. The efficacies are better than marketed GLP-1 analogs or single-targeted FGF21 analogs.
Implications of all the available evidence
Our findings further demonstrate that the two endocrine hormones GLP-1 and FGF21 can have additive and potentially synergistic functions in vivo. The superior and pleiotropic effects of the GLP-1/FGF21 dual agonist in preclinical mice models suggest that the dual agonist may be used to treat T2D, obesity and potentially NASH.
Alt-text: Unlabelled box
1. Introduction
Diabetes is a chronic metabolic disease that affects the health of increasing number of patients. It is characterized by high blood glucose level and is often associated with various cardiovascular risks such as insulin resistance, obesity, hypertension and dyslipidemia. Diabetes has become one of the leading global diseases that threaten human health [1,2]. In Diabetic patients, besides glycemic control, cardiovascular disease (CVD) and other comorbidities and complications such as diabetic nephropathy, diabetic retinopathy, diabetic foot, obesity and non-alcoholic fatty liver disease (NAFLD) also need to be dealt with [2]. Therefore, the development of drugs that can comprehensively improve the internal metabolism, provide patients with multiple benefits including liver condition improvement and cardiovascular benefit while controlling the blood-glucose and body weight, are urgently needed.
Fibroblast growth factor 21 (FGF21) is a secreted endocrine that regulates glucose and lipid metabolism [3], [4], [5]. Its biological activities mainly involved in lowering blood glucose and improving blood lipid profiles. FGF21 is secreted primarily in the liver, but it is also expressed in other tissues associated with lipid and glucose metabolism, such as adipose tissue, pancreas and skeletal muscle. Kharitonenkov et al. firstly reported the metabolic regulation function of FGF21 [6]. Subsequent studies found that FGF21 could independently regulate the glucose metabolism and glucose level [7,8]. FGF21 was also shown to be highly effective in alleviating insulin resistance, improving the function and quality of islet insulin cells, and optimizing the lipoprotein profile [6,9]. It is therefore considered an important potential therapeutic target for type 2 diabetes and non-alcoholic fatty liver disease (NAFLD) [4,10].
FGF21 functions by binding through its C terminal portion to the transmembrane co-receptor β-Klotho, and interacting with fibroblast growth factor receptor (FGFR) through the N-terminal portion [11], to form a stable FGF21/β-Klotho/FGFR complex, which activates downstream receptor tyrosine kinase activities [12,13]. It has been reported that the binding of FGF21 to its co-receptor β-Klotho play critical roles in its biological activity [14,15]. Wild-type FGF21 has a very short half-life, with only 0.5 to 2 h in vivo [16]. The recombinant FGF21 has poor stability and many deficiencies such as high aggregation tendency and high sensitivity to protease cleavage. These properties affected the clinical application of wild-type FGF21 [17]. In addition, because the three-dimensional structure of the complex between full length FGF21 and its receptors has not been defined yet, modifications of its sequence to obtain more active and stable variants have been inefficient [14].
In addition to the many insulin products used in diabetes treatment, Glucagon-like peptide-1 (GLP-1) emerged as a new class of agents for glycemic control in diabetic patients. GLP-1 is a 30 or 31 amino acid long peptide hormone secreted by the intestine and has the activity to lower the blood glucose level by promoting glucose-dependent insulin secretion as well as inhibiting glucagon secretion [18,19]. GLP-1 analogs have been widely used in the clinical treatment of type 2 diabetes and has also shown therapeutic potential in obesity and NASH. Recently, there are studies demonstrate that FGF21 and GLP-1 have interplays in hepatic glucose metabolism and regulation in hepatocytes, in which GLP-1 analogs can stimulate hepatic FGF21 production [20,21]. Additionally, while GLP-1 lowers body weight mainly through inhibiting energy intake, FGF21 has been shown to stimulate energy expenditure [22], suggesting that these two hormones have complementary effects on body weight control. Together, these findings indicate that GLP-1 and FGF21 could have synergistic functions in vivo.
In this study, we optimized the pharmaceutical property of FGF21 not only by extending its half-life, but also by directly enhancing its affinity to β-Klotho through mutagenesis and screening. In addition, we further harnessed the potential synergistic effects of FGF21 and GLP-1 by constructing GLP-1 receptor and FGF receptor dual agonists with the high affinity FGF21. We demonstrate that one of these dual agonists, namely GLP-1-Fc-FGF21 D1, had potent and sustained effects on glucose lowering, weight loss and lipid profile improvement. This novel dual-targeted agonist is thus a new promising therapeutic candidate for the treatment of T2D, obesity and NASH.
2. Methods
2.1. Screening of FGF21 with high β-klotho binding affinity
The extracellular domain of the human β-klotho protein (R&D Systems, Cat: 5889-KB) was biotinylated (EZ-link sulfo-NHS-LC-Biotin, Thermo scientific, Cat: 21335), immobilized on streptavidin-coated magnetic Dynabeads M-280 (Invitrogen, Cat: 11206D), and panned with FGF21 mutant phage display library (constructed in house). After panning, phage with high β-Klotho binding affinity were eluted and used to infect TG1 bacterial cells (Lucigen, Cat: 60502-2). Phage were then rescued with helper phage M13KO7 (Nordic BioSite, Cat: BU-00034). Individual colonies were picked and cultured in 96-well plates overnight. The supernatant was obtained by centrifugation and detected by Elisa. The clones with strong signal were selected for sequencing.
2.2. Construction of fusion protein expression plasmids
To make Fc-FGF21 expressing plasmids DNA encoding Fc-FGF21 fusion proteins (Table 1) were synthesized at Suzhou Genewiz Biotechnology Co., LTD. The Fc region was 228 amino acid portion from IgG4 according to DrugBank (DB09045 Dulaglutide). The synthesized DNA was cut by HindIII (Takara, Cat: 1615) and EcoRI (Takara, Cat: 1611) and inserted into pcDNA3.4 (Invitrogen, Cat: A14697) and modified in house via polyclonal sites insertion. DNA encoding the dual-targeted GLP-1-Fc-FGF21 fusion proteins (Table 2) were constructed through overlapping PCR amplification reactions. First, DNA with sequence containing a HindIII, a portion of signal peptide coding sequence, the GLP-1 coding sequence and a linker was generated with the primers (auz-f, lfc1-r) and the GLP-1 containing plasmid GLP-1-PUC57 were synthesized from Genscript Biotech Corp. A second sequence containing an overlapping portion of the above linker, Fc-FGF21 sequences and an EcoRI was made by PCR with the primers (lfc1 – F, fgf21-r) and the Fc-FGF21 containing plasmids as templates. (GLP-1-Fc-FGF21 D1, D2 and D3 were constructed by Fc-FGF21 S1, S2 and S3 mutants, respectively). Finally, PCR with the two flanking primers (auz-f, fgf21-r) were used to generate the fusion proteins with HindIII (Takara, Cat: 1615) and EcoRI (TaKaRa, Cat: 1611) which were ligated to pcDNA3.4.
Table 1.
Fc-FGF21 mutants. S1, S2 and S3 were constructed by linking Fc fragment to the N terminal of FGF21 mutants.
| Fc-FGF21 mutants | Fc-FGF21 mutants ID |
|---|---|
| Fc-FGF21 (L98R, S167H, P171A, R175L) | S1 |
| Fc-FGF21 (L98R, S167H, P171A, R175P) | S2 |
| Fc-FGF21 (L98R, S167H, P171A) | S3 |
| Fc-FGF21 (L98R, P171A) | Fc-FGF21(RA) |
Table 2.
GLP-1-Fc-FGF21 mutant fusion proteins. D1, D2 and D3 were constructed by linking S1, S2 and S3 mutants with GLP-1, respectively.
| GLP-1-Fc-FGF21 mutants | GLP-1-Fc-FGF21 mutants ID |
|---|---|
| GLP-1-Fc-FGF21 (L98R, S167H, P171A, R175L) | D1 |
| GLP-1-Fc-Fc-FGF21 (L98R, S167H, P171A, R175P) | D2 |
| GLP-1-Fc-Fc-FGF21 (L98R, S167H, P171A) | D3 |
Primers used in the experiment were synthesized by Suzhou Genewiz Biotechnology Co., LTD., The primer sequences are as follows:
Upstream fragment (including glp-1 part) amplification primer:
Primer auz-f: cccaagcttgccgccaccatgaccagactgaccgtgc
Primer lfc1-r: gccgtacttgctctcagatccaccgcctccgcttc
Downstream fragment (fc-fgf21) amplification primer:
Primers lfc1 - f: gcggaggcggtggatctgagagcaagtacggccc
Primer fgf21-r: ccggaattctcatcagctggcgtagctagggct
SOE PCR primer:
Primer auz-f: cccaagcttgccgccaccatgaccagactgaccgtgc
Primer fgf21- r: ccggaattctcatcagctggcgtagctagggct
2.3. Transfection and generation of stable cells expressing FGF21 derivative proteins
To establish stable expressing cells, 293F cells (Invitrogen, Cat: R79007) were transfected with linearized plasmids expressing the various FGF21 proteins using the FreeStyle™ MAX Reagent transfection kit (Invitrogen, Cat: 16447100) as described with the provided protocol. Stable cells were selected with 400 μg/mL G418 (Merck, Cat: 345810-5GM).
2.4. Protein purification and MS analysis
Cells expressing either Fc-FGF21 or GLP-1-Fc-FGF21 chimeric proteins were centrifuged at 200 xg for 10 min, and the supernatant was collected by centrifugation at 8000 rpm for 30 min. The supernatant with Fc containing proteins were loaded onto Protein A column (GE, Cat: 17040301) with equilibrium solution (20 mM PBS, 0.15 m NaCl, pH7.4). Fc-FGF21 proteins were eluted with 0.1 m citric acid buffer (XiLONG SCIENTIFIC, Cat: 1029054-02-09), pH3.2 while FGF21-GLP-1 fusion proteins were eluted with 0.1 m glycine (BBI Life Science, Cat: G130235), pH3.2 ± 0.2. The eluted proteins were collected, concentrated and analyzed by 10% SDS-PAGE electrophoresis and by mass spectrometry. For Mass spec, the samples were acidified by diluting 1:1 with 0.1% formic acid (Sigma-Aldrich, St Louis, MO) followed by liquid chromatography mass spectrometry analysis (Accurate-Mass Q-TOF LC/MS, Type G6530, Agilent Technologies). The samples were separated over an Agilent Poroshell 300SB-C8 (0.5 × 75 mm) column maintained at 60 °C with a flow rate of 0.6 ml/min. Mobile phase A was water with 0.1% formic acid (Sigma, Cat: 94318), and mobile phase B was acetonitrile with 0.1% formic acid. The mass spectrometer was run in positive MS only mode scanning from 800 to 3000 m/z and data was acquired with MassLynx (Waters) 4.1 software. The TOF-MS signal was summarized and de-convoluted using MaxEnt1 (Waters) program.
2.5. Protein binding analysis
The affinity of the FGF21 derivative proteins with β-Klotho protein were tested by Fortebio as follows: Fusion protein samples were diluted to 40 nM with buffer 100 ml PBS (BD, Cat: 276–117) with 0.1 g BSA (Aladdin, Cat: A116563–25 g) and 20 μl Tween 20 (Sigma, Cat: P1319), then added into the wells of the plate. Using affinity detection OCTET SYSTEM RED 96 SYSTEM, the binding of the fusion proteins can be detected automatically by streptavidin probe (PALL, Cat: 18–5019) which could react with biotinylated (EZ-link sulfo-NHS-LC-Biotin, Thermo scientific, Cat: 21335) β-Klotho protein (R&D Systems, Cat: 5889-KB) and the affinity was caculated.
2.6. In vitro functional assays
Stable HEK293 cell lines expressing GLP-1R and/or β-Klotho were established by transfecting HEK293 cells (ATCC Cat# CRL-1573, RRID: CVCL_0045) with GLP-1R and/or β-Klotho expression plasmids (constructed in house) [14,17,23]. HEK293 cells expressing both GLP-1R and β-Klotho cells were established by transfecting HEK293/GLP-1R cells with β-Klotho expression plasmids.
Flow cytometry was performed to analyze the expression of GLP-1R and/or β-Klotho protein on the surface of HEK293 cells. HEK293/GLP-1R or HEK293/GLP-1R-β-Klotho cell line was stained with Mouse Anti-Human GLP-1R Monoclonal Antibody (R&D Systems, RRID: AB_2109906) or isotype control antibody Mouse IgG Flow Cytometry Isotype Control (R&D Systems, Catalog # MAB0041). Cells were incubated for 40 minutes in the dark and washed three times in PBS buffer (supplemented with 0.5% BSA), followed by Anti-Mouse IgG (FITC) secondary antibody (abcam, RRID: AB_955241), and analyzed via flow cytometry. Rabbit Anti-Human β-Klotho monoclonal antibody (R&D Systems, Cat #MAB58891) or isotype control antibody (R&D Systems, Cat # AB-105-C), and anti-Rabbit IgG (PE) secondary antibody (R&D Systems, Cat # F0110) were used for β-Klotho expression. BD FACSVerse cell analysis machine was used to detect FITC dyes (530/30 nm filter) and PE dyes (575/26 nm filter). BD Cell Quest Software (BD Biosciences) were used for data acquisition and analysis. For ERK (extracellular regulated protein kinases) phosphorylation assay, HEK293/β-Klotho cells were seeded in 96 well plate overnight in DMEM medium (Gibco, Cat: 10569010) with 10% fetal bovine serum (FBS) (Gibco, Cat: 16000–044). Then remove culture media was removed and the cells were starved in DMEM medium (Gibco, Cat: 10569010) for 4 h. After incubation for 20 min with FGF21 derivative proteins native FGF21 (abcam Cat# ab133137), Dulaglutide, GLP-1-Fc-FGF21 D1 and Fc-FGF21 S1 (made in house), the cells were lysed and the activity of ERK phosphorylation was measured by reading plate on Envision 2104 plate reader, using AlphaLISA® SureFire® Ultra™ p-ERK 1/2 (Thr202/Tyr204) Assay Kit (PerkinElmer, Cat: ALSU-PERK-A500).
For GLP-1R activation, cells were prepared in 96 wells the day before the experiment, before incubation with FGF21 derivative proteins for 30 min. The cells then were lysed and cAMP concentration was measured using a CISBIO cAMP detection kit (Cisbio Cat: 62AM6PEC) with an HTRF compatible reader at an excitation wavelength of 337 nm to detect the fluorescence values of 665 nm and 620 nm.
All the data were analyzed using GraphPad Prism 5.
2.7. Ethics statement
Animal studies were performed according to protocol procedures approved by the Institutional Animal Care and Use Committee at the Laboratory Animal Center of Sunshine Lake Pharma Co., LTD, and the animals received humane care.
2.8. Pharmacokinetics studies in mice and rats
Sprague-Dawley (SD) rats (Shanghai SLAC Laboratory Animal Co., Ltd) and C57BL6 mice with SPF (Shanghai SLAC Laboratory Animal Co., Ltd) were group-housed and allowed a 1-week acclimation before study initiation. The animals were maintained on a 12 h/12 h light/dark cycle with ad libitum access to food and water during the experiment and divided into groups of 5 according to body weight. PK of GLP-1-Fc-FGF21 D1 was evaluated in SD rats (n = 5) and C57BL6 mice (n = 5) at 0.5 mg/kg and 1.0 mg/kg following SC injections, respectively. Plasma samples were collected before and at the indicated times post dosing and were stored at -80 °C in 0.5% EDTA-K2 (Genview, DH513-2) until used for analysis by Elisa. To determine the drug's concentration, D1 was captured by an anti-GLP-1 (7-37) and GLP-1 (7-36) amide (N-terminal specific) antibody (Bioporto, Cat: ABS 033-10), and detected with a secondary mouse anti-human IgG Fc-HRP antibody (Southern Biotech, Cat: 9200-05). The experimental data were recorded with mean +/- standard deviation and statistically analyzed by EXCEL. All pharmacokinetic parameters were determined from individual animal data using non-compartmental analysis using software WinNonlin (Version 6.3, Pharsight, CA).
2.9. Study of Fc-FGF21 of blood-glucose reducing effect in ob/ob mouse model
Six to seven-week old ob/ob mice with SPF (the model animal research center of Nanjing University) were maintained on a 12 h/12 hour light/dark cycle with ad libitum access to food and water. Animals were group-housed and allowed a 1-week acclimation before study initiation. The mice were randomly divided into groups of 8 according to blood glucose level and body weight. Mice were subcutaneously injected with indicated doses of testing agents or equal volume of PBS. The mice were given free diet and drinking water during the experiment. Blood glucose of the mice in each group was monitored and measured with Accu-Chek Performa (Roche). The experimental data were analyzed statistically.
2.10. Study of blood-glucose reducing effect in db/db mouse model
Db/db male mice with SPF (Jiangsu Gem Pharmatech Biotechnology Co., LTD) at age of 6 to 7 weeks were maintained on a 12 h/12 h light/dark cycle with ad libitum access to food and water. Animals were group-housed and allowed a 1-week acclimation before study initiation. The mice were divided into groups of nine to ten according to their blood glucose level and body weight. Mice were subcutaneously injected once a week for two weeks with indicated doses of testing agents or equal volume of PBS. The mice were given free diet and drinking water during the experiment. Blood was taken from the tip of the tail of the mice, and the blood glucose of the mice in each group was monitored and measured with Accu-Chek Performa (Roche). The experimental data were analyzed statistically.
2.11. Repeated dose study in HFD induced ob/ob mice
Six to seven-week old ob/ob mice with SPF (the model animal research center of Nanjing University) were selected and fed with high-fat diet (R&D, Cat: D12492) for 2 weeks. The mice were randomly divided into groups of 8 according to blood glucose level and body weight. Mice were subcutaneously injected with testing agents or PBS twice a week (day 0 and day 3) for 4 consecutive weeks, 9 injections in total. The mice were given free diet and drinking water during the experiment. Blood glucose, fasting blood glucose measured with Accu-Chek Performa (Roche), blood lipids changes, hepatic function index AST/ALT, the change of TG/CHO content in liver tissue, liver weight and the liver tissue pathology change, etc. were measured and analyzed by using assay kits for AST (Roche, Cat: 20764949322), ALT (Roche, Cat: 20764957322), TG (Roche, Cat: 20767107322), CHO (Roche, Cat: 3039773190) on a Cobas c311 automatic biochemical detector (Roche). The experimental data were analyzed statistically.
2.12. Histological analysis
Mice liver samples were fixed in 10% (w/v) phosphate-buffered formalin (Fisher Scientific, Cat: SF100-4) for 48 h. After dehydration through a graded series of ethanol solutions, the tissues were embedded in paraffin wax (Macklin, Cat: P815421). Serial frontal sections were cut and stained with hematoxylin and eosin (H&E) (abcam, Cat: ab245880).
2.13. Statistical analysis
The experimental data were recorded with ±SD and statistically analyzed by EXCEL. Data collected in this experiment were in the format of Mean ± standard deviation (Mean ± SD), and Student's t-test was used for statistical analysis. The data were summarized and graphically expressed in tables, and GraphPad Prism5 statistical software was used for figure generation.
2.14. Role of funders
CC, SL, YL, LL, XL, XG, JY, BG, XC, WL and XT from funding company HEC Pharm R&D Co., Ltd participated in the study design, data collection, data analysis, interpretation and writing of the report. The National natural science fund of China had no role in study design, data collection, analysis, interpretation of data, writing of the report or in the decision to submit for publication. The corresponding author had full access to all the data in the study and has final responsibility for the decision to submit for publication.
3. Results
3.1. Screening of FGF21 with high β-klotho binding affinity and construction of Fc-FGF21 and GLP-1-Fc-FGF21 fusion proteins
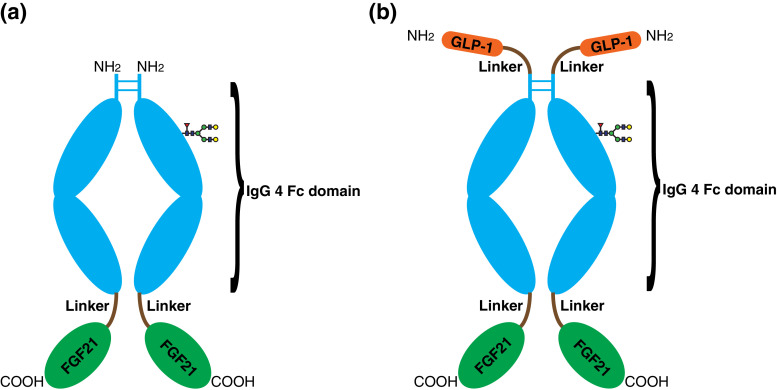
To obtain FGF21 analogs with enhanced binding affinity to β-Klotho, we screened phage libraries displaying FGF21 with random mutations in its DNA sequences. The library size was estimated to be about 108 clones. After panning, phages containing FGF21 with high β-klotho binding affinity were enriched and detected. Ten monoclonal enriched phages were sequenced and five mutations were found in these clones, S167H, R175P/L/H, G113R, L114Q, R135C. S167H was found in nine of the ten sequenced clones while R175 was mutated to different amino acids in three different clones. Protein expression analysis showed that FGF21 bearing G113R, L114Q and R135C mutations were not stable (data not shown), so these mutations were not characterized further. It should be pointed out that previous study has shown that FGF21 with L98R and P171A changes could prevent aggregation in vitro and degradation in vivo, respectively, without interfering its binding to β-klotho [17]. This FGF21 (L98R, P171A) is used as our starting construct and will be referred as FGF21 (RA) thereafter. Consistent with the screening results, structural analysis shows that Serine167 and Arginine175 are located in close vicinity to the β-klotho binding site of FGF21, indicating that these two residues are involved in the high affinity binding of FGF21 with β-klotho (Supplementary Fig. 1). Accordingly, we designed three FGF21 variants S1 (S167H, R175L), S2 (S167H, R175P) and S3 (S167H). To better characterize the constructed FGF21 variants in vitro and in vivo, we further fused these variants to the C-terminus of IgG4 Fc through a linker peptide, generating three Fc-FGF21 proteins (Fig. 1a). The resulting Fc fusion proteins, namely Fc-FGF21 S1, Fc-FGF21 S2 and Fc-FGF21 S3, were expressed and the homodimer formation were confirmed to have molecular weight of 94 KDa by SDS-PAGE and mass spectrometry analysis (Supplementary Fig. 2a and 2c).
Fig. 1.
Schematic representation of FGF21 fusion proteins structures. (a) Fc-FGF21 S1, S2 and S3. The linker consists of a 15 amino acids linked by poly(G4S)3. (b) GLP-1-Fc-FGF21 D1, D2 and D3.
To exploit the multi-functions of GLP-1 in glucose and lipid metabolism, we further linked a GLP-1 analog to the Fc-FGF21 constructs via a peptide linker to the N-terminal Fc portion to make a dual target protein GLP-1-Fc-FGF21 (Fig. 1b). The hypothesis is that the two different mechanisms of glucose and lipid control might have synergistic effect. The dual target fusion proteins, namely GLP-1-Fc-FGF21 D1, D2 and D3 (corresponding to Fc-FGF21 S1, Fc-FGF21 S2 and Fc-FGF21 S3, respectively), were confirmed to have molecular weight of 103 KDa by SDS-PAGE and MS analysis (Supplementary Fig. 2b and 2c). It was noted that GLP-1-Fc-FGF21 D2 showed obvious degradation, thus this molecule was omitted and GLP-1-Fc-FGF21 D1 and D3 were further studied for their β-klotho binding affinity, in vitro activity and in vivo efficacy in animal models.
3.2. FGF21 fusion proteins have high affinity to β-Klotho
To further probe the binding ability of mutants screened by phage display to β-Klotho protein after the formation of fusion protein, the affinities of the Fc- and GLP-1- fusion FGF21 proteins to the β-Klotho receptor were measured by Fortebio assay. As shown in Table 3 and Supplementary Fig. 3, compared with FGF21 (RA), the mutants did show improved β-Klotho binding affinity, and Fc-Fc-FGF21 S1 has the best affinity with β-Klotho. The addition of GLP-1 did not negatively affect the binding of FGF21 to β-Klotho. In fact, the affinity of GLP-1-Fc-FGF21 D1 and D3 had higher affinity than the single-targeted counterparts, especially GLP-1-Fc-FGF21 D1. Fc-FGF21 S1 and GLP-1-Fc-FGF21 D1 were then chosen for further investigation in functional assays.
Table 3.
Summary of the kinetic and affinity constants (KD) for the interaction between FGF21 mutants and β-Klotho using streptavidin (SA) biosensors.
| Sample ID | KD (M) |
|---|---|
| Fc-FGF21 (RA) | 7.67E-10 |
| Fc-FGF21 (S167H,R175L) S1 | 3.16E-10 |
| Fc-FGF21 (S167H) S3 | 5.90E-10 |
| GLP-1-Fc-FGF21 (S167H,R175L) D1 | 9.24E-11 |
| GLP-1-Fc-FGF21 (S167H) D3 | 2.40E-10 |
3.3. In vitro potencies of the single- and dual-targeted Fc fusion proteins
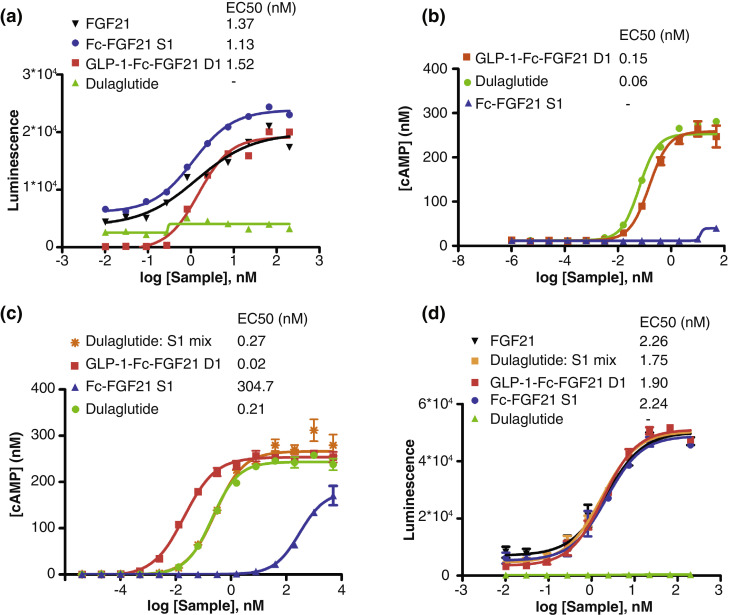
The fusion protein GLP-1-Fc-FGF21 D1 was further investigated and compared with Fc-FGF21 S1 and Dulaglutide (a GLP-1-Fc fusion protein) in cell based assays for their dual function in activating through the FGF21 and the GLP-1 pathways. For these purposes, we constructed reporter HEK293 cell lines expressing β-Klotho alone, expressing GLP-1 receptor (GLP-1R) alone and expressing both GLP-1R and β-Klotho. The result of flow cytometry analysis demonstrated expression of GLP-1R, β-Klotho, or GLP-1R/ β-Klotho in HEK293 cells (Supplementary Fig. 4). FGF21 functions to activate the signaling cascade downstream of FGF receptors in a β-Klotho-dependent manner in vitro while GLP-1 can activate through the GLP-1R and downstream pathways. As expected, Fc-FGF21 S1 functions as FGF21 derivatives and led to the phosphorylation of ERK1/2 in HEK293 cells expressing β-Klotho (Fig. 2a, 2d), but not in HEK293 expressing only GLP-1R (Fig. 2b). Dulaglutide, the GLP-1 analog, on the other hand, activated cAMP production in GLP-1R expressing HEK293 cells (Fig. 2b and 2c). More importantly, the dual GLP-1-Fc-FGF2 D1 protein activated through both GLP-1R and β-Klotho in all three cell lines (Fig. 2a-d). From Fig. 2a, the ability of GLP-1-Fc-FGF21 D1 (EC50 1.52 nM) to stimulate ERK1/2 phosphorylation was similar to that of Fc-FGF21 S1 (EC50 1.13 nM) and native FGF21 (EC50 1.37 nM) when functions through the FGF21 receptor. From Fig. 2b, GLP-1-Fc-FGF21 D1 and Dulaglutide had similar profiles when stimulating cAMP production in HEK293 cells expressing GLP-1R alone. However, the activity of GLP-1-Fc-FGF21 D1 was attenuated by 2~3 fold compared to Dulaglutide with EC50 0.15 nM and 0.06 nM, respectively.
Fig. 2.
Dual target GLP-1-Fc-FGF21 D1 functions through both GLP-1R and FGF21R/β-Klotho pathways. GLP-1-Fc-FGF21 D1, Fc-FGF21 S1 and Dulaglutide were investigated in cell based assays for their trans-activating activity. (a) ERK phosphorylation was measured in HEK293 cells expressing β-Klotho treated with native FGF21, GLP-1-Fc-FGF21 D1, Fc-FGF21 S1 or Dulaglutide. (b) cAMP secreted from HEK93 cells expressing GLP-1R treated with GLP-1-Fc-FGF21 D1, Fc-FGF21 S1 or dulaglutide. (c) HEK93 cells expressing GLP-1R and β-Klotho were treated with GLP-1-Fc-FGF21 D1, Fc-FGF21 S1, Dulaglutide or a 1:1 mixture of Fc-FGF21 S1 and Dulaglutide and the secreted cAMP were measured. (d) ERK phosphorylation was measured in HEK293 cells expressing GLP-1R and β-Klotho treated with GLP-1-Fc-FGF21 D1, Fc-FGF21 S1, Dulaglutide or a 1:1 mixture of Fc-FGF21 S1 and Dulaglutide. Data are showed as mean +/- standard errors of means, n=3.
From Fig. 2c and 2d, in HEK293 cells expressing both GLP-1R and β-Klotho, the results were more complex. As expected, the GLP-1 analog Dulaglutide and Fc-FGF21 S1 both dose dependently activated cAMP secretion through binding to their respective receptor GLP-1R and β-Klotho. We found that the ability of GLP-1-Fc-FGF21 D1 (EC50 1.90 nM) to stimulate ERK1/2 phosphorylation was similar to that of Fc-FGF21 S1 (EC50 2.24 nM) and Dulaglutide mix S1 in the cells expressing both GLP-1 and β-klotho. However, the activation through GLP-1R by Dulaglutide is much more potent than that through β-Klotho by its ligand Fc-FGF21 S1 with EC50 0.21 nM and 304.7 nM, respectively. The activity of the dual-target GLP-1-FGF21 D1 fusion protein was much higher than that of Fc-FGF21 S1 since it has the GLP-1 moiety. Most importantly GLP-1-Fc-FGF21 D1 fusion protein was about 10 times more potent than Dulaglutide with an EC50 0.02 nM, suggesting synergistic effect. Furthermore, when Dulaglutide and single-target protein Fc-FGF21 S1 were mixed at an equal molar ratio, the mixture has activity similar to that of dulaglutide (0.27 nM compare to 0.21 nM), suggesting that simple mixing of FGF21 and GLP-1 will not result in synergistic effect in this system. The results showed that association of dual-target protein GLP-1-Fc-FGF21 D1 with β-Klotho and GLP-1R simultaneously could synergistically increase the cAMP level and achieve stronger activation compared to single-target proteins or the mixture.
3.4. High affinity Fc-FGF21 S1 and S3 has improved anti-diabetic activity than Fc-FGF21 (RA) in vivo
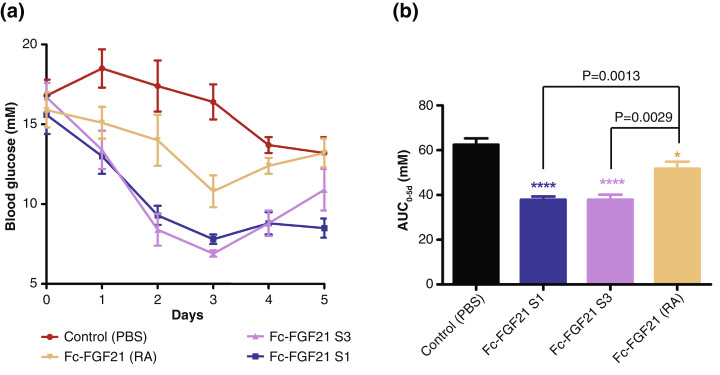
To assess whether the higher binding affinity of Fc-FGF21 S1 and S3 to β-Klotho can lead to improve in vivo activity, owing to the limited glucose lowering ability of FGF21, Fc-FGF21 S1 and S3 were compared to Fc-FGF21 (RA) in ob/ob diabetic mice with a mild hyperglycaemia for their glucose lowering activity. A single subcutaneous injection of the Fc-FGF21 fusion proteins led to significant reduction of blood glucose level in ob/ob mice (Fig. 3a). Importantly, both Fc-FGF21 S1 and S3 showed better efficacy than Fc-FGF21 (RA) during the whole administration cycle, especially after day 2 when glucose reducing effect was more obvious, suggesting that the enhanced receptor binding affinity can indeed lead to improve in vivo function of FGF21. The results also showed that the duration of glucose lowering in Fc-FGF21 S1 treated mice is slightly longer than that treated with Fc-FGF21 S3 (Fig. 3a). The change in AUC for blood glucose by Fc-FGF21 S1 and S3 were similar and both were significantly decreased comparing with Fc-FGF21 (RA) (Fig. 3b).
Fig. 3.
Fc-FGF21 S1 and S3 has improved anti-diabetic effects than Fc-FGF21 (RA) in type 2 diabetic ob/ob mice. Drugs (20 nmol/kg) were given via single subcutaneous injection (n=8) and treated for the indicated time. Random blood glucose was measured (a); and glucose AUC were calculated (b). Data are presented as mean +/- standard deviation, and Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 (vs. control).
3.5. GLP-1/FGF21 dual agonist have improved anti-diabetic activity than single-targeted Fc-FGF21 or Dulaglutide in vivo
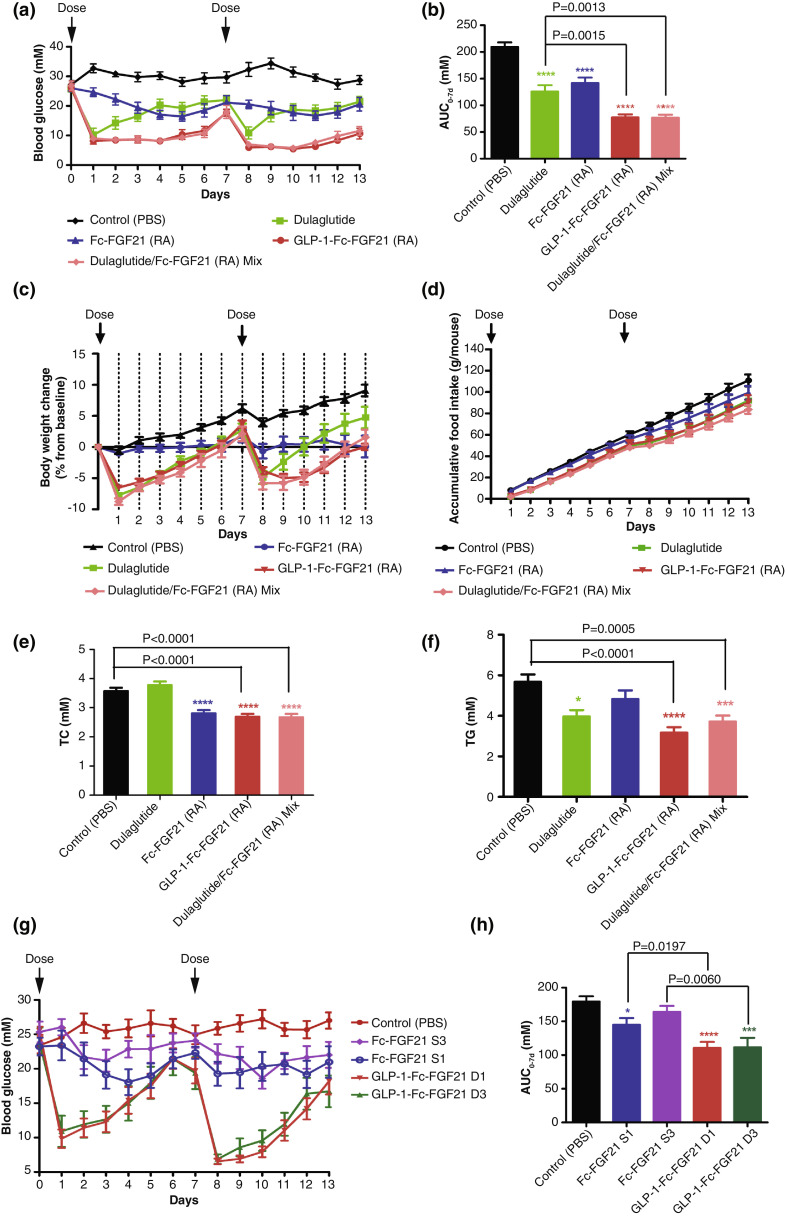
Since both FGF21 and GLP-1 regulate glucose and lipid metabolism and they further have interplays in their function, it is anticipated that the dual-targeted GLP-1-FGF21 construct will have superior activity in vivo [20,21]. To fully characterize the improved anti-diabetic activity of the dual agonist, we assessed the activities of the series of constructs in the highly hyperglycaemic db/db mice model. First, we want to investigate whether indeed the dual protein is better than GLP-1 and FGF21 alone. Spontaneous diabetic db/db mice were subcutaneously injected once weekly for two weeks with 40 nmol/kg single-targeted Fc-FGF21 (RA), dual-targeted GLP-1-Fc-FGF21 (RA), Dulaglutide (40 nmol/kg), or Dulaglutide/Fc-FGF21 (RA) mixture (40 nmol/kg: 40 nmol/kg). As shown in Fig. 4a, both single-targeted and dual-targeted proteins could reduce blood glucose. The kinetics after a single injection of Fc-FGF21 (RA), which lowered glucose gradually for 5 days before a slight rebound, was different from that of Dulaglutide, which reduced glucose quickly for the first day and rebounded quickly. Interestingly, the dual-targeted GLP-1-Fc-FGF21 (RA) quickly reduced glucose level and the effect was sustained to keep the glucose at low level for 4 days with a later rebound, a characteristic of both Dulaglutide and FGF21. Indeed, when Fc-FGF21 and Dulaglutide were used as mixture, the glucose reduction profile was the same as the GLP-1-Fc-FGF21 (RA) (Fig. 4a). Overall, GLP-1-Fc-FGF21 (RA) had statistically significant lower glucose AUC (63.07% reduction from vehicle) than Fc-FGF21 (RA) (32.30%) and Dulaglutide (39.95%) (Fig. 4b). In addition, we can see that during the first dose, the dual agonist showed similar body weight and food intake reductions with that of dulaglutide but it had more robust and sustained glycemic control effect (Fig. 4c and 4d). At day 13, the serum triglyceride levels and total cholesterol levels of GLP-1-Fc-FGF21 (RA) and Dulaglutide/Fc-FGF21 (RA) mixture were significantly decreased (Fig. 4e and 4f).
Fig. 4.
Dual target GLP-1-Fc-FGF21 has better anti-diabetic effects than single target Fc-FGF21 and Dulaglutide in type 2 diabetic db/db mice. (a) The db/db mice were treated for two weeks with 40 nmol/kg Fc-FGF21 (RA), GLP-1-Fc-FGF21 (RA) (40nmol/kg), dulaglutide (40nmol/kg) or 40nmol/kg: 40nmol/kg mixture of dulaglutide/Fc-FGF21 (RA) mixture or PBS by subcutaneous injection once a week. (b) Blood glucose and glucose AUC was monitored daily (n=10). (c) Body weight change was measured daily and is expressed as change from initial body weight (n=10). (d) Food intake(n=10). (e) Blood lipid, TC (total cholesterol), n=10. (f) Blood lipid, TG (Triglyceride), n=10. (g) The db/db mice were treated for one week with Fc-FGF21 S1 (40nmol/kg), Fc-FGF21 S3 (40nmol/kg), GLP-1-Fc-FGF21 D1 (30nmol/kg), GLP-1-Fc-FGF21 D3 (30nmol/kg), or PBS (as control) via single subcutaneous injection (n=9). (h) Blood glucose and glucose AUC was monitored daily (n=9). Data are shown as mean +/- standard deviation, Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 (vs. control).
The dual-targeted proteins GLP-1-Fc-FGF21 D1, D3 in which the FGF21 has higher affinity for β-klotho, were further studied and compared with single-targeted FGF21 derivatives in a separate experiment (Fig. 4g). Again, to test the dual agonist in anti-diabetic activity, db/db diabetic mice were subcutaneously injected twice in two weeks, and the blood glucose were measured. As expected, single-targeted FGF21 derivatives Fc-FGF21 S1 and Fc-FGF21 S3 gradually decreased glucose as in Fig. 4a, while dual GLP-1-Fc-FGF21 D1 and D3 quickly reduced glucose after injection. It was noted that the glucose rebound in mice treated with GLP-1-Fc-FGF21 D1 and D3 were earlier than that seen in Fig. 4a, likely due to the lower dose (30 nmol/kg) used in this experiment. When glucose AUC were analyzed, the proteins with higher β-Klotho binding, namely Fc-FGF21 S1 and GLP-1-Fc-FGF21 D1, had better glucose lowering activity than Fc-FGF21 S3 and GLP-1-Fc-FGF21 D3 (Fig. 4h). The results again demonstrated that GLP-1-Fc-FGF21 dual agonists are superior to single-targeted Fc-FGF21 derivatives. Because dual agonist D1 showed higher β-Klotho binding affinity as well as better in vivo performance than D3, it was therefore chosen for further investigation.
3.6. Pharmacokinetics characterization of GLP-1-Fc-FGF21 D1 in mice and rats
To further develop the candidate dual targeting protein, the PK properties of GLP-1-Fc-FGF21 D1 was assessed in mice (at 1 mg/kg) and rats (at 0.5 mg/kg) following SC administration. As shown in Supplementary Fig. 5 and Supplementary Table 4, the fusion protein's terminal half-life (in terms of active GLP-1) was 30.3 and 25.9 h in mice and rats, respectively. The time at which the maximum serum concentration was observed (Tmax) following a single SC dose was 7.8 and 12 h post-dose in mice and rats, respectively. These data support longer dosing regimen.
3.7. Dual-targeted protein has potent and sustained effect on reducing blood glucose and body weight in HFD ob/ob mice model
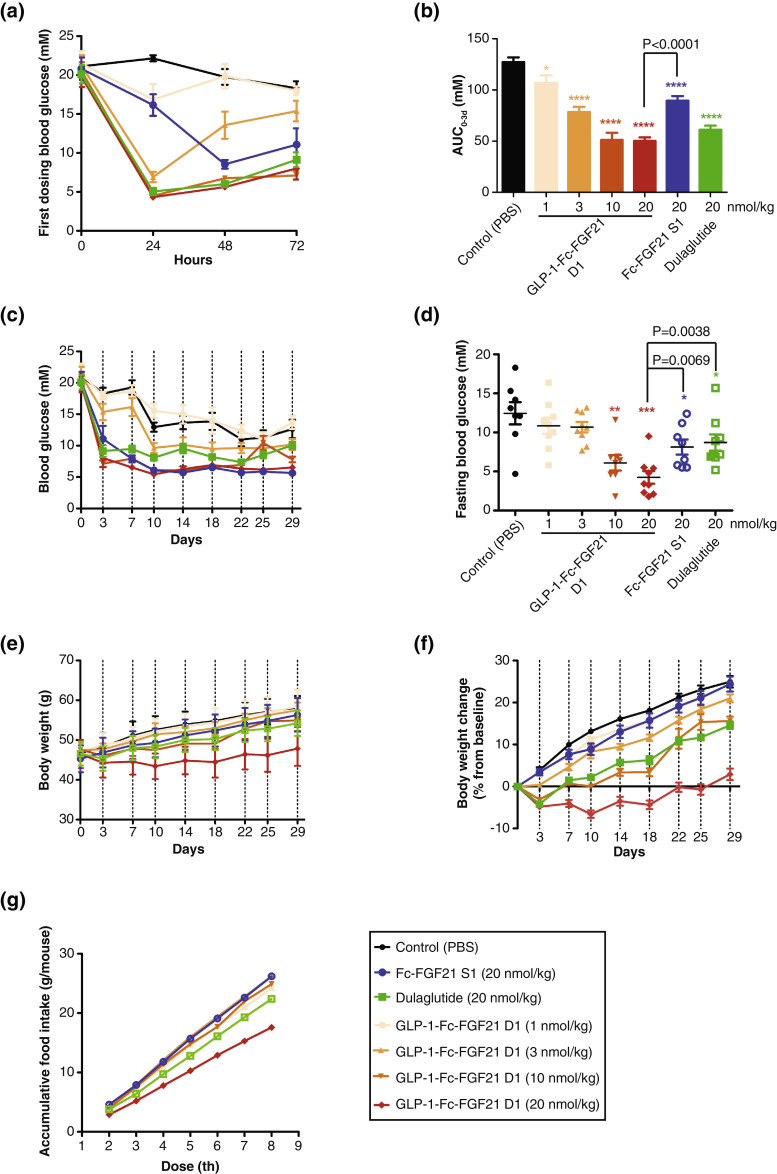
GLP-1-Fc-FGF21 D1 was further studied in high-fat diet (HFD) induced ob/ob mice to test its anti-diabetic and anti-obesity effects. After 2 weeks feeding with HFD, the ob/ob mice were treated with GLP-1-Fc-FGF21 D1 (1 nmol/kg, 3 nmol/kg, 10 nmol/kg and 20 nmol/kg), Fc-FGF21 S1 (20 nmol/kg) or Dulaglutide (20 nmol/kg). The treatment was done by subcutaneous injection twice a week (day 0 and day 3) for 4 consecutive weeks, blood glucose levels were monitored before each injection. In addition, after the first dosing, the mice were monitored daily for their blood glucose level (Fig. 5a and 5b). As shown in Fig. 5a and 5c, GLP-1-Fc-FGF21 D1 dose-dependently reduced blood glucose and the profile was similar to that of Dulaglutide with a quick decrease and a slow rebound. Fc-FGF21 S1 reduced glucose at a slower rate, especially the first day, a profile that was consistent with data in db/db mice (Fig. 4) attributing to FGF21 activity [24]. GLP-1-Fc-FGF21 D1 had better activity at both 10 nmol/kg and 20 nmol/kg than either Fc-FGF21 S1 and Dulaglutide at 20 nmol/kg, and the blood-glucose reducing effect lasted at least for 3 days. Although mice in the PBS control had declining glucose over the course of the experiment, drug treatment with Fc-FGF21 S1, Dulaglutide or GLP-1-Fc-FGF21 D1 significantly reduced glucose levels. Furthermore, GLP-1-Fc-FGF21 D1 showed a continuous and steady decrease in blood glucose after the 6th day, especially at the 20 nmol/kg dose, while the glucose level rebounded in the Dulaglutide treated mice after three weeks (Fig. 5c). The two doses of GLP-1-Fc-FGF21 D1 did not show significant difference, indicating 10 nmol/kg dose had reached maximum efficacy in this experiment setting.
Fig. 5.
GLP-1-Fc-FGF21 D1 has long-lasting reduced glucose and body weight gain in type 2 diabetic ob/ob mice with HFD-induced NASH. The diabetic mice were treated twice a week (day 0 and day 3) for 4 consecutive weeks via subcutaneous injection. (a) Blood glucose after first dosing was monitored (n=8) at different times. (b) Day 0 to day 3 Glucose AUC were calculated and shown. (c) Random blood glucose were monitored twice a week before each injection (n=8). (d) Fasting blood glucose was measured after the last dosing (n=8). (e) Body weight gain was measured twice a week (n=8). (f) Body weight was measured twice a week and is expressed as change from initial body weight (n=8). (g) Food intake was measured. (n=8). Data are shown as mean +/- standard deviation, Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001 (vs. control).
Fasting blood glucose levels were also measured at the end of the treatment. As shown in Fig. 5d, GLP-1-Fc-FGF21 D1 had dose dependently decreased fasting glucose. The 20 nmol/kg GLP-1-Fc-FGF21 D1 also decreased fasting blood glucose significantly better than Fc-FGF21 S1 and Dulaglutide at equivalent doses (Fig. 5c and 5d).
As shown in Fig. 5e and 5f, GLP-1-Fc-FGF21 D1 attenuated body weight gain in a dose-dependent manner and was more effective than both Dulaglutide and FGF21 S1. The body weight control effect of GLP-1-Fc-FGF21 D1 was achieved mainly through the inhibition of food intake (Fig. 5g). Taken together, these data showed that the GLP-1 and FGF21 dual-targeted fusion protein could provide more superior blood glucose and body weight control effects than single-targeted analogs.
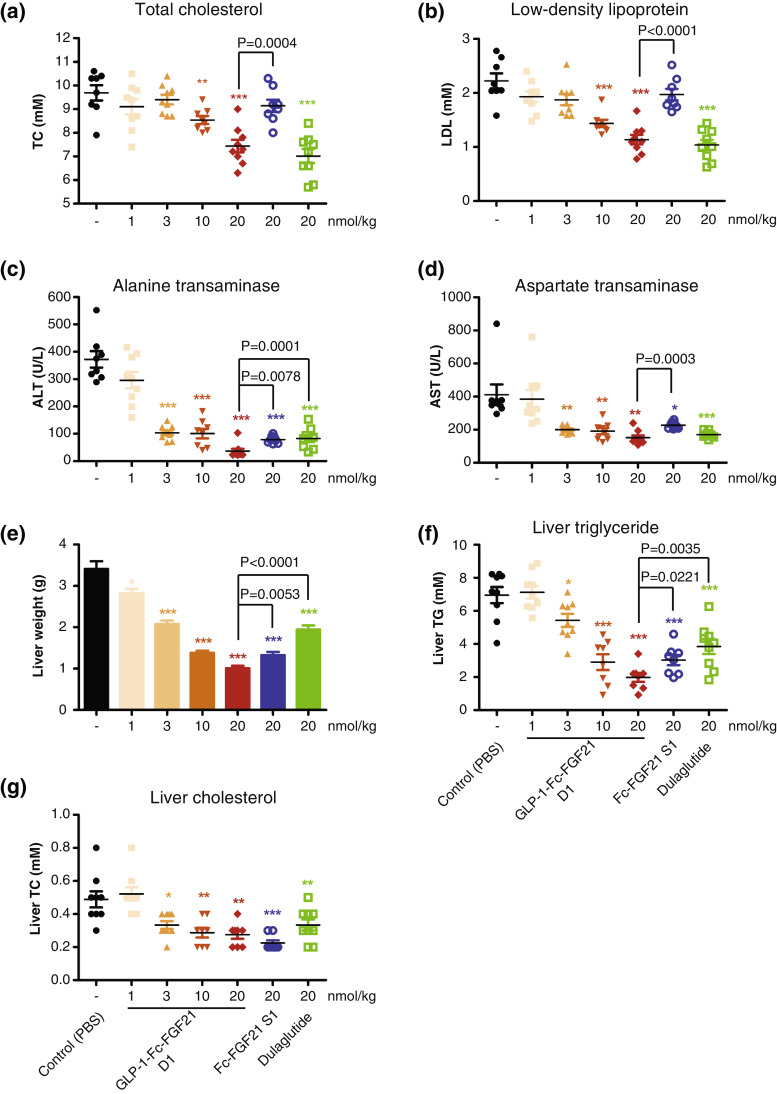
3.8. Dual-targeted protein improves lipid profile and liver function in HFD induced ob/ob mice
Since both GLP1 and FGF21 play multiple functions in gluconeogenesis and lipid metabolism, the chimeric proteins Fc-FGF21 S1 and GLP-1-Fc-FGF21 D1 were investigated for their effect on lipid profile and liver function in HFD induced ob/ob mice. After 4 weeks of treatment, Dulaglutide significantly reduced serum TC and LDL at 20 nmol/kg concentration. Fc-FGF21 S1 (20 nmol/kg), on the other hand, had minimum activity, which may due to the relatively low dose [25]. Importantly, GLP-1-Fc-FGF21 D1 significantly reduced serum TC and LDL concentrations in a dose dependent manner. The improvement of serum lipids by GLP-1-Fc-FGF21 D1 was similar to that by dulaglutide, but much better than Fc-FGF21 S1 (P < 0.001, unpaired two-tailed Student's t-test) at the equivalent doses (Fig. 6a and 6b).
Fig. 6.
Profile of lipoproteins and liver function markers in ob/ob mice with HFD-induced NASH. Mice were treated with the indicated single or dual target proteins by subcutaneous injection twice a week (day 0 and day 3) for 4 consecutive weeks (n=8). At the end of the treatment on day 29 multiple measurements were done. (a) blood lipid, TC. (b) LDL levels. (c) Blood ALT. (d) AST levels. (e) Liver weight. (f) Liver TG. (g) liver TC levels. Data are shown as mean +/- standard deviation, Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001 (vs. control). TC, total cholesterol; LDL, low-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Liver TG, Liver triglyceride; Liver TC, Liver total cholesterol.
Liver inflammation and NASH-related biomarkers and hepatic lipid profile were also examined. GLP-1-Fc-FGF21 D1 dose dependently decreased ALT and AST (P < 0.001, unpaired two-tailed Student's t-test) and the decrease was more profound than that by Dulaglutide (Fig. 6c and 6d). Compared with normal mice, the ob/ob diabetic mice had enlarged livers (3.4 g vs 0.9 g). However, treatment with Dulaglutide, Fc-FGF21 S1 and GLP-1-Fc-FGF21 D1 all significantly reduced the liver weight back to normal at the 20 nmol/kg doses (Fig. 6e). Both GLP-1-Fc-FGF21 D1 and Fc-FGF21 S1 demonstrated better efficacy than Dulaglutide (Fig. 6e). GLP-1-Fc-FGF21 D1 also decreased liver TG and TC contents in a dose dependent manner with similar or better efficacy than Dulaglutide or Fc-FGF21 S1 at equivalent doses (Fig. 6f and 6g). These results suggest that GLP-1/FGF21 dual agonist is superior to mono agonists in improving liver function and reducing liver fat.
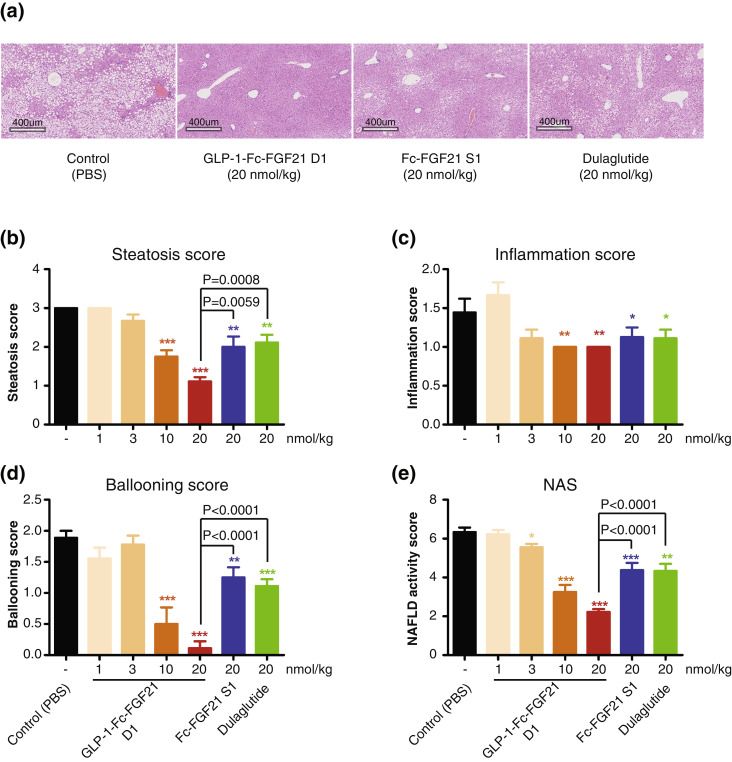
3.9. Dual-targeted protein improves non-alcoholic steatohepatitis in HFD ob/ob mice
The ob/ob mice fed with high fat diet for 6 weeks developed Non-Alcoholic Steatohepatitis as determined by non-alcoholic fatty liver disease (NAFLD) activity score (Fig. 7). In this experiment, the hepatic steatosis score for the vehicle mice was 3.0 ± 0.0, inflammation score was 1.4 ± 0.5, ballooning score was 1.9 ± 0.3 and the overall NAS score was 6.3 ± 0.7. These numbers are higher than the requirement of NASH model standard (NAS>5) demonstrating a successful NASH model. Again, compared with the vehicle control group, all three agents Fc-FGF21 S1, GLP-1-Fc-FGF2 D1 and Dulaglutide significantly reduced liver steatosis, inflammation and hepatocellular ballooning while GLP-1-Fc-FGF21 D1 improved these parameters in a dose dependent manner and showed greater improvements than GLP-1 or FGF21 mono-agonists at equivalent doses, resulting in the lowest NAFLD activity score (Fig. 7). In hepatic steatosis and ballooning measurements, GLP-1-Fc-FGF2 D1 was significantly better at the 20nmol/kg concentration than either Dulaglutide or Fc-FGF21 S1 resulting in a significantly better overall NAS. Even at a lower dose (10 nmol/kg), GLP-1-Fc-FGF2 D1 still showed better activity. These results indicate that GLP-1-Fc-FGF2 D1 targeting both GLP-1 and FGF21 pathways can provide better anti-NASH efficacy than either Dulaglutide or Fc-FGF21 S1 targeting a single pathway. Whether this superior effect can translate in patients is worthy of clinical investigation.
Fig. 7.
GLP-1-Fc-FGF21 D1 dual protein attenuated NASH progression in ob/ob mice with HFD-induced NASH. Liver samples from mice sacrificed at the end of experiment in Fig. 6 were sectioned and anaylized for NASH markers (n=8). (a) Histological sections of liver tissues stained with H&E from animals. The scale bar is 400 micrometers. (b) Hepatic steatosis score. (c) Inflammation score. (d) Ballooning score. (e) NAS score. Data are shown as mean +/- standard deviation, Student's t-test was used for statistical analysis. *P<0.05; **P<0.01; ***P<0.001 (vs. control).
4. Discussion
Several properties of wild type human FGF21 have limited its clinical application as a therapeutic agent. FGF21 has a very short half-life and is vulnerable to protease degradation in vivo, it also tends to aggregate in vitro [26,27]. To overcome these limitations, previous studies have generated FGF21 variants with resistance to degradation and aggregation [17]. For example, the leucine98 to arginine mutation (L98R) could efficiently prevent the in vitro aggregation of FGF21, while the proline171 to glycine or alanine mutations (P171G or P171A) could largely eliminate protease degradation. Fusion to IgG Fc is also used to further enhance the in vivo half-life. We found that the effect of stimulating ERK1/2 phosphorylation in vitro and blood-glucose reducing in ob/ob mouse models in vivo of Fc-FGF21 (RA) is similar to that of Fc-FGF21 (RG) (data not shown). However, these mutations and modifications mainly improved the physicochemical properties of FGF21, but did not improve FGF21 intrinsic activity. FGF21 functions through binding to the co-receptor β-Klotho with its C-terminal tail [28], [29], [30]. Based on structural analysis of the FGF21-CT and β-Klotho complex, we hypothesize that the FGF21 to β-Klotho binding affinity could be improved through mutagenesis, resulting in FGF21 variants with better bioactivity. Indeed, using high-throughput phage display screening, we identified mutations that could contribute to higher β-Klotho binding affinity. Importantly, two of the frequently mutated positions, serine167 and arginine175, are located right in the β-Klotho binding tail of FGF21.
We constructed three FGF21 variants based on the variant FGF21 (RA) (L98R, P171A) and incorporated the identified mutations, generating Fc-FGF21 S1 and S3. As expected, the newly constructed variants showed increased β-Klotho binding affinity comparing to FGF21 (RA). More importantly, the higher binding affinity successfully translated to better in vivo blood glucose lowering efficacy, indicating that increased β-Klotho binding can lead to improved bioactivity of FGF21. And the glucose lowering effect of Fc-FGF21 S1 is similar to that of a recently identified variant Fc-FGF21 (RGE) in ob/ob mouse models (data not shown) [31].
Owing to FGF21’s robust glycemic control efficacies in various pre-clinical models, initial clinical developments of this molecule have focused on its anti-diabetic and anti-obesity potential. Unexpectedly, early clinical results revealed that these FGF21’s benefits were poorly translated into humans, with mild to no effects on glycemic control or body weight reduction [25,32]. One possible explanation behind this gap is that the activity and stability of previous FGF21 analogs were not sufficient for optimal clinical efficacy [33,34]. Interestingly, FGF21 analogs have shown robust efficacy in lipid profile and fibrosis biomarker improvements in clinical settings, making these analogs promising candidates for NASH treatment [33,34]. Indeed, an Fc fusion FGF21 analog, efruxifermin, has provided impressive anti-NASH/anti-fibrosis efficacy in a phase 2 clinical trial and thus brought this class of agents to the forefront for the treatment of NASH (ClinicalTrials.gov Identifier: NCT03976401), a disease currently with no FDA approved therapy. However, FGF21’s poor clinical efficacy in glycemic control and body weight reduction could limit its application to other related metabolic diseases, such as T2D and obesity (ClinicalTrials.gov Identifier: NCT03976401).
GLP-1 has a variety of physiological functions, such as increasing glucose-dependent insulin secretion, protecting islet β-cells, delaying gastric emptying, reducing appetite, inhibiting lipid production or lipid accumulation in the liver and stimulating inflammatory responses. Similarly, FGF21 plays multiple physiological roles in metabolic pathways, can improve lipid metabolism disorder and exert anti-fibrosis effect [35]. Its effect on blood glucose is mild and continuous, which is different from that of insulin [4,5]. Previous study has implicated the potential functional interplay between GLP-1 and FGF21 in vivo, leading us to the hypothesis that a single molecule GLP-1/FGF21 dual agonist may provide synergistic effects on glycemic control, body weight lowering and liver function improvement. We tested this hypothesis by developing and characterizing a novel GLP-1-Fc-FGF21 fusion protein that can target both the GLP-1 and FGF21 pathways. Surprisingly, the dual targeting agonist exhibited potent and sustained efficacy in lowering glucose levels.
Based on our improved Fc-FGF21 S1 and S3 single-targeted analogs, which have 2.5- and 1.3-fold higher affinity than FGF21 (RA), we generated two corresponding GLP-1-Fc-FGF21 dual agonists D1 and D3 and characterized their in vitro potency and in vivo efficacy. Interestingly, the addition of GLP-1 to the N terminal of the Fc with Fc-FGF21 S1 and Fc-FGF21 S3 made them bind β-Klotho with even higher affinity; β-Klotho binding affinity for GLP-1-Fc-FGF21 D1 and GLP-1-Fc-FGF21 D3 was 8.3 and 3.2-fold higher than that for Fc- FGF21 (RA), with KD of 92 pM and 240pM, respectively.
Since these proteins have both GLP-1 and FGF21 moieties, they can activate through both GLP-1 receptor GLP-1R and β-Klotho. In HEK293 cells with only GLP-R but lacking β-Klotho, the FGF21 moiety negatively (2.3-fold decrease in EC50) affected the activation through the GLP-1 pathway, an effect which may due to the conformational difference of a larger protein. In HEK293 cells expressing both GLP-1R and β-Klotho, the data is more interesting. GLP-1-Fc-FGF21 D1 (EC50 0.021 nM) is 10 fold more active than Dulaglutide (EC50 0.21 nM) in term of cAMP activation pathway. Given that cAMP production from FGF21 pathway is minimum in this system, the high potency of GLP-1-Fc-FGF21 D1 seems to result from synergistic effect of both GLP-1 and FGF21 in a single molecule setting. To support this hypothesis, simple mixing of Fc-FGF21 S1 and Dulaglutide had exactly the same EC50 as Dulaglutide. One simple explanation for the synergistic effect could be that high affinity binding of GLP-1-Fc-FGF21 D1 to β-Klotho recruited the GLP-1 to the cell surface and increased its local concentration. This recruiting effect should also exist in tissues where cells are in close proximity but with only one of the receptors. The hope is that in patients this synergistic effect could be observed. However, in vivo functional comparison of dual agonist and mono agonists mixture in mice models showed nearly identical efficacy, suggesting that the local concentration boost phenomenon may not exist physiologically.
When comparing single target FGF21 with dual GLP-1/FGF21 agonists in animal models, dual targeted GLP-1-Fc-FGF21 D1, D3 consistently demonstrated superiority to single targeted Fc-FGF21 S1, S3 and Dulaglutide in lowering blood glucose levels. The increased efficacy not only reflected a longer duration of action but also a greater magnitude of effects. The addition of the GLP-1 moiety made the dual target protein behave like GLP-1 early after injection but kept the blood glucose steadily low due to the FGF21 activity in ob/ob and db/db mice (Fig. 4 and Fig. 5).
We also showed that FGF21 activation acts synergistically with GLP-1 activation to allow greater weight loss in mice than what can be achieved with single GLP-1 receptor agonist Dulaglutide and Fc-FGF21 S1. The reduction in bodyweight also corresponded with a reduction in food intake. Although additional mechanistic studies are required to fully understand the effects of GLP-1-Fc-FGF21 D1 on energy balance, it seems likely that the synergistic actions of the FGF21 and GLP-1 receptors occurs at the level of the CNS to suppress appetite and reduce calorie intake. More importantly, in the HFD induced ob/ob NASH model, GLP-1-Fc-FGF21 D1 significantly decreased the level of ALT and AST and showed better activity than Dulaglutide, especially in lowering ALT (Fig. 6c). Fc-FGF21 S1 did not show activity in reducing TC and LDL, but reduced liver TG and TC (Fig. 7e and 7f). Again, in terms of TG levels, the dual target construct GLP-1-Fc-FGF21 D1 had achieved statistically significant reduction than Fc-FGF21 S1 and Dulaglutide. The analysis of liver pathological features showed that the dual-targeted protein D1 significantly reduced liver steatosis, inflammation and hepatocellular ballooning compared to the vehicle control group. The NAS score decreased to 3.3 ± 1.0 and 2.2 ± 0.4 at the dose of 10 nmol/kg and 20 nmol/kg, respectively. The dual-target protein could significantly improve NASH in a dose-dependent manner, and worked better than Dulaglutide and Fc-FGF21 S1. Because the ob/ob mice in our experiment have not developed fibrosis, the dual agonist's anti-fibrosis effect could not be evaluated. But considering that a similar Fc-FGF21 analog (Efruxifermin) has exhibited such efficacy clinically and that mechanisms of FGF21 and GLP-1 function are complementary, it is speculated that the dual-targeted GLP-1/FGF21 would also have anti-fibrosis activity. In summary, we generated FGF21 analogs with improved β-Klotho binding affinity and bioactivity and constructed a novel GLP-1/FGF21 dual agonist. As we were preparing this manuscript, we noticed that another group reported a similar GLP-1/FGF21 dual agonist fusion protein [36], compared with the reported molecule GLP1-ELP-FGF21, our molecule GLP1-Fc-FGF21 exhibited potent and sustained glycemic control effects in diabetic mice models with a much lower dose. Additionally, it also performed superior body weight reduction, lipid profile improvement and anti-NASH effect in high-fat diet-induced ob/ob model. Whether this superior effect can translate in patients is worthy of clinical investigation. If the synergistic effect could be successfully translated into clinical practice, this novel GLP-1/FGF21 dual agonist thus represents a novel mechanism of action, distinct from currently available therapies and has the great potential to become a comprehensive therapy for anti-diabetic, anti-obesity and anti-NASH treatments.
Contributors
CC and LG conceived the study, SL and QP proposed the method, conducted the experiments and analyzed the data, YL, LL, XL, XG, JY, BG, XC, WL, XT conducted the experiments and analyzed the data, SL, QP, JY and BG wrote the manuscript, CC and LG revised the manuscript. All authors reviewed the report and approved the final version.
Data sharing statement
The data supporting the findings of this study are available within the article and/or the supplementary materials. The data not shown can be available from the corresponding author CC and LG, upon reasonable requests.
Declaration of Competing Interests
CC, SL, YL, LL, XL, XG, JY, BG, XC, WL and XT are/were HEC Pharm R&D Co., Ltd employees and report personal fees from HEC Pharm R&D Co., Ltd, outside the submitted work, at the time the research was performed. LG and QP report grants from National natural science fund of China (Grant/Award Number: 81670763), during the conduct of the study.
Acknowledgements & funding sources
CC, SL, YL, LL, XL, XG, JY, BG, XC, WL and XT were supported by HEC Pharm R&D Co., Ltd. LG and QP were supported by National natural science fund of China (Grant/Award Number: 81670763). We would like to thank HEC Biologics Process Development Platform for their laboratory support. We also thank Peng Jiang and Qing wei Gong for their technical support on Mass spectrometry .
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103202.
Contributor Information
Chao Chen, Email: chchao1987@gmail.com.
Lixin Guo, Email: glxwork2016@163.com.
Appendix. Supplementary materials
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW. IDF Diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharitonenkov A, Shanafelt AB. Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs. 2008;22(1):37–44. doi: 10.2165/00063030-200822010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wang WF, Li SM, Ren GP, Zheng W, Lu YJ, Yu YH. Recombinant murine fibroblast growth factor 21 ameliorates obesity-related inflammation in monosodium glutamate-induced obesity rats. Endocrine. 2015;49(1):119–129. doi: 10.1007/s12020-014-0433-5. [DOI] [PubMed] [Google Scholar]
- 5.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 6.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matuszek B, Lenart-Lipinska M, Duma D, Solski J, Nowakowski A. Evaluation of concentrations of FGF-21 - a new adipocytokine in type 2 diabetes. Endokrynol Pol. 2010;61(1):50–54. [PubMed] [Google Scholar]
- 8.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150(9):4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22(4):1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yie J, Wang W, Deng L, Tam LT, Stevens J, Chen MM. Understanding the physical interactions in the FGF21/FGFR/beta-Klotho complex: structural requirements and implications in FGF21 signaling. Chem Biol Drug Des. 2012;79(4):398–410. doi: 10.1111/j.1747-0285.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 13.Harmer NJ, Ilag LL, Mulloy B, Pellegrini L, Robinson CV, Blundell TL. Towards a resolution of the stoichiometry of the fibroblast growth factor (FGF)-FGF receptor-heparin complex. J Mol Biol. 2004;339(4):821–834. doi: 10.1016/j.jmb.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215(1):1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104(18):7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148(2):774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 17.Hecht R, Li YS, Sun J, Belouski E, Hall M, Hager T. Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS One. 2012;7(11):e49345. doi: 10.1371/journal.pone.0049345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch IB. The future of the GLP-1 receptor agonists. JAMA. 2019;321(15):1457–1458. doi: 10.1001/jama.2019.2941. [DOI] [PubMed] [Google Scholar]
- 19.Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59(7):1765–1770. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Yang K, Yang J, Xiao W, Le Y, Yu F. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine. 2019;41:73–84. doi: 10.1016/j.ebiom.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Zhang L, Wang C, Liu H, Boden G, Yang G. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012;7(11):e48392. doi: 10.1371/journal.pone.0048392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA. Fibroblast growth factor 21 and browning of white adipose tissue. Front Physiol. 2019;10:37. doi: 10.3389/fphys.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84(10):3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharitonenkov A, Adams AC. Inventing new medicines: the FGF21 story. Mol Metab. 2014;3(3):221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 2016;23(3):427–440. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, Chan R. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J Biol Chem. 2016;291(11):5986–5996. doi: 10.1074/jbc.M115.710582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Li Y. Fibroblast growth factor 21, the endocrine FGF pathway and novel treatments for metabolic syndrome. Drug Discov Today. 2014;19(5):579–589. doi: 10.1016/j.drudis.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Lemon B, Li X, Gupte J, Weiszmann J, Stevens J. C-terminal tail of FGF19 determines its specificity toward Klotho co-receptors. J Biol Chem. 2008;283(48):33304–33309. doi: 10.1074/jbc.M803319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, Dickinson CD. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219(2):227–234. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E. Structures of beta-klotho reveal a 'zip code'-like mechanism for endocrine FGF signalling. Nature. 2018;553(7689):501–505. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanislaus S, Hecht R, Yie J, Hager T, Hall M, Spahr C. A novel Fc-FGF21 with improved resistance to proteolysis, increased affinity toward beta-klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology. 2017;158(5):1314–1327. doi: 10.1210/en.2016-1917. [DOI] [PubMed] [Google Scholar]
- 32.Kharitonenkov A, Beals JM, Micanovic R, Strifler BA, Rathnachalam R, Wroblewski VJ. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One. 2013;8(3):e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong JQ, Rossulek M, Somayaji VR, Baltrukonis D, Liang Y, Hudson K. Pharmacokinetics and pharmacodynamics of PF-05231023, a novel long-acting FGF21 mimetic, in a first-in-human study. Br J Clin Pharmacol. 2015;80(5):1051–1063. doi: 10.1111/bcp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18(3):333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kim EK, Lee SH, Lee SY, Kim JK, Jhun JY, Na HS. Metformin ameliorates experimental-obesity-associated autoimmune arthritis by inducing FGF21 expression and brown adipocyte differentiation. Exp Mol Med. 2018;50(1):e432. doi: 10.1038/emm.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilroy CA, Capozzi ME, Varanko AK, Tong J, D'Alessio DA, Campbell JE. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Sci Adv. 2020;6(35):eaaz9890. doi: 10.1126/sciadv.aaz9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.