Abstract
Microalgal polysaccharides (PSs) may be an effective elicitor agent that can efficiently protect plants against biotic stresses. In this study, wee investigates, the effect of PS obtained from microalgae and cyanobacteria (D. salina MS002, P. tricorontum MS023, Porphyridium sp. MS081, Desmodesmus sp., D. salina MS067 and A. platensis MS001) on the biochemical and metabolomics markers linked to defense pathways in tomato plants. The phenylalanine ammonia lyase (PAL), chitinase, 1,3-beta-glucanase and peroxidase (POX) activities have been improved in tomato plants leaves treated by polysaccharides extracted from P. triocnutum (238.26%); Desmodesmus sp. (19.95%); P. triocnutum (137.50%) and Porphyridium sp. (47.28%) respectively. For proteins, polyphenols and H2O2, the maximum effect was induced by D. salina 067 (55.01%), Porphyridium sp. (3.97%) and A. platensis (35.08%) respectively. On the other hand, Gas Chromatography-mass spectrometry (GC–MS) metabolomics analysis showed that PSs induced the modification of metabolite profile involved in the wax construction of tomato leaves, such as fatty acids, alkanes, alkenes and phytosterol. PS treatments improved the accumulation of fatty acids C16:3, C18:2 and C18:3 released from the membrane lipids as precursors of oxylipin biosynthesis which are signaling molecules of plant defense. In addition, PS treatment induced the accumulation of C18:0 and Azelaic acid which is a regulator of salicylic acid-dependent systemic acquired resistance. However, molecular and metabolic studies can determine more precisely the mode of action of microalgal polysaccharides as biostimulants/elicitors plant defense.
Subject terms: Biochemistry, Biotechnology, Plant sciences
Introduction
Plants in their environment are exposed to divers biotic and abiotic stresses1. Traditionally, synthetic pesticides and fertilizers are almost indispensable for the control of plant diseases and crop production. The excessive use of these synthetic chemical products is of global concern, because of their harmful effect on human health and the environment (European Commission 2012)2,3. In addition, the overuse of chemical products is the main cause of resistance development in pathogens4. In this context, the promotion of eco-friendly alternatives appeared necessary for the reduction of the use of chemicals in agriculture and hence of the attenuation of their environment effect5,6.
In the last decades, the sustainable agriculture has gained increasing interest on integrated crop management strategy where a promote the use of alternatives tools such as crop rotation, planting time, tillage and the use of biological products3,7,8. A lot of attention has been focused on the development of plant biostimulants to improve crop growth, yield and quality by enhancing plant nutrition, reducing abiotic stress impact and promoting plant growth7,9,10. Detailed reports are available on the induction of defense resistance in plants via a variety of biological inducers such as plant extracts, essential oils, bacteria and fungus, algal extracts and polysaccharides11. These elicitors have no direct toxic effect on pathogens and pests, but they have the ability to induce signaling pathways related to plant defense against biotic and abiotic stresses1, which makes them amenable as replacement for traditional agrochemicals in a sustainable crop production system. Algal polysaccharides are macromolecules with structural complexity linked to chemical groups with the high potential biological activities make algae polysaccharides an ideal bioresource of plant resistance inducers12.
Microalgae are photosynthetic eukaryotic and prokaryotic microorganisms with great biological and biochemical diversity, which have a potential as a promising bio-resource for different applications such as pharmaceutical, nutraceutical, food and feed, aquaculture, bioenergy and the environment13–19. In the agriculture field, the algal polysaccharide have a biostimulant activities of plant growth, nutrients uptake and biotic and abiotic stresses tolerance16,20–24. Recently studies, have demonstrated the biostimulant effect of microalgal extracts on the seed germination, seedling growth and yields of Lettuce and tomato plants24–28. However, until now, the use of microalgal extracts as biostimulants of natural plant defense mechanisms related to abiotic and biotic stresses is an undergoing initiation24,28,29. In the same way24, have demonstrated that D. salina exopolysaccharide have the potential to induce the metabolic and biochemical pathways related to natural plant defense in tomato plants under salt stress. Lipopolysaccharides and exopolysaccharides are essential structures maintained in pathogenic, non-pathogenic and saprophytic microorganisms, under the name of MAMPS/PAMPS (microbe-/pathogen-associated molecular patterns). These molecules are rapidly detected by the membrane receptors of the host plant, in order to induce various defense responses30.
Divers studies have demonstrated the potential of different extracts and metabolites—among them polysaccharides—of microalgae as biostimulants and biofertilizers of plant growth and crop production12,26,29,31–34. In the present study, we investigated polysaccharides Extracts of 6 Microalgae and Cyanobacteria species for their Bioelicitors effects on the different biochemical and metabolomics pathways related to the plants defense to biotic and abiotic stress of Solanum lycopersicum L. The aim of this research is to demonstrate the potential of microalgae polysaccharides as new bioactive materials for bioproduct development in agriculture.
Results
Microalgae culture and polysaccharides characterization
As shown in Table 1 microalgae growth differed between strains, A. platensis showed the fastest growth, followed by D. salina MS067, D. salina MS002, Porphyridium sp., P. tricornutum, and Desmodesmus sp. with the biomass production of 1.22, 1.13, 0.91, 0.60, 0.45 and 0.42 g L−1 respectively (Table 1). The choice of these strains for our project was based on the adaptation and the feasibility of their culture at large-scale. The microalgae polysaccharide content was recorded as 12,7%, 5.52%, 4.10% and 2.58% for P. tricornutum, Porphyridium sp., D. salina 002 and A. platensis respectively, while their partial characterization indicated that they were composed of neutral sugars (5.0–35.350%), sulfates (4.051–11.541%), uronic acids (3.602–42.865%), proteins (2.701–22.068%), amino acids (0.017–0.206%) and β-glucans (0.008–0.698%). P. tricornutum exhibited the maximum production of polysaccharides (12.7%) composed of 32,3%, 6,75% and 0,698% of uronic acid, sulfate content and beta-glucan content respectively. Whereas D. salina and Porphyridium sp. polysaccharides exhibited the highest sulfate content with 11.5% et 10.4% respectively (Table1, Supplementary Fig. S1).
Table 1.
Biomass production and composition of polysaccharides extracted from Moroccan microalgae.
| Strains | Biomass (g L−1) | PS (%/biomass) | Total sugars (%/PS) | Sulfate (%/PS) | Uronic acid (%/PS) | Protein (%/PS) | Amino acid (%/PS) | Beta Glucan (%/PS) |
|---|---|---|---|---|---|---|---|---|
| A. platensis | 1.22 | 2.590 ± 0.40 | 17.275 ± 1.05 | 5.306 ± 0.37 | 3.602 ± 1.58 | 2.746 ± 0.21 | 0.206 ± 0.0 | 0.149 |
| D. salina002 | 1.13 | 4.103 ± 0.13 | 33.050 ± 0.92 | 11.541 ± 0.92 | 23.951 ± 2.62 | 4.202 ± 0.02 | 0.083 ± 0.0 | 0.008 |
| Porphyridium sp. | 0.6 | 5.529 ± 0.15 | 35.350 ± 0.05 | 10.427 ± 0.54 | 13.808 ± 5.45 | 2.701 ± 0.01 | 0.017 ± 0.0 | 0.108 |
| P. tricoruntum | 0.45 | 12.70 ± 2.66 | 5.003 ± 0.00 | 6.751 ± 0.59 | 32.331 ± 3.14 | 22.068 ± 0.13 | 0.019 ± 0.0 | 0.698 |
| Desmodesmus sp. | 0.42 | 7.2 ± 0.6 | 5.04 ± 0.00 | 5.27 ± 0.10 | 42.865 ± 10.4 | 11.850 ± 0.08 | 0.196 ± 0.0 | 0.043 |
| D. salina 067 | 0.91 | 6.17 ± 0.44 | 5.0 ± 0.00 | 4.051 ± 0.24 | 23.951 ± 5.15 | 15.744 ± 0.09 | 0.181 ± 0.0 | 0.037 |
Microalgal polysaccharides effect on tomato plants defense pathways
To investigate the effect of microalgae crude PSs, different enzymatic and biochemical markers were used in this study such as PRs proteins (Chitinase and 1,3-β,Glucanase) and PAL (Phenylalanine-Ammonia-Lyase) activities, ROS (H2O2) forms, peroxidase (POX) activity and polyphenols content.
Microalgal polysaccharides effect on protein content, PAL, chitinase and 1,3-β,glucanase activities
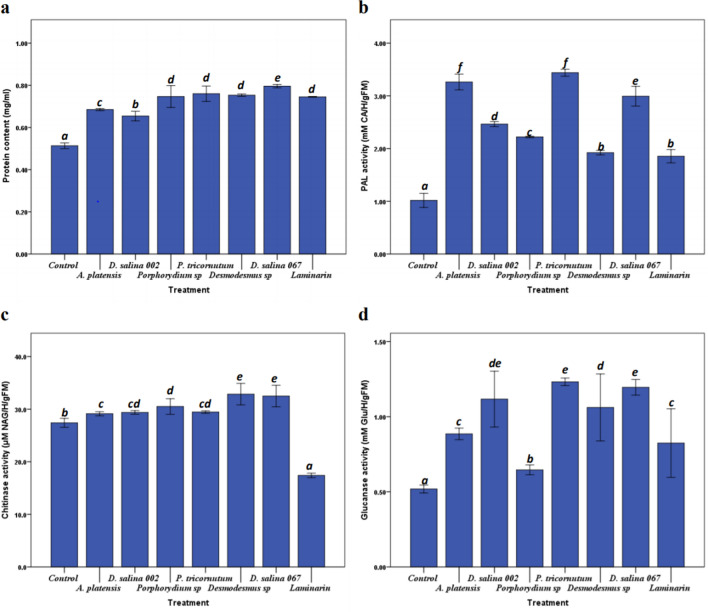
The results in Fig. 1a show that all PS treatments had a highly significant (p < 0.05) effect on protein content in treated plants compared to non-treated control plants. The maximum effect was induced by D. salina 067 polysaccharides with 55.01% improvement compared to the control.
Figure 1.
The effect of crude PS on protein content (a), PAL (b), chitinase (c) and β-1,3-Glucanase (d), activities. Tomato plants were injected with 1 mg mL−1 of PS. H2O and Laminarin (1 mg mL−1) were used as negative and positive controls respectively. Values are the mean (± SE) of five replicates. Different letters indicate significant difference between treatments (p < 0.05) according to Turkey-test, One-way ANOVA.
The enzymatic activity of PAL is highly significant (p < 0.05) in plants treated with different PS extracts (Fig. 1b). The percentages of minimal and maximal improvement (82.46% and 238.26%) in treated plants were induced by Laminarin and P. triocnutum polysaccharides respectively. In the same way, the chitinase activity showed the high significant (p < 0.05) enhancement in treated plants compared to control plant (Fig. 1c). The chitinase activity induced by polysaccharides from Desmodesmus sp., D. Salina 067, Porphyridium sp., P. tricornutum, D. salina 002 and A. platensis was increased by 19.95%, 18.63%, 11.36%, 7.51%, 7.26% and 6.35% respectively compared to control plants (Fig. 1c). Results presented in Fig. 1d, shows significant (p < 0.05) effect of polysaccharides on 1,3-β,Glucanase activity compared to the control plants. It was also observed that the maximum percentage improvement (137.50%) was induced by P. tricocnutum polysaccharides followed by D. salina MS067and D. salina MS002, which had a highly significant (p < 0.05) effect compared to Laminarin-treated plants (positive control).
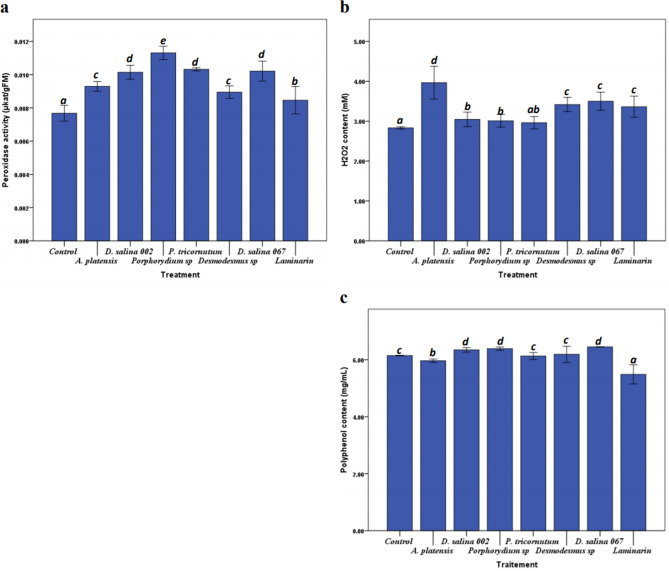
Microalgal polysaccharides effect on peroxidase activity, H2O2 and Polyphenols
The results presented in Fig. 2a, shows that the plants treated by PS of Porphorydium sp., P. triocnutum, D. salina 067 and D. salina MS002 had a very significant (p < 0.05) effect on peroxidase activity compared to controls plants. The maximum improvement of peroxidase activity was noted in plants treated by Porphyridium sp. polysaccharides with percentage difference of 41% compared to non-treated plants. The evaluation of the effect of microalgae polysaccharides on oxidative burst was estimated by the determination of the amount of H2O2 accumulated in tomato plants leaves. Figure 2b, shows that all PS treatments except that of P. triocuntum, had a significant (p < 0.05) effect on H2O2 content in tomato plants leaves. On the other hand, A. platensis PS treatment had a much higher effect on H2O2 content compared to the Laminarin treatment -positive control- and with a percentage improvement of 35.08% compared to control plants. While, the polyphenol compounds in tomato plants leaves showed little improvement in plant treated by microalgae polysaccharides compared to control plants (Fig. 2c).
Figure 2.
Peroxidase activity (a), H2O2 (b) and polyphenols content (c) in the tomato plants leaves after Polysaccharide treatment. Tomato plants were injected with 1 mg mL−1 of PS, and H2O or Laminarin (1 mg mL−1) as a negative and positive controls respectively. Values are the mean (± SE) of five replicates. Different letters indicate significant difference between treatments (p < 0.05) according to Turkey-test, One-way ANOVA.
GC–MS metabolomics analysis of tomato plants’ response to microalgae polysaccharide treatment:
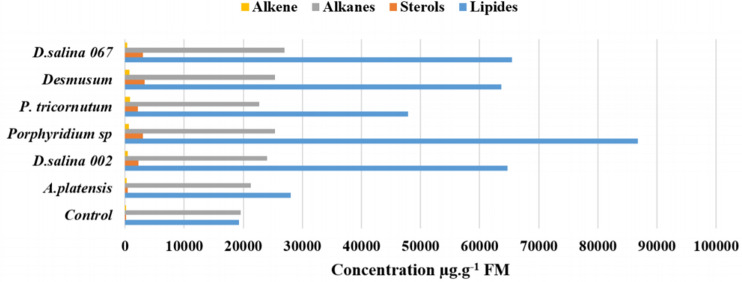
For Metabolic fingerprinting, the GC–MS was used to identify metabolomic change in tomato plants under different microalgae polysaccharides treatment. The Fig. 3 shows a significant redistribution of several metabolites in treated plants such as lipids, sterols, alkanes, alkenes and other molecules. The lipids, sterols, alkanes and alkenes content in tomato leaves were improved by (31.22–77.79%), (79.40–97.11%), (8.20–27.75%) and (57.11–88.70) respectively compared to non-treated plants. It can be seen that the distribution of the metabolites was related to the nature of the polysaccharide. Also, the maximum biosynthesis of lipids, the sterols, alkanes and alkenes were induced by the PS extracts obtained from Porphyridium sp., Desmodesmus sp., D. salina 067 and P. tricornutum respectively (Fig. 3).
Figure 3.
Metabolites profile of tomato plants leaves treated by polysaccharides. Tomato plants were injected with 1 mg mL−1 of PS, and H2O as Control.
Microalgae polysaccharides effect on lipid and sterol profiles in tomato plants:
Lipid profile analysis using GC–MS revealed 23 FAMEs dispersed over 3 categories of fatty acids namely: Very long chain fatty acids (VLCFA), unsaturated fatty acids (UFA) and saturated fatty acids (SFA). Fatty acids highly induced by the various PS treatments were linolenic acid (C18:3), palmitic acid (C16:0), roughanic acid (C16:3) and stearic acid (C18:0), with percentage of 50%, 30%, 5% and 10% respectively (Table 2). Meanwhile, VLCFAs included behenic acid (C22:0), tricosylic acid (C23:0), and lignoceric acid (C24:0) (Table 2).
Table 2.
Lipids profile of tomato plants leaves treated by microalgae polysaccharides.
| Control | A. platensis | D. salina 002 | Porphyridium sp. | P. tricornutum | Desmodesmus sp. | D. salina 067 | ||
|---|---|---|---|---|---|---|---|---|
| Lipids (µg g−1) | ||||||||
| Azelaic acid | C9:0 | 41.92 | 146.66 | 197.80 | 119.48 | 147.91 | 200.17 | 167.70 |
| Lauric acid | C12:0 | 97.49 | 395.19 | 563.56 | 478.36 | 322.19 | 409.64 | 431.97 |
| Myristic acid | C14:0 | 73.63 | 326.23 | 453.98 | 439.97 | 355.99 | 457.66 | 402.26 |
| Palmitic acid | C16:0 | 5843.54 | 6935.68 | 15,763.06 | 24,434.95 | 15,946.61 | 21,679.42 | 19,086.40 |
| Roughanic acid | C16:3 | 1304.35 | 2578.48 | 4848.55 | 5445.68 | 2576.28 | 3408.04 | 4179.01 |
| Margaric acid | C17:0 | 364.80 | 631.64 | 1183.71 | 2393.29 | 1046.90 | 1324.10 | 1375.93 |
| heptadecatrienoate | C17:3 | 0.00 | 238.68 | 4121.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| Stearic acid | C18:0 | 1586.44 | 1506.31 | 3697.84 | 9229.94 | 3559.71 | 5338.57 | 5703.57 |
| Linoleic acid | C18:2 | 107.59 | 0.00 | 0.00 | 0.00 | 290.99 | 0.00 | 0.00 |
| Linolenic acid | C18:3 | 9389.95 | 14,068.73 | 31,142.12 | 40,959.72 | 21,391.22 | 27,192.53 | 30,833.82 |
| Nonadecylic acid | C19:0 | 0.00 | 0.00 | 285.90 | 0.00 | 0.00 | 0.00 | 0.00 |
| Arachidic acid | C20:0 | 164.04 | 330.37 | 603.88 | 934.11 | 606.33 | 980.57 | 810.08 |
| Eicosatrienoic acid | C20:3 | 0.00 | 175.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| heneicosylic acid | C21:0 | 0.00 | 0.00 | 0.00 | 244.97 | 127.76 | 0.00 | 0.00 |
| Behenic acid | C22:0 | 92.25 | 220.70 | 357.39 | 588.86 | 408.30 | 678.46 | 511.46 |
| Tricosylic acid | C23:0 | 0.00 | 70.66 | 102.65 | 0.00 | 117.95 | 181.00 | 133.78 |
| Lignoceric acid | C24:0 | 69.81 | 190.21 | 431.75 | 747.03 | 448.26 | 722.36 | 632.84 |
| Lipid derivatives (µg g−1) | ||||||||
| 9-Hexadecenoic acid, methyl ester | 0.00 | 0.00 | 79.13 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Nonanoic acid, 9-oxo-, methyl este | 0.00 | 0.00 | 0.00 | 0.00 | 118.26 | 143.42 | 208.67 | |
| Pentanoic acid, 4-oxo-, methyl ester | 142.98 | 213.71 | 0.00 | 0.00 | 0.00 | 961.30 | 0.00 | |
| Hexadecanoic acid, 2-hydroxy-, methyl ester | 0.00 | 0.00 | 930.06 | 0.00 | 0.00 | 0.00 | 1010.16 | |
| Methyl 10-trans,12-cis-octadecadienoate | 0.00 | 0.00 | 0.00 | 0.00 | 395.57 | 0.00 | 0.00 | |
| Methyl 9-cis,11-trans-octadecadienoate | 0.00 | 0.00 | 0.00 | 794.14 | 0.00 | 0.00 | 0.00 | |
| Total | 19,278.79 | 28,029.14 | 64,762.54 | 86,810.50 | 47,860.24 | 63,677.24 | 65,487.65 | |
| Total (µg g−1) | ||||||||
| VLCFA | 162.06 | 481.56 | 891.79 | 1335.89 | 974.51 | 1581.82 | 1278.08 | |
| UFA | 10,801.89 | 17,061.80 | 40,111.82 | 46,405.40 | 24,258.49 | 30,600.57 | 35,012.82 | |
| SFA | 8171.86 | 10,272.07 | 22,749.74 | 38,275.07 | 22,113.41 | 30,390.14 | 27,977.91 | |
| Sterols (µg g−1) | ||||||||
| Stigmastan-3,5,22-trien | 55.70 | 0.00 | 1032.85 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Stigmasta-3,5-diene | 39.04 | 74.84 | 875.03 | 1023.03 | 710.37 | 1017.92 | 997.53 | |
| Cholesta-3,5-diene | 0.00 | 29.23 | 366.53 | 639.52 | 368.08 | 0.00 | 551.78 | |
| Stigmastan-6,22-dien, 3,5-dedihydro- | 0.00 | 151.18 | 0.00 | 0.00 | 1129.49 | 0.00 | 1447.17 | |
| beta.-Sitosterol | 0.00 | 204.77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Cholesta-6,22,24-triene, 4,4-dimethyl- | 0.00 | 0.00 | 0.00 | 1353.16 | 0.00 | 0.00 | 0.00 | |
| Stigmasteryl tosylate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1747.20 | 0.00 | |
| Cholest-5-en-3-ol (3.beta.)-, nonanoate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 516.56 | 0.00 | |
| Total | 94.75 | 460.02 | 2274.40 | 3015.71 | 2207.95 | 3281.69 | 2996.47 | |
Some changes in the fatty acid profile were also recorded after treatment with Microalgae PS (Table 2). PS treatment triggered the disappearance of 9-Hexadecenoic acid methyl esters which were present only in control plants, but induced the biosynthesis of new molecules such as Nonanoic acid, 9-oxo-, methyl ester, Pentanoic acid, 4-oxo-, methyl ester, Hexadecanoic acid, 2-hydroxy-, methyl ester, Methyl 10-trans, 12-cis-octadecadienoate and Methyl 9-cis, 11-trans-octadecadienoate.
On the other hand, microalgae PS treatment induced a remodulation of the distribution of sterol metabolites in tomato plant leaves. Generally, there was a disappearance of Stigmastan-3,5,22-trien and Stigmasta-3,5-diene in all treated plants compared to control plants. PS treatment induced the synthesis of new sterol metabolites namely: Cholesta-3,5-diene; Stigmastan-6,22-dien, 3,5-dedihydro-; beta.-Sitosterol; Cholesta-6,22,24-triene, 4,4-dimethyl-; Stigmasteryl tosylate and Cholest-5-en-3-ol (3.beta.)-,nonanoate. The synthesis of these latter depends on the composition of the PS extract used. For example, stigmasteryl tosylate, was highly accumulated (1747.20 μg g−1 FM) in plants treated with Desmodesmus sp. polysaccharides. Whereas, Stigmastan-6.22-dien, 3,5-dedihydro, Cholesta-6,22,24-triene, 4,4-dimethyl, Stigmastan-6, 22-dien, 3,5-dedihydro, Stigmastan-3,5,22-trien, and beta.-Sitosterol were induced in plants treated by D. salina 067, Porphyridium sp., P. tricornutum, D. salina 002 and A. platensis polysaccharides respectively (Table 2).
Microalgae polysaccharide effect on alkanes and alkenes profile
The results presented in Table 3 show that the effect of microalgae PS treatment on the profile of alkanes and alkenes in tomato plants. For Alkanes, heptadecane was accumulated to concentrations reaching 2866.68, 2673.91, 1743.36 and 1716.06 μg g−1 of fresh biomass of the treated plants with crude polysaccharides of Desmodesmus sp., Porphyridium sp., D. salina MS067, D. salina MS002 respectively compared to control plants (351 μg g−1). While a new biosynthesis of eicosane had been induced in plants treated by PS from D. salina MS067, P. tricornutum, D. salina MS002, A. platensis, and Desmodesmus sp. with 2590.71, 1622.30, 1034.08, 840.30 and 161.03 μg g−1 of biomass plants respectively. Also, our results show synthesis of Very long chain alkane (VLCA), C25-C33 in plants treated with polysaccharides which were not observed in control plants. In the same way, PS treatment induced the accumulation of 7-Hexadecene and 1-Octadecene in the treated plants. The most synthesized alkene in treated plants was 1-Octadecene (503.18 μg g−1), induced by PS from Desmodesmus sp., Followed by 9-Nonadecene (399.36 μg g−1), then Z-8-Hexadecene (286 μg g−1) and cetene (279.08 μg g−1), these alkenes were induced respectively by P. tricornutum, D. salina 067 and Porphyridium sp.
Table 3.
The effect of microalgal polysaccharides on the biosynthesis and profile of alkanes and alkenes in tomato plants leaves.
| Control | A. platensis | D. salina 002 | Porphyridium sp. | P. tricornutum | Desmodesmus sp. | D. salina 067 | ||
|---|---|---|---|---|---|---|---|---|
| Alkanes (µg g−1) | ||||||||
| Heptadecane | C17 | 351.69 | 0.00 | 1716.06 | 2673.91 | 0.00 | 2866.68 | 1743.36 |
| Octadecane | C18 | 31.56 | 0.00 | 234.35 | 0.00 | 1253.18 | 156.65 | 0.00 |
| Eicosane | C20 | 0.00 | 840.30 | 1034.08 | 0.00 | 1622.30 | 161.03 | 2590.71 |
| Heneicosane | C21 | 0.00 | 0.00 | 518.91 | 0.00 | 112.51 | 161.55 | 0.00 |
| Docosane | C22 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 155.86 | 0.00 |
| Tricosane | C23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 177.99 |
| Tetracosane | C24 | 35.77 | 709.66 | 0.00 | 1829.03 | 0.00 | 329.94 | 173.74 |
| Pentacosane | C25 | 0.00 | 0.00 | 0.00 | 0.00 | 18.83 | 0.00 | 0.00 |
| Hexacosane | C26 | 0.00 | 77.81 | 0.00 | 0.00 | 211.84 | 61.92 | 0.00 |
| Heptacosane | C27 | 20.76 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Octacosane | C28 | 0.00 | 179.19 | 449.78 | 161.42 | 0.00 | 155.86 | 188.26 |
| Nonacosane | C29 | 0.00 | 217.76 | 0.00 | 74.07 | 0.00 | 0.00 | 0.00 |
| Triacontane | C30 | 0.00 | 141.34 | 483.66 | 0.00 | 0.00 | 0.00 | 325.46 |
| Hentriacontane | C31 | 0.00 | 0.00 | 0.00 | 988.70 | 0.00 | 2034.48 | 388.44 |
| Dotriacontane | C32 | 0.00 | 177.29 | 0.00 | 295.25 | 463.09 | 513.11 | 416.66 |
| Tritriacontane | C33 | 0.00 | 0.00 | 0.00 | 0.00 | 257.45 | 0.00 | 0.00 |
| Alkanes derivatives (µg g−1) | ||||||||
| Trans-2,3-Epoxydecane | 313.55 | 119.83 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 1-Chloroeicosane | 0.00 | 62.39 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Hexadecane, 1-iodo- | 0.00 | 0.00 | 211.69 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Octadecane, 1-iodo- | 0.00 | 0.00 | 637.95 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Bis-siloxane | 28.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Cycloeicosane | 0.00 | 0.00 | 0.00 | 603.16 | 0.00 | 0.00 | 0.00 | |
| Cyclotriacontane | 0.00 | 0.00 | 0.00 | 0.00 | 44.20 | 0.00 | 0.00 | |
| Cyclotetrasiloxane, octamethyl- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1587.31 | |
| Cyclopentasiloxane, decamethyl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 412.08 | |
| Cyclohexasiloxane, dodecamethyl- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 160.65 | |
| -17-((2R,5R)-5-Ethyl-6-methylheptan-2-yl)- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 113.94 | |
| Total | 781.69 | 21,263.07 | 24,023.98 | 25,363.04 | 22,720.90 | 25,334.59 | 27,016.10 | |
| Alkenes (µg g−1) | ||||||||
| 7-Hexadecene | 39.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 1-Octadecene | 54.51 | 118.82 | 351.31 | 0.00 | 124.67 | 503.18 | 0.00 | |
| 1-Pentadecene | 0.00 | 100.15 | 72.24 | 0.00 | 0.00 | 0.00 | 58.63 | |
| 1-Tetradecene | 0.00 | 0.00 | 0.00 | 127.38 | 112.57 | 0.00 | 0.00 | |
| Cetene | 0.00 | 0.00 | 0.00 | 279.08 | 194.58 | 234.31 | 0.00 | |
| 2-Dodecen-1-yl | 0.00 | 0.00 | 0.00 | 196.61 | 0.00 | 0.00 | 0.00 | |
| 9-Nonadecene | 0.00 | 0.00 | 0.00 | 0.00 | 399.36 | 0.00 | 0.00 | |
| Z-8-Hexadecene | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 286.04 | |
| Total | 93.91 | 218.97 | 423.54 | 603.06 | 831.18 | 737.49 | 344.67 | |
Other metabolites
The results presented in Table 4, shows that the microalgae PS treatment induced the synthesis of new metabolites and the disappearance of other metabolites detected in untreated plants. All of the studied microalgae PS inhibited the biosynthesis of Isopropyl triacontyl ether, while they induced the synthesis of other molecules such as Neophytadiene, E-14-Hexadecenal, phenol, 4-methoxy-2-[2-(5-nitro-pyridinyl) diazenyl]- and Isopropyl octacosyl ether and others as shown in (Table 4).
Table 4.
Effect of polysaccharides treatments on profile of others metabolites (µg g−1) in tomato plants.
| Metabolites | Control | A. platensis | D. salina 002 | Porphyridium sp. | P. tricornutum | Desmodesmus sp. | D. salina 067 |
|---|---|---|---|---|---|---|---|
| Citric acid, trimethyl ester | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 293.84 |
| 2,4-Di-tert-butylphenol | 25.54 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 128.33 |
| E,E,Z-1,3,12-Nonadecatriene-5,14-diol | 59.39 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Isopropyl triacontyl ether | 101.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trim | 0.00 | 70.54 | 0.00 | 0.00 | 73.48 | 80.41 | 104.24 |
| Neophytadiene | 0.00 | 406.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| alpha.-Tocospiro B | 0.00 | 123.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Tris(2,4-di-tert-butylphenyl) phosphate | 0.00 | 38.64 | 0.00 | 0.00 | 162.00 | 100.49 | 75.05 |
| E-14-Hexadecenal | 0.00 | 0.00 | 295.24 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2-Nitroso-1-naphthol-4-sulfonic acid | 0.00 | 0.00 | 0.00 | 157.36 | 0.00 | 0.00 | 0.00 |
| phenol, 4-methoxy-2-[2-(5-nitro-pyridinyl)diazenyl]- | 0.00 | 0.00 | 0.00 | 1439.15 | 831.34 | 1358.66 | 0.00 |
| 3H-indole, 2-methyl-3-phenyl- | 0.00 | 0.00 | 0.00 | 203.07 | 0.00 | 0.00 | 0.00 |
| Phenol, 2,5-bis(1,1-dimethylethyl) | 0.00 | 0.00 | 0.00 | 0.00 | 90.95 | 0.00 | 0.00 |
| Diethyl Phthalate | 0.00 | 0.00 | 0.00 | 0.00 | 91.48 | 0.00 | 0.00 |
| 1-(4-Fluorophenyl)-2-[(4-hydroxy-6 -methylpyrimidin-2-yl)thio]ethan-, | 0.00 | 0.00 | 0.00 | 0.00 | 686.86 | 0.00 | 0.00 |
| Methyl 2-octylcyclopropene-1-heptanoate | 0.00 | 0.00 | 0.00 | 0.00 | 771.24 | 0.00 | 0.00 |
| 1,8-Dioxa-5-thiaoctane,8-(9-borabicyclo[3.3.1]non-9-yl)-3-(9-‘ | 0.00 | 0.00 | 0.00 | 0.00 | 138.55 | 0.00 | 0.00 |
| 4H-1,2,4-Triazole-3-thiol, 4-(2-phenylethyl)-5-(4-pyridyl)- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 111.78 |
| 2-Propenoic acid, 3-(4-hydroxyphenyl)-, methyl ester | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 258.53 |
| Carbonic acid, octadecyl 2,2,2-trichloroethyl ester | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 344.35 |
| Isopropyl octacosyl ether | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 504.51 |
| Cyclododecyne | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 359.98 | 0.00 |
| Cyclooctene, 3-ethenyl- | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 448.70 | 0.00 |
| Methyl 9-cis,11-trans-octadecadien | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 780.51 | 0.00 |
| 6-Nitro-2,3-dimethylpyrrolo[3,2-g]quinoline | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 93.51 | 0.00 |
| Undec-10-ynoic acid, undec-2-en-1-yl ester | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 321.13 | 0.00 |
| Protopine | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1066.43 | 0.00 |
| Dioncophyllin A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 89.98 | 0.00 |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Total | 186.26 | 639.19 | 295.24 | 1799.58 | 2845.89 | 4699.80 | 1526.78 |
Discussion
To further evaluate the effect of microalgae polysaccharides on plant tolerance against stress, a biochemical, enzymatic and metabolomics studies on treated plants compared to control plants were performed. In the present study, a bio-elicitor, laminarin, was used as a positive control for its potential to induce plant defense responses; improvement of resistance to viral, fungal and bacterial infections, induction of accumulation of salicylic acid (SA) and induces expressions of ethylene (ET)- and SA-dependent PR proteins35,36. In general, it was observed in this study that certain polysaccharides treatments had a similar effect compared to laminarin, however, we see that other treatments have a highly significant effect on studied parameters. For instance, all the treatments exhibited great effect on chitinase, peroxidase, and polyphenols; whereas all treatments except D. salina 067 had great effect on PAL activity. On the other hand, polysaccharides extracted from P. tricornutum and D. salina induced significantly Glucanase activity; and A. platensis polysaccharides enhance H2O2 production. From these results, it can be concluded that microalgae PS can be good candidates for the induction of plant defense. The activities of PAL, POX, 1,3-β Glucanase and chitinase activities and H2O2 content are frequently studied in plant defense research and are known to correlate with the occurrence of induced resistance, as well as defending the host plant against microbial phytopathogens37.
Our result showed a strong positive correlation between peroxidase (POX) and PAL activities in response to microalgae polysaccharides treatment (Fig. 4a,b). These enzymes are key defense proteins related to plants resistance to diseases38. PAL, is the key enzyme of the phenylpropanoid synthesis pathway, and increase in PAL activity is accompanied by the biosynthesis of active metabolites such as Phytoalexins, lingnins, flavonoids, phenolics compounds and salicylic acid39–41. Another study has shown seaweeds algino-oligosaccharides could induce the enzyme activities of PAL and POX in rice plants as defense against Magnaporthe grisea42. Similarly, the treatment of Olive and Tomato plants by Ulvan significantly induced PAL activities against Verticillium wilt and lycopersicicum respectively43,44. Other studies concluded that there is a correlation and synergy between the stimulation of PAL and POX activities and the induction of tomato resistance to Fusarium oxysporum f, the causative agent of wilt disease, after plant treatment by Pseudomonas fluorescens Pf1 isolates45.
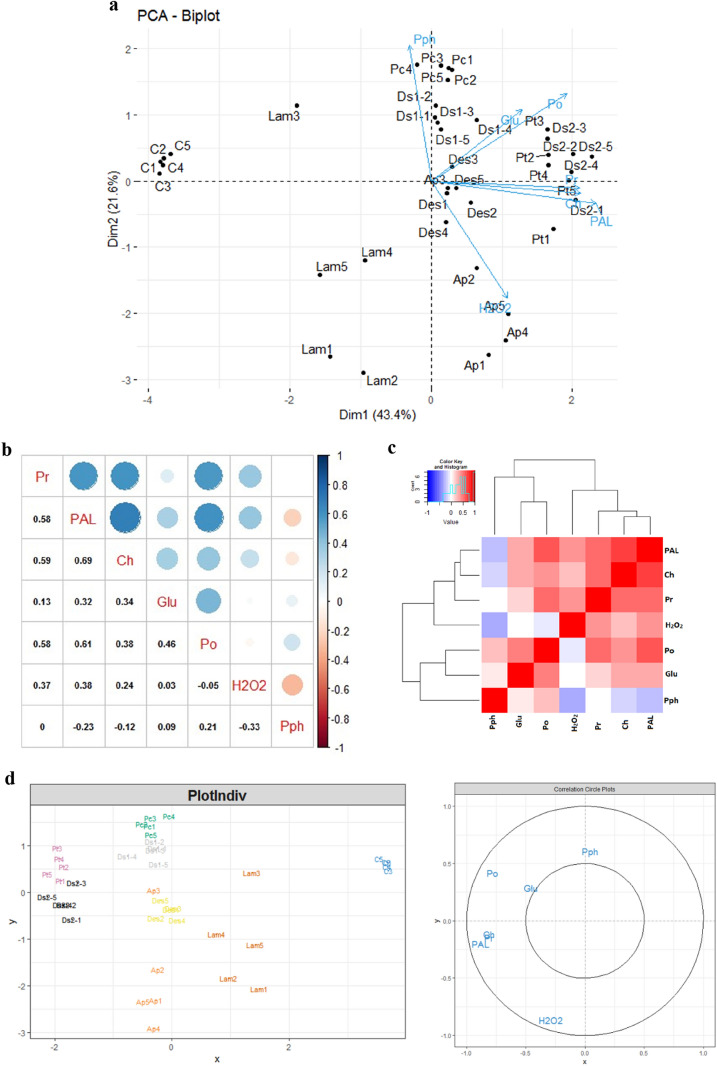
Figure 4.
Biplot of principal component analysis of variables and treatments (a); Correlation between variables (b); Heatmap hierarchal clustering of studied variables (c); PLS-DA for biochemical variables and treatments (d). C: Control; A.p: A.platensis; P.c: Porphyridium sp.; P.t: P. tricornutum; D.s1: D. salina 002; D.s2: D. salina 067; Des: Desmodesmus sp.; Lam: Laminarin; Pr: protein; PAL; Phenyl-alanine amonia-lyase; Ch: Chitinase; Glu: Glucanase; PO: Peroxidase; Pph: Polyphenol.
In response to a pathogen attacks, plants accumulate reactive oxygen species (ROS), the main chemical element of oxidative bursts46. Faced with the oxidative burst induced by a high accumulation of H2O2, plants have mechanisms that allow them to keep the H2O2 levels in homeostasis. The latter is controlled by detoxifiers or scavenging enzymes including; SOD (Superoxide dismutase), CAT (Catalase), APX (Ascorbate peroxidase) and POX (peroxidase)47–49. These enzymes coordinate the elimination of different ROS50. This explains our result, which revealed a negative correlation between peroxidase activity and H2O2 accumulation (Fig. 4a,b). That is to say that in the first step, the PS treatment induced the synthesis of H2O2, then at a certain time, H2O2 levels reached a high concentration which induced the synthesis of POX, in our case, Guaiacol peroxidase, which is found in different cell compartment, capable of producing phenoxy compounds by the reduction of H2O2. The polymerization of phenoxy compounds lead to the formation of cell wall components such as lignin51. On the other hand, peroxidases are involved in other physiological processes, namely the synthesis of phytoalexins, the formation of suberin, cross-linking of cell wall components and the synthesis of nitrogen species reagents (RNS) and the induction of the hypersensitive response, which is the programmed death of the host cells in the site of infection, for the limitation of pathogen propagation and the induction of the pathogenesis related proteins52. The transformation of phenolic compounds by peroxidases also explains our results which we noted that the high accumulation of POXs -after microalgae polysaccharides treatment- accompanied by a low accumulation of phenolic compounds in treated plants (Fig. 2a,c).
glucanase (Glu) and chitinase (Chi) are PR proteins which have the first enzymatic attacks of the plant against pathogens by degrading β-1,3-glucan and chitin present in the fungal cell wall, thus stimulating host defense responses38. These two enzymes intervene in the cases of the wounds and the infections by phytopathogens separately or in combination by reinforcing the defense of the plant against the stresses53. Our results showed that there is a strong accumulation of PR Proteins; Chitinase and Glucanase with a highly significant positive correlation (Fig. 4a,b). This correlation explains why the PS treatments are able to induce both the acquired systemic resistance (SAR) and the induced systemic resistance (ISR). The accumulation of chitinase is accompanied by the overexpression of its gene (PR3) which occurs in the jasmonic acid and ethylene compounds linked to the ISR. The induction of glucanase is ensured by the overexpression of its gene (PR2). The latter, the SA-dependent marker gene, is involved in the induction of SAR54–56. Several studies have shown that there is a great synergy between the accumulation of these two hydrolytic enzymes and the induction of plant defense by pathogens. The study done by57, showed that treatment of tobacco plants with sulfated Fucan Oligosaccharides which are reported as elicitors of the local and systemic response against Tobacoo Mosic Viruss, strongly induced the synthesis of Glucanase and Chitinase. In addition, the enhancement of Chitinase and Glucanase activities after treatment of tomato plants with Pseudomonas fluorescens Pf1 induced their resistance to Fusarium oxysporum f. sp. Lycopersici45.
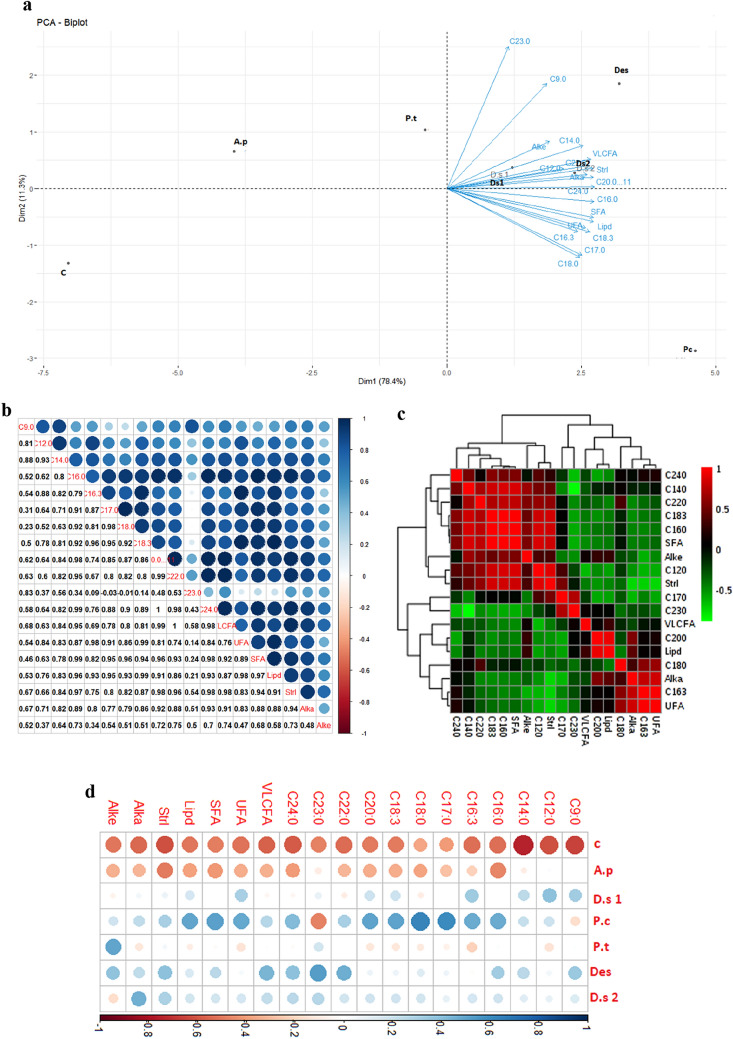
The PCA analysis revealed that the first three components accounted for 43.4%, 21.6%, and 12.1% of the variance, for a total of 77% (Fig. 4a). We believe that the first two major components provided sufficient information to corroborate the key findings presented here. Generally, the correlation is positive between the different variables (Protein, PAL, Glucanase, Chitinase and Peroxidase) (Fig. 4a,b). Hierarchical clustering spawned two large groups; Group 1: which contains proteins, Chitinase PAL and H2O2; and Group 2: which contains Glucanase, Peroxidase and Polyphenols (Fig. 4c). PLS-DA analysis generated some specificity between the type of treatment used and the type of variable induced. In this sense, it was found that the protein level and the glucanase and PAL activities were induced more by polysaccharides extracted from P. tricornutum and D. salina 067. In addition, the enzymatic activities of peroxidase and chitinase and the Polyphenols content were stimulated more by the polysaccharide-based treatment of Porphyridium sp. and D. salina 002. However, it can be seen that the accumulating effect of H2O2 is induced by A. platensis. Polysaccharides extracted from Desmodesmus sp. have a similar effect on all studied variables (Fig. 4d). This moderation of induction of enzymatic activities by microalgal PSs has prompted us to hold the combination of treatments as a perspective for the study of their effect on plant defense pathways.
The analyses, ACP-Biplot, correlation and Heatmap clustering of the metabolic variables, reveal (1): the correlation of the variables studied; (2): the specificity between the metabolite induced vis-à-vis the treatment used; (3): treatments inducing similar biochemical parameters as well as metabolomics parameters. The variables chosen for this analysis were: C9:0 (Azelaic acid), C12:0 (Lauric acid), C14:0 (Muristic acid), C16:0 (Palmitic acid), C16:3 (Roughanic acid), C17:0 (Margaric acid), C18:0 (Stearic acid), C18:3 (Linolenic acid), C20:0 (Arachidic acid), C22:0 (Behenic acid), C23:0 (Tricosylic acid) C24:0 (Lignoceric acid), VLCFA, UFA, SFA, Total Lipid, Total Sterol, Total Alkane, and Total Alkene.
The results in (Fig. 5) show that in general the metabolomics variables are positively correlated with each other (Fig. 5a,b). Heatmap, in Fig. 5d, shows that the polysaccharide treatments from Porphorydium sp., Desmodesmus sp., D. salina 067 have a significant effect on the induction of the synthesis of different metabolites, followed by D. salina 002. On the basis of biochemical and metabolomics results, it can be seen that treatments based on Porphorydium sp., P. tricornutum, Desmodesmus sp. and D. salina 067 have a great potential to induce the different parameters studied.
Figure 5.
PCA-Biplot of metabolites and treatments (a), Correlation between metabolites (b), Heatmap Heatmap hierarchal clustering of metabolomics variable (c), and Heatmap of metabolites and treatments (d), after plant treatment by microalgae PS extract. C: Control; A.p: A. platensis; P.c: Porphyridium sp.; P.t: P. tricornutum; D.s1: D. salina 002; D.s2: D.salina 067; Des: Desmodesmus sp.
The cuticle is composed of cutin polyester and cuticular wax; it allows plants’ resistance to biotic and abiotic stresses58. Generally, the cutin polymer is formed primarily of esterified and oxygenated fatty acids (FA) linked to C16 and C1859. Cuticular wax, which is composed of mixed complexes, contains mainly a variety of alkanes, primary and secondary alcohols, aldehydes, ketones and esters which are derived from very long chain fatty acids (C20–C34)60. All this explains our study findings, a strong accumulation of two precursors (fatty acids, C16 and C18) of VLCFA (C20–C24) and VLCA (C24–C33), induced by microalgae polysaccharide treatments. The strain that induced the maximum synthesis of two precursor’s palmitic acid C16:0 (9.781 μg mL−1) and stearic acid C18:0 (3.694 μg mL−1), was P. creuntum. Meanwhile, the appearance of VLCA (C24–C33), explains that VLCFAs (C25–C34), were transformed totally during the synthesis of VLCA (C24–C33).
Our results show that there is a positive correlation between the synthesis of (C16, C18), VLCFAs and VLCAs. This correlation is explained by the transformation of C16 and C18—through the elongation pathway—into VLCFAs60. The latter undergo an acyl-reduction and a decarocarbonylation—via the VLCAs—for the transformants into wax esters and ketones respectively. Then the latter are transported to the cuticle60. Among the main roles of the cuticle; it is a component that protects plants against desiccation and blocks the penetration of toxins and even pathogens61,62. The work done by63, showed that Arabidopsis plants exposed to water stress induced high synthesis content of cuticular wax accompanied with a very high accumulation of VLCAs, suggesting that the alkane biosynthesis plays an important role in abiotic stress tolerance. The cuticular permeability increases when the biosynthesis of VLCAs decreases64. Our results are comparable to that of29, who reported that C. reinarditi and C. sorokiniana polysaccharides significantly induced the synthesis of C16, C18, VLCFAs and VLCAs in tomato plants.
Plants, when infected by pathogens, trigger a series of metabolic redistributions that potentially contribute to induced tolerance or even increase sensitivity65.
Polyunsaturated fatty acids (PUFAs) are the primordial components of the plasma membrane. Their release is achieved by lipases after pathogen attack66. These FAs play a major role in plant-pathogen interactions; acting directly as free fatty acids or as fatty acid-derivatives (oxilipins)66.
In our study, increased synthesis of C18:2 as PUFAs in tomato plants after treatment with Desmodesmus sp. PSs, is similar to the results of67 which showed that increase in C18:2 synthesis in avocado fruits elicits increased resistance to the fungal pathogen, Colletotrichum gloesporioides. In our results we also see a strong accumulation of PUFAs; Roughanic acid (C16:3) and Linoleic acid (C18:3) after treatment with different PSs treatments. The maximum values of these two FAs are (2.180 μg mL) and (16.395 μg mL−1) respectively, obtained at treatment with Porphyridium sp. Trienoic fatty acids, are PUFAs that play a crucial role in adapting plants to abiotic and biotic stress. A study by68 showed that the high accumulation of linoleic acid (C18:3) in Arabidopsis leaves after inoculation with Pseudomonas syringaepv. tomato DC3000 (avrRpm1), activates NADPH-Oxidase, which is responsible for the synthesis of reactive oxygen species ROS68.
The strong accumulation of C18:3 in plants treated with PSs was accompanied by the accumulation of azelaic acid (AA, C9:0). Also, there was an induction of C18:2 synthesis in treated plants compared with control plants. Our results are similar to that of29,69, who demonstrated that after 24 h of treatment with microalgae polysaccharides, leaf content of C18:1, C18:2 and C18:3 was increased, leading to an induced accumulation of azelaic acid. These three unsaturated octadecanoic are azelaic acid (AA) precursors. Contact of host plants with a pathogen induces their defragmentation from the plasma membrane by phospholipases. After this, AGs undergo oxidation at carbon-9 to synthesize AA (71–74). The latter has been shown to induce local and systemic acquired resistance (SAR) in Arabidopsis plants against Pseudomonas syringae via the induction of salicylic acid accumulation69,70.
Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast71. Stigmasterol are phytosterols derived from β-sitosterol by the cytochrome P450 CYP710A1 via C22 desaturation65. In our results, we observed that the sterol metabolites induced by the different PS extracts are Stigmasta-3,5-diene, Cholesta-3,5-diene and Stigmastan-6,22-dien, 3,5. The accumulation of stigmasterol was higher in plants treated with PS extracts compared to control plants. These results have been confirmed by the Wang’s study71, who reported that phytosterols play a vital role in plant defense against pathogenic bacteria through nutrient flow regulations from the cytoplasm to the apoplastic space. During the invasion of plants by pathogenic bacteria, the virulence factors of the bacterium block the expression of AtCYP710A1 gene (gene responsible for transformation of β-Sitosterol into Stigmasterol), thus repression of the production of stigmasterol., all its going to induce a reduction of flow of nutrients from cytoplasm to the host cell's appoplast71.
Conclusion
In general, microalgae polysaccharides have a bio-elicitor effect on certain biochemical and metabolic pathways of tomato plant defense. In this study PS treatment induced the accumulation of ROS (H2O2) and the antioxidant activity of (POX). PS treatments also improved PAL activity, a key enzyme in the activation of the phytoalexin pathway. Pathogenesis-related proteins, such as β-1.3-Glucanase and Chitinase, which are key protein markers for plant defense against biotic stresses were also induced. Moreover, metabolomics study revealed that PS treatment induced the modification of metabolite profiles in tomato plant leaves. Induced metabolites included C18:2 and C18:3 and Azelaic acid which is a regulator of salicylic acid-dependent systemic acquired resistance. On the other hand, treated plants by PS exhibited higher accumulation of C16:0 and C18:0, VLCFAs and VLCAs which are metabolites linked to the strengthening of the cuticle by wax synthesis in order to inhibit the penetration of toxins from pathogens. These metabolites are also involved in the synthesis of sphingolipid complexes, the basal unit of the membrane lipid bilayer. Finally, PSs also induced the accumulation of sitosterol and stigmasterol derivatives. Sitosterol and stigmasterol play a key role in regulating nutrient flow to the apoplast, making plants resistant to pathogens. Meanwhile, Principal Component Analysis (PCA) showed that PSs from P. tricoruntum MS023 and D. salina MS067 significantly improved chitinase and PAL activities and proteins content. Whereas, the PS treatments of Porphyridium sp. MS081 and D. salina MS002 enhanced the POX and β-1,3-glucanase activities and Polyphenols content. On the basis of biochemical and metabolomics results, it can be seen that treatment with polysaccharides obtained from Porphorydium sp., P. tricornutum, Desmodesmus sp. and D. salina 067 have a great potential to induce the different studied parameters. This moderation of induction of enzymatic activities by microalgal PSs has prompted us to hold the combination of treatments as a perspective for the study of their effect on plant defense pathways.
Our results show that polysaccharide treatments based on microalgae can be used as a biotechnological alternative in sustainable agriculture to protect plants against pathogens and abiotic stresses and to also minimize the excessive use of chemical pesticides.
Materials and methods
Microalgae culture conditions and growth measurements
Microalgae strains used in this study (freshwater: A. platensis MS001, seawater: D. salina MS002, P. tricornutum MS023, Porphyridium sp. MS081, Desmodesmus sp. and D. salina MS067) were maintained in the microalgae collection at MAScIR (Moroccan Foundation for Advanced Science, Innovation and Research). These strains were cultivated in Walne’s medium with pH 8.2 at 25 °C for seawater strains and A. platensis in Zarrouk medium at pH 9 and 30 °C. All cultures were grown in 500 mL Erlenmeyer’s in orbital shaker at 130 rpm with continuous illumination (150 ± 10 μmol m−2 s−1). The biomass was harvested after 24 days of culture by centrifugation at 4200 rpm for 10 min and rinsed twice with a ammonium formate solution of 30 g L−1. The biomass is stored at -20 °C.
Polysaccharides production and partial characterization
1 g of dry microalgae biomass powder was suspended in 40 mL of distilled water and incubated at 90 °C for 2 h with stirring. The mixture was centrifuged at 4000 rpm for 15 min. After recovering the supernatant, the extraction process was repeated twice, and then the supernatants were mixed with two volumes of absolute ethanol, stirred vigorously and left overnight at 4 °C. The precipitated polysaccharides were recovered in the pellet by centrifugation at 11,000 rpm for 10 min. The PS pellet was washed three times with absolute ethanol and lyophilized72. The total neutral sugar and uronic acids contents of the crude PS were determined according to Blumenkrantz and DuBois methods respectively73,74. Sulfate content in crude PS was determined by the barium chloride-gelatin method75. The protein content was determined according to the Bradford method using crystalline bovine serum albumin (BSA) as standard76.
Microalgae polysaccharides effect on tomato plant defense
Bioassays for tomato growth and treatments
In this set of experiments, we used Solanum lycopersicum L. Var. Jana F1 (tomato). Seeds (purchased from “Syngenta Morocco”) were surface-disinfected using sodium hypochlorite solution (1%) containing 10 µL of Tween-20 for 20 min and then rinsed with sterile water.
Sterile seeds were germinated on Murashige and Skoog (MS) + agar medium in magenta boxes, in the dark for 4 days and in the light for 3 days and the seedlings were transplanted into 12 cm diameter pots containing a mixture of sandy soil/peat (60/40, w/w). The tomato seedlings were grown in a phytotron at 26 °C, 16 h/8 h Light/dark cycle and 240 μmol m−2 s−1 illumination intensity.
Plants were irrigated every day with 10 mL of water, alternated twice a week with a nutrient solution containing: 8 μM MnCl2, 0.5 μM CuSO4·5H2O, 1.4 μM ZnSO4, 46 μM H3BO3, 0.25 μM Na2MoO4·2H2O, 0.6 μM Fe-EDTA, 4.1 mM KNO3, 0.9 mM K2SO4, 1 mM MgSO4·7H2O and 1.5 mM KH2PO477.
The polysaccharide treatments were prepared with a ratio of 0.1% PS in distilled water. The pH was adjusted to 6 using HCl or NaOH diluted to 1 M.
The injection was performed using a microsyringe above the cotyledon leaves with a volume of 10μL of the 0.1% PS solution. 30 days after transplantation, the plants were injected with 10 μL of 1 mg mL of microalgae polysaccharides, 10 μL of Laminarin diluted to 1 mg mL−1 as the positive control) and 10 μL of distilled water (dH2O) for the negative control. 24 h after treatment, leaves were harvested according to treatment groups and grinded in liquid nitrogen. The samples were then stored at − 80 °C.
Biochemical and enzymatic parameters
Extraction and enzyme activity assay
100 mg of the tomato plant leaf biomass was homogenized in 1 mL of 100 mM Tris–HCl (pH 8.5) containing 14 mM of β-mercaptoethanol. The mixture was centrifuged at 13,000 rpm and 4 °C for 30 min. The supernatant, which contains the proteins was collected. Protein assay is determined according to Bradford method by using Bovine Serum Albumin (BSA) for the standard curve76.
The activity of phenylalanine ammonia-lyase was determined according to the modified methods previously described by Mozzetti and EL Modafar78,79.
The chitinase activity was determined according to modified Pegg method80.
Glucanase activity based on Chandrasekaran method81. The ground biomass was homogenized in 2 mL of 0.1 M Tris–HCl buffer (pH 7) containing 1% v/v of polyvinylpolypyrrolidone. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was recovered in 1.5 mL Eppendorf tubes and stored at − 20 °C. The reaction mixture contained 50 μL of the enzymatic extract, 50 μL of laminarine (10 mg mL−1) prepared in 50 mM of sodium acetate buffer, (pH 5.0) and incubated at 37 °C for 1 h on a heat block. The reaction was stopped by the addition of 1.5 mL of dinitrosalicylic acid (DNSA) and the mixture was incubated at 100 °C for 5 min a heat block. Absorbance was measured at 530 nm and 1,3-glucanase activity was calculated using the standard glucose curve. The activity of Glucanase was expressed as µM N-Acytelglucosamine H−1 g−1 FM.
The POX activity was carried out, following the method described by Chakraborty82. The substrate was prepared with 5 mL of 1% guaiacol, 5 mL of 0.3% H2O2 mixed in 50 mL of 0.05 M sodium phosphate buffer (pH 6.5). The reaction mixture was prepared with 2.95 mL of the substrate and 0.05 mL of the enzyme extract and the absorption change was measured at 470 nm for 3 min. POX activity was determined by the increase in the absorbance due to guaiacol oxidation and expressed as change in the absorption of the reaction mixture min−1 mg−1 of protein (E = 26.6 mM−1 cm−1) µkat g−1 FM.
The H2O2 determination according to Sanders method83. Tomato plants were harvested, immediately grinded in liquid nitrogen and stored at − 80 °C until H2O2 determination assay. The frozen grinded leaf biomass (150 mg) was directly homogenized in 1 mL solution containing 0.25 ml Trichloroacetic acid (TCA) (0.1% (w:v)), 0.5 mL KI (1 M) and 0.25 ml 10 mM potassium phosphate buffer (10 mM, pH adapted to studied tissue) at 4 °C for 10 min. At the same time, for every sample, a control was prepared with H2O instead of KI for tissue coloration background. Good care was taken to protect samples and solutions from light. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C. H2O2 content was measured at 350 nm sing a UV/VIS spectrophotometer. A calibration curve obtained with H2O2 standard solutions prepared in 0.1% TCA was used for quantification.
The total phenolic content in tomato leaf extracts was determined by using Folin-Ciocalteu reagent84.
Metabolomics analysis
To investigate the effect of microalgae polysaccharides on the redistribution of metabolites in tomato plants, GC–MS metabolomics analysis in this study according to Kamthan method85 with some modifications. The metabolites analyzed are lipids, alkanes, alkenes, sterols and other metabolites.
300 mg of frozen leaf biomass was added to glass vials with cap. 10 µL of internal standard, Dodecane (0.75 g mL−1) was added to each tube, to which 4 mL of chloroform and methanol mixture with a ratio of (v/v; 2/1) was also added. The mixture was vortexed and incubated at 85 °C for 2 h (2 h on heat block), vortexed and then incubated at 60 °C in an ultrasound bath for). After this, 1 mL of H2O was added to the vials and the mixtures were thoroughly vortexed. The organic phase after phase shift was recovered by using a separating funnel and dried by evaporation with nitrogen gas. For acid transesterification, 500 µL of Methanol/sulfuric acid (6%, v/v) was added to the dried organic phase residue. The mixture was incubated on a heat block for 2 h at 85 °C, and then for 1 h in an ultrasound bath. After that, the nitrogen gas was used to drying the mixture, then 250 µL and 750 µL of H2O and Chloroform respectively was added to the mixture. The lower phase was then collected using a separating funnel.
For the metabolomic analysis of volatiles compounds, the gas chromatography (GC) (Agilent 7890A Series) coupled to mass spectrometry (MS) was used in this study. 4 μL of the samples were injected into the 123-BD11 column (15 m × 320 μm × 0.1 μm) by 1/4 split mode using helium as carrier gas at 3 mLmin−1. The temperature was 230 and 150 °C in ion source and quadrupole respectively, and oven temperature program was started at 30 °C and finished at 360 °C. The NIST 2014 MS Library was used for molecules identification.
Statistical analysis
The R software (R Core Team 2013)86 was used to statistical analysis, graphical generation and corrplot and ggplot (Hadley Wickham 2016)87 packages integration. To determine the overall biochemical and metabolomics parameters variation, we used The Principal Component Analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were used studied the variation of metabolomic and biochemical parameters. The correlation between treatment and variables were studied by prcomp function and MixOmics package. The statistical analysis was studied using SPSS, one-way ANOVAs by Tukey HSD test.
Supplementary Information
Acknowledgements
Special thanks for the financial support of MESRSFC and CNRST for the realization of this project under the best conditions (Grant Number: PPR2).
Author contributions
H.E.A. conceived the experiments, F.R., R.B. and Y.K. conducted the experiments, H.E.A., L.S., K.Y. and F.R. analyzed the results. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78820-2.
References
- 1.Xin Z, et al. A disease resistance elicitor laminarin enhances tea defense against a piercing herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019;9:814. doi: 10.1038/s41598-018-37424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durango D, Pulgarin N, Echeverri F, Escobar G, Quiñones W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules. 2013;18:10609–10628. doi: 10.3390/molecules180910609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorg. Med. Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 4.da Cruz Cabral L, Pinto VF, Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 6.Baulcombe D. Reaping benefits of crop research. Science (80-) 2010;327:761. doi: 10.1126/science.1186705. [DOI] [PubMed] [Google Scholar]
- 7.Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in plant science: a global perspective. Front. Plant Sci. 2017;7:2049. doi: 10.3389/fpls.2016.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesel L, et al. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front. Plant Sci. 2014;5:655. doi: 10.3389/fpls.2014.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocira S, et al. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018;9:388. doi: 10.3389/fpls.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam) 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 11.Walters DR, Ratsep J, Havis ND. Controlling crop diseases using induced resistance: challenges for the future. J. Exp. Bot. 2013;64:1263–1280. doi: 10.1093/jxb/ert026. [DOI] [PubMed] [Google Scholar]
- 12.Stadnik MJ, de Freitas MB. Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014;39:111–118. doi: 10.1590/S1982-56762014000200001. [DOI] [Google Scholar]
- 13.Chiaiese P, Corrado G, Colla G, Kyriacou MC, Rouphael Y. Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018;9:1782. doi: 10.3389/fpls.2018.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagwell CE, et al. Discovery of bioactive metabolites in biofuel microalgae that offer protection against predatory bacteria. Front. Microbiol. 2016;7:516. doi: 10.3389/fmicb.2016.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi P, Geng S, Feng T, Wu H. Effects of Ascophyllum nodosum extract on growth and antioxidant defense systems of two freshwater microalgae. J. Appl. Phycol. 2017 doi: 10.1007/s10811-017-1287-z. [DOI] [Google Scholar]
- 16.Renuka N, Guldhe A, Prasanna R, Singh P, Bux F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol. Adv. 2018;36:1255–1273. doi: 10.1016/j.biotechadv.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg JN, et al. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the Microalga C. sorokiniana. PLoS One. 2014;9:e92460. doi: 10.1371/journal.pone.0092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eustance E, Wray JT, Badvipour S, Sommerfeld MR. The effects of limiting nighttime aeration on productivity and lipid accumulation in Scenedesmus dimorphous. Algal Res. 2015;10:33–40. doi: 10.1016/j.algal.2015.04.002. [DOI] [Google Scholar]
- 19.Becker EW. Micro-algae as a source of protein. Biotechnol. Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Zou P, et al. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019;10:48. doi: 10.3389/fpls.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Shi J, Hu D, Song B. A polysaccharide found in Dendrobium nobile Lindl stimulates calcium signaling pathway and enhances tobacco defense against TMV. Int. J. Biol. Macromol. 2019;137:1286–1297. doi: 10.1016/j.ijbiomac.2019.06.179. [DOI] [PubMed] [Google Scholar]
- 22.Khompatara K, Pettongkhao S, Kuyyogsuy A, Deenamo N, Churngchow N. Enhanced resistance to leaf fall disease caused by phytophthora palmivora in rubber tree seedling by Sargassum polycystum extract. Plants. 2019;8:168. doi: 10.3390/plants8060168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook J, Zhang J, Norrie J, Blal B, Cheng Z. Seaweed extract activates innate immune responses in Arabidopsis thaliana and protects host against bacterial pathogens. Mar. Drugs. 2018;16:221. doi: 10.3390/md16070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arroussi EL, H. , et al. Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum) J. Appl. Phycol. 2018;30:2929–2941. doi: 10.1007/s10811-017-1382-1. [DOI] [Google Scholar]
- 25.Faheed F, Fattah Z. Effect of chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008;4:165–169. [Google Scholar]
- 26.Garcia-Gonzalez J, Sommerfeld M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016;28:1051–1061. doi: 10.1007/s10811-015-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barone V, et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.) J. Appl. Phycol. 2017 doi: 10.1007/s10811-017-1283-3. [DOI] [Google Scholar]
- 28.Wuang SC, Khin MC, Chua PQD, Luo YD. Use of spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016;15:59–64. doi: 10.1016/j.algal.2016.02.009. [DOI] [Google Scholar]
- 29.Farid R, et al. Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Appl. Biochem. Biotechnol. 2019;188:225–240. doi: 10.1007/s12010-018-2916-y. [DOI] [PubMed] [Google Scholar]
- 30.Blainski JML, et al. Exopolysaccharides from Lactobacillus plantarum induce biochemical and physiological alterations in tomato plant against bacterial spot. Appl. Microbiol. Biotechnol. 2018;102:4741–4753. doi: 10.1007/s00253-018-8946-0. [DOI] [PubMed] [Google Scholar]
- 31.Michalak I, et al. Evaluation of supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016;7:1591. doi: 10.3389/fpls.2016.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronga D, et al. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9:192. doi: 10.3390/agronomy9040192. [DOI] [Google Scholar]
- 33.Dineshkumar R, et al. Microalgal liquid biofertilizer and biostimulant effect on green gram (Vigna radiata L.) an experimental cultivation. Biomass Convers. Biorefinery. 2020 doi: 10.1007/s13399-020-00857-0. [DOI] [Google Scholar]
- 34.Mutale-joan C, et al. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020;10:2820. doi: 10.1038/s41598-020-59840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera J, Castro J, Gonzalez A, Moenne A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs. 2011;9:2514–2525. doi: 10.3390/md9122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ménard R, et al. β-1,3 glucan sulfate, but not β-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and arabidopsis. Plant Cell. 2004;16:3020. doi: 10.1105/tpc.104.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Li J, Hua D. Effect of calcium nutrition on resistance of tomato against bacterial wilt induced by Ralstonia solanacearum. Eur. J. Plant Pathol. 2013;136:547–555. doi: 10.1007/s10658-013-0186-7. [DOI] [Google Scholar]
- 38.Jin L, Cai Y, Sun C, Huang Y, Yu T. Exogenous l-glutamate treatment could induce resistance against Penicillium expansum in pear fruit by activating defense-related proteins and amino acids metabolism. Postharvest Biol. Technol. 2019;150:148–157. doi: 10.1016/j.postharvbio.2018.11.009. [DOI] [Google Scholar]
- 39.Dixon RA, et al. The phenylpropanoid pathway and plant defence—a genomics perspective. Mol. Plant Pathol. 2002;3:371–390. doi: 10.1046/j.1364-3703.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, et al. Multiple pre-harvest treatments with acibenzolar-S-methyl reduce latent infection and induce resistance in muskmelon fruit. Sci. Hortic. (Amsterdam) 2011;130:126–132. doi: 10.1016/j.scienta.2011.06.024. [DOI] [Google Scholar]
- 41.Singh R, Rastogi S, Dwivedi UN. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010;9:398–416. doi: 10.1111/j.1541-4337.2010.00116.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, et al. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015;29:1–7. doi: 10.1080/13102818.2014.981368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ama I, et al. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018;13:248–255. doi: 10.1080/17429145.2018.1471528. [DOI] [Google Scholar]
- 44.Modafar CE, et al. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. (Amsterdam) 2012;138:55–63. doi: 10.1016/j.scienta.2012.02.011. [DOI] [Google Scholar]
- 45.Vellaisamy R, Thiruvengadam R, Ramasamy S. Induction of defense-related proteins in tomato roots treated with pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp lycopersici. Plant Soil. 2002;239:55–68. doi: 10.1023/A:1014904815352. [DOI] [Google Scholar]
- 46.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signaling. J. Exp. Bot. 2013;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 47.Barna B, Fodor J, Harrach BD, Pogany M, Kiraly Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. PPB. 2012;59:37–43. doi: 10.1016/j.plaphy.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 49.Niu L, Liao W. Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016;7:230. doi: 10.3389/fpls.2016.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar V, Khare T, Kishor P. Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma. 2014;251:1149–1165. doi: 10.1007/s00709-013-0581-0. [DOI] [PubMed] [Google Scholar]
- 51.Misra N, Gupta AK. Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J. Plant Physiol. 2006;163(1):11–18. doi: 10.1016/j.jplph.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Almagro L, et al. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- 53.Wu CT, Leubner-Metzger G, Meins FJ, Bradford KJ. Class I beta-1,3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol. 2001;126:1299–1313. doi: 10.1104/pp.126.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pieterse CM, van Wees SC, Hoffland E, van Pelt JA, van Loon LC. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pieterse CMJ, et al. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derksen H, Rampitsch C, Daayf F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013;207:79–87. doi: 10.1016/j.plantsci.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Klarzynski O, et al. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant. Microbe Interact. 2003;16:115–122. doi: 10.1094/MPMI.2003.16.2.115. [DOI] [PubMed] [Google Scholar]
- 58.Kim H, Choi D, Suh MC. Cuticle ultrastructure, cuticular lipid composition, and gene expression in hypoxia-stressed Arabidopsis stems and leaves. Plant Cell Rep. 2017;36:815–827. doi: 10.1007/s00299-017-2112-5. [DOI] [PubMed] [Google Scholar]
- 59.Heredia A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta. 2003;1620:1–7. doi: 10.1016/S0304-4165(02)00510-X. [DOI] [PubMed] [Google Scholar]
- 60.Raffaele S, Leger A, Roby D. Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant Signal. Behav. 2009;4:94–99. doi: 10.4161/psb.4.2.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziv C, Zhao Z, Gao YG, Xia Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018;9:1088. doi: 10.3389/fpls.2018.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reina-Pinto JJ, Yephremov A. Surface lipids and plant defenses. Plant Physiol. Biochem. 2009;47:540–549. doi: 10.1016/j.plaphy.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Kosma DK, et al. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009;151:1918–1929. doi: 10.1104/pp.109.141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bourdenx B, et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011;156:29–45. doi: 10.1104/pp.111.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griebel T, Zeier J. A role for β-sitosterol to stigmasterol conversion in plant-pathogen interactions. Plant J. 2010;63:254–268. doi: 10.1111/j.1365-313X.2010.04235.x. [DOI] [PubMed] [Google Scholar]
- 66.Walley JW, Kliebenstein DJ, Bostock RM, Dehesh K. Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 2013;16:520–526. doi: 10.1016/j.pbi.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Madi L, Wang X, Kobiler I, Lichter A, Prusky D. Stress on avocado fruits regulates Δ9-stearoyl ACP desaturase expression, fatty acid composition, antifungal diene level and resistance to Colletotrichum gloeosporioides attack. Physiol. Mol. Plant Pathol. 2003;62:277–283. doi: 10.1016/S0885-5765(03)00076-6. [DOI] [Google Scholar]
- 68.Yaeno T, Matsuda O, Iba K. Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J. 2005;40:931–941. doi: 10.1111/j.1365-313X.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 69.Yu K, et al. A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Rep. 2013;3:1266–1278. doi: 10.1016/j.celrep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 70.Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 71.Wang K, Senthil-Kumar M, Ryu C-M, Kang L, Mysore KS. Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol. 2012;158:1789–1802. doi: 10.1104/pp.111.189217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu C, Pan S. The comparison and analysis of three extraction methods for polysaccharides in purslane. J. Food Nutr. Res. 2014;2:401–405. doi: 10.12691/jfnr-2-7-12. [DOI] [Google Scholar]
- 73.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 74.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 75.Lloyd AG, Tudball N, Dodgson KS. Infrared studies on sulphate esters. III. O-sulphate esters of alcohols, amino alcohols and hydroxylated amino acids. Biochim. Biophys. Acta. 1961;52:413–419. doi: 10.1016/0006-3002(61)90397-3. [DOI] [PubMed] [Google Scholar]
- 76.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 77.Claussen W. Proline as a measure of stress in tomato plants. Plant Sci. 2005;168:241–248. doi: 10.1016/j.plantsci.2004.07.039. [DOI] [Google Scholar]
- 78.Mozzetti C, Ferraris L, Tamietti G, Matta A. Variation in enzyme activities in leaves and cell suspensions as markers of incompatibility in different Phytophthora-pepper interactions. Physiol. Mol. Plant Pathol. 1995;46:95–107. doi: 10.1006/pmpp.1995.1008. [DOI] [Google Scholar]
- 79.El Modafar C, Tantaoui A, El Boustani E-S. Differential induction of phenylalanine ammonia-lyase activity in date palm roots in response to inoculation with Fusarium oxysporum f. sp. albedinis and to elicitation with fungal wall elicitor. J. Plant Physiol. 2001;158:715–722. doi: 10.1078/0176-1617-00258. [DOI] [Google Scholar]
- 80.Pegg GF, Vessey JC. Chitinase activity in Lycopersicon esculentum and its relationship to the in vivo lysis of Verticillium albo-atrum mycelium. Physiol. Plant Pathol. 1973;3:207–222. doi: 10.1016/0048-4059(73)90083-0. [DOI] [Google Scholar]
- 81.Murugesan C, Belachew S, Yoon E, Chun S. Expression of β-1,3-glucanase (GLU) and phenylalanine ammonia-lyase (PAL) genes and their enzymes in tomato plants induced after treatment with Bacillus subtilis CBR05 against Xanthomonas campestris pv. vesicatoria. J. Gen. Plant Pathol. 2016;83:7–13. [Google Scholar]
- 82.Chakraborty N, Ghosh S, Chandra S, Sengupta S, Acharya K. Abiotic elicitors mediated elicitation of innate immunity in tomato: an ex vivo comparison. Physiol. Mol. Biol. Plants. 2016;22:307–320. doi: 10.1007/s12298-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Junglee S, Urban L, Huguette S, Lopez F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014;5:730–736. doi: 10.4236/ajac.2014.511081. [DOI] [Google Scholar]
- 84.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 85.Datta A, Kamthan A, Kamthan M, Chakraborty N, Chakraborty S. A simple protocol for extraction, derivatization, and analysis of tomato leaf and fruit lipophilic metabolites using GC-MS. Protoc. Exch. 2012 doi: 10.1038/protex.2012.061. [DOI] [Google Scholar]
- 86.R Core Team. R: A Language and Environment for Statistical Computing (2013).
- 87.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.