Abstract
In a previous study, a method of obtaining mean erythrocyte age () from HbA1c and average plasma glucose (AG) was proposed. However, the true value of the hemoglobin glycation constant ( dL/mg/day), required for this model has yet to be well characterized. Another study also proposed a method of deriving from erythrocyte creatine (EC). Utilizing these formulae, this study aimed to determine a more accurate estimate of . One hundred and seven subjects including 31 patients with hemolytic anemia and 76 subjects without anemia were included in this study. EC and HbA1c data were analyzed, and using HbA1c, AG and the newly-derived constant, were compared to using traditional in three patients whose data were taken from previous case studies. A value of dL/mg/day was determined for . using HbA1c, AG and were found to no be significantly different (paired t-test, ) to using traditional . enables the estimation of from HbA1c and AG.
Subject terms: Diabetes, Diagnostic markers
Introduction
HbA1c is widely used as both an indicator of glycemic control, as well as a diagnostic index, for diabetes in clinical settings1,2. Hemoglobin glycation is assumed to obey a three compartment model (Fig. 1). The rate constant of the total glycation reaction () is as follows.
| 1 |
Although HbA1c is generally indicative of recent glycemic control over the past 1–2 months, it is known to show reduced correlation to glycemic control status in the presence of diseases which result in a shortened erythrocyte lifespan such as hemolytic anemia3.
Figure 1.
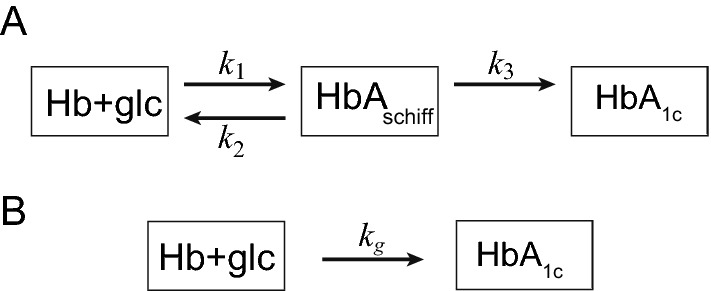
Hemoglobin glycation. (A) HbA1c is produced from Schiff base by amadori rearrangement. (B) Simplified two compartment model. is aldimine complex (intermediate product). , are kinetic constants.
Erythrocyte creatine (EC) is a good marker that reflects the mean erythrocyte age4. We proposed a method that compensates glycated albumin (GA)/IFCC-HbA1c ratio for hemolysis by EC5.
We have recently proposed a simple method to obtain mean erythrocyte age () from HbA1c and average glucose (AG)6, which has theoretically derived based on -like function model of erythrocyte lifespan7:
| 2 |
This formula provides meaningful information for the diagnosis of anemia. We estimated to be 6–10 dL/mg/day based on past literature6. However, a more accurately estimated value of would provide more useful information.
The relationship between and EC was previously established based on a model and the data8 from 21 patients, which included EC and , as following9:
| 3 |
This study aimed to determine the accurate value of from EC-derived and HbA1c.
Results
Participant characteristics
Participant demographics are shown in Table 1. All participants had no more than 16% GA. There was no significant difference in the GA of anemic and non-anemic subjects. However HbA1c, Hb, EC and their derivatives showed significant variation between the two groups.
Table 1.
Participants characteristics.
| Non-hemolysis | Hemolysis | p | |
|---|---|---|---|
| n (M/F) | 76 (30/46) | 31 (17/14) | 0.1463 |
| Age (years) | 62.3 ± 7.9 | 45.6 ± 15.0 | |
| HbA1c (%) | |||
| iA1c (mmol/mol) | |||
| GA (%) | 0.151 | ||
| GA/iA1c | |||
| Hb (g/dL) | |||
| EC (μmol/g Hb) | |||
| EC- (days) |
Results are expressed as mean ± standard deviation (SD). Sex ratio was examined by test. Other items were examined by t-test (bilateral).
GA, glycated albumin; EC, erythrocyte creatine.
The demographic information on the 3 patients from the previous cases are shown in Table 2.
Table 2.
Characteristics of three reported patients with latent hemolysis and DM in literature.
| Case | Herranz10 | Ishii11 | Hiratani12 |
|---|---|---|---|
| Age/sex | 30F | 72M | 58F |
| Disease | AIHA | AIHA | HSt |
| DM | Type 1 | Type 2 | Type 2 |
| HbA1c (%) | 5.4 | 6.5 | 5.8 |
| GA (%) | – | 26.1 | 23.3 |
| Hb (g/dL) | Normal | 13.5 | 11.5 |
| Ret (%) | Normal | 1.3 | 1.3 |
| Hpt (mg/dL) | Normal | 82 | 58 |
These patients showed normal Hb, reticulocyte, and haptogloblin.
AIHA, autoimmune hemolytic anemia; HSt, hereditary stomatocytosis, DM, diabetes mellitus; GA, glycated albumin; Hb, hemoglobin; Ret, reticulocyte; Hpt, haptoglobin.
Estimation of
EC derived and are shown in Fig. 2. A linear relationship was successfully observed.
Figure 2.
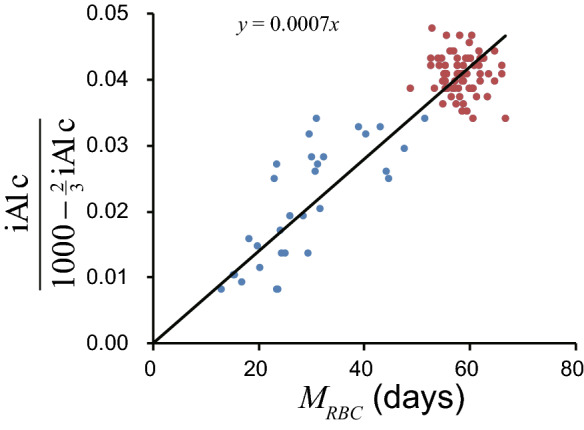
Relationship between EC derived and iA1c/(1000−(2/3)iA1c). Red circles denote non-hemolytic participants and blue circles denote hemolytic patients. Black line denotes regression line through origin.
calculated by the two methods outlined previously, for non-hemolytic participants and the entire study population are seen in Table 3. All 4 numbers can be approximated to . Figure 2 shows that data from severe hemolytic patients is less stable. Thus, the value derived from the direct method for calculating is likely to be the least accurate. Excluding this value as an outlier, the 3 remaining figures were 6.94–6.99 (average ). Therefore, considering significant figures, can be said to be .
Table 3.
estimation.
| Population | Slope | Straight forward |
|---|---|---|
| The whole | ( | |
| Non-hemolytic | ( |
Results obtained by the direct method are expressed as mean ± standard deviation (SD).
Confirmation of derived
The using the derived , and using half-life are shown in Table 4.
Table 4.
of 3 cases in literature.
derived from iA1c were , derived from half-life were . Paired t-test: t, ; df, 2; p (bilateral), 0.4514. Thus, derived from iA1c and using half-life were not significantly different.
Discussion
Based on EC-derived and HbA1c data, a more accurate value for the constant was obtained. Though was previously determined to be 6–10 dL/mg/day6, the more accurate value of improves the usefulness of the proposed model allowing closer approximation of based on AG and iA1c.
Moreover, the validity of has been confirmed through comparison of derived from iA1c and with derived from half-life. Of the three patients with hemolytic anemia and comorbid DM analyzed, data from two patients showed a remarkable correlation with the model derived figures. Data from one patient showed a 1.47 times difference in values however, this may be attributable to the use of SMBG instead of CGM, and the difficulty of standardizing data containing elution.
Variant hemoglobin should be distinguished from hemolysis when determined by Eq. (2) is low. Glycated variant hemoglobin will exhibit different peaks in HPLC from normal HbA1c, resulting in erroneously low values for HbA1c (some variants show an artefactually high value). It has previously been reported that variant hemoglobin can be detected by the dissociation between HbA1c measured by HPLC and by immunoassay13. Moreover, some variant hemoglobins such as Hb Himeji14 have different values from normal Hb. In patients with these variant hemoglobins, Eq. (2) is likely to provide a falsely low .
There are a number of limitations to this study. The data used to calculate a more specific estimate of contained EC and HbA1c, but lacked CGM data, necessitating the use of 100 mg/dL as an approximation of AG. However, participants were confirmed to be free of DM through GA, an indicator of glycemic control that is independent of mean erythrocyte age, with a cut off of GA no more than 16%. Further study with more complete data including CGM, HbA1c and EC would provide an even more definitive value for . Another limitation is that the value for derived in this study is totally dependent on Eq. (3) that derives from EC. This equation was based on old published data8, which used less sensitive and poorly specific chemical methods of measuring creatine which were prone to cross-reactivity with other guanidino compounds. This may reduce the reliability of the system. In contrast, in this study creatine was measured using an enzymatic method which was sensitive and specific to creatine in erythrocytes which uses 10-N-methylcarbamoyl-3,7-bis(dimethylamino) phenothiazine (MCDP), an N-methylcarbamoyl derivative of methylene blue, with a high molar absorption coefficient ()4, as a chromogen.
Methods
Participants
One hundred and seven subjects including 31 patients with hemolytic anemia and 76 subjects without anemia were included in this study. All samples were prepared and analyzed in accordance with the protocols approved by the institutional committees at Kumamoto University and other collaborating institutions.
Patients with hemolytic anemia were recruited from 115 patients who were older than 20 years old and required laboratory tests including complete blood counts and reticulocyte counts (Ret) for clinical reasons. Those who were suspected of having diabetes mellitus (DM) based on history, a low 1,5-Anhydroglucitol (1,5-AG) value (male, < 14.9 μg/mL; female, < 12.4 μg/mL), or had comorbid liver or renal diseases, were excluded, as liver and renal diseases affect HbA1c and GA. EC, HbA1c, GA, haptoglobin, and other biochemical screening items were measured using the existing plasma samples from these patients. Use of existing plasma samples from anemic patients without written consent was approved by the institutional review board.
Participants without anemia were recruited from medical examination checkup recipients at Takagi Hospital. Those who had anemia, DM, liver disease, renal disease or who were pregnant were excluded to avoid confounding effects on HbA1c or GA value. We provided the healthy volunteers with detailed information about the study, and all participants without anemia provided written informed consent to participate.
Data interpretation
EC was measured enzymatically in accordance with a previous report4, HbA1c was measured by high performance liquid chromatography (HPLC) method15, and GA was measured by enzymatic method using albumin-specific protenase, ketoamine oxidase, and albumin assay reagent (Lucica GA-L; Asahi Kasei Pharma Co., Tokyo, Japan)16.
HbA1c expressed in International Federation of Clinical Chemistry (IFCC) units (iA1c) was used for calculations in this study. While the National Glycohemoglobin Standardization Program (NGSP) is used to express HbA1c in many clinical research and medical care settings, NGSP is measured by an old standardized method and at the time of conception, HPLC was not able to distinguish true HbA1c from other products. HPLC technology later advanced, however the derived HbA1c value is adjusted to NGSP in the interest of consistency. IFCC provides a strict definition of iA1c as hemoglobin with a glycated valine in the N-terminal -chain. Thus, iA1c value is preferred value for estimation of hemoglobin glycation.
To acquire iA1c from HbA1c expressed in NSGP unit, we used the following equation17:
| 4 |
| 5 |
was acquired from EC by the aforementioned Eq. (3).
An AG value of 100 mg/dL was substituted for plasma glucose values derived using CGM. This number was based on the average AG of non-diabetic participants and the previously reported findings from a study which showed the median AG in healthy subjects to be reported to be 101.0 (96.3–106.0) mg/dL18 and another ADAG (A1c-derived average glucose) study which found that the AG of the non-diabetic group of their study was similarly 100 mg/dL19,20.
was also determined using half-life. As the reference range for half-life was described as 28–30 days10, 30 ± 5 days11, and 26–40 days12, was calculated by multiplying half-life and 2.14 (= 60/28), 60 days being the normal value for .
Data analysis
EC and data were analyzed using a spreadsheet software, Excel 365 (Microsoft Corporation, Redmond, WA, USA).
Estimation of
The following two methods were used to estimate . The slope method—the following Eq. (6) derived from Eq. (2) shows that the slope of the line connecting a point and the origin is .
| 6 |
Estimating the slope of the regression line through the origin by the least square model:
| 7 |
where , are and of each participant, respectively.
The direct method—the of each participant was calculated by the following equation:
| 8 |
Then, average and standard deviation of each was calculated.
Confirmation of derived
The method of obtaining from AG and iA1c was applied to data from three patients with latent hemolysis who were presented in a previous case studies10–12.
Data of Herranz10 and Ishii11 showed changes in HbA1c during the course of the study. Therefore, was calculated separately for each period. For the Ishii case11, AG was calculated by averaging self-monitoring of blood glucose (SMBG) data for each period. The Hiratani study12 examined erythrocyte lifespan measurement during hospitalization in Oct 1999 and CGM in Feb 2016. While HbA1c and plasma glucose concentrations fluctuate routinely, RBC lifespan remain comparatively constant, especially when influenced by a certain diseases (stomatocytosis). Furthermore, supply of was ceased in Japan in 2015 and thus it can no longer be used to study erythrocyte lifespan.
Ethical approval and consent to participate
The work was conducted in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan and conformed to the Helsinki Declaration. All samples were prepared and analyzed in accordance with the protocols approved by the institutional committees at Kumamoto University and other collaborating institutions.
Acknowledgements
The authors would like to thank Ms. Natalie Okawa for English language editing of this manuscript.
Abbreviations
- AG
Average plasma glucose
- CGM
Continuous glucose monitoring
- DM
Diabetes mellitus
- EC
Erythrocyte creatine
- GA
Glycated albumin
- Hb
Hemoglobin
- HPLC
High performance liquid chromatography
- Hpt
Haptoglobin
- IFCC
International Federation of Clinical Chemistry
Hemoglobin glycation constant
- MCDP
10-N-methylcarbamoyl-3,7-bis (dimethylamino) phenothiazine
Mean erythrocyte age
- NGSP
National Glycohemoglobin Standardization Program
- Ret
Reticulocyte
- SMBG
Self-monitoring of blood glucose.
Author contributions
M.Ka. contributed to study design, discussing the results, statistical analysis, project administration, the figures and initial draft manuscript preparation. T.O. contributed to organization of data acquisition, measurement of erythrocyte creatine, and advised the project. S.T., Y.M., H.M., Y.O., T.I. contributed to acquisition of data. K.H. advised the project. M.Ko. contributed to the conceptualization, study design, data curation, discussing the results, statistical analysis, and project administration. All the authors discussed the project and have read and approved the final manuscripts.
Data availability
The data supporting the findings can be obtained on reasonable request to the corresponding author.
Competing interests
M.Kameyama received research funds from Fujifilm Toyama Cemical Co., Ltd., Nihon Medi-Physics Co. Ltd., and Daiichi-Sankyo Co., Ltd. TO received research funding from Asahi Kasei Pharma.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koenig RJ, et al. Correlation of glucose regulation and hemoglobin A1c in diabetes mellitus. N. Engl. J. Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Glycemic targets in Standards of Medical Care in Diabetes—2017. Diabetes Care40, S48–S56. 10.2337/dc17-S009 (2017). [DOI] [PMC free article] [PubMed]
- 3.Panzer S, et al. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59:1348–1350. doi: 10.1182/blood.V59.6.1348.1348. [DOI] [PubMed] [Google Scholar]
- 4.Okumiya T, et al. Sensitive enzymatic assay for erythrocyte creatine with production of methylene blue. Clin. Chem. 1998;44:1489–1496. doi: 10.1093/clinchem/44.7.1489. [DOI] [PubMed] [Google Scholar]
- 5.Koga M, et al. HbA1c adjusted by erythrocyte creatine is a useful glycemic control indicator in patients with hemolysis. Clin. Biochem. 2019;73:77–81. doi: 10.1016/j.clinbiochem.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Kameyama M, Takeuchi S, Ishii S. Steady-state relationship between average glucose, HbA1c and RBC lifespan. J. Theor. Biol. 2018;447:111–117. doi: 10.1016/j.jtbi.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha RP, et al. Models for the red blood cell lifespan. J. Pharmacokinet. Pharmacodyn. 2016;43:259–274. doi: 10.1007/s10928-016-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehr J, Knob M. Comparison of red cell creatine level and reticulocyte count in appraising the severity of hemolytic processes. Blood. 1979;53:966–976. doi: 10.1182/blood.V53.5.966.966. [DOI] [PubMed] [Google Scholar]
- 9.Kameyama M, Koga M, Okumiya T. A novel method for calculating mean erythrocyte age using erythrocyte creatine. Aging (Albany NY) 2020;12:8702–8709. doi: 10.18632/aging.103193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herranz L, Grande C, Janez M, Pallardo F. Red blood cell autoantibodies with a shortened erythrocyte life span as a cause of lack of relation between glycosylated hemoglobin and mean blood glucose levels in a woman with type 1 diabetes. Diabetes Care. 1999;22:2085–2086. doi: 10.2337/diacare.22.12.2085. [DOI] [PubMed] [Google Scholar]
- 11.Ishii C, Tane N, Negishi K, Katayama S. A case of type 2 diabetes who showed discrepancy between plasma glucose and HbA1c due to latent autoimmune hemolytic anemia (in Japanese) J. Jpn. Diabetes Soc. 2001;44:157–160. doi: 10.11213/tonyobyo1958.44.157. [DOI] [Google Scholar]
- 12.Hiratani K, Natazuka T, Suemori S, Wada H, Koga M. A case of stomatocytosis in a type 2 diabetic patient accompanied with falsely low HbA1c levels due to latent hemolysis (in Japanese) J Japan Diab Soc. 2016;59:719–723. doi: 10.11213/tonyobyo.59.719. [DOI] [Google Scholar]
- 13.Miyazaki A, Kohzuma T, Kasayama S, Koga M. Classification of variant forms of haemoglobin according to the ratio of glycated haemoglobin to glycated albumin. Ann. Clin. Biochem. 2012;49:441–444. doi: 10.1258/acb.2012.011192. [DOI] [PubMed] [Google Scholar]
- 14.Koga M, et al. Aldimine formation reaction, the first step of the maillard early-phase reaction, might be enhanced in variant hemoglobin, Hb Himeji. Ann. Clin. Lab. Sci. 2015;45:643–649. [PubMed] [Google Scholar]
- 15.Kashiwagi A, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol. Int. 2012;3:8–10. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin. Chim. Acta. 2002;324:61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 17.Hoelzel W, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin. Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 18.Tsujino D, et al. Daily glucose profiles in Japanese people with normal glucose tolerance as assessed by continuous glucose monitoring. Diabetes Technol. Ther. 2009;11:457–460. doi: 10.1089/dia.2008.0083. [DOI] [PubMed] [Google Scholar]
- 19.Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci. Transl. Med. 2016;8:359ra130. doi: 10.1126/scitranslmed.aaf9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan DM, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings can be obtained on reasonable request to the corresponding author.


