Abstract
The incidence of laparoscopy-related shoulder pain reaches 90% in women. We evaluated the effect of lidocaine patch 5% on the shoulder pain after laparoscopic cholecystectomy (LC) in female patients. Total 63 female patients were randomly allocated to patch group (n = 31) and control group (n = 32). Patch group received lidocaine patch 5% and dressing retention tape on both shoulder, and control group received only dressing retention tape. Abdominal and shoulder pains were evaluated with rating on numeric rating scale (0 = no pain and 10 = the worst pain) at baseline and at 30 min, 6 h, 24 h, and 48 h after surgery. There were no significant differences in patient characteristics and operation details. The overall incidence of shoulder pain was significantly lower in patch group than in control group (42% vs. 78%, P = 0.005). The severity of shoulder pain also was significantly reduced in patch group compared to control group at 24 h and 48 h after surgery (P = 0.01 and P = 0.015, respectively). Complications related to lidocaine patch were not found except nausea. Lidocaine patch 5% reduced the incidence and severity of postoperative shoulder pain in female patients undergoing LC without complications.
Subject terms: Gastroenterology, Health care, Medical research, Signs and symptoms
Introduction
Laparoscopic cholecystectomy (LC) has become a standard treatment for gall bladder disease because of advantages such as smaller incision, shorter hospital stays and faster recovery compared with open cholecystectomy1. Although LC is considered as a less painful procedure, patients may experience shoulder pain after undergoing LC. Shoulder pain after surgery occurs rarely in open surgery, but its incidence rises to 30–60% in general laparoscopic surgery, reaching 90% in women2–4. Some patients unexpectedly may experience severe pain in laparoscopic surgery than in aggressive, major surgeries4,5. However, laparoscopy-related shoulder pain is poorly responsive to analgesics4. Therefore, the efforts to prevent the laparoscopy-related shoulder pain are essential.
Although the mechanism has not been fully clarified, laparoscopy-related shoulder pain is generally considered to develop due to diaphragmatic irritations from direct injury, stretching, or CO2 gas2,3,6. Clinically, diaphragmatic irritation manifests as referred pain in the shoulder arising from the phrenic nerve4,7. Interventions to reduce shoulder pain after LC aim to minimize diaphragmatic irritation through low-pressure pneumoperitoneum8, intraperitoneal instillation of analgesics9, drain suction10, active gas aspiration11 or phrenic nerve block12. However, local anesthesia applied to the area of referred pain, and not initial area, has also been shown to be effective in reducing referred pain in the tibialis muscle13; further, trigger point injection or a eutectic mixture of local anesthetics (EMLA) cream applied to the shoulders, not the diaphragm, significantly reduced shoulder pain after laparoscopic hysterectomy14.
Lidocaine patch 5% is a topical analgesic that interrupts pain signals in peripheral nociceptors with minimal systemic absorption and few adverse effects15. In a randomized controlled study of myofascial pain syndrome, lidocaine patch 5% decreased the symptoms of pain and the sensation of the skin as effectively as trigger point injection16. We hypothesized that application of lidocaine patch 5% to the shoulder could also reduce the severity of shoulder pain after LC.
The aim of this study was to evaluate the analgesic effect of lidocaine patch 5% on shoulder pain after LC in female patients.
Methods
This randomized, double-blinded, prospective, parallel-group study was conducted with patients undergoing LC at the Ajou University Health System between February 2017 and September 2017. The Ajou Hospital Institutional Review Board affiliated to Ajou University School of Medicine (protocol number: AJIRB-MED-CT4-16-076) approved the study protocol (ClinicalTrial.gov, NCT02827136, 11/07/2016). This study was conducted in proportion to relevant guidelines and regulations. After obtaining written informed consent from all participants, female patients with American Society of Anesthesiologists (ASA) physical status I, II or III aged 19–85 years, were included. LC included both elective and emergence surgeries performed in the day time (8:00–17:00). Exclusion criteria were as follows: histories of trauma, infection, surgery, or chronic pain involving the shoulders, hypersensitivity to local anesthetics, chronic abuse of opioids, impaired liver or renal dysfunction, or denial to participate in this study.
Interventions
Participants (n = 64) were randomized to one of two groups by randomization generator (http://www.random.org) at 1:1 ratio by J.E.K.: the patch group (n = 32) and the control group (n = 32). Assigned group was concealed in a sealed, opaque envelope. Immediately before anesthesia induction, the envelope was opened by an independent investigator who performed all interventions but was not participated in outcome assessment. The anesthesia provider, patients, and preoperative and postoperative outcome assessors did not know the assigned group throughout the study period.
None of the patients received premedication. On arrival to the operating room, basic monitoring including pulse oximetry, electrocardiography, and non-invasive blood pressure measurement was performed. Before anesthesia induction, lidocaine patches (10 × 14 cm; Lidotop, Teikoku Seiyaku Co., Kagawa, Japan) were applied to both shoulders of patients in the patch group; then, the lidocaine patches were covered with dressing retention tape (12 × 15 cm; Hypafix, BSN Medical GmbH, Hamburg, Germany). In the control group, only dressing retention tape (12 × 15 cm; Hypafix) was applied, also to both shoulders. The patients’ shoulders were covered with clothes; thus, the outcome assessors could not see it. For anesthesia induction, intravenous (IV) propofol 2 mg/kg and remifentanil 0.3 μg/kg were started and rocuronium 0.8 mg/kg was followed. After endotracheal intubation, mechanical ventilation was initiated. For maintenance of anesthesia, remifentanil was infused at a rate of 0.05–0.10 μg/kg/min, and sevoflurane 2–2.5% was used within a range of bispectral index score 40–60. In case of mean arterial pressure (MAP) < 60 mmHg or heart rate (HR) < 40 beats/min, IV ephedrine 4 mg or atropine 0.5 mg was administered, respectively. Approximately 10 min prior to the end of surgery, IV propacetamol 1 g was administered for postoperative analgesia. At the end of surgery, sevoflurane were discontinued, and the fresh gas flow was increased to 5 L/min. To reverse residual neuromuscular blockade, IV neostigmine 50 μg/kg plus glycopyrrolate 10 μg/kg were injected after confirming the train-of-four count > 2 using a nerve stimulator. After confirming adequate tidal volume, patients were extubated with maintaining the remifentanil infusion of 0.05 μg/kg/min to prevent the emergence cough. Then, the patients were transferred to a post-anesthesia care unit (PACU).
All procedures were carried out by two skilled surgeons with same method. LC were performed through three abdominal ports (10-mm infraumbilical camera, 5-mm subxipoid, and 5-mm right lateral subcostal ports). CO2 gas was inflated through infraumbilical Veress needle. Abdominal insufflation pressure was set at 12 mmHg. Drain was not inserted, and local anesthetics were not injected at peritoneum or port site.
Data collection
The primary outcome of this study was the severity of shoulder pain after surgery. Preoperative variables included demographics, ASA physical status, and diagnosis. Intraoperative variables included anesthesia time, operation time, and amounts of crystalloid and bleeding. Hemodynamic data such as HR and MAP were collected at five time points: at baseline, at pneumoperitoneum, at 20 min and 30 min after pneumoperitoneum, and at the end of surgery. Pain included the abdominal pain, and overall, right, and left shoulder pains. The incidence of shoulder pain was evaluated based on the overall value of shoulder pain and defined as the number of patients who had a pain score that was higher than the value at baseline. “> abdominal pain” was defined as the number of patients who had worse shoulder pain compared with abdominal pain during the 48 h following surgery. “Alleviated pain” was defined as the number of patients who had less shoulder pain compared to value at baseline. The severity of pain was quantified on a numeric rating scale (NRS) ranging from 0 to 10 (0 = no pain and 10 = the worst pain) at five time points: at baseline, and at 30 min, 6 h, 24 h, and 48 h after surgery. The pain score at 48 h after surgery was investigated by phone call with the patient. Nausea was graded into four (1 = none, 2 = mild, 3 = moderate, and 4 = severe). IV ramosetron 0.3 mg was administered to with vomiting or nausea grade ≥ 3 or 4. The lidocaine patches and/or dressing retention tape were removed by the ward’s attending nurse at 12 h following surgery. Complications related to lidocaine patch 5% (skin erythema, pruritus, blisters, contact hypersensitivity, nausea, headache, and arrhythmia) were evaluated by the ward’s attending nurse at ward until discharge from the hospital.
Postoperative pain treatment
On arrival to the PACU, IV fentanyl 1 μg/kg was administered as a rescue analgesic in patients reporting an NRS ≥ 5. At the ward, IV ketorolac 30 mg was administered at 8 h intervals on the day of surgery. In addition, IV nefopam 20 mg was administered as a rescue analgesic in patients reporting an NRS ≥ 5. At the postoperative day 1, the patient discharged with prescription drug, which was consist of oral acetaminophen/tramadol 325/37.5 mg at three times a day.
Statistical analysis
To calculate the sample size, we focused on the severity of shoulder pain after surgery. In a previous study, the pain score of shoulder pain after LC was 4.43 ± 1.417. Considering that a mean difference of 1.2 in pain score was significant18, 29 participants were required in each group for a type I error of 5% and a power of 90%. Considering a 10% dropout rate, a total of 64 patients (32 per group) were required.
Data are shown as mean ± standard deviation (or standard error), median (interquartile range), or number of patients (proportion). Normality of distribution was assessed with the Kolmogorov–Smirnov test. Parametric and nonparametric data were analyzed using Student’s t-test and the Mann–Whitney test, respectively. Categorical data were analyzed using the chi-square test or Fisher’s exact test. Repeated measured data were analyzed using the linear mixed model. When the interaction was statistically significant, the adjusted P value was obtained with Bonferroni correction. P < 0.05 was considered statistically significant. SPSS for Windows (version 25.0, SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
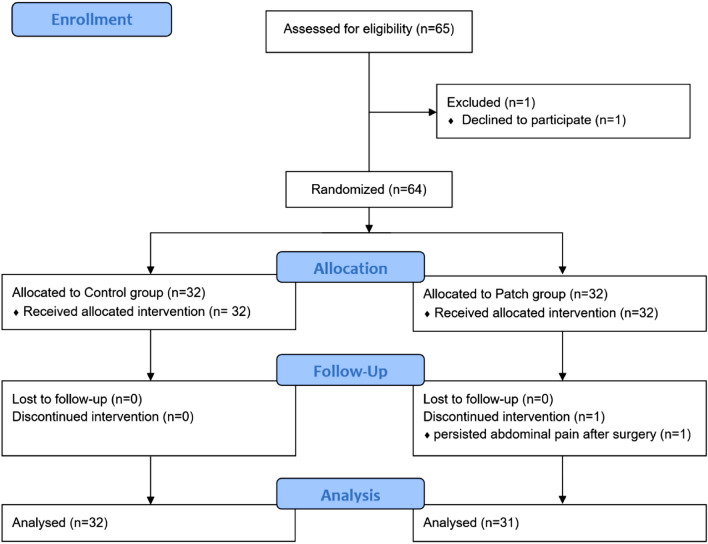
Of the 64 patients included in this study between February 2017 and September 2017, one patient in the patch group dropped out due to persisting intolerable abdominal pain; finally, the data of 63 patients were analyzed (Fig. 1). There were no significant differences in the patient characteristics and operation details between the two groups (Table 1). Intraoperative HR and MAP were comparable throughout the study period (Fig. 2).
Figure 1.
Flow diagram.
Table 1.
Patient’s characteristics and operation details.
| Control group (n = 32) | Patch group (n = 31) | P value | |
|---|---|---|---|
| Age (years) | 52 (42–63) | 47 (40–61) | 0.527 |
| Height (cm) | 158 (153–163) | 159 (155–161) | 0.581 |
| Weight (kg) | 61.3 ± 10.8 | 58.1 ± 9.8 | 0.229 |
| BMI (kg/m2) | 24 (22–27) | 23 (21–25) | 0.284 |
| ASA physical status (1/2/3) | 18/13/1 | 19/12/0 | > 0.999 |
| Diagnosis | 0.743 | ||
| Adenomyomatosis or polyps | 9 (28%) | 12 (39%) | |
| Cholecystitis | |||
| Mild | 12 (38%) | 10 (32%) | |
| Moderate | 2 (6%) | 3 (10%) | |
| Severe | 9 (28%) | 6 (19%) | |
| Crystalloid (mL) | 300 (275–400) | 300 (275–400) | 0.916 |
| Bleeding (mL) | 10 (10–20) | 15 (5–20) | 0.938 |
| Total dose of remifentanil (μg) | 400 (320–600) | 350 (280–400) | 0.055 |
| Operation time (min) | 50 (40–65) | 50 (35–57.5) | 0.229 |
| Anesthesia time (min) | 85 (70–97.5) | 80 (65–90) | 0.348 |
Values are presented as mean ± standard deviation, median (interquartile range) or number (proportion).
BMI body mass index, ASA American Society of Anesthesiologists.
Figure 2.
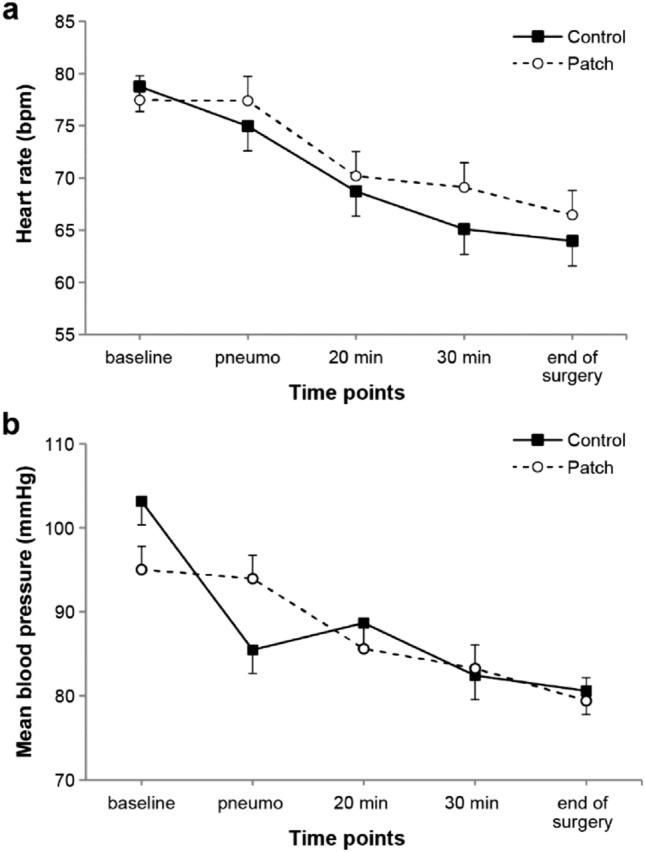
Changes of heart rate (a) and mean blood pressure during surgery (b). Values were expressed as mean ± standard error. Baseline before anesthesia induction, pneumo at pneumoperitoneum, 20 min 20 min after pneumoperitoneum, 30 min 30 min after pneumoperitoneum, end of surgery 10 min before the end of surgery.
The overall incidence of shoulder pain was significantly lower in the patch group than in the control group (42% vs. 78%, P = 0.005, Table 2). The incidence of shoulder pain at each time point except the baseline was also lower in the patch group. The number of patients showing more severe shoulder pain than abdominal pain was higher in the control group (P = 0.041), and the number of patients showing less shoulder pain compared to baseline was higher in the patch group (P = 0.024).
Table 2.
Incidence of shoulder pain.
| Control group (n = 32) | Patch group (n = 31) | P value | |
|---|---|---|---|
| Incidencea | |||
| Overall | 25 (78%) | 13 (42%) | 0.005 |
| Baseline | 4 (13%) | 7 (23%) | 0.337 |
| 30 min after surgery | 6 (19%) | 0 | 0.024 |
| 6 h after surgery | 15 (47%) | 6 (19%) | 0.032 |
| 24 h after surgery | 22 (69%) | 11 (35%) | 0.012 |
| 48 h after surgery | 20 (63%) | 8 (26%) | 0.005 |
| > Abdominal painb | 12 (37%) | 4 (13%) | 0.041 |
| Alleviated painc | 0 | 5 (16%) | 0.024 |
Values are presented as number (proportion).
aIncidence was defined as the number of patients having higher shoulder pain compared with baseline.
bThe number of patients having worse shoulder pain compared with abdominal pain.
cThe number of patients having less shoulder pain compared with baseline.
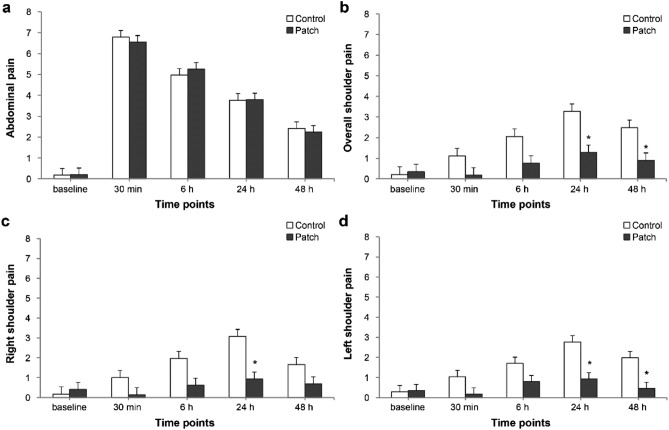
Abdominal pain showed a peak of severity at 30 min after surgery and gradually decreased thereafter in both groups (Pgroup*time = 0.868; Fig. 3a). Overall shoulder pain showed a peak of severity at 24 h after surgery in both groups (Fig. 3b). In addition, overall shoulder pain tended to be significantly different between the two groups over time (Ptime < 0.001) and was significantly lower in the patch group than in the control group at 24 h and 48 h after surgery [mean value (SE); 1.3 (0.4) vs 3.3 (0.4), Padjusted = 0.01 and 0.9 (0.4) vs 2.5 (0.4), Padjusted = 0.015 at 24 h and 48 h, respectively]. Right shoulder pain was lower in the patch group at 24 h after surgery (Padjusted = 0.01; Fig. 3c), and left shoulder pain was lower in the patch group at 24 h and 48 h after surgery (Padjusted = 0.005 for both; Fig. 3d) compared with control group.
Figure 3.
Changes of abdominal pain (a), and overall (b), right (c), and left shoulder pain (d) during the first 48 h after surgery. Values were expressed as mean ± standard error. Baseline before anesthesia induction, 30 min 30 min after surgery, 6 h 6 h after surgery, 24 h 24 h after surgery, 48 h 48 h after surgery. *P < 0.05 compared with the control group.
Right shoulder pain did not differ from left shoulder pain in either group (Pgroup*time = 0.613 and Pgroup*time = 0.449 in the control group and patch group, respectively; Fig. 4).
Figure 4.
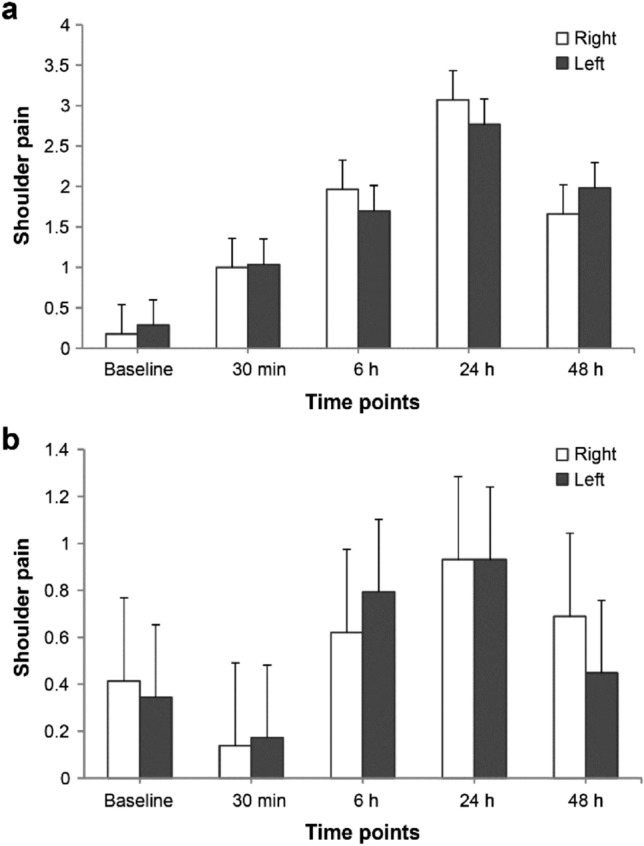
Comparison between right and left shoulder pain in control group (a) and patch group (b). Values were expressed as mean ± standard error. Baseline before anesthesia induction, 30 min 30 min after surgery, 6 h 6 h after surgery, 24 h 24 h after surgery, 48 h 48 h after surgery.
The recovery data were comparable between the two groups (Table 3). Nausea developed in 24 patients (12 patients in each group) during PACU or ward stay; no other complications related to the use of lidocaine patch 5% or dressing retention tape were found.
Table 3.
Recovery profiles.
| Control group (n = 32) | Patch group (n = 31) | P value | |
|---|---|---|---|
| In PACU | |||
| Nausea | 26/0/1/5 | 21/3/2/5 | 0.323 |
| Vomiting | 2 (6%) | 2 (7%) | > 0.999 |
| Patient requesting antiemetics | 7 (22%) | 8 (26%) | 0.714 |
| Patient requesting analgesic | 25 (78%) | 25 (81%) | 0.805 |
| Rescue fentanyl dose (μg) | 57 (11–68) | 54 (46–67) | 0.803 |
| Duration of PACU stay (min) | 40 (30–50) | 40 (40–50) | 0.190 |
| At ward | |||
| Complications | |||
| Fever | 5 (16%) | 3 (10%) | 0.708 |
| Urinary retention | 2 (6%) | 1 (3%) | > 0.999 |
| Nausea | 8 (25%) | 4 (13%) | 0.222 |
| Vomiting | 3 (9%) | 2 (7%) | > 0.999 |
| Hypotension | 0 | 1 (3%) | 0.492 |
| Patient requesting antiemetics | 2 (6%) | 3 (10%) | 0.672 |
| Patient requesting analgesic | 17 (53%) | 19 (61%) | 0.513 |
| Hospital stay after surgery (day) | 1 (1–2) | 1 (1–1) | 0.468 |
Values are presented as median (interquartile range) or number (%).
PACU post-anesthesia care unit.
Discussion
This study demonstrated the beneficial analgesic effect of lidocaine patch 5% on decreasing shoulder pain after LC in female patients. The incidence of shoulder pain in the patch group was significantly reduced up to approximately 50% of that in the control group. The severity of shoulder pain also was significantly reduced in the patch group at 24 h and 48 h after surgery. The number of patients showing more severe shoulder pain than abdominal pain was higher in the control group, and the number of patients having less shoulder pain compared to baseline was higher in the patch group.
Although still unclear, the most probable mechanism of laparoscopy-related shoulder pain is the excitation of the phrenic nerve due to diaphragmatic or peritoneal irritation2,3,6,19. The phrenic nerve originates from the anterior branch of cervical spinal nerve roots C3–C5 and provides sensory innervation to the mediastinal pleura, pericardium, and peritoneal surfaces of the diaphragm7,12. The main nerve C4 also provides cutaneous innervation to the shoulder. Regarding the misinterpretation of the origin of input from the referred pain area20,21, diaphragmatic irritation during laparoscopy can provoke referred shoulder pain. Based on this “misinterpretation theory,” numerous strategies have been developed to reduce laparoscopy-related shoulder pain by minimizing diaphragmatic irritation. These interventions are sometimes effective, but the results are conflicting and there is no consensus on preventive measures.
A “pre-local hyper-excitability theory” has been proposed, in which stimuli in the initial area cause the hyper-excitation of the connective nerve between the referred area and initial area, consequently inducing the increased sensitivity of the referred area22. According to this theory, the primary pathogenesis is peripheral sensitization rather than central sensitization. In experimental studies on healthy volunteers, referred pain was partially decreased when the input from the peripheral receptors in the referred area was blunted, though conflicting results have been published20,21. For example, an EMLA cream over the referred skin area reduced the intensity of referred pain by 22.7%23, and a complete nerve block in the referred area reduced it by 40%13. In clinical studies on patients undergoing laparoscopic surgery, treatment on the shoulder effectively decreased referred shoulder pain after laparoscopy. For example, pretreatment using a trigger point injection or an EMLA cream on the shoulder significantly reduced the incidence and severity of shoulder pain after laparoscopy14. Moreover, transcutaneous electrical nerve stimulation on the shoulder alleviated shoulder pain during laparoscopy24. Based on these studies, we hypothesized that the application of a lidocaine patch to the shoulder could also reduce referred shoulder pain after laparoscopic surgery. In the present study, lidocaine patch 5% was applied to the referred pain area (the shoulder); consequently, the incidence and severity of shoulder pain after LC were reduced significantly.
Lidocaine patch 5% is a skin patch approved for the treatment of post-herpetic neuralgia. It is also used for localized and painful conditions such as vascular access, pain caused by trauma fracture, wound pain after surgery, and arthritis18,25. Each patch contains 700 mg of lidocaine in aqueous base, but only 2–3% of the dose is absorbed; the peak plasma level is 0.13 μg/mL (toxic level, 5 μg/mL), thus showing minimal adverse effects26. In a previous study, application of an EMLA cream on the shoulders reduced laparoscopy-related shoulder pain to an NRS score of < 114, which was more effective than the lidocaine patch 5% used in present study (mean NRS scores of 1.3 and 0.9 at 24 h and 48 h after surgery, respectively). One of differences between the EMLA cream and the lidocaine patch is that EMLA produces local anesthesia by blocking large sensory fibers15 and the lidocaine patch exerts an analgesic effect by blocking the small sensory fibers without causing local anesthesia. Thus, the skin under the lidocaine patch has a normal sensation15. Despite the low analgesia potency, the lidocaine patch might be better for surgical patients than the EMLA cream due to the lack of numbness and occlusive dressing.
The peak shoulder pain score in this study was 1.3 at 24 h after surgery in the patch group. This was lower than the scores ranging from 1.9 to 4.2 in studies focusing on lessening diaphragmatic irritation during LC12,17,27. In addition, the present study only included female patients who have a lower pain threshold than male28. This is interesting finding that shoulder intervention showed more effective analgesia than diaphragmatic intervention during LC, because referred pain has been known to be mainly associated with central components (initial area) and not with peripheral components (referred area).
In the present study, shoulder pain after LC was reduced until 48 h after surgery despite the application of the lidocaine patch during the first 12 h. Lidocaine patch 5% has a half-life of 6–8 h15. In patients with myofascial pain syndrome, the effect of lidocaine patch 5% applied to three focal sites throughout the body for 4 days was superior to that of a placebo patch until day 9 after the beginning of treatment16. Similarly, in an area limited to the upper trapezius, a lidocaine patch applied for 7 days also relieved pain more effectively than a placebo patch for a period of 2 weeks29. There are two possible explanations for the long analgesic period of the lidocaine patch. First, after long-term application, lidocaine patch 5% decreases epidermal nerve fiber density without affecting pressure pain and threshold for heat- and cold-induced pain in the skin of healthy volunteers30. Second, central sensitization might play a role in persistent complaints in patients with shoulder pain31, although being poorly investigated. In the present study, the antinociceptive effect of lidocaine patch 5% that was initiated before the pneumoperitoneum might inhibit the central sensitization of the shoulder to some degree.
Right and left shoulder pain did not differ in the patch and control groups in present study. Shoulder pain after LC is generally more frequent in the right side2. During laparoscopic hysterectomy, right shoulder pain was more severe than left shoulder pain32. In contrast, Schoeffler et al. reported that more severe shoulder tip pain is noted in the left side in reference with protection of the right side of the diaphragm through the liver33. Further research is required to evaluate which side is more affected.
This study has several limitations. First, the sample size might be small when considering the simple intervention. Further studies are needed to verify our findings in a larger sample size. Second, shoulder pain scores were not evaluated by dividing separately during rest and movement. Third, when patients requested rescue analgesics, the main site of complaint was not evaluated. Fourth, more-than-mild pain (NRS ≥ 4) has considerable clinical significance. Regretfully, the number of patients with shoulder pain of NRS ≥ 4 was similar in this study (10 [31%] vs. 4 [13%] in the control vs. patch groups, P = 0.08). Fifth, at the time of patch removal by a nurse, the patient might have not remained blinded. Shame patches may be needed for complete blinding. Sixth, longer follow-up time of patients would be needed, because post-laparoscopic pneumoperitoneum was detected on upright chest radiographs in patients undergoing LC within the first week after surgery34.
In conclusion, lidocaine patch 5% reduced the incidence and severity of postoperative shoulder pain in female patients undergoing LC. Application of lidocaine patch 5% on the shoulder can be a simple, non-invasive, and effective analgesic method without adverse effects.
Acknowledgements
The authors would like to thank Hye Sun Lee, MS, Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Republic of Korea.
Author contributions
Conception and design: J.E.K.; Provision of study materials or patients: S.K.M.; Collection and assembly of data: H.Y.K. and M.Y.C.; Data analysis and interpretation: J.B.C. and G.M.L.; Manuscript writing: H.Y.K. and J.B.C.; Final approval of manuscript: all authors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ha Yeon Kim and Jong Bum Choi.
References
- 1.Keus, F., Gooszen, H. G. & van Laarhoven, C. J. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Datab. Syst. Rev.1, 1–23 (2010). [DOI] [PMC free article] [PubMed]
- 2.Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur. J. Surg. Acta Chirurgica. 2001;167:84–96. doi: 10.1080/110241501750070510. [DOI] [PubMed] [Google Scholar]
- 3.Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: Factors affecting the incidence and severity. J. Laparoendosc. Adv. Surg. Tech. Part A. 2010;20:677–682. doi: 10.1089/lap.2010.0112. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Song T, Kim KH, Lee KW. Incidence, natural course, and characteristics of postlaparoscopic shoulder pain. Surg. Endosc. 2018;32:160–165. doi: 10.1007/s00464-017-5651-5. [DOI] [PubMed] [Google Scholar]
- 5.Gerbershagen HJ, et al. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 6.Joris J, Thiry E, Paris P, Weerts J, Lamy M. Pain after laparoscopic cholecystectomy: Characteristics and effect of intraperitoneal bupivacaine. Anesth. Analg. 1995;81:379–384. doi: 10.1097/00000539-199508000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Morelot-Panzini C, Le Pimpec-Barthes F, Menegaux F, Gonzalez-Bermejo J, Similowski T. Referred shoulder pain (C4 dermatome) can adversely impact diaphragm pacing with intramuscular electrodes. Eur. Respir. J. 2015;45:1751–1754. doi: 10.1183/09031936.00220614. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee HK, et al. Impact of standard-pressure and low-pressure pneumoperitoneum on shoulder pain following laparoscopic cholecystectomy: A randomised controlled trial. Surg. Endosc. 2017;31:1287–1295. doi: 10.1007/s00464-016-5108-2. [DOI] [PubMed] [Google Scholar]
- 9.El-Labban GM, et al. Intraincisional vs intraperitoneal infiltration of local anaesthetic for controlling early post-laparoscopic cholecystectomy pain. J. Minimal Access Surg. 2011;7:173–177. doi: 10.4103/0972-9941.83508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseinzadeh F, Nasiri E, Behroozi T. Investigating the effects of drainage by hemovac drain on shoulder pain after female laparoscopic surgery and comparison with deep breathing technique: a randomized clinical trial study. Surg. Endosc. 2020 doi: 10.1007/s00464-00019-07339-z. [DOI] [PubMed] [Google Scholar]
- 11.Atak I, et al. Active gas aspiration to reduce pain after laparoscopic cholecystectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2011;21:98–100. doi: 10.1097/SLE.0b013e318213c301. [DOI] [PubMed] [Google Scholar]
- 12.Yi MS, et al. Effect of ultrasound-guided phrenic nerve block on shoulder pain after laparoscopic cholecystectomy—A prospective, randomized controlled trial. Surg. Endosc. 2017;31:3637–3645. doi: 10.1007/s00464-016-5398-4. [DOI] [PubMed] [Google Scholar]
- 13.Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. Referred pain is dependent on sensory input from the periphery: A psychophysical study. Eur. J. Pain (London, England) 1997;1:261–269. doi: 10.1016/S1090-3801(97)90035-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim JE, Kim JY, Lee HS, Seok S, Kil HK. Analgesic effect of trigger point injection and EMLA for shoulder pain in patients undergoing total laparoscopic hysterectomy: A randomized controlled study. Medicine (Baltimore) 2019;98:e14087. doi: 10.1097/MD.0000000000014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: A review of the literature. J. Clin. Pharmacol. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 16.Affaitati G, et al. A randomized, controlled study comparing a lidocaine patch, a placebo patch, and anesthetic injection for treatment of trigger points in patients with myofascial pain syndrome: Evaluation of pain and somatic pain thresholds. Clin. Ther. 2009;31:705–720. doi: 10.1016/j.clinthera.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Yasir M, et al. Evaluation of post operative shoulder tip pain in low pressure versus standard pressure pneumoperitoneum during laparoscopic cholecystectomy. Surgeon J. R. Coll. Surg. Edinburgh Ireland. 2012;10:71–74. doi: 10.1016/j.surge.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Khanna M, Peters C, Singh JR. Treating pain with the lidocaine patch 5% after total knee arthroplasty. PM & R J. Injury Funct. Rehabilit. 2012;4:642–646. doi: 10.1016/j.pmrj.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br. J. Surg. 2000;87:273–284. doi: 10.1046/j.1365-2168.2000.01374.x. [DOI] [PubMed] [Google Scholar]
- 20.Arendt-Nielsen L, Svensson P. Referred muscle pain: Basic and clinical findings. Clin. J. Pain. 2001;17:11–19. doi: 10.1097/00002508-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kosek E, Hansson P. Perceptual integration of intramuscular electrical stimulation in the focal and the referred pain area in healthy humans. Pain. 2003;105:125–131. doi: 10.1016/S0304-3959(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 22.Farasyn A. Referred muscle pain is primarily peripheral in origin: The "barrier-dam" theory. Med. Hypotheses. 2007;68:144–150. doi: 10.1016/j.mehy.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 23.Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. Quantification of local and referred pain in humans induced by intramuscular electrical stimulation. Eur. J. Pain (London, England) 1997;1:105–113. doi: 10.1016/S1090-3801(97)90068-9. [DOI] [PubMed] [Google Scholar]
- 24.Asgari Z, et al. A comparative study between transcutaneous electrical nerve stimulation and fentanyl to relieve shoulder pain during laparoscopic gynecologic surgery under spinal anesthesia: A randomized clinical trail. Pain Res. Manag. 2018;2018:9715142. doi: 10.1155/2018/9715142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng YJ. Lidocaine skin patch (Lidopat 5%) is effective in the treatment of traumatic rib fractures: A prospective double-blinded and vehicle-controlled study. Med. Principles Practice Int. J. Kuwait Univ. Health Sci. Centre. 2016;25:36–39. doi: 10.1159/000441002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87:7–17. doi: 10.1016/S0304-3959(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu T, et al. Low-pressure pneumoperitoneum versus standard pneumoperitoneum in laparoscopic cholecystectomy, a prospective randomized clinical trial. Surg. Endosc. 2009;23:1044–1047. doi: 10.1007/s00464-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 28.Frot M, Feine JS, Bushnell MC. Sex differences in pain perception and anxiety. A psychophysical study with topical capsaicin. Pain. 2004;108:230–236. doi: 10.1016/j.pain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Cohen H, Jones HW. The reference of cardiac pain to a phantom left arm. Br. Heart J. 1943;5:67–71. doi: 10.1136/hrt.5.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrfritz A, et al. Differential effects on sensory functions and measures of epidermal nerve fiber density after application of a lidocaine patch (5%) on healthy human skin. Eur. J. Pain (London, England) 2011;15:907–912. doi: 10.1016/j.ejpain.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Noten S, et al. Central pain processing in patients with shoulder pain: A review of the literature. Pain Pract. Off. J. World Inst. Pain. 2017;17:267–280. doi: 10.1111/papr.12502. [DOI] [PubMed] [Google Scholar]
- 32.Choi JB, et al. Pain characteristics after total laparoscopic hysterectomy. Int. J. Med. Sci. 2016;13:562–568. doi: 10.7150/ijms.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoeffler P, Diemunsch P, Fourgeaud L. Ambulatory celioscopy. Cah. Anesthesiol. 1993;41:385–391. [PubMed] [Google Scholar]
- 34.Millitz K, et al. Pneumoperitoneum after laparoscopic cholecystectomy: Frequency and duration as seen on upright chest radiographs. AJR Am. J. Roentgenol. 1994;163:837–839. doi: 10.2214/ajr.163.4.8092019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.