Abstract
Human papillomavirus (HPV) is the leading cause of cervical cancer and has been implicated in several other cancer types including vaginal, vulvar, penile, and oropharyngeal cancers. Despite the recent availability of a vaccine, there are still over 310,000 deaths each year worldwide. Current treatments for HPV-mediated cancers show limited efficacy, and would benefit from improved understanding of disease mechanisms. Recently, we developed a Drosophila ‘HPV 18 E6’ model that displayed loss of cellular morphology and polarity, junctional disorganization, and degradation of the major E6 target Magi; we further provided evidence that mechanisms underlying HPV E6-induced cellular abnormalities are conserved between humans and flies. Here, we report a functional genetic screen of the Drosophila kinome that identified IKK—a regulator of NF-κB—as an enhancer of E6-induced cellular defects. We demonstrate that inhibition of IKK reduces Magi degradation and that this effect correlates with hyperphosphorylation of E6. Further, the reduction in IKK suppressed the cellular transformation caused by the cooperative action of HPVE6 and the oncogenic Ras. Finally, we demonstrate that the interaction between IKK and E6 is conserved in human cells: inhibition of IKK blocked the growth of cervical cancer cells, suggesting that IKK may serve as a novel therapeutic target for HPV-mediated cancers.
Subject terms: Cancer, Cell biology, Microbiology
Introduction
Human papillomaviruses (HPV) are small, double-stranded circular DNA viruses that are obligate epitheliotrophs: they specifically infect either squamous or mucocutaneous epithelia. The latter HPV types can be categorized into two classes: low-risk HPVs and high-risk HPVs. The former induce large and obvious benign lesions—condylomata—of the infected tissues. The latter are carcinogenic, causing most cervical cancers, as well as being strongly implicated in oropharyngeal, anal, vulvar, vaginal and penile cancers1. Among all high-risk HPVs, HPV 16 and HPV 18 are the most prominent types, causing more than 70% of all invasive cervical cancers. According to World Health Organization (WHO), every year 570,000 women are diagnosed with cervical cancer, with a current mortality rate of more than 50%2,3.
The availability of effective vaccines against the most prevalent high-risk HPVs is expected to eventually reduce HPV-dependent tumors. However, the number of new HPV-induced cancer cases is not predicted to decline appreciably for the next few decades. Economic and cultural barriers hinder widespread immunization in the middle- and low-income countries that account for the majority of cervical cancers4. Furthermore, chronic HPV infection can require several decades to provoke transformation5,6. Existing vaccines are only prophylactic against new HPV infections and are not effective against preexisting HPV infections, nor can they inhibit cancer progression and malignancy7. As a result of these hurdles, effective treatment for HPV-induced cancer in general remains a major unmet clinical need. Current treatments for invasive HPV-induced cancers are primarily radiation and chemotherapy, which show limited effectiveness and the survival rates of, for example, advanced-stage cervical cancer patients is low8,9.
HPV 16 and HPV 18 direct cellular transformation through the persistent expression of two viral early genes, E6 and E710,11. E6 and E7 oncoproteins cause cellular transformation through elimination of key tumor suppressors P53, and Rb respectively12–14. High-risk HPV E6s contain a PDZ binding motif (PBM) at the extreme C terminus that is absent in low-risk E6s15. Interaction of the PBM with the PDZ domains of key host cellular PDZ domain proteins, including Magi, Dlg and Scribble, targets these proteins for ubiquitination and subsequent proteasome-mediated destruction16–19. These PDZ domain-containing proteins are important components of tight junctions and cell polarity-controlling complexes20–22. This action of E6 requires the assistance of the host E3 ubiquitin ligase, UBE3A and is necessary for cellular transformation23,24. Transgenic mice expressing high-risk E6 and E7 in the skin develop cancers at high frequency; those expressing E7 and an E6 deficient in the PBM do not develop cancers. The failure to induce cellular transformation is independent of P53, as PBM-deleted E6 retains the ability to inactivate P5325. However, beyond the cellular targets of HPV oncogenes, we have a limited understanding of how persistent expression of E6 and E7 can lead to dysplasia and cancer.
Drosophila has proven a strongly useful platform for modeling human diseases including cancer, owing in part to high conservation of genes and signaling pathways, and the availability of a broad array of genetic tools26–31. One particular advantage of Drosophila disease models is their use in functional genetic screens designed to discover novel targets and pathways that mediate human disease32–34. In this study we used a recently developed Drosophila model of HPV18 E6 + UBE3A35 in a screen to identify kinases active in aspects of E6/UBE3A-induced transformation. We report that reduced activity of ‘inhibitor of nuclear factor kappa-B kinase subunit beta’ (IKKβ)—which regulates the innate immune response—strongly suppressed E6 + UBE3A-mediated cellular abnormalities, as well as rescuing degradation of E6 targets. We provide evidence that the IKK-mediated suppression of Magi degradation is due to phosphorylation of E6, which was previously shown to block the interaction of E6 with PDZ domain proteins. Further, reduction in IKK suppressed the cellular transformation caused by the cooperative action of HPVE6 and oncogenic Ras. Finally, we used a targeted inhibitor to demonstrate that reduced IKKβ activity results in strongly reduced growth of cultured human cervical cancer cells of established cell lines. Together, our results support the view that targeting IKKβ is a candidate strategy for developing novel lead therapeutics for HPV-induced cancer.
Material and methods
Fly strains
The following fly stocks were used: UAS-hUBE3A36, UAS-HPV18-E635, GMR-Gal4, Kinome set, IKK1/TM6B,Tb, UAS-IKK RNAi attp40, UAS-Ras64BV14 from Bloomington Drosophila stock center.
In vitro assays
To examine the effect of IMD 0354 on the growth of HaCat (HPV−ve), HeLa (HPV 18 +ve), CaSki and SiHa (both HPV 16 +ve), the cells were plated out and allowed to adhere overnight. Next day their culture medium was replaced and different concentrations of IMD 0354 (Santa Cruz Biotechnologies, Santa Cruz, CA) (final concentrations 250, 500, 750, 1000, 1250 nM, using DMSO to equalize input volumes and as negative control 0 nM) were added to the plates. Cells were counted 48 h later using a haemocytometer. The assay was repeated 3 times. One-way ANOVA test using the Prism program was used for statistical analysis.
Cell cycle and apoptosis analysis
HaCat, HeLa and CaSki cells were plated out at equal density, and allowed to attach overnight. The next day they were treated with 500 nM IMD354 (or DMSO alone) for 3 h, then harvested by typsinisation and centrifugation (the medium and all washes were also centrifuged to collect any floating or dead cells). The cell pellet was resuspended in citrate buffer with 0.1% NP-40, RNase and Propidium iodide, and incubated for 30 min in the dark at room temperature. FACS analyses were then performed (FACScalibur, Becton Dickinson) and the cell cycle profiles were analysed (10,000 events per run). The assays were performed four times. Analysis: the % of events recorded in the subG1 (Essentially apoptosis), G1, S and G2 peaks for each run were compared between treated and untreated samples for each cell type and the fold change with treatment was calculated.
Western blot analysis
HPV18-positive HeLa cells were seeded on 6 cm dishes and allowed to attach overnight. Fresh medium was then added, containing either 0, 100 nM or 500 nM IMD 0345 (in DMSO). After 5 h the cells were harvested in 2× SDS-PAGE gel loading buffer, run on SDS-PAGE, and analyzed by Western Blot. The membrane was probed with antibody specific for phosphorylated E6, as described previously37,38. Following incubation with primary antibody, the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako) were used, followed by enhanced chemiluminescence (ECL) detection according to the manufacturer's instructions (GE Healthcare). Original blots provided in supplementary information.
Immunohistochemistry
For immunolabeling pupal eyes, 40–42 h after puparium formation, were dissected in PBS and fixed in 4% formaldehyde. Fixed tissues were washed three times in PBS solution containing 0.1% Triton-X-100 and blocked in 5% normal goat serum for 1 h before incubation with primary antibodies. The primary antibodies used in this study were rabbit anti-Magi 1:20039, rabbit anti-Baz 1:1000, rat anti-DE-cadherin 1:50 (Developmental Studies Hybridoma Bank). The appropriate secondary antibodies were conjugated Alexa488, Alexa594, and Alexa 647 (Invitrogen).
Results
Kinome screen identified IKKβ as a mediator of E6 + hUBE3A-mediated defects
The fly eye is a compound eye consisting of 750 unit eyes. These ‘ommatidia’ are clusters of sensory neurons arranged in a precisely repeated hexagonal pattern formed by the precise arrangement of supporting pigment cells. Formation of this highly organized pattern requires precisely regulated cell proliferation, cell differentiation and programmed cell death; disruption of any of these processes leads to a disorganized, rough eye phenotype that is readily scored under a light microscope40.
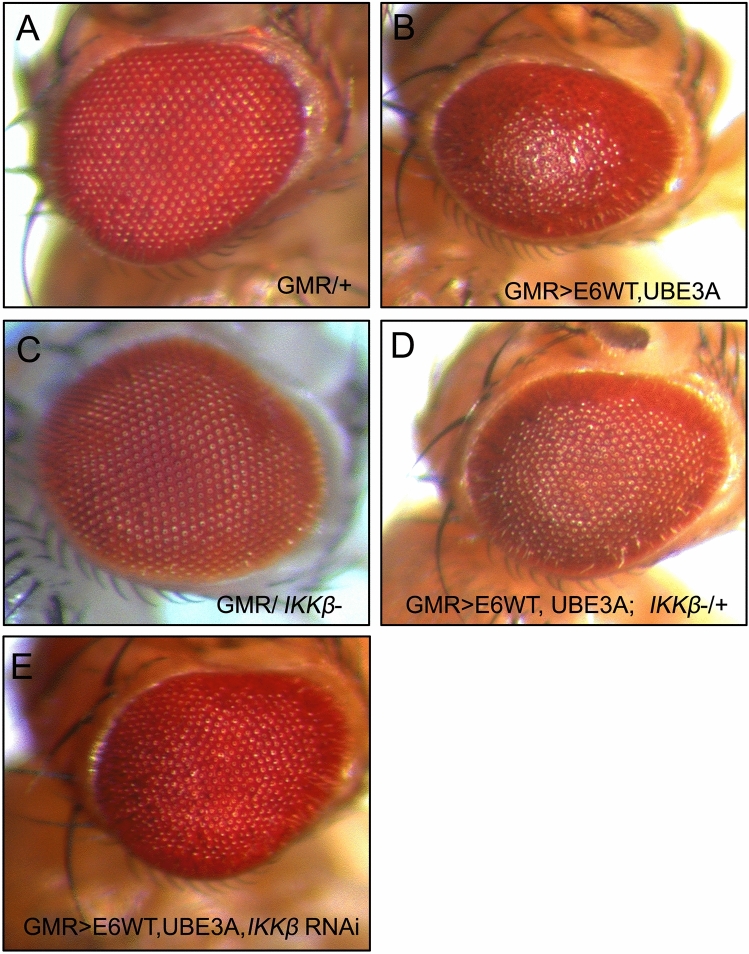
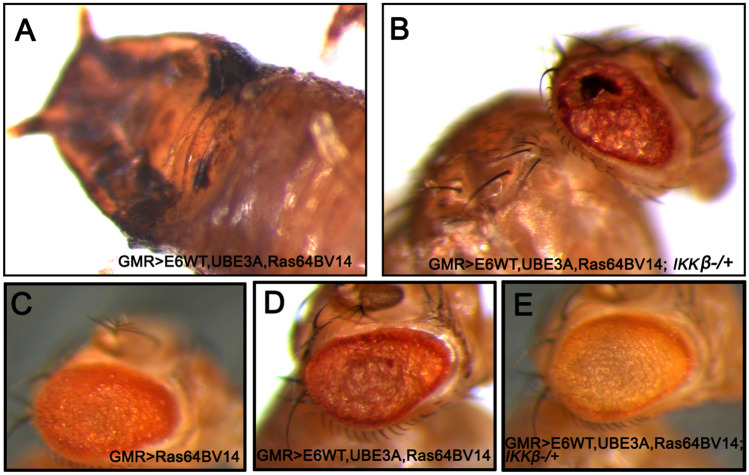
We have previously shown that co-expression of E6 and human UBE3A (hUBE3A) in the developing fly eye leads to a disorganized, rough eye phenotype. To identify loci that modify the E6 + hUBE3A-induced eye defects, we used mutations in the kinome to perform a dominant genetic modifier screen. Flies with stable integration of the transgenes GMR-Gal4, UAS-E6, and UAS-hUBE3A (referred to as GMR>E6/hUBE3A) were crossed to flies heterozygous for a mutant kinase. Comparing GMR>E6/hUBE3A; kinase+/− to GMR> E6/hUBE3A flies, we screened 195 kinases and identified IKKβ as the strongest suppressor of the E6 + hUBE3A-mediated rough eye phenotype (Fig. 1D, compare with Fig. 1B; Fig. 1A,C are controls). Rescue was evident in 100% of flies (n = 40) with GMR>E6/hUBE3A; IKKβ+/− genotype and to the same extent shown in Fig. 1D. The rescue by reduced IKKβ activity was further confirmed with an RNA-interference transgene targeting IKKβ (Fig. 1E).
Figure 1.
Reduction of IKKβ suppresses the E6 and UBE3A co-expression phenotypes. (A) Adult eye expressing GMR-Gal4 showing an intact eye morphology. (B) GMR-Gal4-driven co-expression of E6 and UBE3A causes rough eye morphology. (C) One mutated copy of IKKβ gene in GMR-Gal4 expressing eye has no effect on the eye morphology. (D) One mutated copy of IKKβ gene suppresses the E6 + UBE3A-induced rough eye defects. (E) Expression of IKKβ RNAi suppresses the E6 + UBE3A-induced rough eye defects.
Inhibiting IKKβ in cervical cancer cells blocked their growth
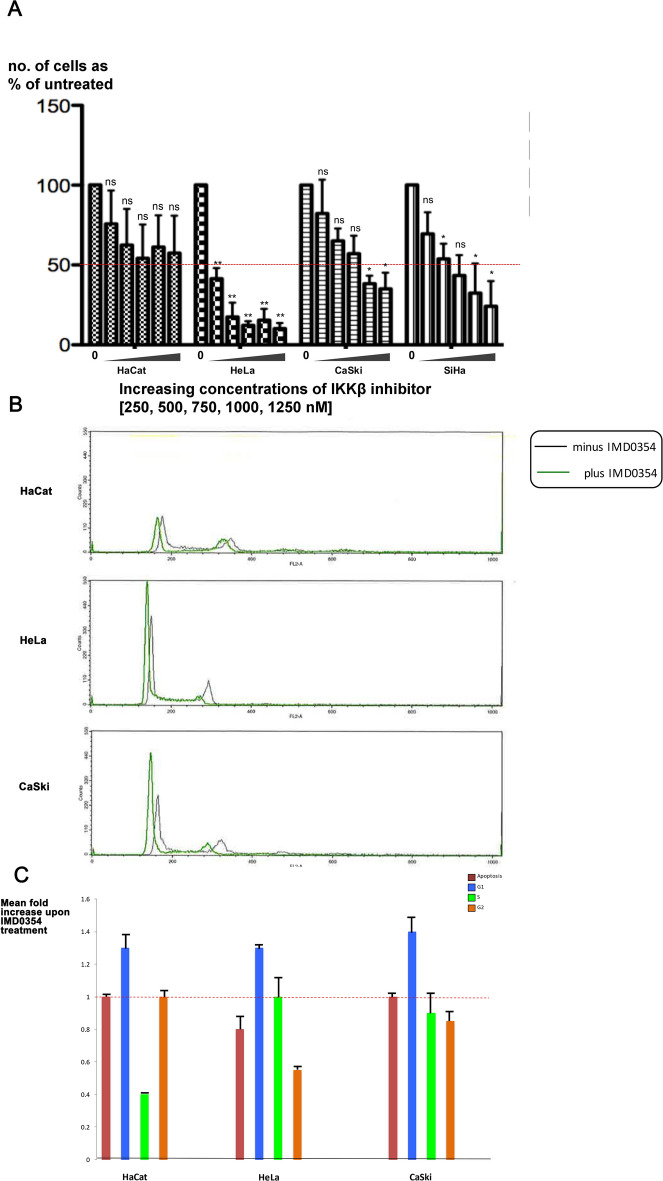
IKKβ is a serine/threonine kinase that is highly conserved between flies and humans. It regulates the innate immune pathway by activating NF-κB, which in turn activates the expression of antimicrobial peptides to fight against pathogens41–43. To determine whether IKKβ also has a functional link to E6 + UBE3A in human cells, we assessed the effect of the synthetic IKKβ inhibitor IMD 0354 (N-(3,5-Bis-trifluoromethylphenyl)-5-chloro-2-hydroxybenzamide)44 on the growth of HaCat (HPV−ve), HeLa (HPV 18 +ve), CasKi (HPV 16 +ve), and SiHa (HPV 16 +ve) cells. IMD 0354 is a non–adenosine triphosphate–binding (ATP-binding) competitive selective IKKβ inhibitor that prevents ATP attachment to IKKβ38. Different concentrations of the inhibitor were tested ranging from 250, 500, 750, 1000, to 1250 nM. IMD 0354 reduced growth of all four cell types; HeLa cells were most strongly affected, with a significant effect starting at the lowest concentration of 250 nM. While IMD 0354 had a significant effect on the HPV 18 and 16 positive cells, it had a minor effect on the growth of HaCat cells that lack HPV (Fig. 2A). This result suggested that the IKKβ-mediated mechanism of E6 + UBE3A-induced cellular abnormalities is conserved between humans and fruit flies. To understand whether the reduced cell number resulting from inhibition of IKKβ is due to arrest in cell cycle or to apoptosis, we treated these cells with 500 nM of IMD 0354 and analyzed their cell cycle profile 3 h post treatment. We found no increase in apoptotic cell death for HeLa and CaSki cells in comparison with HaCat cells. However, the majority of cells were found to exhibit a G1 cell cycle arrest (Fig. 2B,C). This finding indicated that the reduction in cell numbers upon IKKβ inhibition is due to a halt in cell growth and not to an increase in apoptotic cell death.
Figure 2.
Inhibition of Human IKKβ in cervical cancer cells blocks their growth. (A) Five different concentrations (250, 500, 750, 1000, 1250 nM) of human IKKβ inhibitor, IMD 0354, were applied to HPV-negative (HaCat), HPV 18-positive (HeLa), and HPV 16-positive (CaSki and SiHa) cells and the effect was measured by number of surviving cells after 48 h. The inhibitor blocks the growth of HPV 16 and 18 cervical cancer cells without a significant effect on the HaCat cells, showing a most significant effect on HeLa cells: reducing the initial number of cells to 40%, at starting concentration of 250 nM and 10% at 1250 nM. Data are presented as mean ± SD (n = 3); not significance (n.s.) = P > 0.05; *P < 0.05, **P < 0.01. One-way ANOVA test using the Prism program was used for statistical analysis. (B) HPV-negative (HaCat), HPV 18-positive (HeLa), and HPV 16-positive (CaSki) cells were treated with 500 nM of human IKKβ inhibitor, IMD 0354, or with DMSO as control. After 3 h cells were trypsinized and stained with Propidium Iodide, and the cell cycle profiles were analysed by FACS. n = 4. Representative cell cycle profiles are shown for each cell type and condition. (C) The cell cycle FACS profiles were analysed to determine the number of cells in subG1 (i.e. apoptotic), G1, S and G2M phases of the cell cycle. The histogram shows the results from four experiments expressed as the fold change in cell number upon IMD 0354 treatment, relative to DMSO treatment. Standard deviations are shown. The assay was repeated 4 times, n = 4.
Reducing IKKβ suppressed the cellular defects caused by co-expression of E6 and hUBE3A
As reduction in IKKβ suppressed the rough eye phenotype caused by co-expression of E6 and hUBE3A, we examined the eye tissue of these flies 40 h after puparium formation, the time point at which the E6 + hUBE3A effect becomes apparent. In the normal pupal eye, each ommatidium consists of eight photoreceptor cells covered by four glial-like cells or cone cells and two primary pigment cells. Neighboring ommatidia are separated from each other by a lattice of secondary and tertiary pigment cells and the sensory bristle cells. This interweaving lattice of pigment cells organizes the ommatidial array into a precise pattern of repeated hexagons45.
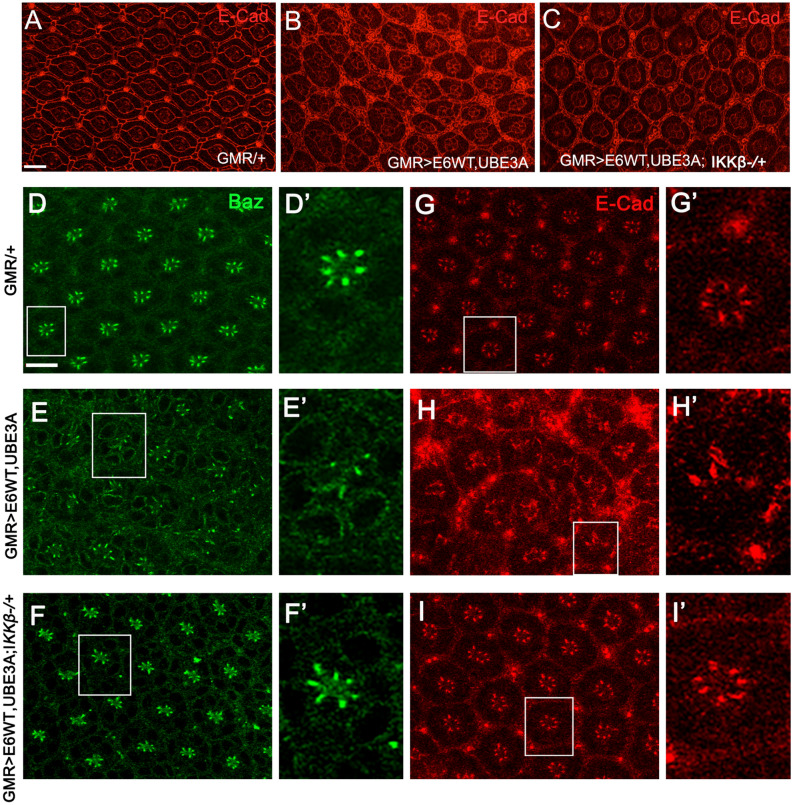
Pupal eyes expressing E6 and hUBE3A exhibit severe morphological defects, including fusion of neighboring ommatidia, increase in the number of pigment cells and cone cells, and a severe alteration in the stereotyped pattern of ommatidia. In comparison, removing a single genomic copy of IKKβ (GMR>E6/hUBE3A; IKKβ+/−) led to a strong phenotypic rescue: pigment and cone cell defects were reduced and the overall organization of the ommatidial array improved (Fig. 3A–C). These observations suggest that E6 interferes with a molecular mechanism involving IKKβ and that this mechanism plays an important role in E6-induced cellular abnormalities.
Figure 3.
Reduction of IKKβ suppresses the cellular abnormalities and restores the cell polarity and junctional integrity disrupted by E6 + UBE3A co-expression. (A–C) Pupal eyes showing E-Cad immunolabeling. (A) Expression of GMR-Gal4, showing a normal stereotype pattern of ommatidia. (B) Co-expression of E6 and UBE3A causes severe abnormalities, including increase in number of pigment cells, bristle cells, and fusion of neighboring ommatidia. (C) A mutated copy of the IKKβ gene suppresses the E6 + UBE3A abnormalities. (E–H′) Co-expression of E6 and UBE3A causes disruption of cell polarity and junctional complex, as shown by loss of Bazooka (Baz) and E-Cad from photoreceptor cells. This is in comparison with (D–G′) where Baz and E-Cad are both localized correctly in each photoreceptor. (F–I′) A mutated copy of IKKβ gene in eyes expressing E6 and UBE3A restores the polarity and junctional integrity as is shown by the correct localization of Baz and E-Cad. Scale bars indicate 10 µm. Insets are digitally magnified 200%. Results shown are representative of those of 5 different flies.
Reducing IKKβ suppressed the junctional and polarity defects caused by co-expression of E6 and hUBE3A
We have previously shown that E6, in cooperation with UBE3A, perturbs the integrity of junctional and polarity complexes35. Therefore, we hypothesized that reducing IKKβ activity might suppress these defects. Immunolabeling for junctional marker E-cadherin and polarity marker Bazooka (the homolog of human Par-3) revealed that, in comparison with pupal eyes expressing E6 and UBE3A, in which both the junctional and polarity complexes were perturbed in ommatidia (Fig. 3 E–H′), GMR>E6/hUBE3A; IKKβ+/− pupal eyes showed no disruption of junctional and polarity complexes and the integrity of these complexes was restored to the extent seen in control eye tissues (Fig. 3F–I′ compared to D–G′). These observations suggest that alterations in IKKβ significantly contribute to the E6-induced cellular junctional and polarity disorganization.
Reduced IKKβ activity suppressed E6 + hUBEA-induced degradation of PDZ domain proteins
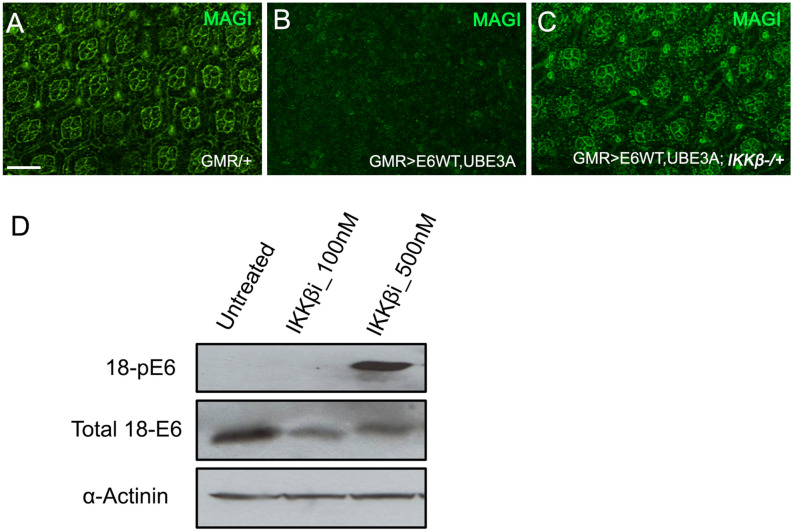
Proteasomal degradation of PDZ domain-containing proteins, including Magi, Dlg, and Scribble, was shown to be crucial for the cancerous effect of HPV 16 and 18 E625. We have previously shown that HPV 18 E6, with the addition of human UBE3A, targets the fly counterparts of these proteins for ubiquitin-mediated proteasomal destruction35. As a reduction in IKKβ levels suppressed the cellular defects caused by E6 plus hUBE3A, we asked whether the E6-mediated degradation of PDZ domain proteins was altered. To address this question we examined the level of Magi, as Magi has been identified as a major degradation target of HPV18 E6 in human and Drosophila19,35. Immunolabeling of pupal eyes for Magi revealed that, whereas GMR>E6/hUBE3A eyes exhibited a complete loss of Magi, GMR>E6/hUBE3A; IKKβ+/− pupal eyes exhibited no detectable loss of Magi (Fig. 4A–C). This result suggests that reducing IKKβ activity suppresses the E6-induced degradation of Magi, and that rescue of Magi degradation is likely to play a role in suppression of E6-induced cellular defects.
Figure 4.
Reduced activity of IKKβ causes hyperphosphorylation of E6 and suppression of E6 + UBE3A-mediated degradation of PDZ domain protein, Magi. (A–C) Pupal eyes showing Magi immunolabeling. (B) Co-expression of E6 + UBE3A results in loss of Magi from cone cells and pigment cells compared with (A) in which only the Gal4 driver is expressed. (C) A mutated copy of the IKKβ gene suppresses the E6 + UBE3A-mediated loss of Magi. (D) Western blot of HeLa cell extracts shows that inhibition of IKKβ using a concentration of 500 nM of the inhibitor IMD 0354 results in phosphorylation of E6; compare that to lack of E6 phosphorylation in HeLa cells grown with no inhibitor (untreated) or with a low concentration of 100 nM. Scale bar indicates 10 µm. Results shown in (A–C) are representative of those of 5 different flies. Result shown in (D) is a representative of n = 3.
Reducing IKKβ resulted in hyperphosphorylation of E6
Phosphorylation of the HPV 18 E6 PBM was previously demonstrated to block its interaction with PDZ domain proteins Dlg and Magi37,46,47. To assess whether phosphorylation-mediated regulation of E6 plays a role in suppressing Magi degradation, we treated cells expressing HPV 18 E6 with the IKKβ inhibitor IMD 0354 (at 100 and 500 nM) and compared with untreated cells for E6 phosphorylation. Western blot analysis was performed using an antibody to detect phosphorylated E6. We found that inhibition of IKKβ resulted in extensive phosphorylation of E6, which was absent in untreated cells (Fig. 4D). This result is consistent with previous findings, and suggests that the lack of Magi degradation in cells expressing E6 + hUBE3A with mutated IKKβ could be due to the loss of PDZ recognition by E6.
Reducing IKKβ suppressed the cooperative effect of Ras and E6 + hUBE3A
Previous studies have shown that the HPV oncogenes E6 and E7 alone are insufficient to direct oncogenic transformation, and that other factors including genetic alterations contribute to HPV-induced tumorigenesis in humans and mice48. Mutations in Ras family proteins have been implicated in HPV-related cancers, and we have previously shown that cooperation between E6 and oncogenic isoforms of Drosophila Ras (Ras64BV14) promotes cellular transformation and malignancy in fly epithelia35,48–50. Therefore, we asked whether reducing IKKβ activity can suppress the cellular transformation caused by the cooperation of oncogenic Ras and E6. Co-expression of oncogenic Ras, Ras64BV14 with E6, and hUB3A in eye imaginal discs (GMR>E6/hUBE3A/Ras64BV14) at 25 °C resulted in overgrowth and pupal lethality (Fig. 5A). However, when the level of IKKβ was reduced (GMR>E6/hUBE3A/Ras64BV14; IKKβ+/−), the lethality was rescued and flies developed to adulthood. These adult flies, however, still exhibited significant abnormalities in eye morphology, suggesting incomplete rescue (Fig. 5B).
Figure 5.
Reduction of IKKβ suppresses the cellular transformation caused by cooperative action of Ras and E6 + UBE3A. (A,B) Expression of transgenes at 25 °C. (B) A mutated copy of IKKβ gene in eyes expressing E6, UBE3A and an oncogenic Ras, suppresses pupal lethality and the severe defects caused by cooperative action of E6, UBE3A and oncogenic Ras (A). (C–E) Expression of transgenes at 22 °C. (C) Expression of oncogenic Ras in the eye causes cellular transformation. (D) Co-expression of E6 and UBE3A with oncogenic Ras enhances the Ras phenotype. (E) A mutated copy of IKKβ gene suppresses the severe eye abnormalities mediated by the synergistic effect of oncogenic Ras, E6 and UBE3A. Results shown are representative of those of 5 different flies.
Gal4 activity is reduced at lower temperatures51. At 22 °C, GMR>E6/hUBE3A/Ras64BV14 flies developed to adulthood, exhibiting enhanced transformed eye morphology in comparison to expression of oncogenic Ras alone (Fig. 5D compared to 5C). Reducing IKKβ activity significantly suppressed the transformed eye morphology in these conditions (Fig. 5E), further demonstrating that IKKβ is important for the cooperative action of E6 and oncogenic Ras.
Discussion
In this study we identify IKKβ as a mediator of the HPV 18 E6 and hUBE3A-induced cellular defects in both fly and human cancer models. This is consistent with previous studies demonstrating that HPV 16 E6 interacts with components of the innate immune pathway, including IKKβ, and it activates the NF-κB transcription factor52,53. The role of the innate immune system in HPV infection and cancer progression is not well understood. Activation of the innate immune system, particularly the Toll pathway, has been associated with regression and clearance of HPV 16 infection54. This is consistent with other studies demonstrating that HPV 16 oncogenes repress the expression of a key innate immune sensor, Toll like receptor 9 (TLR9)55, suggesting that persistent infection may reflect a defect in the host innate immune system. Conversely, activation of the innate immune system, and consequent inflammation, contributes to HPV-induced tumor progression56. Several studies have shown an increase in the level of inflammatory cytokines in individuals with reduced HPV clearance57–59. Additionally, upregulation of several TLRs and components of the NF-κB signaling pathway have been implicated in HPV-related cancers. It has been suggested that the malignant phenotype of cervical cancer cells requires the function and activation of IKKβ/NF-κB signaling60,61. IKKβ phosphorylates the inhibitor of NF-κB, resulting in its ubiquitination and proteasomal degradation. This action of IKKβ frees the NF-κB, which in turn enters the nucleus and activates the transcription of pro-inflammatory, pro-cell proliferation and anti-apoptotic genes62,63. Increased expression of IKKβ and its association with an aggressive phenotype has been reported in several types of cancers including head-and-neck, ovarian and liver cancers64–66. It is notable that IKKβ's role in cancer is not only limited to its function in regulation of the NF-κB pathway; IKKβ can also phosphorylate p53, resulting in its ubiquitination and subsequent degradation. Inactivation or loss of p53 has been identified in more than 50% of cancers, including HPV-induced cancers: high-risk HPV E6s target p53 for ubiquitination and proteasomal degradation14,67. IKKβ-mediated loss of p53 can be suppressed by inhibition of IKKβ in cancer cells68,69. The inhibitory function of IKKβ on p53 was further found in HPV38E6E7 human keratinocytes. IKKβ phosphorylates and stabilizes a dominant-negative inhibitor of p53, ∆Np73 resulting in repression of p53-regulated genes such as p21. This inhibitory effect of IKKβ on p53 can be suppressed upon its inactivation, which results in destabilization and degradation of ∆Np7370. Another phosphorylation target of IKKβ is fork-head transcription factor FOXO3a. IKKβ has been found to stimulate cell cycle progression and proliferation of breast cancer cells, through phosphorylation of FOXO3a, leading to its exclusion from nucleus and subsequent degradation in the proteasome. This function of IKKβ could be further overridden by the overexpression of FOXO3a71. All these findings suggest that IKKβ may contribute to HPV-induced cellular abnormalities in several ways, some through the innate immune pathway, and some independent of it.
IKKβ inhibitors have served as potent anti-tumor agents inhibiting cell proliferation and invasiveness, as well as inducing cell death. Their activity has been demonstrated in several cancer types including breast, colon, ovarian, oral, prostate, liver, melanoma, and leukemia65,66,72–76. One such inhibitor is IMD 0354, which selectively inhibits IKKβ by preventing ATP attachment to IKKβ. This will in turn block phosphorylation of IκBα, thus preventing nuclear translocation and activation of NF-κB38. Although IMD 0354 was initially designed to inhibit inflammation77, it has recently proved to have strong anticancer properties78,79 in several cancers. IMD 0354 inhibits breast cancer cell proliferation, progression of mast cells, and it induces apoptosis in prostate, lung and colon cancers44,80–82. In our study we found that IMD 0354 inhibited the growth of HPV 16+ and 18+ cervical cancer cell lines. This result is consistent with previously reported function of IMD 0354 in cancer cells and hence suggests a therapeutic potential for IKKβ inhibitors to treat HPV-induced cancers. Although both HPV16+ and HPV18+ cervical cancer cell lines responded to IMD 0354, however, IMD 0354-mediated inhibition of IKKβ exhibited greater effectiveness on HeLa cells (HPV 18+) compared with SiHa and CaSki cells (HPV 16+). It has previously been demonstrated that these cell lines differ in a number of molecular pathways, and in their response to treatments such as therapeutic agents that induce cell death83–85. HeLa cells exhibit greater apoptotic cell death in response to cisplatin than SiHa and CaSki cells do, a difference that is due to the higher levels of p53 and p21 in HeLa cells84. Additionally, these cell lines show distinct proteomic profiles for pathways connected to p53 activation, mitochondrial function and oxidative stress85,86. These findings suggest that the stronger effect of IMD 0354 in HeLa relative to SiHa and CaSki cells could in part be due to their difference in molecular pathways affected by IKKβ. Additionally, IMD 0354 caused an extensive phosphorylation of E6, which was absent in untreated cells. This finding is intriguing, as phosphorylation of E6 on the PDZ binding motif results in disruption of its interaction with PDZ domain-containing proteins46,47. This is consistent with our results, as degradation of Magi was inhibited when IKKβ was reduced. It would be of interest to understand the mechanism by which inhibition of IKKβ results in E6 hyperphosphorylation.
In summary this study suggests an important role for IKKβ in E6-induced cellular abnormalities and that targeting IKKβ could be a potential therapeutic option for cervical cancer and, perhaps, for other HPV-related cancers for which there is currently no effective treatment.
Supplementary Information
Acknowledgements
We would like to thank department of biology for their support and the Samuel Roberts Noble Microscopy Laboratory for assistance in confocal imaging. This study was partially supported by Oklahoma Shared Clinical and Translational Resources grant funded by NIH.
Author contributions
M.P.B. wrote the manuscript and prepared Figs. 1, 3, 4A–C and 5. M.T., J.V.T., and A.V. prepared Figs. 2 and 4D. R.L.C. provided reagents. Experiments for Figs. 2 and 4D were performed in L.B. laboratory. B.Z., R.L.C. and L.B. all intellectually contributed to the project and revised the manuscript. Additionally, all authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80193-5.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sanjose S, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller JT, Muller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217–225. doi: 10.1016/S1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol. Biomark. Prev. 2013;22:553–560. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skeate JG, Woodham AW, Einstein MH, Da Silva DM, Kast WM. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum. Vaccin Immunother. 2016;12:1418–1429. doi: 10.1080/21645515.2015.1136039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas TL. Cancer prevention: HPV vaccination. Semin. Oncol. Nurs. 2016;32:273–280. doi: 10.1016/j.soncn.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su WH, Chuang PC, Huang EY, Yang KD. Radiation-induced increase in cell migration and metastatic potential of cervical cancer cells operates via the K-Ras pathway. Am. J. Pathol. 2012;180:862–871. doi: 10.1016/j.ajpath.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Pectasides D, Kamposioras K, Papaxoinis G, Pectasides E. Chemotherapy for recurrent cervical cancer. Cancer Treat. Rev. 2008;34:603–613. doi: 10.1016/j.ctrv.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6-gene and E7-gene of the human papillomavirus type-16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 1989;63:4417–4421. doi: 10.1128/JVI.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho GYF, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J. Natl. Cancer. 1995;I(87):1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 12.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7-oncoprotein is able to bind to the retinoblastoma gene-product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 13.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus type-16 and type-18 E6 proteins with P53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 14.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 15.Spanos WC, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J. Virol. 2008;82:2493–2500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyono T, et al. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell Biol. 2000;20:8244–8253. doi: 10.1128/Mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pim D, Thomas M, Javier R, Gardiol D, Banks L. HPV E6 targeted degradation of the discs large protein: Evidence for the involvement of a novel ubiquitin ligase. Oncogene. 2000;19:719–725. doi: 10.1038/sj.onc.1203374. [DOI] [PubMed] [Google Scholar]
- 19.Kranjec C, Banks L. A systematic analysis of human papillomavirus (HPV) E6 PDZ substrates identifies MAGI-1 as a major target of HPV type 16 (HPV-16) and HPV-18 whose loss accompanies disruption of tight junctions. J. Virol. 2011;85:1757–1764. doi: 10.1128/JVI.01756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laura RP, Ross S, Koeppen H, Lasky LA. MAGI-1: A widely expressed, alternatively spliced tight junction protein. Exp. Cell Res. 2002;275:155–170. doi: 10.1006/excr.2002.5475. [DOI] [PubMed] [Google Scholar]
- 21.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 22.Javier RT, Rice AP. Emerging theme: Cellular PDZ proteins as common targets of pathogenic viruses. J. Virol. 2011;85:11544–11556. doi: 10.1128/JVI.05410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The Hpv-16 E6 and E6-Ap Complex Functions as a Ubiquitin-Protein Ligase in the Ubiquitination of P53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 24.Beaudenon S, Huibregtse JM. HPV E6, E6AP and cervical cancer. BMC Biochem. 2008 doi: 10.1186/1471-2091-9-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J. Virol. 2003;77:6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halder G, Mills GB. Drosophila in cancer research: To boldly go where no one has gone before. Oncogene. 2011;30:4063–4066. doi: 10.1038/onc.2011.128. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert LI. Drosophila is an inclusive model for human diseases, growth and development. Mol. Cell Endocrinol. 2008;293:25–31. doi: 10.1016/j.mce.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Vidal M, Cagan RL. Drosophila models for cancer research. Curr. Opin. Genet. Dev. 2006;16:10–16. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Crozatier M, Vincent A. Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis. Model Mech. 2011;4:439–445. doi: 10.1242/dmm.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maitra U, Ciesla L. Using Drosophila as a platform for drug discovery from natural products in Parkinson's disease. Medchemcomm. 2019;10:867–879. doi: 10.1039/c9md00099b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirzoyan Z, et al. Drosophila melanogaster: A model organism to study cancer. Front. Genet. 2019;10:51. doi: 10.3389/fgene.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito S, et al. A genetic screen in Drosophila for regulators of human prostate cancer progression. Biochem. Biophys. Res. Commun. 2014;451:548–555. doi: 10.1016/j.bbrc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Rossi F, et al. An in vivo genetic screen in Drosophila identifies the orthologue of human cancer/testis gene SPO11 among a network of targets to inhibit lethal(3)malignant brain tumour growth. Open Biol. 2017 doi: 10.1098/rsob.170156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson AM, Bailetti AA, Rodkin E, De A, Bach EA. A genetic screen reveals an unexpected role for Yorkie signaling in JAK/STAT-dependent hematopoietic malignancies in Drosophila melanogaster. G3. 2017;7:2427–2438. doi: 10.1534/g3.117.044172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padash Barmchi M, et al. A Drosophila model of HPV E6-induced malignancy reveals essential roles for magi and the insulin receptor. PLoS Pathog. 2016;12:e1005789. doi: 10.1371/journal.ppat.1005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiter LT, Seagroves TN, Bowers M, Bier E. Expression of the Rho-GEF Pbl/ECT2 is regulated by the UBE3A E3 ubiquitin ligase. Hum. Mol. Genet. 2006;15:2825–2835. doi: 10.1093/hmg/ddl225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boon SS, Tomaic V, Thomas M, Roberts S, Banks L. Cancer-causing human papillomavirus E6 proteins display major differences in the phospho-regulation of their PDZ interactions. J. Virol. 2015;89:1579–1586. doi: 10.1128/JVI.01961-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennikov A, et al. IkappaB kinase-beta inhibitor IMD-0354 beneficially suppresses retinal vascular permeability in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2014;55:6365–6373. doi: 10.1167/iovs.14-14671. [DOI] [PubMed] [Google Scholar]
- 39.Padash Barmchi M, Samarasekera G, Gilbert M, Auld VJ, Zhang B. Magi is associated with the par complex and functions antagonistically with bazooka to regulate the apical polarity complex. PLoS ONE. 2016;11:e0153259. doi: 10.1371/journal.pone.0153259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsachaki M, Sprecher SG. Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev. Dyn. 2012;241:40–56. doi: 10.1002/dvdy.22738. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 43.Vallabhapurapu S, Karin M. Regulation and function of NF-kappa B transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka A, et al. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- 45.Cagan R. Principles of Drosophila eye differentiation. Curr. Top. Dev. Biol. 2009;89:115–135. doi: 10.1016/S0070-2153(09)89005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhne C, Gardiol D, Guarnaccia C, Amenitsch H, Banks L. Differential regulation of human papillomavirus E6 by protein kinase A: Conditional degradation of human discs large protein by oncogenic E6. Oncogene. 2000;19:5884–5891. doi: 10.1038/sj.onc.1203988. [DOI] [PubMed] [Google Scholar]
- 47.Boon SS, Banks L. High-risk human papillomavirus E6 oncoproteins interact with 14–3-3 zeta in a PDZ binding motif-dependent manner. J. Virol. 2013;87:1586–1595. doi: 10.1128/Jvi.02074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenhalgh DA, et al. Transgenic mice expressing targeted HPV-18 E6 and E7 oncogenes in the epidermis develop verrucous lesions and spontaneous, rasHa-activated papillomas. Cell Growth Differ. 1994;5:667–675. [PubMed] [Google Scholar]
- 49.Schreiber K, et al. Strong synergy between mutant ras and HPV16 E6/E7 in the development of primary tumors. Oncogene. 2004;23:3972–3979. doi: 10.1038/sj.onc.1207507. [DOI] [PubMed] [Google Scholar]
- 50.Anwar K, Nakakuki K, Naiki H, Inuzuka M. ras gene mutations and HPV infection are common in human laryngeal carcinoma. Int. J. Cancer. 1993;53:22–28. doi: 10.1002/ijc.2910530106. [DOI] [PubMed] [Google Scholar]
- 51.Duffy JB. GAL4 system in Drosophila: A fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira LB, Haga IR, Villa LL. Human papillomavirus (HPV) 16 E6 oncoprotein targets the Toll-like receptor pathway. J. Gen. Virol. 2018 doi: 10.1099/jgv.0.001057. [DOI] [PubMed] [Google Scholar]
- 53.James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daud II, et al. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int. J. Cancer. 2011;128:879–886. doi: 10.1002/ijc.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan UA, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013;210:1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31:1905–1912. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- 57.Baker R, et al. Increased plasma levels of adipokines and inflammatory markers in older women with persistent HPV infection. Cytokine. 2011;53:282–285. doi: 10.1016/j.cyto.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemp TJ, et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol. Biomarkers Prev. 2010;19:1954–1959. doi: 10.1158/1055-9965.EPI-10-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam JO, et al. Association of serum cytokines with oral HPV clearance. Cytokine. 2016;83:85–91. doi: 10.1016/j.cyto.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich AM, Hussaini HM, Parachuru VP, Seymour GJ. Toll-like receptors and cancer, particularly oral squamous cell carcinoma. Front. Immunol. 2014;5:464. doi: 10.3389/fimmu.2014.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma XF, et al. IKKbeta/NF-kappaB mediated the low doses of bisphenol A induced migration of cervical cancer cells. Arch. Biochem. Biophys. 2015;573:52–58. doi: 10.1016/j.abb.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol. Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 64.Nottingham LK, et al. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. 2014;33:1135–1147. doi: 10.1038/onc.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez L, et al. Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer. Cancer Res. 2010;70:4005–4014. doi: 10.1158/0008-5472.CAN-09-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang R, et al. High expression levels of IKKalpha and IKKbeta are necessary for the malignant properties of liver cancer. Int. J. Cancer. 2010;126:1263–1274. doi: 10.1002/ijc.24854. [DOI] [PubMed] [Google Scholar]
- 67.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 68.Xia Y, et al. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc. Natl. Acad. Sci. USA. 2009;106:2629–2634. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang PM, et al. Loss of IKKbeta activity increases p53 stability and p21 expression leading to cell cycle arrest and apoptosis. J. Cell Mol. Med. 2010;14:687–698. doi: 10.1111/j.1582-4934.2009.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Accardi R, et al. IkappaB kinase beta promotes cell survival by antagonizing p53 functions through DeltaNp73alpha phosphorylation and stabilization. Mol Cell Biol. 2011;31:2210–2226. doi: 10.1128/MCB.00964-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu MC, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/S0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 72.Cilloni D, et al. The NF-kappaB pathway blockade by the IKK inhibitor PS1145 can overcome imatinib resistance. Leukemia. 2006;20:61–67. doi: 10.1038/sj.leu.2403998. [DOI] [PubMed] [Google Scholar]
- 73.Yemelyanov A, et al. Effects of IKK inhibitor PS1145 on NF-kappaB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene. 2006;25:387–398. doi: 10.1038/sj.onc.1209066. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: Involvement of nuclear factor kappaB and mitochondria pathways. Clin. Cancer Res. 2006;12:950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson J, Shi Z, Liu Y, Stack MS. Inhibitors of NF-kappaB reverse cellular invasion and target gene upregulation in an experimental model of aggressive oral squamous cell carcinoma. Oral Oncol. 2014;50:468–477. doi: 10.1016/j.oraloncology.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shao L, Wu L, Zhou D. Sensitization of tumor cells to cancer therapy by molecularly targeted inhibition of the inhibitor of nuclear factor kappaB kinase. Transl. Cancer Res. 2012;1:100–108. doi: 10.3978/j.issn.2218-676X.2012.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sugita A, et al. Antiallergic and anti-inflammatory effects of a novel I kappaB kinase beta inhibitor, IMD-0354, in a mouse model of allergic inflammation. Int. Arch. Allergy Immunol. 2009;148:186–198. doi: 10.1159/000161579. [DOI] [PubMed] [Google Scholar]
- 78.Ochiai T, et al. Inhibition of IkappaB kinase beta restrains oncogenic proliferation of pancreatic cancer cells. J. Med. Dent. Sci. 2008;55:49–59. [PubMed] [Google Scholar]
- 79.Uota S, et al. An IkappaB kinase 2 inhibitor IMD-0354 suppresses the survival of adult T-cell leukemia cells. Cancer Sci. 2012;103:100–106. doi: 10.1111/j.1349-7006.2011.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayakawa Y, et al. Effectiveness of IkappaB kinase inhibitors in murine colitis-associated tumorigenesis. J. Gastroenterol. 2009;44:935–943. doi: 10.1007/s00535-009-0098-7. [DOI] [PubMed] [Google Scholar]
- 81.Kanduri M, Tobin G, Aleskog A, Nilsson K, Rosenquist R. The novel NF-kappaB inhibitor IMD-0354 induces apoptosis in chronic lymphocytic leukemia. Blood Cancer J. 2011;1:e12. doi: 10.1038/bcj.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S, et al. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 2019;9:10003. doi: 10.1038/s41598-019-46447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maliekal TT, Anto RJ, Karunagaran D. Differential activation of Smads in HeLa and SiHa cells that differ in their response to transforming growth factor-beta. J. Biol. Chem. 2004;279:36287–36292. doi: 10.1074/jbc.M404568200. [DOI] [PubMed] [Google Scholar]
- 84.Funaoka K, et al. High-risk HPV-positive human cancer cell lines show different sensitivity to cisplatin-induced apoptosis correlated with the p21Waf1/Cip1 level. Cancer Lett. 1996;108:15–23. doi: 10.1016/s0304-3835(96)04362-5. [DOI] [PubMed] [Google Scholar]
- 85.Filippova M, et al. Cellular levels of oxidative stress affect the response of cervical cancer cells to chemotherapeutic agents. Biomed. Res. Int. 2014;2014:574659. doi: 10.1155/2014/574659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pappa KI, et al. High resolution analysis of the intracellular proteome of cervical cancer cell lines unveils novel regulators of cervical carcinogenesis. Oncol. Rep. 2019 doi: 10.3892/or.2019.7269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.