Lambru et al. argue that trigeminal neurovascular contact with morphological changes is involved in the aetiology of both SUNCT and SUNA. The absence of any significant radiological differences between SUNCT and SUNA adds to evidence suggesting they should be considered a single clinical entity.
Keywords: SUNCT, SUNA, trigeminal autonomic cephalalgia, cluster headache, trigeminal neuralgia
Abstract
Emerging data-points towards a possible aetiological and therapeutic relevance of trigeminal neurovascular contact in short lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and perhaps in short lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA). We aimed to assess the prevalence and significance of trigeminal neurovascular contact in a large cohort of consecutive SUNCT and SUNA patients and evaluate the radiological differences between them. The standard imaging protocol included high spatial and nerve-cistern contrast resolution imaging acquisitions of the cisternal segments of the trigeminal nerves and vessels. MRI studies were evaluated blindly by two expert evaluators and graded according to the presence, location and degree of neurovascular contact. The degree of contact was graded as with or without morphological changes. Neurovascular contact with morphological changes was defined as contact with distortion and/or atrophy. A total of 159 patients (SUNCT = 80; SUNA = 79) were included. A total of 165 symptomatic and 153 asymptomatic trigeminal nerves were analysed. The proportion of neurovascular contact on the symptomatic trigeminal nerves was higher (80.0%) compared to the asymptomatic trigeminal nerves (56.9%). The odds on having neurovascular contact over the symptomatic nerves was significantly higher than on the asymptomatic nerves [odds ratio (OR): 3.03, 95% confidence interval (CI) 1.84–4.99; P < 0.0001]. Neurovascular contact with morphological changes were considerably more prevalent on the symptomatic side (61.4%), compared to the asymptomatic side (31.0%) (OR 4.16, 95% CI 2.46–7.05; P < 0.0001). On symptomatic nerves, neurovascular contact with morphological changes was caused by an artery in 95.0% (n = 77/81). Moreover, the site of contact and the point of contact around the trigeminal root were respectively proximal in 82.7% (67/81) and superior in 59.3% (48/81). No significant radiological differences emerged between SUNCT and SUNA. The multivariate analysis of radiological predictors associated with the symptomatic side, indicated that the presence of neurovascular contact with morphological changes was strongly associated with the side of the pain (OR: 2.80, 95% CI 1.44–5.44; P = 0.002) even when adjusted for diagnoses. Our findings suggest that neurovascular contact with morphological changes is involved in the aetiology of SUNCT and SUNA. Along with a similar clinical phenotype, SUNCT and SUNA also display a similar structural neuroimaging profile, providing further support for the concept that the separation between them should be abandoned. Furthermore, these findings suggest that vascular compression of the trigeminal sensory root, may be a common aetiological factor between SUNCT, SUNA and trigeminal neuralgia thereby further expanding the overlap between these disorders.
Introduction
Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) are considered separate clinical entities encompassed within the trigeminal autonomic cephalalgias (TACs) group. SUNCT and SUNA are characterized by episodes of moderate or severe, unilateral, stabbing or shooting pain in the trigeminal distribution, associated with ipsilateral cranial autonomic signs and symptoms. The head pain episodes generally last between l s and 600 s and can occur spontaneously or be triggered by innocuous cutaneous or intraoral ipsilateral trigeminal stimulation, with a daily frequency of occurrence of at least one a day, though often dozens or hundreds of attacks a day are reported when the disorder is active (Headache Classification Subcommittee of the International Headache Society, 2018). A recent study on the clinical phenotype of SUNCT and SUNA showed the absence of major clinical difference between these two conditions, suggesting that SUNCT and SUNA maybe considered the same clinical entity (Lambru et al., 2019).
Functional neuroimaging studies during SUNCT/SUNA attacks have demonstrated posterior hypothalamic activation. The similarities in the clinical phenotype of the TACs taken together with the hypothalamic activation demonstrated in all these syndromes has led to the suggestion that the posterior hypothalamus plays a central role in the pathophysiology of these disorders. (May et al., 1999; Matharu et al., 2004, 2006; Cohen, 2007). However, SUNCT and SUNA display a broad demographic and clinical overlap with similar responses to medical treatments as trigeminal neuralgia. This overlap has led to growing interest in exploring the presence of shared pathophysiological mechanisms as the basis of these similarities (Lambru and Matharu, 2014). Emerging data points towards a possible aetiological and therapeutic relevance of trigeminal neurovascular contact (NVC) in SUNCT and perhaps in SUNA, similar to trigeminal neuralgia (Barker et al., 1996; Williams and Broadley, 2008; Williams et al., 2010; Antonini et al., 2014; Maarbjerg et al., 2015). Several case reports of trigeminal NVC on the symptomatic side in subjects with SUNCT have been described (Favoni et al., 2013). A small case series of SUNCT and SUNA where dedicated high-resolution MRI trigeminal sections were obtained, showed a much greater proportion of vessels contacting the symptomatic trigeminal nerve compared to the asymptomatic nerve (Williams and Broadley, 2008). Conversely, other series have reported a much lower prevalence of trigeminal NVC. However, in these studies it was not specified whether dedicated views of the trigeminal nerves were obtained (Cohen et al., 2006; Weng et al., 2017).
The aims of this study were to explore the role of trigeminal NVC in SUNCT and SUNA by evaluating the presence, degree, type of vessel and localization of the vascular contact in these patients. We also sought to compare MRI NVC characteristics between the two groups of patients in order to evaluate the presence of any significant radiological differences between SUNCT and SUNA.
Material and methods
Consecutive patients diagnosed by the headache team at the National Hospital for Neurology and Neurosurgery with SUNCT and SUNA between 2007 and 2018 were included. Diagnosis was made by two headache neurologists based on the criteria of the Second edition of International Classification of Headache Disorders (ICHD-2) and ICHD-3 beta (Headache Classification Subcommittee of the International Headache Society, 2004, 2013). With publication of the ICHD-3 criteria in 2018, we subsequently ensured that all patients included in the study fulfilled these criteria (Headache Classification Subcommittee of the International Headache Society, 2018). Exclusion criteria were patients with MRI brain without high-resolution cisternal imaging, patients with abnormal MRI scans other than a trigeminal NVC, patients who could not have the relevant MRI of the brain sequences because of implanted devices (i.e. occipital nerve stimulation) and patients who had an invasive trigeminal procedure prior to the MRI.
MRI protocol and neurovascular conflict definitions
MRI examinations were performed on a 1.5-T GE Signa Excite (GE Medical Systems), 1.5-T Siemens Avanto (Siemens) or 3.0-T Siemens Trio (Siemens) MRI scanner. The standard imaging protocol included high spatial and nerve-cistern contrast resolution imaging acquisitions of the cisternal segments of the trigeminal nerves and vessels, with 3D Fast Imaging Employing Steady-State Acquisition (FIESTA; echo time: 1.5 ms, repetition time: 4.9 ms, number of excitations: 4), 3D Constructive Interference in Steady State (CISS; echo time: 5.3 ms, repetition time: 10.6 ms, excitations: 1), or 3D Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE; echo time: 132 ms, repetition time: 1000 ms, excitations: 2). The slice thickness of all examinations ranged from 0.5 mm to 1 mm. High resolution axial and coronal T2-weighted sequences through the trigeminal nerves were also obtained with acquisitions a slice thickness of 2 mm, in order to assess intrinsic signal hyperintensity. When present, Time-Of-Flight Magnetic Resonance Angiography (TOF MRA) was used to confirm the location of the cerebellar arteries and veins in close proximity to the cisternal segments of the trigeminal nerves on the high-resolution images; high signal within vessels on this sequence were considered arteries and the contrary, veins.
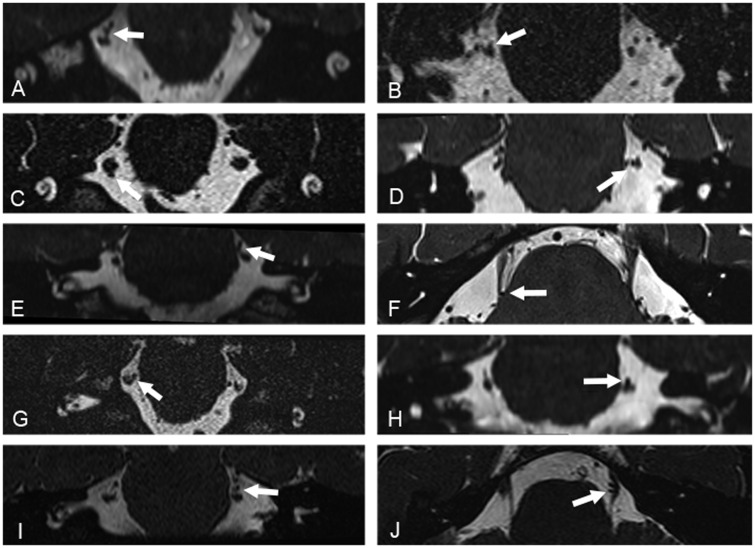
All MRI studies were reviewed and analysed on Coronis high-resolution 3-megapixel monitors (Barco Inc) on IMPAX 6 Picture Archiving and Communication System (AGFA Healthcare NV, Belgium) by an experienced neuroradiologist (I.D.) and a neurosurgeon specialized in performing microvascular decompression procedures (L.Z.) who were blinded to the diagnosis and the laterality of the headache. The inter-operator agreement on MRI evaluations was measured with K-score. The trigeminal nerve on the side of the pain was defined as the symptomatic nerve; the trigeminal nerve contralateral to the side of the pain was defined as the asymptomatic nerve. In patients with side alternating unilateral head pain, both trigeminal nerves were considered symptomatic. NVC with the cisternal segments of the trigeminal nerves was assessed on both sides on multiplanar reformats of the source high-resolution data. NVC was defined on the analysis of imaging by no perceptible cerebrospinal signal intervening between the vascular structure (arterial or venous) and the cisternal segment of the trigeminal nerve. In view of the ongoing debate upon the definition and boundaries of the zone where peripheral myelination transitions to central myelination (‘root entry zone’ or ‘transition zone’) (De Ridder et al., 2002; Peker et al., 2006), sites of NVC on the trigeminal nerve were divided into three segments, namely, proximal, middle and distal. If more than one site was contacted by the vessel, it was defined as mixed. The degree of NVC was graded as with or without morphological changes (Headache Classification Subcommittee of the International Headache Society, 2018). NVC without morphological changes was defined a simple contact. NVC with morphological changes was defined as a contact with distortion and/or atrophy (Fig. 1). Distortion was defined as indentation or displacement of the trigeminal nerve at the site of the neurovascular contact. Atrophy was defined as a reduced volume of the trigeminal nerve at the site of the neurovascular contact. NVC with one or more arteries was defined as an arterial conflict; NVC with one or more veins was defined as a venous conflict; NVC with one artery and one vein was defined as a mixed conflict. Furthermore, the point of contact around the nerve diameter was evaluated and defined as superior, lateral, medial and inferior. If more than one point of contact was present, then the term ‘mixed’ was used.
Figure 1.
High-resolution volumetric 3-D CISS and SPACE MRI acquisitions. Acquisitions were reconstructed in the coronal (A–E and G–I) and axial (F and J) planes, and demonstrate contact of a segment of the superior cerebellar artery with the cisternal segment of the trigeminal nerve (white arrows). Images in the left column show contact with no morphological changes of the corresponding segment of the trigeminal nerve. Images in the right column show contact with morphological changes, with deviation and distortion of the corresponding adjacent cisternal segment of the trigeminal nerve.
The study was approved by Northwick Park Hospital Research Ethics Committee, London, UK (REC no: 11/LO/1709). Patients gave informed consent to participate in this study.
Statistical analysis
Continuous data were summarized by descriptive statistics. Categorical variables are presented with frequency distributions (n, %). We used the binomial approximation to the normal distribution to compare the proportions between symptomatic and asymptomatic nerves. Unadjusted and adjusted multilevel logistic regression models, to account for diagnoses, were used to investigate potential association between the presence of vascular contact, degree of contact, site of contact within the trigeminal sensory root and type of vessel involved, and the symptomatic as opposed to asymptomatic trigeminal nerves in SUNA and SUNCT. Statistical analyses were performed using STATA (Stata Corp. 2001. Stata Statistical Software: Version 12.1, College Station, Texas, USA). Statistical significance level was set to 5% two-sided. A Bonferroni adjustment was applied to P-values to control the type I error inflation as a result of multiple testing. Threshold for significance after Bonferroni correction was 0.003.
Data availability
The data that support the findings of this study are available from the corresponding author.
Results
A total of 224 SUNCT and SUNA patients were assessed within the study period. Of these, the following were excluded: 21 patients did not have high-resolution cisternal imaging MRI scans because of incorrect MRI protocol; 29 had not had trigeminal nerve imaging at our centre and either declined further imaging or were unable to have an MRI scan because of an occipital nerve stimulator in situ; 12 patients were excluded as they were assessed at our centre after a trigeminal procedure had been performed; and three were excluded because of abnormal MRI scans (one patient with mid-brain low grade astrocytoma, two patients with posterior fossa ischaemic strokes). The remaining 159 patients (SUNCT = 80, 50.3% and SUNA = 79, 49.7%) had high-resolution cisternal imaging MRI scans according to our protocol and were included in the study. Table 1 summarizes the demographic and clinical characteristics of the study group.
Table 1.
Demographic and clinical characteristics of SUNCT and SUNA patients
| Demographic/clinical characteristic | |
|---|---|
| Age, years, mean (SD) [range] | 58.7 (±15.4) [20–93] |
| Sex | |
| Male | 78 (49%) |
| Female | 81 (51%) |
| Diagnoses | |
| Chronic SUNCT | 80 (50.3%) |
| Chronic SUNA | 79 (49.7%) |
| Headache duration, years, mean (SD) [range] | 14.9 (±7.19) [3–44] |
| Headache laterality | |
| Right | 90 (56.6%) |
| Left | 63 (39.6%) |
| Unilateral side variable | 6 (3.8%) |
| Headache distribution | |
| V1 | 36 (22.6%) |
| V2 | 23 (14.5%) |
| V1-V2 | 47 (29.6%) |
| V2-V3 | 22 (13.8%) |
| V1-C2 | 5 (3.1%) |
| V1-V2-V3 | 16 (10.1%) |
| V1-V2-C2 | 10 (6.3%) |
| Mean number of daily attacks, mean (SD) [range] | 70.79 (±102.16) [2–700] |
| Mean attack severity (0–10), mean (SD) [range] | 9.42 (±1.42) [6–10] |
| Mean attack duration, s, mean (SD) [range] | 85.92 (±127.16) [1–600] |
| Spontaneous and/or triggered attacks | |
| Spontaneous and triggered | 105 (66.0%) |
| Spontaneous only | 43 (27.0%) |
| Triggered only | 11 (6.9%) |
| Refractory period | |
| No | 75 (47.2%) |
| Yes | 14 (8.8%) |
| Not applicable | 43 (27.0%) |
| Patients not sure | 27 (17.0%) |
| Personal history of migraine | |
| Yes | 87 (54.7%) |
| No | 72 (45.2%) |
C2 = second cervical root; SD = standard deviation; V1 = first division of the trigeminal nerve; V2 = second division of the trigeminal nerve; V3 = third division of the trigeminal nerve.
MRI results: assessor concordance
There was good concordance in categorizing the trigeminal nerves between the two assessors (kappa coefficient = 0.91, P < 0.05). When discrepancies existed, the two assessors arrived at a consensus view that was used for statistical analysis.
Neurovascular contact on the symptomatic and asymptomatic side
Of the 159 patients, 90 (56.6%) had strictly unilateral right-sided attacks (SUNCT = 48; SUNA = 42), 63 (39.6%) had strictly unilateral left-sided attacks (SUNCT = 29; SUNA = 34) and six (3.8%) had unilateral side-alternating attacks (SUNCT = 3; SUNA = 3). A total of 165 symptomatic trigeminal nerves (SUNCT = 83, SUNA = 82) and 153 asymptomatic trigeminal nerves (SUNCT = 77, SUNA = 76) were analysed. The prevalence of NVC on the symptomatic trigeminal nerves was significantly higher (80.0%), compared to the asymptomatic trigeminal nerves (56.9%) (P < 0.0001). The estimates of odds ratio indicated that the presence of an NVC was associated with the symptomatic side [odds ratio (OR): 3.03, 95% confidence interval (CI) 1.84–4.99). NVC with morphological changes was considerably more prevalent on the symptomatic side (n = 81, 61.4%), compared to the asymptomatic side (31.0%) (P < 0.0001). The estimates of odds ratio indicate that the presence of NVC with morphological changes as opposed to NVC without morphological changes is strongly associated with the nerve being symptomatic rather than asymptomatic (OR: 4.50, 95% CI 2.69–7.54). There was a statistically greater proportion of arteries contacting the trigeminal nerves on the symptomatic side (65.9%) compared to the asymptomatic side (44.8%) (P < 0.0001). The presence of an artery as opposed to a vein was a strong predictor for the trigeminal nerve being symptomatic. (OR: 3.51, 95% CI 2.21–5.58). The presence of vascular contacts over the proximal segments of the trigeminal nerves were significantly greater on symptomatic (48.5%) than asymptomatic nerves (43.7%) (P < 0.0001). The estimates of odds ratio indicated that the presence of a proximal contact was associated with the nerve being symptomatic (OR: 2.90, 95% CI 1.82–4.62). Similarly, a superior point of contact of the vessel was more frequently found on symptomatic (34.1%) rather than asymptomatic nerves (31.0%) (P = 0.001). The estimates of odds ratio indicate that the presence of a superior point of contact was associated with the nerve being symptomatic (OR: 2.28, 95% CI 1.43–3.62) (Table 2).
Table 2.
Characteristics of trigeminal neurovascular contacts by symptomatic and asymptomatic nerves in all patients
|
Symptomatic nerve
(n = 165) n (%) |
Asymptomatic nerve
(n = 153) n (%) |
OR (95% CI) * | P-value * | |
|---|---|---|---|---|
| Any contact | ||||
| Yes | 132 (80.0) | 87 (56.9) | 3.03 (1.84–4.99) | <0.0001 |
| No | 33 (20.0) | 66 (43.1) | Reference | |
| Degree of contact | ||||
| Contact | 51 (38.64) | 60 (69.0) | 0.97 (0.62–1.51) | 0.891 |
| Distortion | 33 (25.0) | 15 (17.2) | 4.16 (2.46–7.05) | <0.0001 |
| Atrophy | 3 (2.3) | 1 (1.2) | 5.06 (2.27–11.28) | <0.0001 |
| Mixeda | 45 (34.1) | 11 (12.6) | 4.84 (2.40–9.77) | <0.0001 |
| Type of vessel | ||||
| Vein | 28 (21.2) | 37 (42.5) | 0.82 (0.51–1.33) | 0.423 |
| Artery | 87 (65.9) | 39 (44.8) | 3.51 (2.21–5.58) | <0.0001 |
| Mixed | 17 (12.9) | 11 (12.6) | 1.48 (0.67–3.28) | 0.330 |
| Site of contact | ||||
| Proximal | 64 (48.5) | 38 (43.7) | 2.90 (1.82–4.62) | <0.0001 |
| Middle | 20 (15.15) | 18 (20.7) | 1.81 (1.04–3.14) | 0.036 |
| Distal | 17 (12.9) | 21 (24.1) | 1.15 (0.66–2.00) | 0.616 |
| Mixed | 31 (23.5) | 10 (11.5) | 1.75 (1.01–3.04) | 0.048 |
| Point of contact | ||||
| Superior | 45 (34.1) | 24 (31.0) | 2.28 (1.43–3.62) | 0.001 |
| Medial | 30 (22.7) | 17 (19.5) | 1.92 (1.18–3.11) | 0.008 |
| Lateral | 3 (2.3) | 4 (4.6) | 1.14 (0.46–2.84) | 0.774 |
| Inferior | 12 (9.1) | 17 (19.5) | 0.91 (0.50–1.67) | 0.771 |
| Mixed | 42 (31.8) | 25 (28.7) | 1.75 (1.01–3.04) | 0.048 |
Odds ratio (OR) estimated from multilevel logistic regression with symptomatic status of the nerve as the outcome variable. Ref = reference. aAll mixed degrees of contact were severe contact.
The threshold for statistical significance based on Bonferroni adjustment is 0.003 (i.e. 0.05/17).
Neurovascular conflict in the SUNCT cohort
Amongst SUNCT patients’ severe NVCs were considerably more prevalent on the symptomatic (n = 46, 64.8%) compared to the asymptomatic side (n = 19, 35.2%) (P < 0.0001). NVC with morphological changes as opposed to NVC without morphological changes was a predictor for the nerve being symptomatic (OR: 3.80, 95% CI 1.93–7.46). There was a statistically greater proportion of arteries contacting the trigeminal nerves on the symptomatic side (70.4%) compared to the asymptomatic side (44.4%) (P < 0.0001). The presence of an artery as opposed to a vein was a strong predictor for the trigeminal nerve being symptomatic (OR: 4.06, 95% CI 2.10–7.86; P < 0.0001). The vascular structures were found more often over the proximal segment of the symptomatic (54.9%) compared to the asymptomatic nerves (42.6%) (P = 0.002). Proximal site of contact was associated with the nerve being symptomatic (OR: 2.83, 95% CI 1.49–5.38) (Table 3).
Table 3.
Characteristics of trigeminal NVCs by symptomatic and asymptomatic nerves in SUNCT patients
|
Symptomatic nerve
(n = 83) n (%) |
Asymptomatic nerve
(n = 77) n (%) |
OR (95% CI) * | P-value * | |
|---|---|---|---|---|
| Any contact | ||||
| Yes | 71 (85.5) | 54 (70.1) | 2.52 (1.15–5.51) | 0.021 |
| No | 12 (14.5) | 23 (29.9) | Reference | |
| Degree of contact | ||||
| Contact | 25 (35.2) | 35 (64.8) | 0.75 (0.40–1.39 | 0.357 |
| Distortion | 19 (26.8) | 11 (20.4) | 3.45 (1.73–6.87) | <0.0001 |
| Atrophy | 3 (4.2) | 1 (1.9) | 4.57 (1.62–12.89) | 0.004 |
| Mixeda | 24 (33.8) | 7 (13.0) | 4.07 (1.64–10.11) | 0.003 |
| Type of vessel | ||||
| Vein | 13 (18.3) | 26 (48.2) | 0.54 (0.27–1.04) | 0.065 |
| Artery | 50 (70.4) | 24 (44.4) | 4.06 (2.10–7.86) | <0.0001 |
| Mixed | 8 (11.3) | 4 (7.4) | 1.95 (0.56–6.75) | 0.293 |
| Site of contact | ||||
| Proximal | 39 (54.9) | 23 (42.6) | 2.83 (1.49–5.38) | 0.002 |
| Middle | 12 (16.9) | 12 (22.2) | 1.40 (0.66–2.97) | 0.379 |
| Distal | 7 (9.9) | 15 (27.8) | 0.72 (0.33–1.57) | 0.405 |
| Mixed | 13 (18.3) | 4 (7.4) | 1.44 (0.70–2.94) | 0.323 |
| Point of contact | ||||
| Superior | 22 (31.0) | 10 (18.5) | 2.41 (1.25–4.610) | 0.008 |
| Medial | 18 (25.4) | 12 (22.2) | 1.59 (0.84–3.04) | 0.157 |
| Lateral | 1 (1.4) | 2 (3.7) | 0.76 (0.22–2.59) | 0.660 |
| Inferior | 6 (8.5) | 13 (24.1) | 0.61 (0.28–1.35) | 0.219 |
| Mixed | 24 (33.8) | 17 (31.5) | 1.44 (0.70–2.95) | 0.323 |
Odds ratio (OR) estimated from multilevel logistic regression with symptomatic status of the nerve as the outcome variable.
All mixed degrees of contact were severe contact.
The threshold for statistical significance based on Bonferroni adjustment is 0.003 (i.e. 0.05/17).
Neurovascular contact in the SUNA cohort
Amongst SUNA patients there was a significantly greater proportion of neurovascular contacts on the symptomatic (74.4%) compared to the asymptomatic trigeminal nerves (43.4%) (P < 0.0001). The estimates of odds ratio indicated that the presence of a vascular contact was a strong predictor for the nerve to be symptomatic rather than asymptomatic (OR: 3.79, 95% CI 1.93–7.41). NVC with morphological changes were considerably more prevalent on the symptomatic (n = 35, 57.4%) compared to the asymptomatic nerves (n = 8, 24.2%) (P < 0.0001). The presence of severe NVC was linked to the nerve being symptomatic (OR: 6.33, 95% CI 2.70–14.86). There was a greater proportion of arteries contacting the trigeminal nerves on the symptomatic side (60.7%) compared to the asymptomatic side (45.5%) (P = 0.001). Arterial conflicts as opposed to venous ones were associated with the trigeminal nerve being symptomatic (OR: 3.14, 95% CI 1.62–6.07) (Table 4).
Table 4.
Characteristics of trigeminal NVCs by symptomatic and asymptomatic nerves in SUNA patients
|
Symptomatic nerve
(n = 82) n (%) |
Asymptomatic nerve
(n = 76) n (%) |
OR (95% CI) * | P-value * | |
|---|---|---|---|---|
| Any contact | ||||
| Yes | 61 (74.4) | 33 (43.4) | 3.79 (1.93–7.41) | <0.0001 |
| No | 21 (25.6) | 43 (56.6) | Reference | |
| Degree of contact | ||||
| Contact | 26 (42.6) | 25 (75.8) | 1.28 (0.67–2.42) | 0.454 |
| Distortion | 14 (23.0) | 4 (12.1) | 5.72 (2.43–13.46) | <0.0001 |
| Atrophy | 0 | 0 | 5.90 (1.65–21.16) | 0.006 |
| Mixeda | 21 (34.4) | 4 (12.1) | 6.20 (2.02–19.04) | 0.001 |
| Type of vessel | ||||
| Vein | 15 (24.6) | 11 (33.3) | 1.34 (0.66–2.72) | 0.428 |
| Artery | 37 (60.7) | 15 (45.5) | 3.14 (1.62–6.071) | 0.001 |
| Mixed | 9 (14.8) | 7 (21.2) | 1.22 (0.43–3.44) | 0.714 |
| Site of contact | ||||
| Proximal | 25 (41.0) | 15 (45.5) | 3.07 (1.55–6.08) | 0.001 |
| Middle | 8 (13.1) | 6 (18.2) | 2.42 (1.06–5.53) | 0.036 |
| Distal | 10 (16.4) | 6 (18.2) | 1.84 (0.83–4.05) | 0.132 |
| Mixed | 18 (29.5) | 6 (18.2) | 2.39 (0.97–5.88) | 0.058 |
| Point of contact | ||||
| Superior | 23 (37.7) | 14 (42.4) | 2.15 (1.11–4.187) | 0.024 |
| Medial | 12 (19.7) | 5 (15.2) | 2.51 (1.19–5.33) | 0.016 |
| Lateral | 2 (3.3) | 2 (6.1) | 1.92 (0.46–8.00) | 0.368 |
| Inferior | 6 (9.8) | 4 (12.1) | 1.69 (0.63–4.55) | 0.298 |
| Mixed | 18 (29.5) | 8 (24.2) | 2.39 (0.97–5.88) | 0.058 |
Odds ratio (OR) estimated from multilevel logistic regression with symptomatic status of the nerve as the outcome variable.
All mixed degrees of contact were severe contact.
The threshold for statistical significance based on Bonferroni adjustment is 0.003 (i.e. 0.05/17).
Neurovascular contact: comparison between SUNCT and SUNA cohorts
There was no statistically significant difference in the proportion of neurovascular contacts on the symptomatic nerves between SUNCT [71/83 (85.5%)] and SUNA [61/82 (74.4%)] (P = 0.073). Similarly, there was no statistically significant difference in the proportion of NVC with morphological changes between SUNCT [46/71 (55.9%)] and SUNA [35/61 (42.4%)] (P = 0.102). The comparison between arterial structures (P = 0.052), proximal site of contact (P = 0.030 threshold for significance after Bonferroni correction was 0.003) and superior point of contact (P = 0.824) showed no significant differences between the two groups of patients.
Radiological predictors for the symptomatic or asymptomatic side
The multivariate regression analysis adjusted for diagnosis demonstrated that, among the radiological variables that were associated with the symptomatic nerve in the univariate analysis, NVC with morphological changes remained a significant predictor for the nerve to be symptomatic rather than asymptomatic [OR: 2.80 95% CI 1.44–5.44, P = 0.002]. The other variables that had emerged in the model as potential radiological predictors did not show any significant statistical difference between symptomatic and asymptomatic nerves (Table 5).
Table 5.
Multivariate logistic regression analysis radiological predictors for symptomatic compared to asymptomatic nerves in SUNCT and SUNA
|
Symptomatic nerves
OR (95% CI) * |
P-value | |
|---|---|---|
| Presence of neurovascular contact | 0.800 | |
| Yes | 1.10 (0.53–2.28) | |
| No | Reference | |
| Degree of contact | 0.002 | |
| With morphological changes | 2.80 (1.44–5.44) | |
| Without morphological changes | Reference | |
| Type of vessel | 0.360 | |
| Artery | 1.42 (0.67–2.98) | |
| Not artery | Reference | |
| Site of contact | 0.413 | |
| Proximal | 1.30 (0.69–2.43) | |
| Not proximal | Reference | |
| Point of contact | 0.407 | |
| Superior | 1.31 (0.70–2.45) | |
| Not superior | Reference |
Odds ratios (OR) and P-values obtained from a multilevel regression mode with symptomatic status of the nerve as outcome variable and radiological predictors as explanatory variables.
Discussion
This is the first prospective, blinded MRI study, conducted in the largest cohort of consecutive radiologically evaluated SUNCT and SUNA patients, assessing the significance of trigeminal NVC. The findings demonstrate that trigeminal NVC is a highly prevalent neuroanatomical finding ipsilateral to the side of the pain in SUNCT and SUNA. Furthermore, other factors significantly more prevalent on the symptomatic side include severe degree of NVC, arterial conflict, NVC with the proximal segment of the trigeminal sensory root and NVC with the superior aspect of the nerve. There were no significant differences between SUNCT and SUNA in any of these MRI findings. Among these radiological outcomes, NVC with morphological changes was demonstrated to be a robust predictor of the symptomatic side in SUNCT and SUNA. This finding is likely to explain the high prevalence of arterial NVC with morphological changes, the proximal site and superior point of contact found on the symptomatic side. A high proportion of superior only, supero-medial and supero-lateral points of contact on both symptomatic and asymptomatic sides in SUNCT/SUNA, was also shown in a recent study, which aimed to assess the relation between the location of vascular compression and the pain distribution in trigeminal neuralgia patients operated with trigeminal microvascular decompression (Sindou and Brinzeou, 2020). Most patients in the trigeminal neuralgia study displayed a superior compression (61.7%). In view of the infrequent location of the painful attacks in V1 in trigeminal neuralgia, this and our findings may suggest that a superior trigeminal conflict is a frequent normal neuroanatomical finding. However, in the trigeminal neuralgia study, patients with supero-medial conflict had an OR of 2.7 (95% CI: 1.66–4.41) of manifesting pain in V1 and inferior compression was more likely to manifest with V3 pain (OR: 2.6, 95% CI: 1.21–5.45), supporting the role of the point of conflict and pain in trigeminal neuralgia and indicating the need of evaluating this association in SUNCT/SUNA as well.
The association between NVC with morphological changes and the symptomatic side raises the possibility that these neuroanatomical findings may be implicated in the pain mechanisms of SUNCT and SUNA. The majority of SUNCT cases are considered idiopathic, but a significant minority of them seem to be secondary to intracranial abnormalities. Similar to secondary cases of other TACs and trigeminal neuralgia, cases of SUNCT have been attributed to posterior fossa abnormalities and pituitary adenomas (Cittadini and Matharu, 2009; Chitsantikul and Becker, 2013). Furthermore, growing evidence supports the frequent occurrence of NVC of the trigeminal nerve close to the root entry zone ipsilateral to the side of the pain (Favoni et al., 2013). In a series of 24 SUNCT/SUNA cases, 17 patients were studied with dedicated MRI of the trigeminal nerves. NVC was detected in 15 of 17 patients (88%). In 90% of cases, a vascular loop was impinging on the symptomatic trigeminal nerve, compared to only 7% in which the vascular loop was pressing on the asymptomatic nerve (Williams and Broadley, 2008). The high prevalence of NVC with morphological changes in their series could be explained by the small sample size. Moreover, the authors did not detail the scanning protocol and their definition included cases where only contact with a vascular loop was observed. It is therefore possible that the prevalence of NVC with morphological changes was overestimated. Nonetheless, the significantly greater proportion of NVC with morphological changes on the symptomatic side compared to the asymptomatic side in both that and the current study suggests that this finding is more than a mere chance association.
It is widely accepted that NVC ipsilaterally to the pain side constitutes one of the pivotal neuroanatomical findings in trigeminal neuralgia (Devor et al., 2002; Antonini et al., 2014; Maarbjerg et al., 2015). Trigeminal NVC on the symptomatic side is reported in 47–90% of trigeminal neuralgia cases and it often occurs at the root entry zone by an artery. However, the presence of trigeminal NVC without morphological changes seems not specific for trigeminal neuralgia as it is highly prevalent on both the symptomatic and the asymptomatic side (Love and Coakham, 2001; Antonini et al., 2014; Maarbjerg et al., 2015). Meta-analyses of MRI studies showed that NVC with morphological changes on the symptomatic side is strongly linked with trigeminal neuralgia compared to the control nerves (OR: 13.3; 95% CI 5.8–30.6; P < 0.0001) (Antonini et al., 2014; Maarbjerg et al., 2015; Bendtsen et al., 2019). Similarly, arterial NVC with morphological changes at the root entry zone on symptomatic nerves were found to be more prevalent on the symptomatic nerves compared to the asymptomatic nerves. It was concluded that the strong association between NVC with morphological changes and the symptomatic trigeminal nerve, could be considered a major aetiological factor in trigeminal neuralgia (Maarbjerg et al., 2015). The aetiological importance of trigeminal NVC in trigeminal neuralgia is ultimately corroborated by the long-term efficacy of trigeminal microvascular decompression procedure, the gold standard surgical approach for this condition (Barker et al., 1996). Although the odds ratio for the association between NVC with morphological changes and the symptomatic side in our study is considerable lower compared to those found in classical trigeminal neuralgia studies, our MRI and clinical findings, along with therapeutic similarities reported in previous studies between SUNCT, SUNA and trigeminal neuralgia, support the concept that these conditions share pain generating mechanisms. A focal demyelination of the sensory trigeminal root ipsilaterally to the side of the pain caused by NVC with morphological changes maybe one of the key aetiological mechanisms that explain SUNCT, SUNA and trigeminal neuralgia overlap. Initial encouraging evidence from single case reports and a small case series suggest that trigeminal microvascular decompression in SUNCT and SUNA patients with NVC on the symptomatic nerve, could lead to pain freedom (Sebastian et al., 2013). Our findings may indicate that trigeminal microvascular decompression could be carefully considered in chronic SUNCT/SUNA patients with MRI evidence of ipsilateral NVC with morphological changes. However, in order to explore the aetiological significance of trigeminal NVC, further studies may need to evaluate the efficacy of trigeminal microvascular decompression in patients with MRI evidence of ipsilateral NVC with and without morphological changes, follow them up long term and assess the presence of any surgical outcome differences between those with and without MRI evidence of morphological changes.
In both this and the Maarbjerg et al. (2015) studies, only about half of the patients displayed severe NVC on the symptomatic side. Since studies in trigeminal neuralgia patients have shown a high prevalence of NVC in non-trigeminal neuralgia individuals (Peker et al., 2006), this neuroanatomical finding may not be the only relevant aetiologically factor for these disorders. Other mechanisms may also play a role in the aetiology of pain and associated symptoms for SUNCT and SUNA. Derangement of central trigeminal nociceptive and autonomic modulating structures such as the posterior hypothalamic region have been postulated after functional neuroimaging studies have shown activation of the posterior hypothalamus bilaterally as well as ipsi- and contralateral to the pain in SUNCT and SUNA patients during painful attacks (May et al., 1999; Cohen, 2007). Although the exact meaning of these neuroimaging changes is unknown, the pivotal role of this brain region in the pathophysiology of SUNCT and SUNA is supported by the efficacy of deep brain stimulation of the ventral tegmental area reported in chronic refractory subtypes of these disorders (Miller et al., 2016). Additionally, the efficacy of sodium channels modulators in the prevention of SUNCT and SUNA, along with the described co-occurrence between SUNCT/SUNA and hemiplegic migraine, raises the possibility of ion channel dysfunction being implicated in the pathogenesis of SUNCT and SUNA (Lambru et al., 2012). It is likely that the pathophysiology of these disorders is multifactorial, involving impaired posterior hypothalamic region function, sodium channel dysfunction and pathological processes within the trigeminal sensory root (demyelination and axonal injury) due to NVC.
SUNCT and SUNA are listed separately in the International Classification of Headache Disorders under the umbrella term ‘short-lasting unilateral neuralgiform headache attacks’ (Headache Classification Subcommittee of the International Headache Society, 2018). A recent comparison of the clinical characteristics of SUNA and SUNCT phenotype in a large series of patients found no significant differences between the two phenotypes. This suggests that SUNCT and SUNA may represent a single clinical entity (Lambru et al., 2019). The current study shows similar prevalence and neurovascular contacts characteristics between SUNCT and SUNA, providing structural neuroimaging evidence that these two conditions maybe the same entity, not only on a clinical, but also on a neuroradiological basis.
The study has some limitations, namely the lack of a healthy control group; to obviate this problem and given the prominent unilaterality of these headache disorders, the contralateral side was used as control. The MRI evaluators were not blinded to the diagnosis of short-lasting unilateral neuralgiform headache attacks, albeit that they were blinded to the subclassification of SUNCT or SUNA; this could potentially have induced them to report mostly one NVC per patient. However, a high proportion of bilateral NVC was reported in this series, confirming the accuracy of the MRI evaluations. The strengths of this study include its prospective nature, the large number of patients included for such a rare condition and the presence of two MRI evaluators blinded to the laterality of symptoms.
In conclusion, this study demonstrated that NVC is highly prevalent on the symptomatic side in SUNCT and SUNA. In particular, our findings indicated a robust association between NVC with morphological changes and the symptomatic side, suggesting an important aetiological role of this neuroimaging characteristic in SUNCT/SUNA, similarly to trigeminal neuralgia. Ultimately, long-term evidence of headache freedom after trigeminal microvascular decompression would confirm the clinical value of our findings. The study also demonstrated the absence of any significant radiological differences between SUNCT and SUNA. These findings, along with the absence of clinical differences, support the notion that SUNCT and SUNA may be the same clinical entity and that their separation should be abandoned.
Acknowledgements
The authors thank our headache specialist nurses for their involvement with the patients, as well as the patients and their families for their help with this project.
Funding
No targeted funding was received towards this work. L.Z. and the Unit of Functional Neurosurgery are supported by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre. I.D. is supported by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre.
Competing interests
G.L. has received speaker honoraria, funding for travel and has received honoraria for participation in advisory boards sponsored by Allergan, Novartis, Eli Lilly and TEVA. He has received speaker honoraria, funding for travel from electroCore, Nevro Corp. and Autonomic Technologies. L.Z. has received speaker honoraria from Medtronic and Boston Scientific. M.S.M. serves on the advisory board for Allergan, St Jude Medical and Medtronic and has received payment for the development of educational presentations from Allergan, Merck Sharpe and Dohme Ltd., Medtronic and electroCore. All other authors report no competing interests.
Glossary
- NVC =
neurovascular conflict; SUNA = short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms;
- SUNCT =
short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing
References
- Antonini G, Di Pasquale A, Cruccu G, Truini A, Morino S, Saltelli G, et al. MRI contribution for diagnosing symptomatic neurovascular contact in classic trigeminal neuralgia. A blinded case-control study and meta-analysis. Pain 2014; 155: 1464–71. [DOI] [PubMed] [Google Scholar]
- Barker FG 2nd, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med 1996; 334: 1077–83. [DOI] [PubMed] [Google Scholar]
- Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A.. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol 2019; 26: 831–49. [DOI] [PubMed] [Google Scholar]
- Cittadini E, Matharu MS.. Symptomatic trigeminal autonomic cephalalgias. Neurologist 2009; 15: 305–12. [DOI] [PubMed] [Google Scholar]
- Chitsantikul P, Becker WJ.. SUNCT SUNA and pituitary tumors: clinical and characteristics and treatment. Cephalalgia 2013; 33: 160–70. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Matharu MS, Goadsby PJ.. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)-a prospective clinical study of SUNCT and SUNA. Brain 2006; 129: 2746–60. [DOI] [PubMed] [Google Scholar]
- Cohen AS. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing. Cephalalgia 2007; 27: 824–32. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L.. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery 2002; 51: 427–33. [DOI] [PubMed] [Google Scholar]
- Devor M, Amir R, Rappaport ZH.. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002; 18: 4–13. [DOI] [PubMed] [Google Scholar]
- Favoni V, Grimaldi D, Pierangeli G, Cortelli P, Cevoli S.. SUNCT, SUNA and neurovascular compression: new cases and critical review of the literature. Cephalalgia 2013; 33: 1337–48. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders 2nd edition. Cephalalgia 2004; 24: 1–195. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- Lambru G, Matharu MS.. SUNCT, SUNA and trigeminal neuralgia: different disorders or variants of the same disorder? Curr Opin Neurol 2014; 325–31. [DOI] [PubMed] [Google Scholar]
- Lambru G, Nesbitt A, Shanahan P, Matharu MS.. Coexistence of hemiplegic migraine with SUNCT or SUNA: a case series. Cephalalgia 2012; 32: 258–62. [DOI] [PubMed] [Google Scholar]
- Lambru G, Rantell K, Levy A, Matharu MS.. A prospective comparative study and analysis of predictors of SUNA and SUNCT. Neurology 2019; 93: e1127–e1137. [DOI] [PubMed] [Google Scholar]
- Love S, Coakham HB.. Trigeminal neuralgia: a pathology and pathogenesis. Brain 2001; 124: 2347–60. [DOI] [PubMed] [Google Scholar]
- Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L.. Significance of neurovascular contact in classical trigeminal neuralgia. Brain 2015; 138: 311–9. [DOI] [PubMed] [Google Scholar]
- Matharu MS, Cohen AS, McGonigle DJ, Ward N, Frackowiak RS, Goadsby PJ.. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache 2004; 44: 747–61. [DOI] [PubMed] [Google Scholar]
- Matharu MS, Cohen AS, Frackowiak RS, Goadsby PJ.. Posterior hypothalamic activation in paroxysmal hemicrania. Ann Neurol 2006; 59: 535–45. [DOI] [PubMed] [Google Scholar]
- May A, Bahra A, Buchel C, et al. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: short-lasting neuralgiform headache with conjunctival injection and tearing. Ann Neurol 1999; 46: 791–4. [DOI] [PubMed] [Google Scholar]
- Miller S, Akram H, Lagrata S, Hariz M, Zrinzo L, Matharu M.. Ventral tegmental area deep brain stimulation in refractory short-lasting unilateral neuralgiform headache attacks. Brain 2016; 139: 2631–40. [DOI] [PubMed] [Google Scholar]
- Peker S, Kurtkaya O, Uzun I, Pamir MN.. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 2006; 59: 354–9. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Schweitzer D, Tan L, Broadley SA.. Role of trigeminal microvascular decompression in the treatment of SUNCT and SUNA. Curr Pain Headache Rep 2013; 17: 332. [DOI] [PubMed] [Google Scholar]
- Sindou M, Brinzeou A.. Topography of pain in classical trigeminal neuralgia: insights into somatotopic organization. Brain 2020; 143: 531–40. [DOI] [PubMed] [Google Scholar]
- Weng H-Y, Cohen AS, Schankin C, Goadsby PJ.. Phenotypic and treatment outcome data on SUNCT and SUNA, including a randomised placebo-controlled trial. Cephalalgia 2017; 38: 1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bazina R, Tan L, Rice H, Broadley SA.. Microvascular decompression of the trigeminal nerve in the treatment of SUNCT and SUNA. J Neurol Neurosurg Psychiatry 2010; 81: 992–6. [DOI] [PubMed] [Google Scholar]
- Williams MH, Broadley SA.. SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci 2008; 15: 526–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.