Abstract
In this study we demonstrate that 2 month old APPswe/PS1dE9 mice, a transgenic model of Alzheimer’s disease, exhibited intact short-term memory in Pavlovian hippocampal—dependent contextual fear learning task. However, their long-term memory was impaired. Intra-CA1 infusion of isoproterenol hydrochloride, the β-adrenoceptor agonist, to the ventral hippocampus of APPswe/PS1dE9 mice immediately before fear conditioning restored long-term contextual fear memory. Infusion of the β-adrenoceptor agonist + 2.5 h after fear conditioning only partially rescued the fear memory, whereas infusion at + 12 h post conditioning did not interfere with long-term memory persistence in this mouse model. Furthermore, Intra-CA1 infusion of propranolol, the β-adrenoceptor antagonist, administered immediately before conditioning to their wildtype counterpart impaired long-term fear memory, while it was ineffective when administered + 4 h and + 12 h post conditioning. Our results indicate that, long-term fear memory persistence is determined by a unique β-adrenoceptor sensitive time window between 0 and + 2.5 h upon learning acquisition, in the ventral hippocampal CA1 of APPswe/PS1dE9 mice. On the contrary, β-adrenoceptor agonist delivery to ventral hippocampal CA1 per se did not enhance innate anxiety behaviour in open field test. Thus we conclude that, activation of learning dependent early β-adrenoceptor modulation underlies and is necessary to promote long-term fear memory persistence in APPswe/PS1dE9.
Subject terms: Neuroscience, Systems biology
Introduction
Progressive impairment of memory and subsequent cognitive decline are a major feature of Alzheimer’s disease (AD)1,2. Several studies with AD patients and familial AD mouse models have suggested that memory deficits might be caused by ineffective encoding and consolidation of new information3–6, or disrupted retrieval of stored information7–9. However, how early consolidation processes influence late long-term memory persistence still remained unclear. Changes in the noradrenergic system have been observed early in the progression of AD10–16. Progressive damage to noradrenergic terminals and the locus coeruleus neuronal cell body have been described in multiple APP transgenic models17–20. In all these mouse models, degenerative changes in the locus coeruleus were observed well after the onset of plaque deposition. Interestingly, in the APPswe/PS1dE9 (APP/PS1) mice, exposure of the monoaminergic synaptic terminals or distal axons, especially to oligomeric species of Aβ21,22, could lead to early cortical/hippocampal pathology. APP/PS1 mice exhibit particularly widespread and severe loss of monoaminergic axons23,24. In this study we examined the repercussions of early damage to noradrenergic axon terminals projecting to the hippocampus in 2-month-old APP/PS1 mice. Noradrenaline released in hippocampus is known to modulate synaptic plasticity and memory consolidation through activation of β adrenergic receptors (β-AR)25. It has been recently demonstrated that long-term fear memory at 24 h is impaired in 2-month old APP/PS1 mice prior to onset of amyloidosis26. Progressive plaque deposition is known to occur at approximately 6 months of age in this mouse model27, and is known to parallel behavioural decline28–30.
Long-term persistence of fear memories is dependent on the early and late consolidation windows in the hippocampus. This process is regulated by local β-AR signalling between + 2 and + 6 h31,32 and D1/5 dopamine receptor signalling at the ventral hippocampus (vH) + 12 h33–35 post learning acquisition. The duality of function of hippocampus is attributed along its dorso-ventral axis. Noradrenergic innervation and the endogenous levels of noradrenaline are higher in vH than in dH36,37. The dorsal hippocampus is involved in cognitive processes and vH in emotional or anxiety related behaviour38,39. Distinct subsets of vCA1 neurons to basal amygdala are necessary for contextual fear learning and memory40,41. vCA1 also sends dense, non-overlapping projections to hypothalamus42,43 modulating innately anxiogenic behaviours. Therefore, distinct representation arise within the vCA1 based on the valence of the stimuli (innate vs learned) routing information via projection defined vCA1 neurons40. However, we still do not know the dynamics and the nature of learned fear versus innate anxiety in APPswe/PS1dE9 mice at an age where there is constitutive overexpression of APP and PS1 genes without β-amyloid deposition.
Here we applied contextual fear conditioning (cFC) and open field test (OFT) to investigate the causal role of local vH β-AR dependent signalling in modulating learned fear versus innate anxiety related behaviour. The current study primarily mapped the time course of fear expression after cFC in APP/PS1 mice. Consequently, this measure has proved useful in detecting the time dependent decline in the strength of fear memory after learning acquisition. Further experiments were designed specifically to study the role of β-AR dependent plasticity in the vH during early stages of memory consolidation, that would determine long-term fear memory persistence in 2-month-old APP/PS1 mice. We report that APP/PS1 has a local vH β-AR signalling dependent time window spanning from 0 to + 2.5 h, which is imperative for long-term fear memory persistence. Furthermore, using OFT, we demonstrate that vH β-AR agonist infusion per se does not interfere with innate anxiety responses of APP/PS1 mice.
Materials and methods
Experimental animals
The generation, care, and use of mice as well as all experimental procedures were approved by the Institutional Animal Ethics Committee of the Indian Institute of Science, Bangalore. Transgenic mice B6C3-Tg (APPswe/PS1dE9) 85Dbo/J (https://www.jax.org/strain/005864) were originally obtained from The Jackson Laboratory, and was kindly provided by Prof. Vijayalakshmi Ravindranath, Director, Centre for Brain Research, Bangalore, India26. Wild Type (WT) and APPswe/PS1dE9 (APP/PS1) mice were bred at the Institutional Central Animal Facility, and were housed in standard mouse cages under conventional laboratory conditions (12 h dark and 12 h light cycle, constant temperature and humidity), and were given food and water ad libitum. Behavioural experiments were performed using male APP/PS1 and WT mice. No samples were excluded from any of the experiments described herein, unless otherwise mentioned in the analysis. Animal experiments were designed and followed in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. WT and APP/PS1 mice were assigned randomly to the respective groups based on the genotype. Different litters of the same age group were taken and were divided into control and experimental groups.
Behavioural procedures
All experiments were carried out with male mice that were 55–65 days old (~ 30 g) at the beginning of the experiment. Mice were housed individually for 3 days, and were handled for 5 min everyday prior to behavioural testing. All behavioural experiments were conducted at approximately the same time during the light cycle (9:00–15:00) by one constant experimenter.
Contextual fear conditioning (cFC)
For cFC26, the training context was rectangular in shape. Identity of the context was maintained with the presence of 2% acetic acid (vol/vol). The conditioning chamber was cleaned with 70% ethanol before and after each session. Mice were allowed to explore the apparatus for 1 min, and then received 3 foot shocks (2 s and 0.6 mA each, intershock interval 30 s). Contextual fear memory was assessed by returning the mice to the training context 24 h or one month after fear conditioning and analysing freezing during a test period of 2 min.
Open field test (OFT)
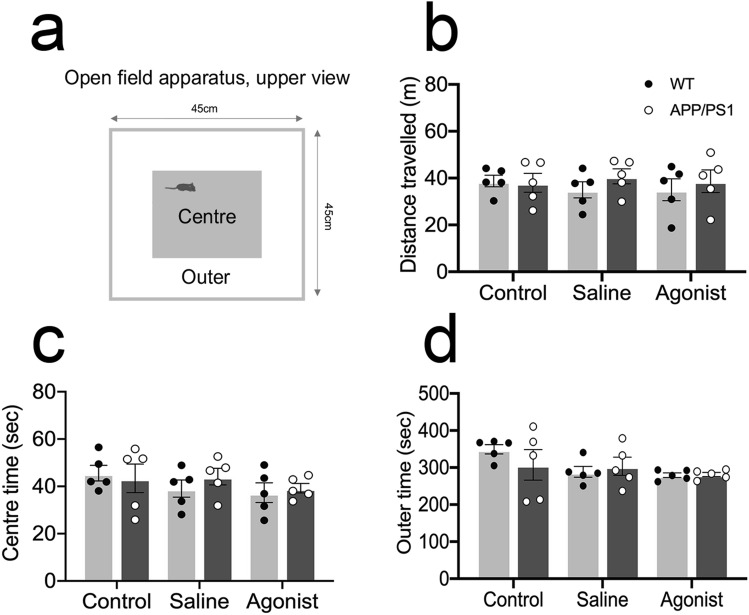
Mice were released in the corner of a 45 × 45 cm open field arena (Fig. 3a). Their activity was recorded with a camera mounted on the ceiling above the center of the open field arena for 10 min. At the end of testing, mice were returned to their home cage. The arena was partitioned into outer periphery and center region, and the amount of time spent and the distance travelled were extracted using a previously validated open-source toolbox for automated phenotyping of mouse behaviour44.
Figure 3.
Infusion of β-adrenergic receptor agonist at—0 h do not modulate innate anxiety levels in 2-month-old APP/PS1 mice: (a) Open-field test apparatus. WT and APP/PS1 mice were tested for distance travelled (b), time spent in the center (c), and time spent in the outer periphery (d). n = 5; two-way ANOVA followed by Sidak’s post hoc test. Data are presented as mean ± S.E.M.
Pharmacology in vivo
All the local treatments were carried out with the help of cannulas (33G). Guide cannulae (26 gauge, Plastics One) were implanted to the skull with dental cement. Mice were then given 1 week to recover from surgery. 32-gauge stainless steel injectors attached to 5-μl Hamilton syringes were inserted into the guide cannulae to deliver the following drugs—Isoproterenol Hydrochloride (0.25 μg per side; Sigma Aldrich, India); ( ±)-Propranolol hydrochloride (0.5 μg per side; Sigma Aldrich, India), and 0.9% saline. Coordinates relative to bregma are as follows: vH (AP − 3.0, ML ± 2.6, DV − 3.2). A total volume of 200 nl was injected; the injector was left for another minute to allow diffusion into the tissue.
Statistical analysis
Data analysis was performed using Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). No statistical methods were used to predetermine sample sizes. Our sample sizes are similar to those generally employed in the field33. The sample size per group is mentioned in the respective figure legends. Statistical analyses were designed using the assumption of normal distribution and similar variance among groups. They were performed using unpaired two-tailed Student’s t-test for paired comparisons, and two-way ANOVA followed by post hoc tests was performed when two factors were compared. Results are presented as mean ± S.E.M. The significance of the results was accepted at P < 0.05. Eta squared (η2) are reported as a measure of effect size45.
Results
2-month-old APP/PS1 mice exhibit long-term, but not short-term fear memory loss
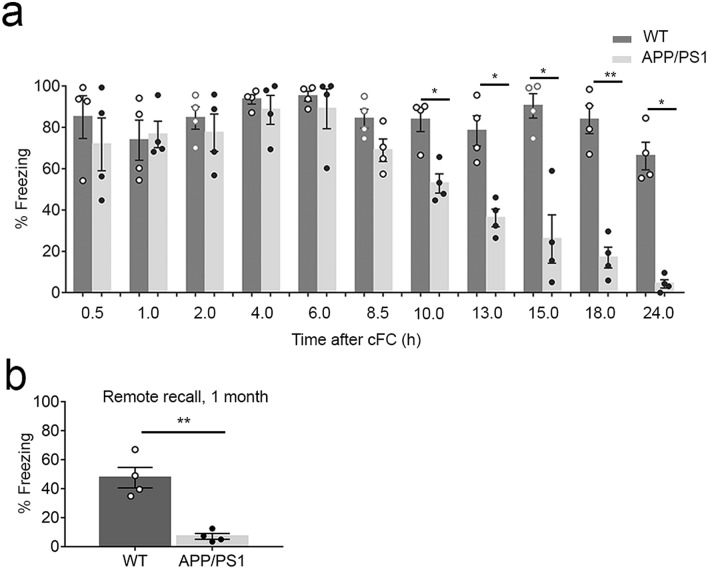
In order to determine whether freezing impairments observed at 24 h in 2-month-old APP/PS126 are due to a failure to form short-term memory, we tested APP/PS1 for recall of fear at different time points post cFC from + 0.5, + 1, + 2, + 4, + 6, + 8.5, + 10, + 13, + 15, + 18 till + 24 h. (+) indicates the timepoint after cFC. This experiment was conducted with 11 groups of WT and APP/PS1 mice, with n = 4 mice per group per time point. We designated the time at which the learning protocol was completed (in cFC: the last of the three foot shocks in the training context) as the time of acquisition, or time + 0 h. Freezing was intact in APP/PS1 mice tested between + 0.5 and + 8.5 h, indicating an intact short-term memory. However, strength of the memory displayed a steep decline from + 10 h till + 24 h in APP/PS1 mice as compared to WT (Fig. 1a: Two-way ANOVA followed by Sidak’s post hoc test: time effect: F (3.466, 20.80) = 12.66, P < 0.0001; genotype effect: F (1, 6) = 108.2, P < 0.0001; interaction: F (10, 60) = 7.287, P < 0.0001). Recall at a remote time point (1 month) post cFC acquisition also indicated a weak fear memory in APP/PS1 as compared to WT mice (Fig. 1b: two-tailed unpaired Student's t test; 1 month : t(6) = 5.503, P = 0.0015, η2 = 0.8346). These results clearly indicate that short-term memory is intact in 2-month-old APP/PS1 mice.
Figure 1.
Long-term fear memory, not short-term is impaired in 2-month-old APP/PS1 mice: (a) Time course of percentage time spent freezing to the context during memory recall at different time points post fear conditioning. There is a gradual decline of strength of fear memory + 10 h onwards in APP/PS1 mice compared to WT. n = 4, Two-way ANOVA followed by Sidak’s post hoc test. (b) Percentage time spent freezing to the context during remote memory recall after one month of cFC. n = 4; Two tailed unpaired Students t test; Data are presented as mean ± S.E.M. *P = 0.0332, **P = 0.0021.
β-AR activation during acquisition or immediately post acquisition promotes long-term fear memory persistence in APP/PS1 mice
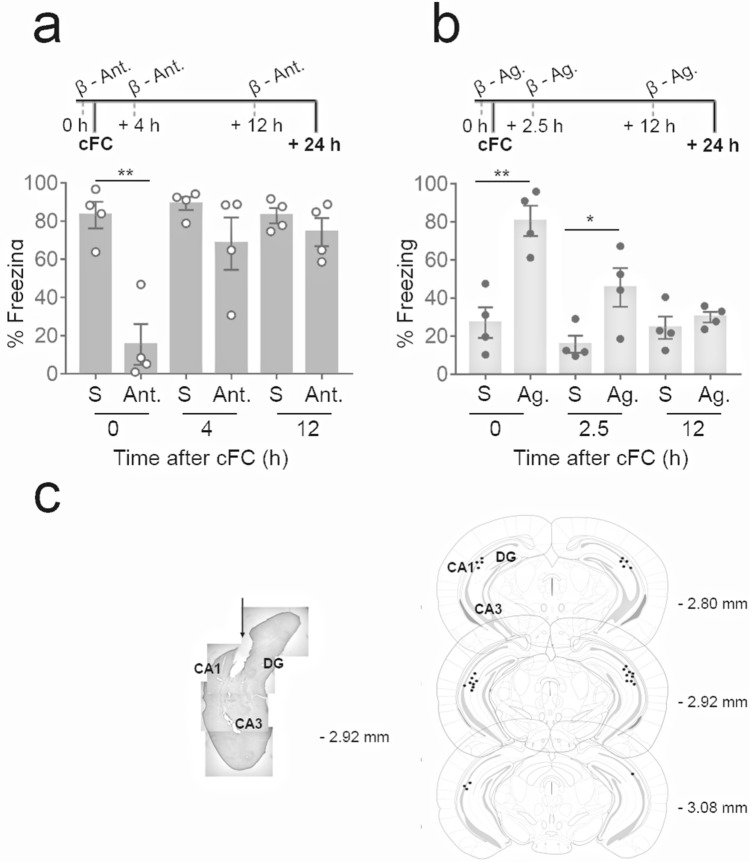
To determine whether endogenous local β-AR signalling is necessary to ensure longevity of short-term memory during and following learning, we delivered β-AR antagonist (±)-Propranolol hydrochloride to the vH CA1 (Fig. 2c) at different time points in APP/PS1 mice (Fig. 2a). A previous report suggested that the injection of protein synthesis inhibitor anisomycin to the CA1 impaired long-term memory supporting the existence of an early phase of synaptic plasticity occurring at mossy fiber terminals during cFC46. Moreover, the vH was chosen due to its reciprocal connections to the amygdala, mediating fear memory38–41,46,47, and due to its early involvement in fear memory consolidation and expression unlike dorsal hippocampus33,48. β-AR antagonist delivered to vH CA1 10 min before acquisition (− 10 min) prevented long-term memory consolidation in WT mice (Fig. 2a: two-tailed unpaired Student’s t test; + 0 h: t(6) = 5.331, P = 0.0018, η2 = 0.8257). However, β-AR antagonist delivery did not suppress long-term fear memory when delivered at + 4 h and + 12 h after acquisition (Fig. 2a: two-tailed unpaired Student's t test; + 4 h: t(6) = 1.484, P = 0.1884, η2 = 0.2684; + 12 h: t(6) = 1.026, P = 0.3443, η2 = 0.1494). Furthermore, β-AR agonist delivered to vH immediately before acquisition at + 0 h produced increased freezing to context at + 24 h compared to saline treated APP/PS1 animals (Fig. 2b: two-tailed unpaired Student's t test; + 0 h: t(6) = 4.714, P = 0.0033, η2 = 0.7874), and were undistinguishable from fear memory of WT mice. However, β-AR agonist delivery at + 2.5 h only partially enhanced the persistence of long-term fear memory (Fig. 2b: two-tailed unpaired Student’s t test; + 2.5 h: t(6) = 2.684, P = 0.0364, η2 = 0.5455; + 0 h vs. + 2.5 h: t(6) = 2.693, P = 0.0359, η2 = 0.5473). Furthermore, the β-AR agonist failed to rescue long-term fear memory persistence when delivered + 12 h after acquisition (Fig. 2b: two-tailed unpaired Student’s t test; + 12 h: t(6) = 0.8798, P = 0.4128, η2 = 0.1143). Previous studies have shown that, under local in vivo delivery conditions comparable to those used here, the agonist is effective up to 2.5–5 min following delivery49. These results clearly indicate that long-term memory persistence is determined by a unique early time window (+ 0 to + 2.5 h) in the vH CA1 post learning acquisition in APP/PS1 mice. Moreover, it indirectly indicates early noradrenergic dysfunction in this animal model.
Figure 2.
Infusion of β-adrenergic receptor agonist at—0 h modulates strength of memory in 2-month-old APP/PS1 mice: (a) β-AR antagonist delivery to vH at + 0 h, but not at + 4 h or + 12 h decreased freezing in the WT mice (S: 0.9% Saline; Ant: β-AR antagonist). (b) Time windows at which local delivery of β-AR agonist to vH influence long-term consolidation of fear memory (S: 0.9% Saline; Ag: β-AR agonist) in APP/PS1 mice. Long-term fear memory consolidation specifically depends on vH β-AR signaling around + 0 h. Infusion of β-AR agonist at + 2.5 h, but not at + 12 h post cFC partially rescues fear memory in APP/PS1 mice. (c) Mouse brain coronal section (left) with location of vH (DG, CA3, CA1). To the right, numbers: antero-posterior coordinates caudal to bregma. Representation of the tips of the injectors (black circles) aiming the vH CA1. Injection sites from 15 mice into CA1 region of vH. n = 4; Two tailed unpaired Students t test; Data are presented as mean ± S.E.M. *P = 0.0332, **P = 0.0021.
β-AR activation did not interfere with innate anxiety-related behaviour in APP/PS1 mice
The OFT assess anxiety-like behaviours in response to a novel environment. Distance travelled is a measure of ambulatory movement, whereas the amount of time spent in the center zone versus the outer zone is a measure of anxiety levels due to the rodent’s natural aversion or avoidance behaviour to open spaces50. Two-way ANOVA followed by Sidak’s post hoc test did not reveal any significant differences between APP/PS1 and WT mice, in the amount of time each mouse spent in the open center zone (Fig. 3c: treatment effect: F (2, 24) = 1.232, P = 0.3096; genotype effect: F (1, 24) = 0.2396, P = 0.6289; interaction: F (2, 24) = 0.4229, P = 0.6599), the outer zone (Fig. 3d: treatment effect: F (2, 24) = 2.632, P = 0.09626; genotype effect: F (1, 24) = 0.2594, P = 0.6152; interaction: F (2, 24) = 0.9806, P = 0.3896) and the total ambulatory movement (Fig. 3b: treatment effect: F (2, 24) = 0.08025, P = 0.9231; genotype effect: F (1, 24) = 0.8361, P = 0.3696; interaction: F (2, 24) = 0.3744, P = 0.6916) following β-AR agonist delivery to vCA1 immediately before taking the mice to OFT.
Discussion
In this study, we describe the temporal course of behavioural expression of fear memory upon cFC, to determine the dynamics of β-AR modulation and memory consolidation in the vH CA1 of 2-month old APP/PS1 mice. We demonstrate a unique β-AR dependent time window of early memory consolidation (+ 0–2.5 h) that is crucial for long-term fear memory persistence in APP/PS1 mice. Our findings are consistent with previous reports using 7-month-old APP/PS1 mice that are impaired in long-term but not in short-term contextual fear memory9. The APP/PS1 mouse model exhibits constitutive overexpression of APP and PS1 genes, elevated levels of toxic soluble oligomeric Aβ, and synaptic deficits are reported at 1–1.551 and 3.5 months of age in the cortex and hippocampus52–54. This might underlie the mild behavioural impairments observed in our study with 2-month-old APP/PS1 mice. We further demonstrate that β-AR agonist per se does not modulate the baseline innate anxiety levels of APP/PS1.
Pharmacological studies using male Sprague–Dawley rats have indicated that between 0 and 6 h after a learning experience, the noradrenergic system is activated to reinforce long-term memory processing in the hippocampus31,32 and prefrontal cortex55–57. Our results indicate the existence of a local β-AR signalling dependent early consolidation window between + 0 and 2.5 h which is required to ensure long-term fear memory persistence. Consistent with this notion, the late consolidation window which has been reported to be D1/5R dependent at + 12 h33–35 was unaffected by administration of β-AR antagonist.
Apart from being involved in consolidation of long-term fear memory, vH neurons also carry a representation of innately anxiogenic stimuli. Manipulation of the vH afferents or efferents are also known to directly impact anxiety-related behaviour58–60. Furthermore, anxiolytic effects of propranolol have been well documented in animal models of anxiety61,62. Therefore, the increased freezing to context induced by β-AR agonist may have emerged from β-AR agonist induced changes in innate anxiety levels of APP/PS1. This led us to perform open field experiment with APP/PS1 mice following vCA1 β-AR agonist infusion to investigate whether the β-AR agonist potentiated the consolidation of the fear memory trace, or enhanced innate anxiety levels to promote avoidance behaviour. Intriguingly, we found the concentration of β-AR agonist which we administered in our studies did not have a significant role in mediating an avoidance behaviour to the context in APP/PS1.
It was recently demonstrated that β2-AR expressed in astrocytes are the primary mediators of fear based contextual memory consolidation, rather than β1-AR which are primarily located in the neurons63. These findings indicate the differential contribution of β1-ARs and β2-ARs in mediating hippocampal memory formation and associated processes. More importantly, β2-AR dysregulation in astrocytes has been implicated in AD64. These findings further suggest that the β-AR agonist may facilitate strengthening of memory trace by gating synaptic plasticity in vCA1 synapses through distinct pathways for learned versus innate anxiety, based on the affective valence of the inputs.
Memory stabilization after learning also involves temporal molecular changes65. De novo protein synthesis occurring within 1 h or around 4 h after learning acquisition is known to be important for memory stabilization66. A recent study67 demonstrated translational repression of specific genes such as neurensin-1 (Nrsn1) and Mitogen-activated protein kinase 6 (Mapk6) around 5–10 min post cFC acquisition without a change in mRNA levels. Furthermore, at later time points of 30 min and 4 h, they observed gene suppression especially of a group of genes which were positively controlled by Estrogen Receptor 1 (ESR1). Overexpressing Nrsn1 or activating ESR1 in the hippocampus post learning acquisition was sufficient to impair contextual fear memory formation. Interestingly, signalling pathways of ESRs are intertwined with those of the β-ARs68 pointing to the possibility of functional convergence and their interdependence at around 2.5 h in the vH modulating long-term memory consolidation. However, we still do not know the mechanisms underlying these upstream regulations which might be pertinent for long-term memory persistence.
The most progressive hypothesis with regard to memory deficits in AD is the ‘retrieval deficit’ where the store of memory remains relatively intact during early AD and the deficits are associated with inability to access and modify this information long-term7–9. In contrast, our results support the notion that early consolidation deficits caused by absence of appropriate β-adrenergic neuromodulation may be responsible for memory deficits in early AD. Thus, β-adrenergic neuromodulation may be necessary to recruit and maintain system wide networks for long-term memory persistence in APP/PS1 mice. Accordingly, the unique time window for memory persistence uncovered in this study may represent a new therapeutic window to alter memory networks and promote better quality control of mechanisms that are required for long-term memory consolidation.
Acknowledgements
This work was supported by funds obtained from the Centre for Brain Research (CBR) and Pratiksha Trust. Author is grateful to Prof. Vijayalakshmi Ravindranath, Director, CBR for the support throughout the study. Author specially thanks the Scientific Advisory Committee at CBR for their insightful inputs. Author would also like to specially thank Mr. Radeesh R, Mr. Muniraju N and the staff at Central Animal Facility, IISc.
Author contributions
S.K. devised, carried out all the behavioural experiments, analyzed all experiments and wrote the manuscript.
Data availability
Requests for raw data can be addressed to the corresponding author.
Competing interests
The author declares no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249:288–290. doi: 10.1007/PL00014176. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011;3:77. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granholm E, Butters N. Associative encoding and retrieval in Alzheimer’s and Huntington’s disease. Brain Cogn. 1988;7:335–347. doi: 10.1016/0278-2626(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harbor Perspect. Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquier F, Grymonprez L, Lebert F, Van der Linden M. Memory impairment differs in frontotemporal dementia and Alzheimer's disease. Neurocase. 2001;7:161–171. doi: 10.1093/neucas/7.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Beglopoulos V, et al. Early detection of cryptic memory and glucose uptake deficits in pre-pathological APP mice. Nat. Commun. 2016;7:11761. doi: 10.1038/ncomms11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. Psychol. Aging. 1997;12:183–188. doi: 10.1037/0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- 8.Finke K, Myers N, Bublak P, Sorg C. A biased competition account of attention and memory in Alzheimer's disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20130062. doi: 10.1098/rstb.2013.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy DS, et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grudzien A, et al. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr. Opin. Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Del Tredici K. Alzheimer’s pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- 14.Andrés-Benito P, et al. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol. Appl. Neurobiol. 2017;43:373–392. doi: 10.1111/nan.12386. [DOI] [PubMed] [Google Scholar]
- 15.Kelly SC, et al. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017;5:8. doi: 10.1186/s40478-017-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theofilas P, et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer’s Dementia. 2017;13:236–246. doi: 10.1016/j.jalz.2016.06.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis BM, et al. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:1934–1944. doi: 10.1038/npp.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalinin S, et al. The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2012;33:1651–1663. doi: 10.1016/j.neurobiolaging.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.German DC, Nelson O, Liang F, Liang CL, Games D. The PDAPP mouse model of Alzheimer's disease: Locus coeruleus neuronal shrinkage. J. Compar. Neurol. 2005;492:469–476. doi: 10.1002/cne.20744. [DOI] [PubMed] [Google Scholar]
- 20.Guérin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol. Aging. 2009;30:272–283. doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 22.Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, et al. Passive (Amyloid-β) immunotherapy attenuates monoaminergic axonal degeneration in the AβPPswe/PS1dE9 mice. J. Alzheimer’s Dis. 2011;23:271–279. doi: 10.3233/JAD-2010-101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Dell TJ, Connor SA, Guglietta R, Nguyen PV. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 2015;22:461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kommaddi RP, et al. Aβ mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer's disease. J. Neurosci. 2018;38:1085–1099. doi: 10.1523/JNEUROSCI.2127-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankowsky JL, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J. Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y, et al. Retinoic acid attenuates beta-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. J. Neurosci. 2008;28:11622–11634. doi: 10.1523/JNEUROSCI.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 31.Ji JZ, Wang XM, Li BM. Deficit in long-term contextual fear memory induced by blockade of beta-adrenoceptors in hippocampal CA1 region. Eur. J. Neurosci. 2003;17:1947–1952. doi: 10.1046/j.1460-9568.2003.02620.x. [DOI] [PubMed] [Google Scholar]
- 32.Zheng J, Luo F, Guo NN, Cheng ZY, Li BM. β1-and β2-adrenoceptors in hippocampal CA3 region are required for long-term memory consolidation in rats. Brain Res. 2015;1627:109–118. doi: 10.1016/j.brainres.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Karunakaran S, et al. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat. Neurosci. 2016;19:454–464. doi: 10.1038/nn.4231. [DOI] [PubMed] [Google Scholar]
- 34.Katche C, Cammarota M, Medina JH. Molecular signatures and mechanisms of long-lasting memory consolidation and storage. Neurobiol. Learn. Mem. 2013;106:40–47. doi: 10.1016/j.nlm.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 36.Hortnagl H, Berger ML, Sperk G, Pifl C. Regional heterogeneity in the distribution of neurotransmitter markers in the rat hippocampus. Neuroscience. 1991;45:261–272. doi: 10.1016/0306-4522(91)90224-C. [DOI] [PubMed] [Google Scholar]
- 37.Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J. Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez JC, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron. 2018;97:670–683. doi: 10.1016/j.neuron.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jimenez JC, et al. Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat. Commun. 2020;11:3492. doi: 10.1038/s41467-020-17270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canteras NS. The medial hypothalamic defensive system: Hodological organization and functional implications. Pharmacol. Biochem. Behav. 2002;71:481–491. doi: 10.1016/S0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 43.Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: A PHAL anterograde tract-tracing study in the rat. J. Comp. Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- 44.Patel TP, et al. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front. Behav. Neurosci. 2014;8:349. doi: 10.3389/fnbeh.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011;6:135. doi: 10.1016/j.edurev.2010.12.001. [DOI] [Google Scholar]
- 46.Remaud J, et al. Anisomycin injection in area CA3 of the hippocampus impairs both short-term and long-term memories of contextual fear. Learn. Mem. 2014;21:311–315. doi: 10.1101/lm.033969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelikowsky M, Bissiere S, Fanselow MS. Contextual fear memories formed in the absence of the dorsal hippocampus decay across time. J. Neurosci. 2012;32:3393–3397. doi: 10.1523/JNEUROSCI.4339-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Procaccini DE, Sawyer JE, Watt KM. Pharmacology of Cardiovascular drugs. In: Ungerleider RM, Meliones J, Jacobs J, Cooper DS, editors. Critical Heart Disease in Infants and Children. 3. Amsterdam: Elsevier; 2019. pp. 192–212. [Google Scholar]
- 50.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad F, et al. Reactive oxygen species-mediated loss of synaptic Akt1 signaling leads to deficient activity-dependent protein translation early in Alzheimer's disease. Antioxid. Redox Signal. 2017;27:1269–1280. doi: 10.1089/ars.2016.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shemer I, et al. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 2006;23:2035–2047. doi: 10.1111/j.1460-9568.2006.04733.x. [DOI] [PubMed] [Google Scholar]
- 53.Hu YS, et al. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1DeltaE9 mice. FASEB J. 2010;24:1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/S0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 55.Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: Beta-adrenergic receptor involvement in the late phase. Learn. Mem. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- 56.Tronel S, Feenstra MGP, Sara SJ. Noradrenergic action in prefrontal cortex in the late stage of memory consolidation. Learn. Mem. 2004;11:453–458. doi: 10.1101/lm.74504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 58.Felix-Ortiz AC, et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padilla-Coreano N, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–866. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol. Biochem. Behav. 1998;59:387–397. doi: 10.1016/S0091-3057(97)00457-7. [DOI] [PubMed] [Google Scholar]
- 62.Gorman AL, Dunn AJ. Beta-adrenergic receptors are involved in stress-related behavioral changes. Pharmacol. Biochem. Behav. 1993;45:1–7. doi: 10.1016/0091-3057(93)90078-8. [DOI] [PubMed] [Google Scholar]
- 63.Gao V, et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong JH, et al. β2-adrenergic receptor and astrocyte glucose metabolism. J. Mol. Neurosci. 2012;48:456–463. doi: 10.1007/s12031-012-9742-4. [DOI] [PubMed] [Google Scholar]
- 65.Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harbor Perspect. Biol. 2014;7:a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourtchouladze R, et al. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn. Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- 67.Cho J, et al. Multiple repressive mechanisms in the hippocampus during memory formation. Science. 2015;350:82–87. doi: 10.1126/science.aac7368. [DOI] [PubMed] [Google Scholar]
- 68.Machuki JO, Zhang HY, Harding SE, Sun H. Molecular pathways of oestrogen receptors and β-adrenergic receptors in cardiac cells: Recognition of their similarities, interactions and therapeutic value. Acta Physiol. 2018;222:e12978. doi: 10.1111/apha.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for raw data can be addressed to the corresponding author.