Abstract
As the world population grows, muscle atrophy leading to muscle wasting could become a bigger risk. Long noncoding RNAs (lncRNAs) are known to play important roles in muscle growth and muscle atrophy. Meanwhile, it has recently come to light that many putative small open reading frames (sORFs) are hidden in lncRNAs; however, their translational capabilities and functions remain unclear. In this study, we uncovered 104 myogenic-associated lncRNAs translated, in at least a small peptide, by integrated transcriptome and proteomic analyses. Furthermore, an upstream ORF (uORF) regulatory network was constructed, and a novel muscle atrophy-associated lncRNA named SMUL (Smad ubiquitin regulatory factor 2 [SMURF2] upstream lncRNA) was identified. SMUL was highly expressed in skeletal muscle, and its expression level was downregulated during myoblast differentiation. SMUL promoted myoblast proliferation and suppressed differentiation in vitro. In vivo, SMUL induced skeletal muscle atrophy and promoted a switch from slow-twitch to fast-twitch fibers. In the meantime, translation of the SMUL sORF disrupted the stability of SMURF2 mRNA. Mechanistically, SMUL restrained SMURF2 production via nonsense-mediated mRNA decay (NMD), participating in the regulation of the transforming growth factor β (TGF-β)/SMAD pathway and further regulating myogenesis and muscle atrophy. Taken together, these results suggest that SMUL could be a novel therapeutic target for muscle atrophy.
Keywords: lncRNA SMUL, nonsense-mediated mRNA decay, SMURF2, TGF-β/SMAD pathway, myogenesis
Graphical Abstract
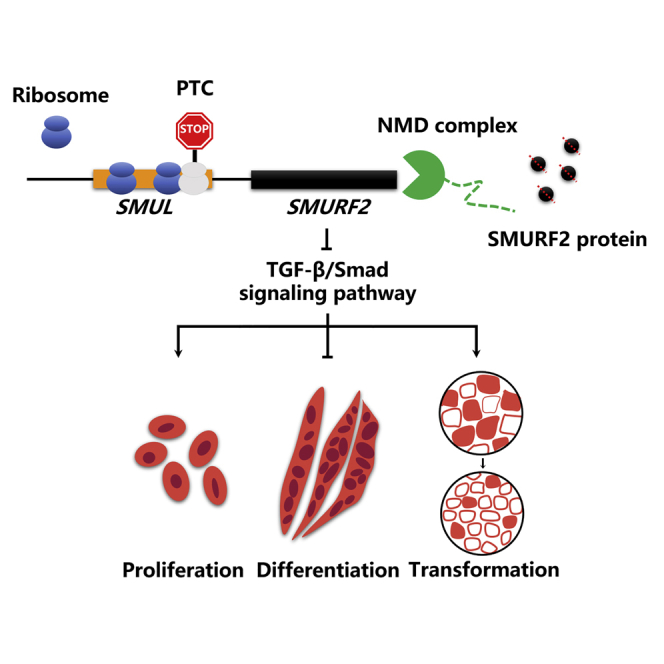
Muscle atrophy has become a major health challenge. Nie and colleagues identified a novel lncRNA, SMUL, that restrains SMURF2 production via nonsense-mediated mRNA decay to modulate myogenesis and induce muscle atrophy, suggesting that SMUL may serve as a potential therapeutic target for muscle atrophy.
Introduction
As the world population expands, muscle atrophy has become a major health challenge. Specifically, muscle atrophy conditions can present themselves during both aging and prolonged periods of muscle inactivity.1,2 Prevention of this condition could provide a significant clinical benefit.
Muscle growth is a highly ordered process in which myoblasts withdraw from the cell cycle, express muscle-specific genes, and prevent the expression of other cell- or tissue-specific genes, including myoblast proliferation and differentiation as well as myotube formation and maturity, which are controlled by a series of myogenic regulatory factors.3, 4, 5 After birth, the number of muscle fibers in animals is basically fixed, and their skeletal muscle development is mainly regulated by the size and composition of muscle fibers. Protein synthesis and catabolism are the main factors affecting skeletal muscle mass.6 When protein synthesis exceeds its degradation, muscle hypertrophy occurs and the cross-sectional area of muscle fiber increases. Furthermore, excessive protein degradation induces muscle atrophy, which leads to a decrease in muscle fiber cross-sectional area and muscle strength. Skeletal muscle is heterogeneous,7 and changes in its mass can often cause the transformation of muscle fiber types. In response to environmental demands, skeletal muscle can remodel by activating signaling pathways to reprogram gene expression to sustain muscle performance.7 Recently, multiple studies have found that long noncoding RNAs (lncRNAs) can determine epigenetic or transcriptional regulation in skeletal muscle development.8, 9, 10, 11, 12
Increasing high-throughput studies have revealed the presence of pervasive transcription across 70%–90% of the human genome. Tens of thousands of genomic loci produce transcripts, but more than 98% of these, known as noncoding RNAs (ncRNAs), are not translated to protein.13 Noncoding transcripts can generally be divided into small noncoding RNAs and lncRNAs on the basis of their size. Compared with small noncoding RNAs, lncRNAs are a novel class of mRNA-like transcripts, can vary in length from 200 nt to 100 kb, and make up the largest proportion of mammalian noncoding transcriptome.14,15 The translation capacity of lncRNAs has long been overlooked with the traditional approaches to classify the potential protein-coding capacity of RNA transcripts, which have been based on canonical open reading frames (ORFs), codon conservation, and similarity to known protein domains.16,17 The cutoff of 100 aa was an artificial standard for annotation of ORFs as protein-coding transcripts or not. Therefore, the distinction between lncRNAs and mRNAs is not always clear.16,18 Increasing studies revealed that lncRNAs were often associated with ribosomes, providing key clues to unanticipated translation potential of lncRNAs.19,20 Previous studies have demonstrated that lncRNAs can give rise to functional peptides. lncRNA-Six1, located upstream of protein-coding gene Six1, can produce a 7.26-kDa peptide, affecting myoblast proliferation and differentiation.21 Myoregulin (MLN) is a conserved 46-aa peptide encoded by a skeletal muscle-specific lncRNA, and colocalized with sarcoplasmic/endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA). MLN decreases Ca2+ release in skeletal muscle and affects muscle performance.22 To date, the function that translation of lncRNAs contributes to muscle atrophy is unclear.
Smad ubiquitin regulatory factor 2 (SMURF2) is a homology to E6 carboxyl terminus (HECT) domain-containing E3 ubiquitin ligase that specifies substrates for ubiquitination and degradation by the proteasome.23,24 SMURF2 regulates key biological processes during development and homeostasis via controlling the transforming growth factor β (TGF-β)/mothers against decapentaplegic (SMADs) signaling pathway.25, 26, 27, 28, 29 However, the precise mechanisms regulating SMURF2 abundance remain largely unknown.
Recently, combined with proteomics analysis, some hidden proteins encoded by lncRNA have been discovered.30, 31, 32 To systematically identify the lncRNA with potential translation ability involved in myogenesis, we characterized the lncRNA and mRNA expression profiles as well as peptidomic profiles during chicken primary myoblast (CPM) proliferation and differentiation. Based on these results, a potential translated lncRNA, LNC_004915, located upstream of the SMURF2 gene, was identified and named SMUL (SMURF2 upstream lncRNA). SMUL is highly expressed in skeletal muscle, and its expression decreases with myoblast differentiation. Gain- and loss-of-function analysis revealed that SMUL promoted myoblast proliferation and inhibited myogenic differentiation. In vivo, SMUL induced skeletal muscle atrophy and activated the fast-twitch fiber phenotype. Mechanistically, SMUL induces nonsense-mediated mRNA decay (NMD) of SMURF2 through its translation process and activates the TGF-β/SMAD pathway. Altogether, our studies uncover a functional lncRNA that modulates skeletal muscle development, and they provide a novel therapeutic target for treating muscle atrophy.
Results
Identification and characterization of small ORF (sORF)-encoded lncRNAs by integrated transcriptome and proteomic analysis
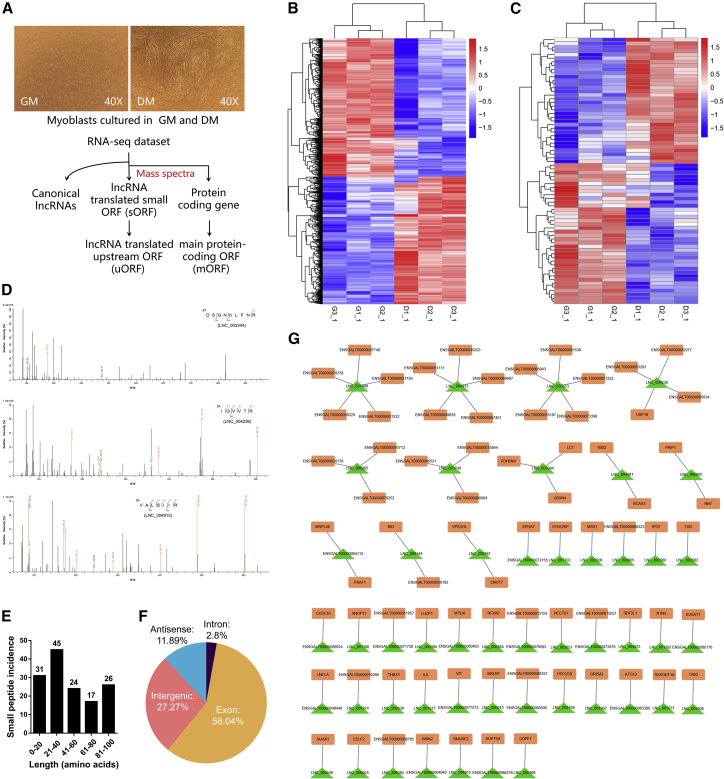
CPMs were cultured in differentiation medium to induce myoblast differentiation in vitro. To systematically identify the myogenesis-associated lncRNAs, RNA sequencing (RNA-seq) was performed during myoblast proliferation (CPMs cultured in growth medium [GM]) and the third day of differentiation (CPMs cultured in differentiation medium [DM]) (Figure 1A). In total, 3,106 differentially expressed genes (DEGs) and 72 differentially expressed lncRNAs (DE-lncRNAs) were identified during myoblast proliferation and differentiation (Figures 1B and 1C; Tables S1 and S2). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis found that the DEGs were enriched in RNA transport, ribosome biogenesis in eukaryotes, biosynthesis of amino acids, and the cell cycle (Figures S1A and S1B).
Figure 1.
Translated lncRNAs are hidden in myogenesis
(A) Overview of experimental strategy for transcriptome (RNA-seq) and peptidome. The upper panel shows microscopic images of CPMs during proliferation (CPMs cultured in growth medium [GM]) and the third day of differentiation (CPMs cultured in differentiation medium [DM]). The lower panel is the discovery pipeline for translated lncRNAs. (B and C) Heatmaps of differentially expressed mRNAs (B) and lncRNAs (C) during myoblast proliferation and differentiation, with rows representing mRNAs or lncRNAs and columns representing different myoblast stages. (D) Mass spectrometry results of small peptide encoded by three translated lncRNAs (LNC_003344, LNC_004236, and LNC_004915) as examples. (E) Amino acid length distribution of lncRNA translated peptides detected by mass spectra. (F) Incidence of lncRNA translated peptides detected by mass spectra in each category within RefSeq mRNAs. (G) uORF regulatory network of translated lncRNAs and their potential target genes. lncRNAs containing peptides identified by mass spectrometry were used for the regulatory network analysis.
To identify the protein-coding potential of lncRNA sORF, we performed an integrated analysis using RNA-seq and proteomics data during CPM proliferation and differentiation. Interestingly, we found 104 myogenic lncRNAs that translated at least one small peptide, indicating that translated sORFs are hidden in myogenic lncRNAs (Figure 1D; Table S3). The length distribution of those small peptides is relatively uniform, with most in the range of 21–40 aa (Figure 1E). Furthermore, more than 80% of those are located on exons (58.04%) and are intergenic (27.27%), implying that they may perform their biological functions by acting as regulatory elements (Figure 1F).
Cis-acting is one of the main ways that lncRNA participates in epigenetic regulation.21,33,34 Upstream ORF (uORF), as an important cis-element, appears in nearly half of mammalian transcripts and extensively regulates downstream gene expression.35,36 To study the regulatory function of translated lncRNA, we constructed a uORF (which is transcribed by translated lncRNA) regulatory network (Figure 1G). A total of 49 translated lncRNA-transcribed ORFs were found to locate on the same chain and within 1.5 kb upstream of the main protein-coding ORF (mORF), which is predicted to function as a uORF (Figure 1G). SMURF2 is well known to modulate the TGF-β/SMAD pathway, which widely regulates animal development.25, 26, 27, 28, 29 Therefore, the LNC_004915-SMURF2 pair served as a candidate for further study.
lncRNA SMUL is reduced in myoblast differentiation and has translation ability
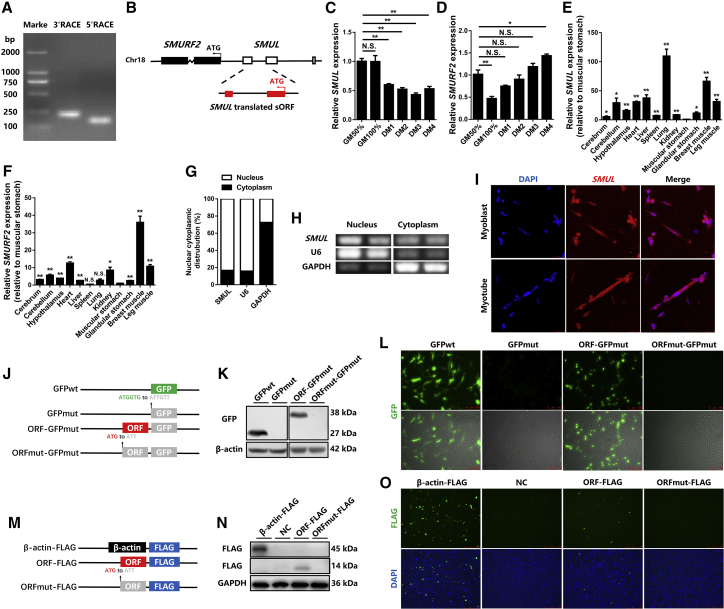
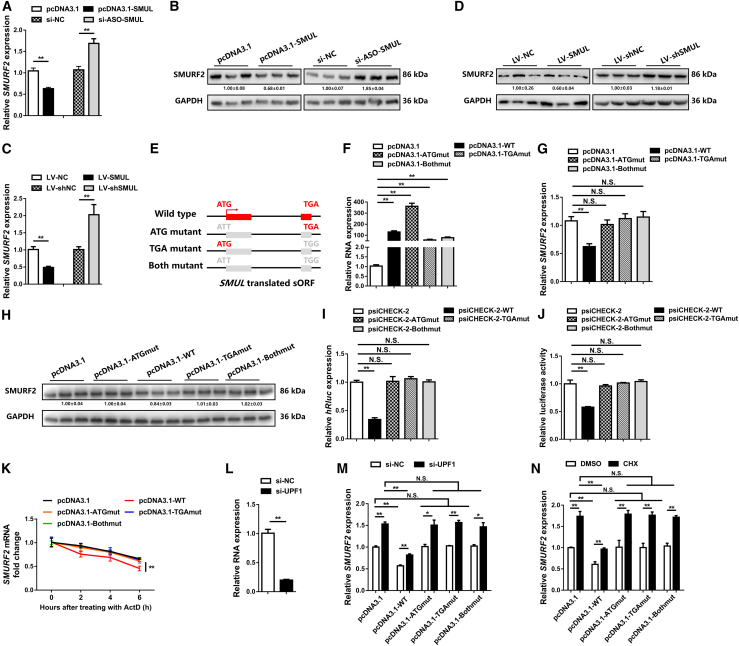
To evaluate the full-length sequence of SMUL (LNC_004915), the 5′ and 3′ ends of this lncRNA were determined by a rapid amplification of cDNA ends (RACE) system (Figure 2A). The Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) showed that SMUL was 1,061 nt long, located at chromosome 18 from position 6,970,951 to 6,967,249, relatively conserved in Numida meleagris, Meleagris gallopavo, and Coturnix japonica, and that its 3′ end was separated from the SMURF2 coding sequence (CDS) by 1,241 bp (Figure 2B; Figures S2A and S2B). SMUL was highly expressed in polyadenylated RNA, demonstrating that SMUL is a polyadenylated lncRNA, which is similar to mRNA, and has a poly(A) tail (Figure S2C). The expression level of SMUL was significantly downregulated during myogenic differentiation, whereas its target gene (SMURF2) was gradually increased with the differentiation of myoblasts (Figures 2C and 2D). In the meantime, SMUL and SMURF2 were highly expressed in breast and leg muscle (Figures 2E and 2F), implying that they may play an important role in skeletal muscle development. Cell-fractionation assays demonstrated that SMUL is mainly present in the nuclei of CPMs (Figures 2G and 2H). A similar result was also confirmed by in situ RNA hybridization (Figure 2I). Interestingly, our peptidome data revealed that SMUL encoded a small peptide, suggesting that SMUL has translation potential.
Figure 2.
lncRNA SMUL is downregulated in myogenic differentiation and has translation capacity
(A) Results of SMUL 5′ RACE and 3′ RACE. (B) Schematic image of the locus for SMUL (white), SMUL translated sORF (red), and SMURF2 (black). Thin arrows represent the direction of transcription. (C and D) Relative SMUL (C) and SMURF2 (D) expression during CPM differentiation (n = 4). (E and F) Tissue expression profiles of SMUL (E) and SMURF2 (F). The horizontal axis and vertical axis indicate different tissues and their relative expression values, respectively (n = 4). (G and H) The distribution of SMUL in the cytoplasm and nuclei of CPMs was determined by quantitative real-time PCR (G) and semi-quantitative real-time PCR (H). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 serve as cytoplasmic and nuclear localization controls, respectively. (I) RNA in situ hybridization of SMUL in chicken primary myoblasts. Special FISH probes against SMUL were modified by Cy3 (red). The nucleus was stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue). (J) Diagram of the GFP fusion constructs used for transfection. The initiation codon ATGGTG of the GFP (GFPwt) gene is mutated to ATTGTT (GFPmut). The initiation codon ATG of the SMUL sORF is mutated to ATT. (K and L) Western blotting with anti-GFP (K) and GFP fluorescence (L) were performed following 48 h after transfection of the indicated plasmids. (M) Diagram of the FLAG fusion constructs used for transfection. The initiation codon ATG of the SMUL sORF is mutated to ATT. (N and O) Western blotting with anti-FLAG (N) and immunostained using anti-FLAG (O) were performed following 48 h of transfection of the indicated plasmids. Data are presented as mean ± SEM. Statistical significance of differences between means was assessed using an independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
To verify this prediction, a series of constructs was generated in which a GFP mutant (GFPmut) ORF (in which the start codon ATGGTG was mutated to ATTGTT) was fused to the C terminus of the ORF and the ORF mutant (ORFmut) in the full-length SMUL transcript (Figure 2J). Western blotting analysis using anti-GFP antibodies showed that SMUL-GFP fusion protein exhibited the predicted relative molecular masses in SMUL ORF-GFPmut-transfected cells, but not in SMUL ORFmut-GFPmut-transfected cells (Figure 2K). Substantial expression of the SMUL-GFP fusion protein was observed in SMUL ORF-GFPmut-transfected cells, while mutation of the SMUL ORF start codon (in which the start codon ATG was mutated to ATT) abolished the expression of the SMUL-GFP fusion protein (Figure 2L). Meanwhile, we further cloned a FLAG epitope tag in-frame with the C terminus of the ORF and the ORFmut within the full-length SMUL transcript (Figure 2M). These results are consistent with those found using the SMUL-GFP fusion protein (Figures 2N and 2O), indicating that SMUL, which is annotated as an lncRNA, has translation capacity.
SMUL negatively regulates muscle development and induces muscle atrophy
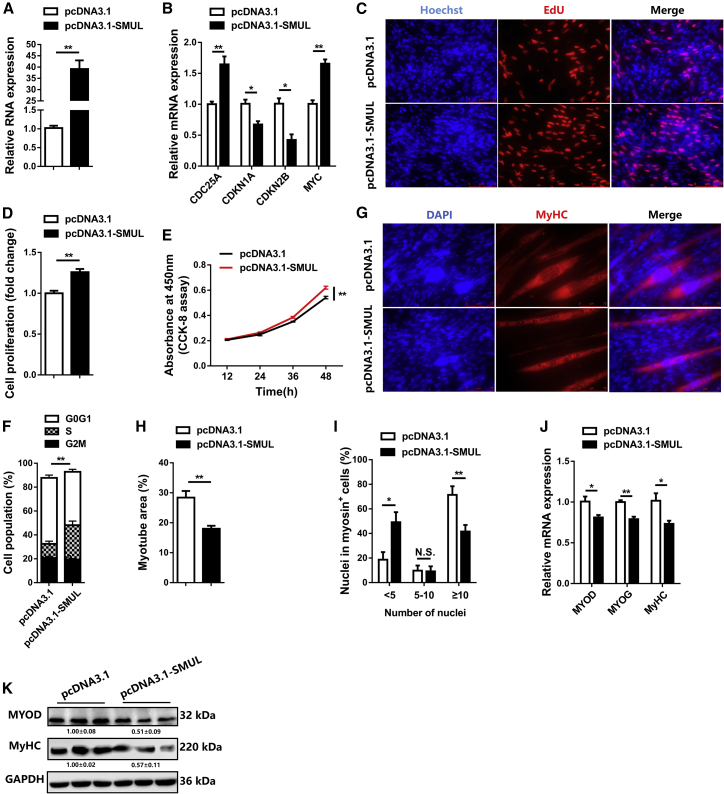
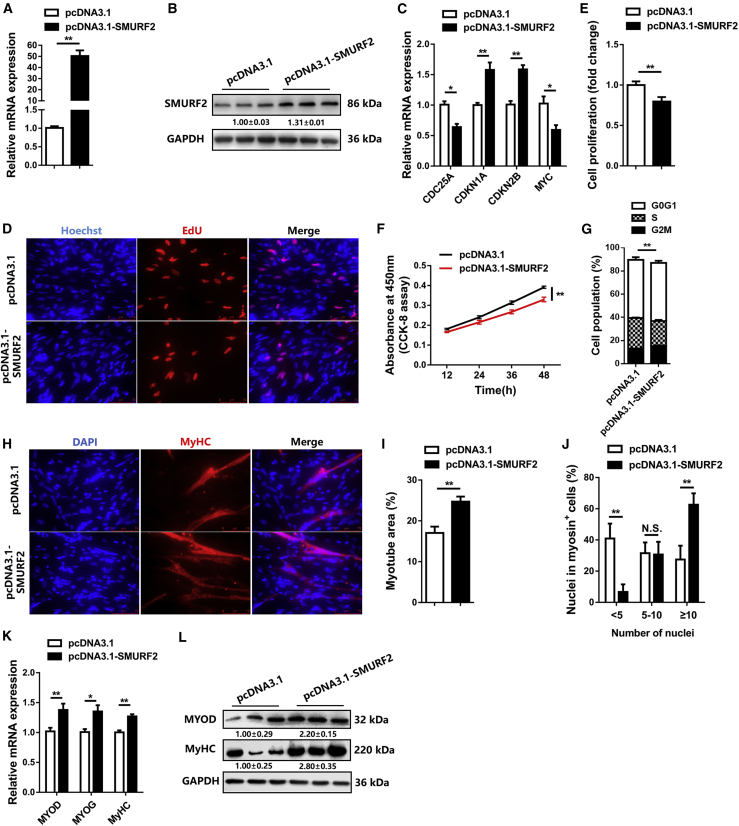
In order to determine the function of lncRNA SMUL in myogenesis, the overexpression vector of SMUL at full length was constructed and transfected into CPMs (Figure 3A). Overexpression of SMUL increased the expression level of cell cycle-promoting genes, including CDC25A and MYC, while it decreased the expression level of cell cycle-inhibiting genes such as CDKN1A and CDKN2B (Figure 3B). Using 5-ethynyl-2′-deoxyuridine (EdU) and Cell Counting Kit-8 (CCK-8) assays, we found that SMUL promoted myoblast proliferation (Figures 3C–3E). Furthermore, SMUL overexpression reduced the number of cells that progressed to G0/G1, thus increasing the number of S phase cells (Figure 3F). Meanwhile, the opposite result was observed by SMUL lncRNA interference (Figures S3A–S3F). To further investigate the potential function of SMUL in myoblast differentiation, immunofluorescence staining was performed. Immunofluorescence staining showed that overexpression of SMUL suppressed myotube formation and myoblast fusion (Figures 3G–3I). Moreover, the expression levels of myoblast differentiation marker genes, including MYOD, MYOG, and MyHC, were significantly downregulated with SMUL overexpression (Figures 3J and 3K). In contrast, SMUL interference facilitated myoblast differentiation (Figures S3G–S3K).
Figure 3.
SMUL facilitates myoblast proliferation but suppresses myoblast differentiation in vitro
(A) Relative SMUL expression after introducing SMUL into CPMs (n = 4). (B) Relative mRNA levels of several cell cycle genes after overexpression of SMUL (n = 4). (C) Proliferation of transfected CPMs was assessed by 5-ethynyl-2′-deoxyuridine (EdU) incorporation (n = 3). (D) Proliferation rate of myoblasts with SMUL overexpression (n = 3). (E) CCK-8 assays were performed in CPMs with SMUL overexpression (n = 6). (F) Cell cycle analysis of myoblasts at 48 h after overexpression of SMUL (n = 4). (G–I) MyHC immunostaining (G) (n = 3), myotube area (%) (H) (n = 3), and myoblast fusion index (I) (n = 3) of CPMs transduced with SMUL overexpression. Cells were differentiated for 72 h after transfection. The nuclei were visualized with DAPI. (J and K) Relative mRNA (J) (n = 4) and protein (K) (n = 3) expression levels of myoblast differentiation marker genes from pcDNA3.1-SMUL-transfected CPMs. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to GAPDH. Data are expressed as a fold change relative to the control. Results are shown as mean ± SEM. Statistical significances of differences between means were assessed using an independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
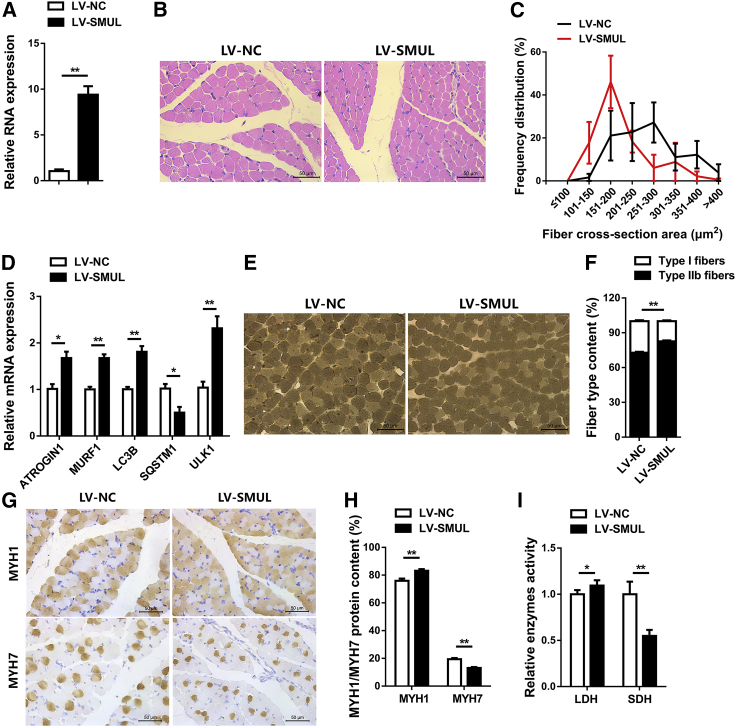
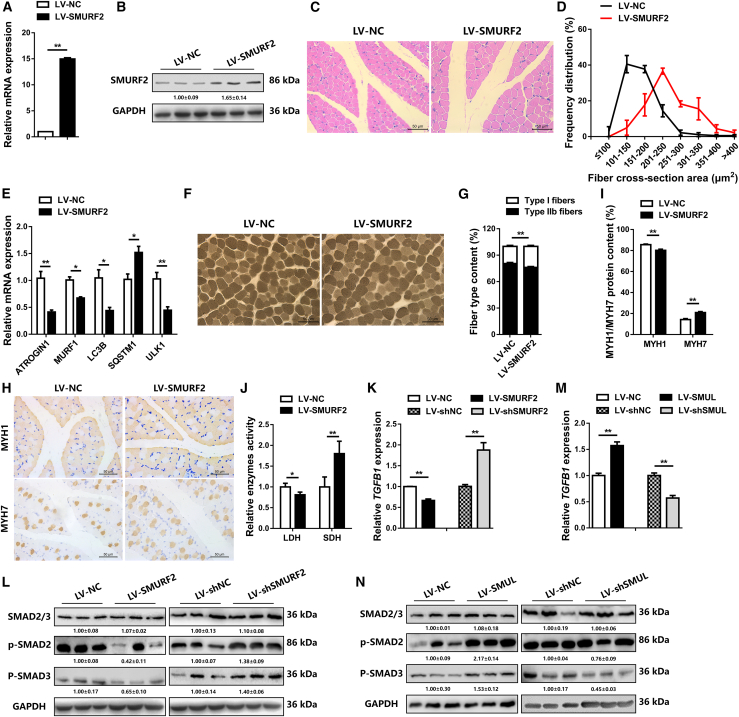
To test whether SMUL regulates skeletal muscle development in vivo, 1-day-old chicks were injected with lentiviral-mediated SMUL overexpression (LV-SMUL) or lentiviral-mediated SMUL knockdown (LV-shSMUL) (Figure 4A; Figure S3K). Overexpression of SMUL not only elevated the proportion of small myofibers (<200 μm2), but it also upregulated the expression level of muscle atrophy marker genes (ATROGIN1 and MURF1) and activated autophagy (Figures 4B-4D). However, on the contrary, knockdown of SMUL promoted muscle fiber hypertrophy (Figures S3M–S3O). Muscle fiber type is closely related to the occurrence of muscle atrophy. Compared with type I (slow-twitch; oxidative) muscle fibers, type II (fast-twitch; glycolytic) muscle fibers are more susceptible to atrophy.37,38 Numerous studies have found that muscle atrophy is often accompanied by the skeletal muscle transition from slow-twitch to fast-twitch fibers.39, 40, 41, 42 Here, overexpression of SMUL not only increased the percentage of type II (fast-twitch; glycolytic) muscle fibers, it also reduced the appearance of type I (slow-twitch; oxidative) muscle fibers (Figures 4E and 4F). Immunohistochemical results also showed that SMUL promoted MYH1/fast protein level and suppressed the expression level of MYH7/slow protein (Figures 4G and 4H). Moreover, the activity of lactate dehydrogenase (LDH) was enhanced, while the activity of succinate dehydrogenase (SDH) was suppressed with SMUL overexpression, suggesting that SMUL promoted the glycolytic capacity of skeletal muscle and induced the fast-twitch muscle phenotype (Figure 4I). In contrast, knockdown of SMUL induced fast-twitch muscle fiber transformation to slow-twitch muscle fibers and suppressed muscle atrophy (Figures S3P–S3T).
Figure 4.
SMUL induces skeletal muscle atrophy and activates fast-twitch muscle phenotype in vivo
(A) Relative SMUL expression in gastrocnemius muscle after infection with SMUL-expressing lentivirus (LV-SMUL) or negative control (LV-NC) (n = 4). (B and C) H&E staining (B) (n = 4) and frequency distribution of fiber cross-section area (CSA) (C) (n = 3) of transverse sections of gastrocnemius muscle infected with the indicated lentivirus. (D) Relative mRNA expression of the atrophy and autophagy-related genes in gastrocnemius muscle after infection with the indicated lentivirus (n = 4). (E and F) ATPase staining (E) (n = 4) and fiber type content (F) (n = 4) of gastrocnemius muscle injected with the listed lentivirus. (G and H) Immunohistochemistry analysis of MYH1/MYH7 (G) (n = 4) and MYH1/MYH7 protein content (H) (n = 4) of gastrocnemius muscle injected with the listed lentivirus. (I) Relative enzymes activity of lactate dehydrogenase (LDH) and succinate dehydrogenase (SDH) in gastrocnemius muscle infected with the indicated lentivirus (n = 4). Data are presented as means ± SEM, and the statistical significance of differences between means was assessed using an independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
SMUL sORF disrupted the stability of SMURF2 mRNA via NMD
To investigate the molecular mechanism of SMUL, we detected the expression level of its target gene (SMURF2). Both in vitro and in vivo, overexpression of SMUL decreased the mRNA and protein levels of SMURF2, while SMURF2 mRNA and protein were upregulated after SMUL knockdown (Figures 5A–5D). Expression vectors of wild-type (WT), ATG mutant (in which the start codon ATG was mutated to ATT), TGA mutant (in which the termination codon TGA was mutated to TGG), and both ATG and TGA mutant (in which both the start codon ATG was mutated to ATT and the termination codon TGA was mutated to TGG) of SMUL sORF were constructed to explore whether SMUL works through its sORF (Figures 5E and 5F). Importantly, only wild-type SMUL sORF inhibited the expression level of SMURF2, while the other mutant-types had no effect (Figures 5G and 5H). To further study the role of SMUL sORF in post-transcriptional regulation, the wild-type and mutant types of SMUL sORF were inserted into the 5′ UTR of hluc of the psiCHECK-2 dual-luciferase reporter vector. A reporter gene assay showed that the SMUL sORF suppressed the translation of the downstream gene (Figures 5I and 5J).
Figure 5.
SMUL sORF disrupts SMURF2 mRNA stability through nonsense-mediated mRNA decay (NMD) mechanism
(A–D) Relative mRNA (A and C) (n = 4) and protein (B and D) (n = 3) expression levels of SMURF2 with SMUL overexpression or knockdown in vitro (A and B) and in vivo (C and D). The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to GAPDH. Data are expressed as a fold change relative to the control. (E) Diagram of the SMUL sORF wild-type and mutant constructs used for transfection. For the ATG mutant, the initiation codon ATG of the SMUL sORF was mutated to ATT. For the TGA mutant, the termination codon TGA of the SMUL sORF was mutated to TGG. For both the ATG and TGA mutant, the initiation codon ATG and termination codon TGA of the SMUL sORF was mutated to ATT and TGG. (F–H) Relative expression levels of SMUL sORF (F) (n = 4) and SMURF2 (G and H) (n = 4 and n = 3) induced by the listed nucleic acids in CPMs. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to GAPDH. Data are expressed as a fold change relative to the control. (I and J) Relative hRlu expression and luciferase activity after wild-type and mutant SMUL sORFs are inserted into the 5′ UTR of the hRluc gene (n = 4 and n = 5). (K) SMURF2 stability assay after transfection with the indicated plasmids (n = 4). (L) Relative expression levels of UPF1 with UPF1 interference (n = 4). (M and N) Relative SMURF2 expression following NMD pathway inhibition via siRNA-mediated depletion of the regulator UPF1 (M) (n = 4) or cycloheximide (CHX)-mediated inhibition of protein synthesis (N) (n = 4) induced by the listed nucleic acids in CPMs. Results are expressed as the mean ± SEM, and the statistical significance of differences between means was assessed using an independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
SMUL sORF is located upstream of the SMURF2 mORF, and its presence restrains SMURF2 production. We hypothesized that SMUL may suppress SMURF2 expression due to the introduction of a premature termination codon (PTC) at the 5′ UTR of SMURF2, which induces NMD. RNA half-life was measured to study whether SMUL disrupts SMURF2 mRNA stability. Both SMUL full-length and SMUL sORF accelerated the degradation of SMURF2 mRNA, while the stability of SMURF2 mRNA was maintained with SMUL lncRNA interference (Figure 5K; Figures S4A and S4B). To validate that NMD occurs, UPF1 (an essential component of the NMD machinery) was knocked down by small interfering RNA (siRNA) (Figure 5L). As predicted, NMD inhibition significantly increased the expression level of the SMURF2 transcript and rescued the inhibitory effect from SMUL full-length and SMUL sORFs (Figure 5M; Figures S4C and S4D). To rule out possible artifacts of UPF1 depletion unrelated to NMD, we also indirectly inhibited NMD by blocking translation with cycloheximide (CHX), which generated similar results (Figure 5N; Figures S4E and S4F).
The biological functions of wild-type and mutant SMUL sORFs in myogenesis were also evaluated. Similar results to SMUL full-length sORF were found with wild-type SMUL sORF overexpression, which contains the same sORF (Figure S5). In contrast, the mutant constructs, both of which do not express SMUL sORF, did not promote myoblast proliferation and restrain myoblast differentiation (Figure S5). Taken together, these data revealed that SMUL sORF suppressed SMURF2 production-regulated myogenesis via NMD.
SMURF2 is involved in myogenesis by negatively regulating the TGF-β/SMAD pathway
To further identify the role of SMURF2 in myoblast proliferation and differentiation, SMURF2 overexpression and knockdown in CPMs were performed (Figures 6A and 6B; Figures S6A and S6B). SMURF2 overexpression significantly suppressed myoblast proliferation (Figures 6C–6F). This also resulted in a large number of G0/G1 cells and few S phase cells (Figure 6G). Furthermore, overexpression of SMURF2 significantly promoted the formation of myotubes and induced myoblast fusion, as well as upregulated the expression levels of myoblast differentiation marker genes (Figures 6H–6L). However, the interference of SMURF2 induced myoblast proliferation and suppressed myoblast differentiation (Figures S6C–S6L).
Figure 6.
SMURF2 inhibits myoblast proliferation and promotes myoblast differentiation
(A and B) Relative mRNA (A) (n = 4) and protein (B) (n = 3) expression levels of SMURF2 with SMURF2 overexpression. The numbers shown below the bands were folds of band intensities relative to control. Band intensities were quantified by ImageJ and normalized to GAPDH. Data are expressed as a fold change relative to the control. (C) The relative mRNA expression of several cell cycle genes induced by SMURF2 overexpression (n = 4). (D) EdU proliferation assays for CPMs with the overexpression of SMURF2 (n = 3); (E) the numbers of proliferative cells were also counted (n = 3). (F) Cell growth was measured after SMURF2 overexpression (n = 6). (G) Cell cycle analysis of CPMs with SMURF2 overexpression (n = 4). (H–J) MyHC staining (H) (n = 3), myotube area (%) (I) (n = 3), and myoblast fusion index (J) (n = 3) of CPMs at 72 h after overexpression of SMURF2. (K and L) Relative mRNA (K) (n = 4) and protein (L) (n = 3) expression of the differentiation marker genes after transfection with pcDNA3.1-SMURF2. The numbers shown below the bands are fold changes of band intensities relative to the control. Band intensities were quantified by ImageJ and normalized to GAPDH. Data are expressed as a fold change relative to the control. Data are presented as means ± SEM. Statistical significance of differences between the means was assessed using an independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
To investigate the function of SMURF2 in skeletal muscle in vivo, lentiviral-mediated SMURF2 overexpression (LV-SMURF2) and lentiviral-mediated SMURF2 knockdown (LV-shSMURF2) were introduced into gastrocnemius muscle of 1-day-old chicks (Figures 7A and 7B; Figures S6M and S6N). Overexpression of SMURF2 promoted muscle fiber hypertrophy, as well as reduced the expression of atrophy-related genes and suppressed autophagy (Figures 7C–7E). In addition, SMURF2 overexpression induced the slow-twitch muscle phenotype and improved the oxidative capacity of gastrocnemius muscle (Figures 7F–7J). Inversely, gastrocnemius muscle fibers were induced to atrophy, and the fast-twitch muscle program was activated with SMURF2 knockdown (Figures S6O–S6V).
Figure 7.
SMURF2 rescues skeletal muscle atrophy and drives a switch from fast-twitch to slow-twitch fibers via the TGF-β/SMAD pathway
(A and B) Relative SMURF2 mRNA (A) (n = 3) and protein (B) (n = 4) expression in gastrocnemius muscle with infection of SMURF2-expressing lentivirus (LV-SMURF2) or LV-NC. (C and D) H&E staining (C) (n = 4) and frequency distribution of CSA (D) (n = 4) of gastrocnemius muscle injected with the listed lentivirus. (E) Relative mRNA expression of the atrophy and autophagy-related genes in gastrocnemius muscle after infection with the indicated lentivirus (n = 4). (F and G) ATPase staining (F) (n = 4) and fiber type content (G) (n = 4) of gastrocnemius muscle infected with the indicated lentivirus. (H and I) Immunohistochemistry analysis of MYH1/MYH7 (H) (n = 4) and MYH1/MYH7 protein content (I) (n = 4) of gastrocnemius muscle injected with the indicated lentivirus. (J) Relative enzymes activity of LDH and SDH in gastrocnemius muscle infected with the listed lentivirus (n = 4). (K and M) Relative TGFB1 mRNA expression in gastrocnemius muscle infected with the indicated lentivirus (n = 4). (L and N) Protein expression levels of the TGF-β/SMAD pathway with infection of the indicated lentivirus (n = 3). Results are expressed as the mean ± SEM. Statistical significance of differences between means was assessed using independent sample t test. ∗p < 0.05, ∗∗p < 0.01.
It is well established that SMURF2 is an upstream regulatory gene of the TGF-β/SMAD pathway, and this pathway is widely involved in the regulation of cell growth, differentiation, and development. We further assessed the TGF-β/SMAD pathway and found that SMURF2 overexpression downregulated the expression of TGFB1 and inhibited the phosphorylation of SMAD2 and SMAD3 (Figures 7K and 7L). Conversely, this pathway was activated with the knockdown of SMURF2 (Figures 7K and 7L). More importantly, the contrary results were found with SMUL overexpression and knockdown (Figures 7M and 7N), clearly demonstrating that SMUL participated in the TGF-β/SMAD pathway by modulating SMURF2.
Discussion
With the development of genome research, it has been found that the transcription of the animal genome is not only ubiquitous, but also incredibly complex. In the past, lncRNA was considered part of the “noise” of the transcribed genome, without translation potential. However, increasing evidence has shown that some lncRNAs function biologically through translation.31,32,43,44 In the present study, we first compared transcriptome and peptidome data during myoblast proliferation and differentiation in chickens and discovered up to 104 lncRNAs that translate at least one small peptide.
So far, only a limited number of lncRNAs have been well characterized, with a diverse array of mechanisms.45 Previous studies on the regulation mechanism of lncRNAs have been mainly focused on how lncRNAs adsorb microRNAs (miRNAs) to regulate gene expression by acting as competing endogenous RNAs (ceRNAs).10, 11, 12,46,47 However, how translated lncRNAs function in concert with their target genes is still not clear. To uncover this novel regulation of lncRNAs and identify the key molecular players in chicken muscle development, in this study we constructed a uORF (which is transcribed by lncRNA) regulatory network.
NMD serves as a cellular surveillance mechanism and can deplete transcripts that show signs of premature translational termination.48 Genome-wide research in multiple organisms49, 50, 51, 52 demonstrated that uORF-bearing transcripts are particularly susceptible to targeted degradation by NMD, attributed to the 5′-near termination events at uORF termination codons. In this study, we found that the termination codon of the lncRNA SMUL-transcribed sORF is located at the 5′ UTR of SMURF2. SMUL’s translation disrupted the stability of SMURF2 and suppressed SMURF2 production. More importantly, SMUL-induced NMD was confirmed directly and indirectly by NMD inhibition models, which demonstrated that lncRNA SMUL-transcribed sORF can work as a uORF, modulating SMURF2 mRNA via NMD by introducing a PTC.
Although several lncRNAs have been shown to have roles in myoblast proliferation and differentiation in vitro,11,12,53,54 little is known about their function in skeletal muscle atrophy and fiber remodeling in vivo. In this study, we found that SMUL is highly expressed in skeletal muscle, and its expression decreases with myoblast differentiation. Gain- and loss-of-function analysis revealed that SMUL promotes myoblast proliferation and suppresses myoblast differentiation in vitro.
lncRNAs are important in regulating gene expression. Thus, lncRNAs have received wide attention as pivotal players in a variety of physiological and pathological processes, including skeletal muscle atrophy.12 The ubiquitin-proteasome system (UPS) and autophagy-lysosomal system are well known to regulate skeletal muscle atrophy.4,55, 56, 57, 58 In this study, in vivo experiments demonstrated that SMUL promotes skeletal muscle atrophy by activating proteasomal degradation and autophagy, as well as drives the transformation of slow-twitch muscle fibers to fast-twitch muscle fibers. From a pathway perspective, SMUL modulates myogenesis and muscle atrophy by restraining SMURF2 to activate the TGF-β/SMAD pathway.
Our findings present a novel model showing that lncRNA can play a regulatory role through its translation process rather than its translation products to modulate myogenesis and muscle atrophy. These findings, which are expected to shape the direction of future developmental research, open a small gate to further study on the lncRNA mechanism of action.
Materials and methods
Ethics statement
All animal studies were sanctioned by the Institutional Animal Care and Use Committee at the South China Agricultural University. All experiments were performed according to the regulations and guidelines established by this committee and by international standards for animal welfare.
Cell culture, transfection, and treatment
CPMs were isolated from leg muscles of embryonic day 11 (E11) as previously described.59 The obtained cells were cultured in growth medium consisting of Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Gaithersburg, MD, USA) with 20% fetal bovine serum (FBS; Gibco). To induce myogenic differentiation, growth medium was removed and replaced with differentiation medium (RPMI 1640 medium containing 2% horse serum) after myoblasts achieved 90% cell confluence. Cells were incubated at 37°C with an atmosphere of 5% CO2.
All transient transfections used Lipofectamine 3000 reagent (Invitrogen, USA) following the manufacturer’s protocol.
To indirectly block NMD, primary myoblasts were treated for 6 h with 100 μg/mL CHX (Sigma-Aldrich, St. Louis, MO, USA) or the vehicle dimethyl sulfoxide (DMSO) as a control. To detect the half-life of SMURF2 mRNA, myoblasts were treated with 2 μg/mL actinomycin D (ActD, Sigma-Aldrich, St. Louis, MO, USA), which could inhibit transcription, and harvested at 0, 2, 4, and 6 h after treatment. GAPDH mRNA was applied as internal control, which was stable within 32 h.
RNA-seq
CPMs during proliferation and differentiation were used for RNA-seq. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quantity and quality were evaluated on an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany), and RNA integrity was further examined using agarose gel electrophoresis. Ribosomal RNA (rRNA) was removed by an Epicenter Ribo-Sero rRNA removal kit (Epicenter, USA), and rRNA free residue was cleaned up by ethanol precipitation. Subsequently, sequencing libraries were generated using the rRNA-depleted RNA by NEBNext Ultra directional RNA library prep kit for Illumina (NEB, USA) following the manufacturer’s recommendations, and sequenced on an Illumina HiSeq 2500 platform by Beijing Novogene Bioinformation Technology. The raw data of RNA-seq were deposited in the Sequence Read Archive (SRA) database under accession no. PRJNA674499.
DEGs were subjected to enrichment analysis of GO functions and KEGG pathways, respectively.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
Proliferating and differentiated CPMs were lysed respectively and then mixed with SDS sample buffer. Proteins were fractionated by SDS-PAGE on 12% Bis-Tris acrylamide NuPAGE gels using 2-(N-morpholino)ethanesulfonic acid (MES) SDS running buffer (Invitrogen). Lower protein bands (lower than 15 kDa) were excised and subjected to in-gel digestion. The resulting peptides were analyzed by a Q Exactive mass spectrometer coupled to a nano-LC (Advance LC, Michrom) via a nano-electrospray source with a column oven set at 37°C (AMR), as previously reported,30 by Gene Denovo Biotechnology (Guangzhou, China). Briefly, samples were dissolved and then separated by an in-house-made 20-cm column (inner diameter, 100 μm, 3-μμ L-column, CERI, Japan) with a linear gradient at a flow rate of 250 nL/min. The Q Exactive was operated in a data-dependent mode with survey scans acquired at a resolution of 70,000 at m/z 400. Up to the top 10 most abundant ions with charge 2+ or 3+ from the survey scan were selected and fragmented by higher energy collision dissociation. The acquired MS/MS spectra were analyzed with the SEQUEST HT algorithm using a database derived from three-frame (forward only) translation (longer than 5 aa) of lncRNA sequences. The mass spectrometry proteomics data were deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository60 with the dataset identifier PXD021030.
Prediction and construction of interaction network
lncRNAs encoding small peptide identified by mass spectrometry were selected for cis-target gene prediction. The sORF of translated lncRNA, which is on the same chain and located within 1.5 kb upstream to an mORF, was predicted to have the regulatory role as uORF. A translated lncRNA sORF-mORF interaction network was constructed using the Cytoscape 3.7.1 program.
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (TaKaRa, Otsu, Japan) as recommended by the supplier. Nuclear and cytoplasmic RNA fractionation was performed by using the Paris kit (Ambion, Life Technologies, USA), following the manufacturer’s protocol. cDNA synthesis for mRNA was carried out using the PrimeScript RT reagent kit with genomic DNA (gDNA) eraser (perfect real time) (TaKaRa, Otsu, Japan). Quantitative real-time PCR with an iTaq Universal SYBR Green supermix kit (Bio-Rad, USA) and analyses with the 2−ΔΔCt method were performed as described previously.61 Chicken β-actin was used as an internal control. Primer pairs for PCR and quantitative real-time PCR are shown in Table S4.
5′ and 3′ RACE
A SMARTer RACE cDNA amplification kit (Clontech, Osaka, Japan) was used to obtain the full-length sequence of SMUL as recommended by the supplier. Nested-PCR reactions were performed. The products of the RACE PCR were cloned into the pJET 1.2/blunt cloning vector (CloneJET PCR cloning kit; Fermentas, Glen Burnie, MD, USA) and sequenced by Sangon Biotech (Shanghai, China). All primers used in RACE are summarized in Table S4.
RNA in situ hybridization
RNA in situ hybridization was performed by using a fluorescence in situ hybridization (FISH) kit (C10910, RiboBio, Guangzhou, China), following the manufacturer’s protocol. Special FISH probes against SMUL, which were modified by Cy3, were also designed and synthesized by Guangzhou RiboBio (Guangzhou, China).
Plasmid construction and RNA oligonucleotides
For EGFP fusion protein construction, the ORF and ORFmut (in which the start codon ATG was mutated to ATT) in the full-length SMUL transcript were amplified and cloned into the pEGFP-N1 mutation vector (pGFPmut) by using the NheI and XhoI restriction sites, in which the GFP start codon (ATGGTG) was mutated to ATTGTT (Clontech, Osaka, Japan).
For FLAG fusion protein construction, the ORF and ORFmut in the full-length SMUL transcript were amplified by PCR and then subcloned into HindIII and XhoI restriction sites of the pcDNA3.1-3xFLAG-C vector.
For overexpression vector construction, the full-length sequence, ORF wild-type, ORF ATG mutant (in which the start codon ATG was mutated to ATT), ORF TGA mutant (in which the termination codon TGA was mutated to TGG), and ORF both ATG and TGA mutant (in which both the start codon ATG was mutated to ATT and the termination codon TGA was mutated to TGG) of SMUL and SMURF2 coding sequence (NCBI: XM_425380.6) were amplified and cloned into the expression plasmid pcDNA-3.1 (Promega, Madison, WI, USA) by using HindIII and XhoI restriction sites.
For psiCHECK-2 dual-luciferase reporter vectors constructed, the ORF wild-type, ORF ATG mutant, ORF TGA mutant, and ORF both ATG and TGA mutant of SMUL were amplified and subcloned into NheI restriction sites in the psiCHECK-2 dual-luciferase reporter vector (Promega, Madison, WI, USA).
For overexpression lentiviral vectors constructed, the full lengths of SMUL and SMURF2 coding sequences were amplified and then cloned into the pLVX-mCMV-ZsGreen-IRES-Puro vector (Addgene, Cambridge, MA, USA) between the SpeI and NotI sites. Short hairpin RNAs (shRNAs) against SMUL or SMURF2 were designed by Shanghai Hanbio Biotechnology and then subcloned into the pLVX-shRNA2-Puro vector (Addgene, Cambridge, MA, USA) by using the BamHI and EcoRI restriction sites.
SMUL is a lncRNA molecule present in the cytoplasm and nucleus. The siRNAs and antisense oligonucleotides (ASOs) that were used for the specific knockdown of SMUL in the cytoplasm and nucleus, respectively, were designed and synthesized by Guangzhou RiboBio (Guangzhou, China). The siRNAs against SMURF2 and UPF1 were also designed and synthesized.
The primers and oligonucleotide sequences used in this study are listed in Tables S4 and S5.
Immunoblotting and immunofluorescence
Western blot analysis was performed as previously described.59 The primary antibodies used were anti-GFP (50430-2-AP, 1:1,000, Proteintech), anti-FLAG (AF519, 1:1,000, Beyotime), anti-MYOD (ABP53067, 1:500, Abbkine), anti-MyHC (B103, 0.5 μg/mL, DHSB), anti-SMURF2 (ab94483, 1 μg/mL, Abcam), anti-SMAD2/3 (70R-51804, 1:500, Fitzgerald), anti-phosphorylated (phospho-)SMAD2 (bs-3419R, 1:500, Bioss), anti-phospho-SMAD3 (bs-3425R, 1:500, Bioss), anti-β-actin (bsm-33036M, 1:1,000, Bioss), and anti-GAPDH (60004-1-Ig, 1:5,000, Proteintech). ProteinFind goat anti-mouse immunoglobulin G (IgG) (H+L), horseradish peroxidase (HRP) conjugate (HS201-01, 1:1,000, TransGen Biotech, Beijing, China) and ProteinFind goat anti-rabbit IgG (H+L), HRP conjugate (HS101-01, 1:500, TransGen Biotech) were used as a secondary antibody.
The immunofluorescence was performed using anti-FLAG (AF519, 1:1,000, Beyotime) or anti-MyHC (B103, 2.5 μg/mL, DHSB). Images were obtained with a fluorescence microscope (DMi8; Leica, Germany). The area of cells labeled with anti-MyHC was measured and calculated as previously described.59
Flow cytometry, EdU, and CCK-8 assays
For the flow cytometry analysis of the cell cycle, myoblasts were seeded in 12-well plates. After a 48-h transfection, the cultured cells in growth media were collected and fixed overnight in 70% ethanol at −20°C. With a cell cycle analysis kit (Thermo Fisher Scientific, USA), the cells were analyzed by a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were processed using FlowJo software (7.6, Tree Star, Ashland, OR, USA).
For the EdU assay, primary myoblasts seeded in 24-well plates were cultured to 50% density and then transfected. Forty-eight hours after transfection, the cells were fixed and stained with a C10310 EdU Apollo in vitro imaging kit (RiboBio, China; 50 μM) as previously described.21 A fluorescence microscope (DMi8; Leica, German) was used to capture three randomly selected fields to visualize the number of EdU-stained cells.
For the CCK-8 assay, primary myoblasts were seeded in a 96-well plate and cultured in growth medium. After being transfected, the proliferation of the cell culture was monitored at 12, 24, 36, and 48 h using the TransDetect CCK (TransGen Biotech, Beijing, China), according to the manufacturer’s protocol. The data for absorbance at 450 nm were read by an iMark microplate absorbance reader (Bio-Rad). All of the data were acquired by averaging the results from six independent repeats.
Lentivirus production and transduction
To generate lentivirus, the recombinant lentiviral expression vectors were co-transfected with packaging plasmid psPAX2 (Addgene, USA) and envelope plasmid pMD2.G (Addgene, USA) into 293T cells using Lipofectamine 3000 reagent. Infectious particles were harvested at 48 and 72 h after transfection, filtered through 0.45-μm polyvinylidene fluoride (PVDF) membranes (Millipore, CA, USA), and concentrated by ultracentrifugation. The viral titer was evaluated by a gradient-dilution method.
Thirty-two 1-day-old chicks were randomly divided into four groups (n = 8): (1) LV-SMUL and LV-NC, (2) LV-shSMUL and LV-shNC, (3) LV-SMURF2 and LV-NC, and (4) LV-shSMURF2 and LV-shNC. Chicks received two intramuscular doses (at days 1 and 7) of lentivirus (106 titers) in two different sites of the gastrocnemius muscle. Thirteen days after the initial injection, chickens were euthanized. Subsequently, gastrocnemius muscles were collected after rapid dissection, then immediately frozen in liquid nitrogen and stored at −80°C.
Hematoxylin and eosin (H&E) staining, myosin-ATPase staining, and immunohistochemistry
For H&E staining, gastrocnemius muscle tissues were immersed in 4% paraformaldehyde and then embedded in paraffin and cut into 4-μm-thick transverse sections. Subsequently, the sections were stained with H&E.
Myosin-ATPase staining was carried out using an ATPase staining solution kit (G2380, Solarbio, Beijing, China), following the manufacturer’s instructions. Myosin-ATPase staining was performed at pH 10.4. Under these alkaline conditions, MyHC I isoforms were inactivated while MyHC IIb isoforms were still functional, resulting in addition of black dye to MyHC IIb-positive muscle fibers.
An SP-POD kit (SP0041, Solarbio, Beijing, China) was used for immunohistochemistry as recommended by the supplier. The primary antibodies included anti-MYH1 (F59, 1:100, DHSB) and anti-MYH7 (S58, 1:300, DHSB) and were used for labeling the signals.
Enzyme activities assay
The glycolytic capacity of skeletal muscle was evaluated by the activity of LDH, while the oxidative capacity of skeletal muscle was evaluated by the activity of SDH. Enzyme activities were measured by commercial assay kits (BC0685 and BC0955) that were purchased from Beijing Solarbio Science & Technology.
Dual-luciferase reporter assay
The psiCHECK-2 recombinant vectors were transfected into primary myoblasts using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) in 96-well plates. At 48 h after transfection, the firefly and Renilla luciferase activities were measured using a fluorescence/multi-detection microplate reader (BioTek, Winooski, VT, USA) and a Dual-Glo luciferase assay system kit (Promega, USA). The firefly luciferase activities were normalized to Renilla luminescence in each well.
Statistical analysis
Each experiment was repeated at least three times, and all data are expressed as means ± SEM. Where applicable, the statistical significance of the data was tested using one-sample or paired t tests. The types of tests and the p values, when applicable, are indicated in the figure legends.
Acknowledgments
This work was supported by the Natural Scientific Foundation of China (U1901206, 31802051), the Local Innovative and Research Teams Project of Guangdong Province (2019BT02N630), the Ten-Thousand Talents Program (W03020593), the Chinese Postdoctoral Science Foundation (2017M622715), the Natural Science Foundation of Guangdong Province (2018A030310209), and the China Agricultural Research System (CARS-41-G03).
Author contributions
Q.N. and X.Z. conceived and designed the study. B.C. and Z.L. performed the experiments, interpreted the data, and wrote the paper. M.M., J.Z., and S.K. performed the experiments. B.A.A., H.X., E.J., and R.A.L. interpreted the data. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.12.003.
Supplemental Information
References
- 1.Sun L., Si M., Liu X., Choi J.M., Wang Y., Thomas S.S., Peng H., Hu Z. Long-noncoding RNA Atrolnc-1 promotes muscle wasting in mice with chronic kidney disease. J. Cachexia Sarcopenia Muscle. 2018;9:962–974. doi: 10.1002/jcsm.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tieland M., Trouwborst I., Clark B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Braun T., Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham M., Rigby P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Sartorelli V., Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci. STKE. 2004;2004:re11. doi: 10.1126/stke.2442004re11. [DOI] [PubMed] [Google Scholar]
- 7.Bassel-Duby R., Olson E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 8.Hubé F., Velasco G., Rollin J., Furling D., Francastel C. Steroid receptor RNA activator protein binds to and counteracts SRA RNA-mediated activation of MyoD and muscle differentiation. Nucleic Acids Res. 2011;39:513–525. doi: 10.1093/nar/gkq833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L., Sun K., Chen X., Zhao Y., Wang L., Zhou L., Sun H., Wang H. Genome-wide survey by ChIP-seq reveals YY1 regulation of lincRNAs in skeletal myogenesis. EMBO J. 2013;32:2575–2588. doi: 10.1038/emboj.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu M., Liu J., Xiao J., Yang L., Cai M., Shen H., Chen X., Ma Y., Hu S., Wang Z. lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 2017;8:14718. doi: 10.1038/ncomms14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma M., Cai B., Jiang L., Abdalla B.A., Li Z., Nie Q., Zhang X. lncRNA-Six1 is a target of miR-1611 that functions as a ceRNA to regulate Six1 protein expression and fiber type switching in chicken myogenesis. Cells. 2018;7:243. doi: 10.3390/cells7120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z., Cai B., Abdalla B.A., Zhu X., Zheng M., Han P., Nie Q., Zhang X. lncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle. 2019;10:391–410. doi: 10.1002/jcsm.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X., Cao G., Jing L., Lin S., Wang X., Zhang J., Wang M., Liu W., Lv C. Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J. Cell. Mol. Med. 2014;18:991–1003. doi: 10.1111/jcmm.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinger M.E., Pang K.C., Mercer T.R., Mattick J.S. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput. Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goffeau A., Barrell B.G., Bussey H., Davis R.W., Dujon B., Feldmann H., Galibert F., Hoheisel J.D., Jacq C., Johnston M. Life with 6000 genes. Science. 1996;274:546–, 563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 19.van Heesch S., van Iterson M., Jacobi J., Boymans S., Essers P.B., de Bruijn E., Hao W., MacInnes A.W., Cuppen E., Simonis M. Extensive localization of long noncoding RNAs to the cytosol and mono- and polyribosomal complexes. Genome Biol. 2014;15:R6. doi: 10.1186/gb-2014-15-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson B.A., Masel J. Putatively noncoding transcripts show extensive association with ribosomes. Genome Biol. Evol. 2011;3:1245–1252. doi: 10.1093/gbe/evr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai B., Li Z., Ma M., Wang Z., Han P., Abdalla B.A., Nie Q., Zhang X. lncRNA-Six1 encodes a micropeptide to activate Six1 in cis and is involved in cell proliferation and muscle growth. Front. Physiol. 2017;8:230. doi: 10.3389/fphys.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson D.M., Anderson K.M., Chang C.L., Makarewich C.A., Nelson B.R., McAnally J.R., Kasaragod P., Shelton J.M., Liou J., Bassel-Duby R., Olson E.N. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath D., Shadan S. The ubiquitin system. Nature. 2009;458:421. doi: 10.1038/458421a. [DOI] [PubMed] [Google Scholar]
- 24.Rotin D., Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2009;10:398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 25.Bonni S., Wang H.R., Causing C.G., Kavsak P., Stroschein S.L., Luo K., Wrana J.L. TGF-β induces assembly of a Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat. Cell Biol. 2001;3:587–595. doi: 10.1038/35078562. [DOI] [PubMed] [Google Scholar]
- 26.Inoue Y., Imamura T. Regulation of TGF-β family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99:2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang L.Y., Yamashita M., Coussens N.P., Tang Y., Wang X., Li C., Deng C.X., Cheng S.Y., Zhang Y.E. Ablation of Smurf2 reveals an inhibition in TGF-β signalling through multiple mono-ubiquitination of Smad3. EMBO J. 2011;30:4777–4789. doi: 10.1038/emboj.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Y., Zhou C.H., Fu D., Shen X.Z. Overexpression of Smad ubiquitin regulatory factor 2 suppresses transforming growth factor-β mediated liver fibrosis. J. Dig. Dis. 2012;13:327–334. doi: 10.1111/j.1751-2980.2012.00592.x. [DOI] [PubMed] [Google Scholar]
- 29.Cao S., Xiao L., Rao J.N., Zou T., Liu L., Zhang D., Turner D.J., Gorospe M., Wang J.Y. Inhibition of Smurf2 translation by miR-322/503 modulates TGF-β/Smad2 signaling and intestinal epithelial homeostasis. Mol. Biol. Cell. 2014;25:1234–1243. doi: 10.1091/mbc.E13-09-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto A., Pasut A., Matsumoto M., Yamashita R., Fung J., Monteleone E., Saghatelian A., Nakayama K.I., Clohessy J.G., Pandolfi P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541:228–232. doi: 10.1038/nature21034. [DOI] [PubMed] [Google Scholar]
- 31.Bazin J., Baerenfaller K., Gosai S.J., Gregory B.D., Crespi M., Bailey-Serres J. Global analysis of ribosome-associated noncoding RNAs unveils new modes of translational regulation. Proc. Natl. Acad. Sci. USA. 2017;114:E10018–E10027. doi: 10.1073/pnas.1708433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S., Zhang J., Lian X., Sun L., Meng K., Chen Y., Sun Z., Yin X., Li Y., Zhao J. A hidden human proteome encoded by “non-coding” genes. Nucleic Acids Res. 2019;47:8111–8125. doi: 10.1093/nar/gkz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap K.L., Li S., Muñoz-Cabello A.M., Raguz S., Zeng L., Mujtaba S., Gil J., Walsh M.J., Zhou M.M. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitrova N., Zamudio J.R., Jong R.M., Soukup D., Resnick R., Sarma K., Ward A.J., Raj A., Lee J.T., Sharp P.A., Jacks T. lincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell. 2014;54:777–790. doi: 10.1016/j.molcel.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvo S.E., Pagliarini D.J., Mootha V.K. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. USA. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbosa C., Peixeiro I., Romão L. Gene expression regulation by upstream open reading frames and human disease. PLoS Genet. 2013;9:e1003529. doi: 10.1371/journal.pgen.1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sĭrca A., Susec-Michieli M. Selective type II fibre muscular atrophy in patients with osteoarthritis of the hip. J. Neurol. Sci. 1980;44:149–159. doi: 10.1016/0022-510x(80)90123-9. [DOI] [PubMed] [Google Scholar]
- 38.Fiori M.G., Andreola S., Ladelli G., Scirea M.R. Selective atrophy of the type IIb muscle fibers in rheumatoid arthritis and progressive systemic sclerosis (scleroderma). A biopsy histochemical study. Eur. J. Rheumatol. Inflamm. 1983;6:168–181. [PubMed] [Google Scholar]
- 39.Diffee G.M., Caiozzo V.J., Herrick R.E., Baldwin K.M. Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am. J. Physiol. 1991;260:C528–C534. doi: 10.1152/ajpcell.1991.260.3.C528. [DOI] [PubMed] [Google Scholar]
- 40.Mozdziak P.E., Greaser M.L., Schultz E. Myogenin, MyoD, and myosin heavy chain isoform expression following hindlimb suspension. Aviat. Space Environ. Med. 1999;70:511–516. [PubMed] [Google Scholar]
- 41.Fitts R.H., Riley D.R., Widrick J.J. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol (1985) 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 42.Potthoff M.J., Arnold M.A., McAnally J., Richardson J.A., Bassel-Duby R., Olson E.N. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol. Cell. Biol. 2007;27:8143–8151. doi: 10.1128/MCB.01187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J.Z., Chen M., Chen D., Gao X.C., Zhu S., Huang H., Hu M., Zhu H., Yan G.R. A peptide encoded by a putative lncRNA HOXA-AS3 suppresses colon cancer growth. Mol. Cell. 2017;68:171–184.e6. doi: 10.1016/j.molcel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Nelson B.R., Makarewich C.A., Anderson D.M., Winders B.R., Troupes C.D., Wu F., Reese A.L., McAnally J.R., Chen X., Kavalali E.T. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z.K., Li J., Guan D., Liang C., Zhuo Z., Liu J., Lu A., Zhang G., Zhang B.T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle. 2018;9:613–626. doi: 10.1002/jcsm.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z.K., Li J., Guan D., Liang C., Zhuo Z., Liu J., Lu A., Zhang G., Zhang B.T. Long noncoding RNA lncMUMA reverses established skeletal muscle atrophy following mechanical unloading. Mol. Ther. 2018;26:2669–2680. doi: 10.1016/j.ymthe.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He F., Li X., Spatrick P., Casillo R., Dong S., Jacobson A. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol. Cell. 2003;12:1439–1452. doi: 10.1016/s1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 49.Isken O., Maquat L.E. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 50.Mendell J.T., Sharifi N.A., Meyers J.L., Martinez-Murillo F., Dietz H.C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 51.Iacono M., Mignone F., Pesole G. uAUG and uORFs in human and rodent 5′untranslated mRNAs. Gene. 2005;349:97–105. doi: 10.1016/j.gene.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 52.Ramani A.K., Nelson A.C., Kapranov P., Bell I., Gingeras T.R., Fraser A.G. High resolution transcriptome maps for wild-type and nonsense-mediated decay-defective Caenorhabditis elegans. Genome Biol. 2009;10:R101. doi: 10.1186/gb-2009-10-9-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin J.J., Lv W., Xia P., Xu Z.Y., Zheng A.D., Wang X.J., Wang S.S., Zeng R., Luo H.M., Li G.L., Zuo B. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc. Natl. Acad. Sci. USA. 2018;115:E9802–E9811. doi: 10.1073/pnas.1801471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L., Sun K., Zhao Y., Zhang S., Wang X., Li Y., Lu L., Chen X., Chen F., Bao X. Linc-YY1 promotes myogenic differentiation and muscle regeneration through an interaction with the transcription factor YY1. Nat. Commun. 2015;6:10026. doi: 10.1038/ncomms10026. [DOI] [PubMed] [Google Scholar]
- 55.Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 56.Gomes M.D., Lecker S.H., Jagoe R.T., Navon A., Goldberg A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013;45:2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai B., Ma M., Chen B., Li Z., Abdalla B.A., Nie Q., Zhang X. miR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018;9:367. doi: 10.1038/s41419-018-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma J., Chen T., Wu S., Yang C., Bai M., Shu K., Li K., Zhang G., Jin Z., He F. iProX: an integrated proteome resource. Nucleic Acids Res. 2019;47(D1):D1211–D1217. doi: 10.1093/nar/gky869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.