Abstract
The relationship between anti-neutrophil cytoplasmic antibody (ANCA) titer levels and relapse risk in patients with ANCA-associated vasculitis (AAV) following clinical remission remains controversial. We herein report a case showing ectopic relapse of AAV in the pituitary with no ANCA elevation after renal remission. Magnetic resonance imaging revealed an enlarged pituitary gland. A pituitary biopsy showed geographic necrosis with multinucleated giant cells. We diagnosed this case as relapse of AAV in the pituitary. One month after rituximab therapy, the pituitary gland volume had decreased. The intensification of therapy due to the possibility of vasculitis relapse may facilitate better control of AAV.
Keywords: ANCA, vasculitis, pituitary, relapse, ANCA-associated vasculitis, pituitary gland
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) has an autoimmune response that produces ANCAs. To facilitate more effective clinical management, AAV is categorized based on clinicopathologic and serotypic features (1). Based on the clinicopathologic features, AAV is subdivided into renal-limited vasculitis (RLV), microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). RLV presents with pauci-immune necrotizing glomerulonephritis in the absence of systemic vasculitis. Based on the serotypes, AAV is classified with respect to ANCA type, mainly, ANCA specific for leukocyte proteinase3 (PR3-ANCA) positive, myeloperoxidase (MPO-ANCA) positive, and ANCA-negative.
The relationship between ANCA titer levels and relapse risk in patients with AAV after clinical remission has remained a matter of debate (2). Although the risk of AAV relapse with renal involvement is reportedly associated with ANCA elevation, there have been several cases of AAV relapse in ANCA-negative patients.
We herein report a case of AAV relapse in the pituitary with no ANCA titer elevation after seven years of RLV remission.
Case Report
A 50-year-old Japanese woman with an elevated PR3-ANCA titer was referred to our department due to rapidly progressive glomerulonephritis (RPGN) (PR3-ANCA titer: 22.0, normal 0.0-3.5 U/mL, chemiluminescent enzyme immunoassay: CLEIA).
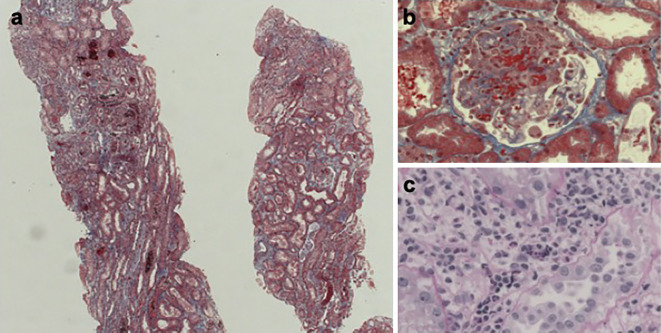
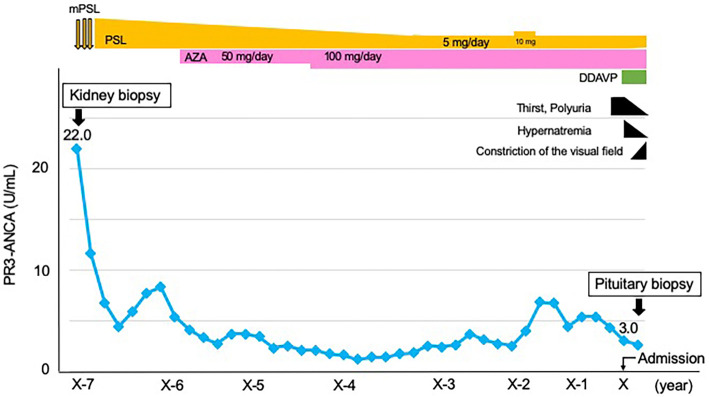
We first conducted a renal biopsy. Electron microscopy and immunofluorescence images showed no evidence of immune complex deposition (data not shown). Light microscopy showed that one of the four glomeruli had a cellular crescent morphology with fibrinoid necrosis (Fig. 1a, b). Many inflammatory cells were observed in the peritubular capillary (Fig. 1c). There was no granuloma on the specimen. She had no symptoms of upper or lower respiratory tract disease. Computed tomography (CT) of the head, chest, and abdomen showed no abnormalities (data not shown). We diagnosed her with RLV, based on Watts's classification (3). She was then administered methylprednisolone pulse therapy (1,000 mg) for 3 consecutive days, followed by prednisolone at a dose of 0.8 mg/kg/day. Later, we tapered the treatment with prednisolone and substituted it with azathioprine. With prednisolone at 5 mg/day and azathioprine at 100 mg/day (1.5 mg/kg/day) as maintenance therapy, RLV remained in remission. The PR3-ANCA titer had not increased since achieving remission for the past five years (Fig. 2). She developed steroid diabetes and we treated with an oral hypoglycemic agent. At 2 years before admission, her PSL dose was temporarily increased to 10 mg/day for 3 months because of slight PR3-ANCA titer elevation but was reduced back to 5 mg/day with the reduction in the PR3-ANCA titer.
Figure 1.
Kidney biopsy results. Kidney sections under light microscopy show four glomeruli, one of which shows a cellular crescent (a: Masson’s trichrome staining, original magnification ×40). The cellular crescent has fibrinoid necrosis (b: Masson’s trichrome staining, original magnification ×400). Peritubular capillaries contain inflammatory cells (c: Periodic acid-Schiff staining, original magnification ×400).
Figure 2.
Clinical course. The x-axis shows the years from the diagnosis of renal-limited vasculitis (RLV). The y-axis shows the level of anti-neutrophil cytoplasmic antibody (ANCA) specific for leukocyte proteinase3 (PR3-ANCA, U/mL). mPSL: methylprednisolone, PSL: prednisolone, AZA: azathioprine, DDAVP: desmopressin acetate hydrate
At 1 month before admission, she suddenly developed thirst and polyuria (10 times a day on average). At her regular visit, she was found to have developed hypernatremia. We suspected diabetes insipidus (DI), and she was admitted to our hospital. A physical examination indicated that she was 145 cm tall, weighed 61.0 kg, and had a body temperature of 37.0℃, blood pressure of 129/94 mmHg, heart rate of 89 beats/min in sinus rhythm, and urinary volume of 3,000 mL/day. She had lost 4.0 kg in the past 3 months. She did not have lymphadenopathy.
Laboratory findings showed hypernatremia (149, normal 135-145 mmol/L) with low urine osmolarity (228, <300 mOsm/L) and low arginine vasopressin levels (AVP, 0.4 pg/mL) measured with a kit (YAMASA Shoyu, Choshi, Japan) (Table). We then conducted a hypertonic saline test with AVP measurement. Hypertonic (5%) saline was infused at 0.05 mL/kg/min for 120 minutes. Blood samples were collected in tubes before (0 min) and 30 minutes, 60 minutes, 90 minutes, and 120 minutes after initiation of the infusion. However, we discontinued the test at 60 minutes due to complaints of fatigue. The results showed that the plasma AVP concentrations remained at low levels while the serum sodium levels increased from 147 mmol/L to 156 mmol/L (Fig. 3). These results confirmed the diagnosis of complete type of central DI (4).
Table.
Laboratory Data at the Onset of Hypernatremia.
| Parameter | Value | Reference range |
|---|---|---|
| White blood cells (/μL) | 6200 | 3300-8400 |
| Neutrophils (%) | 70.0 | 39.8-70.0 |
| Eosinophils (%) | 0.2 | 0.0-5.4 |
| Hemoglobin (g/dL) | 13.9 | 11.0-14.7 |
| Platelets (×104/μL) | 16.4 | 13.0-34.0 |
| Blood urea nitrogen (mg/dL) | 11.4 | 8.0-20.0 |
| Creatinine (mg/dL) | 0.9 | 0.2-0.8 |
| eGFR (mL/min/1.73 m2) | 48.3 | >60 |
| Total serum protein (g/dL) | 6.7 | 6.7-8.3 |
| Serum albumin (g/dL) | 3.8 | 3.9-4.9 |
| Sodium (mmol/L) | 149 | 135-145 |
| Potassium (mmol/L) | 4.1 | 3.5-5.0 |
| Chloride (mmol/L) | 112 | 98-102 |
| Calcium (mg/dL) | 9.8 | 8.6-10.1 |
| Uric acid (mg/dL) | 3.1 | <7.0 |
| AST (U/L) | 27 | 13-33 |
| ALT (U/L) | 29 | 2-27 |
| CRP (mg/dL) | 0.1 | 0.0-0.3 |
| HbA1c (%) | 6.8 | 4.6-6.2 |
| PR3-ANCA (U/mL; CLEIA) | 3.0 | 0.0-3.5 |
| MPO-ANCA (U/mL; CLEIA) | 0.0 | 0.0-3.5 |
| Plasma osmolality (mOsm/L) | 309 | 275-290 |
| Urinary osmolality (mOsm/L) | 228 | 50-1300 |
| Urinary red blood cells (/HPF) | 1-4 | <1-4 |
| Urinary protein-creatinine ratio (g/gCre) | 0.1 | <0.3 |
| AVP (pg/mL) | 0.4 | - |
eGFR: estimated glomerular filtration rate, AST: aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, PR3-ANCA: anti-neutrophil cytoplasmic antibody specific for leukocyte proteinase 3, MPO-ANCA: anti-neutrophil cytoplasmic antibody specific for myeloperoxidase, CLEIA: chemiluminescent enzyme immunoassay, AVP: arginine vasopressin
Figure 3.
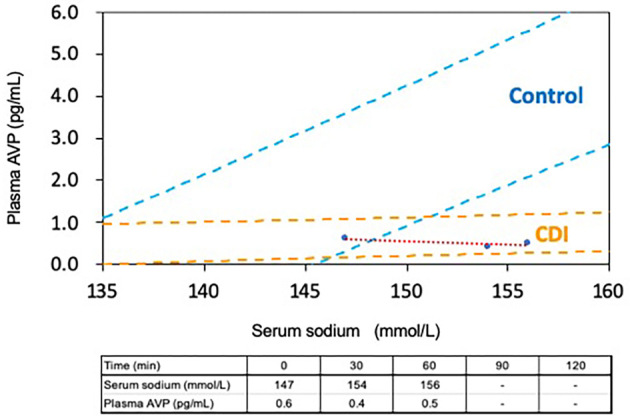
Serum sodium and plasma AVP concentrations during hypertonic saline infusion: The x-axis shows the level of serum sodium (mmol/L). The y-axis shows the level of plasma AVP (pg/mL). AVP: arginine vasopressin, CDI: central diabetes insipidus
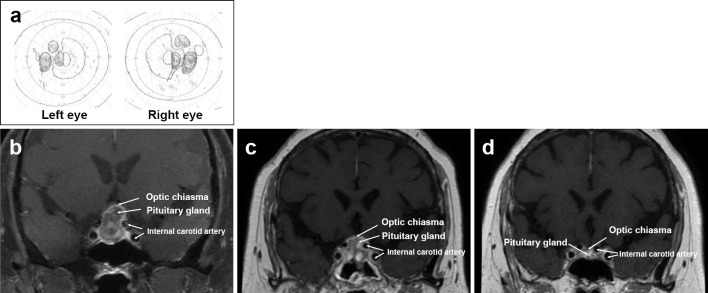
We then checked her basal hormone levels. Laboratory findings with fasting in the early morning were as follows: serum thyrotropin, 0.94 μIU/mL; free triiodothyronine, 2.23 pg/mL; free thyroxine, 1.66 ng/dL; plasma adrenocorticotropic hormone, 7.4 ng/mL; serum cortisol, 1.8 μg/dL; serum growth hormone, 0.31 ng/mL, serum insulin-like growth factor 1, 139.0 ng/mL; serum prolactin, 45.32 ng/mL; serum follicle stimulating hormone, 25.36 mIU/mL; and lutenizing hormone, 0.59 mIU/mL. T1-weighted magnetic resonance imaging (MRI) revealed no innate high intensity in the posterior pituitary (Fig. 4). MRI also showed the enlarged pituitary gland without an optic chiasma compression. CT of the chest and abdomen showed no obvious abnormalities. Initiating desmopressin therapy with desmopressin acetate hydrate (DDAVP), 120 μg/day, resulted in relief from thirst, polyuria, and hypernatremia after 2 weeks (Fig. 2). Her urinary frequency decreased to 5 times a day, and her urinary output also decreased to 1,500 mL/day.
Figure 4.
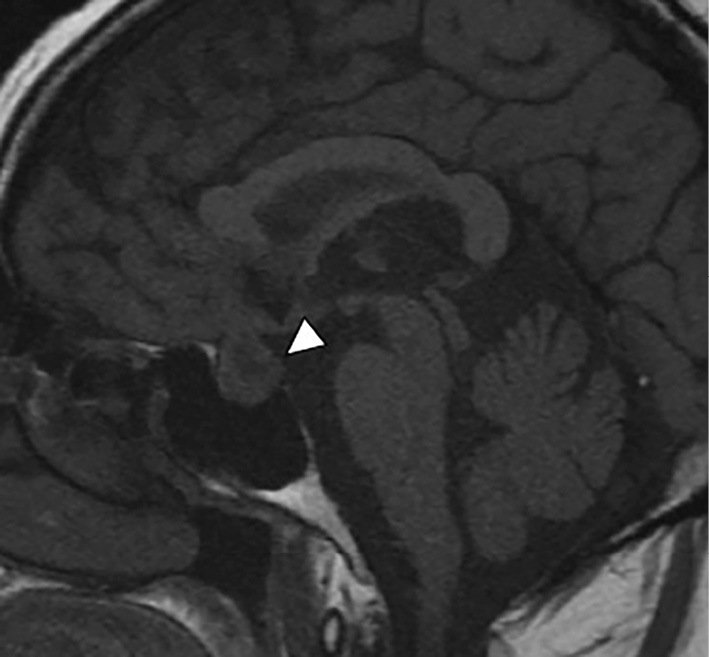
MRI findings at the onset of hypernatremia: An innate high intensity in the posterior pituitary is not detected on T1-weighted MRI (white arrowhead).
One month later, she presented with bitemporal hemianopsia after experiencing difficulty while driving (Fig. 5a). However, she still tested negative for PR3-ANCA. MRI showed an enlarged pituitary gland compressing the optic chiasma, suggesting the presence of tumors or vasculitis (Fig. 5b). We therefore performed a pituitary biopsy.
Figure 5.
MRI findings at the onset of constriction of the visual field and from one to six months after rituximab therapy. The visual field test shows bitemporal hemianopsia (a). The enlarged pituitary gland compresses the optic chiasma (b). One month after rituximab therapy, the pituitary gland is reduced in size. This leads to the amelioration of the displacement of the optic chiasma (c). Six months after rituximab therapy, follow-up MRI shows the further decrease in the pituitary gland size (d).
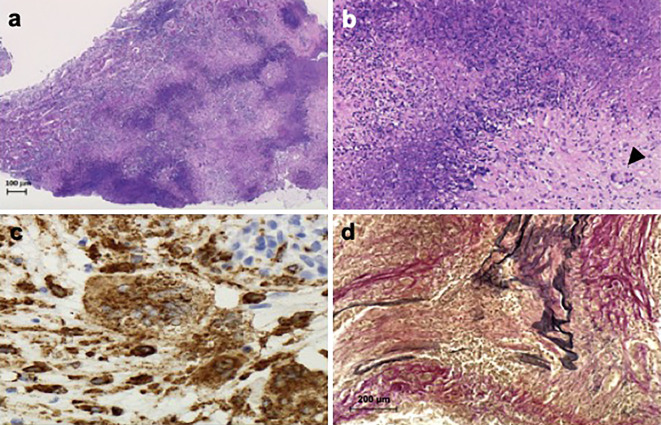
The pituitary biopsy showed geographic necrosis, which comprised epithelioid cells and multinucleated giant cells (Fig. 6a, b). These cells were CD68-positive (Fig. 6c). The middle-sized arteries showed medial thickening and intimal proliferation. Many inflammatory cells had infiltrated into the arterial walls. The internal elastic membrane was ruptured (Fig. 6d). Gram, Grocott, and Ziehl-Neelsen stain results were negative (data not shown). These findings revealed the histopathological characteristics of GPA (5), which confirmed that vasculitis had caused the pituitary enlargement.
Figure 6.
Pituitary biopsy results. Pituitary sections under light microscopy show geographic necrosis [a: Hematoxylin and Eosin (H&E) staining, original magnification ×40]. Epithelioid cells and multinucleated giant cells were observed (b: H&E staining, black arrowhead shows multinucleated giant cells, original magnification ×100). These cells are CD68-positive (c: CD68 staining, original magnification ×400). Middle-sized arteries shown medial thickening and intimal proliferation. Many inflammatory cells have infiltrated into the arterial walls. The internal elastic membrane is ruptured (d: Elastica Van Gieson stain, original magnification ×200).
However, she still had no symptoms of upper or lower respiratory tract disease. She also had no hypercalcemia or lymphadenopathy. We therefore diagnosed the patient with a relapse of vasculitis in the pituitary, with no ANCA elevation after renal remission. We treated her with rituximab at 375 mg/m2 of body surface area, once weekly for 2 weeks. Because of her steroid diabetes, we did not increase the steroid dose. One month after rituximab therapy, we observed a volume reduction in the pituitary gland (Fig. 5c) and confirmed the preserved pituitary size after six months (Fig. 5d). This led to an improved displacement of the optic chiasma and relieved her bitemporal hemianopsia. We continued to treat her with DDAVP at 120 μg/day.
Discussion
Although small temporary increase of PR3-ANCA titer observed at 5 years after diagnosis, RLV remained in remission for 7 years. However, the vasculitis relapsed in the pituitary gland with a constriction of the visual field, which led to disturbance in the patient's daily life.
AAV patients with PR3-ANCA are at a higher risk of relapse than those with MPO-ANCA (2). However, the presence of a relationship between ANCA levels and relapse risk in patients with AAV after clinical remission has been unclear. Although the risk of AAV relapse with renal involvement is reportedly associated with ANCA elevation (6,7), there have been several cases of AAV relapse in ANCA-negative patients. For example, Yamaguchi et al. reported relapse in 4 of 126 cases of AAV patients with negative ANCA titers (7). Watanabe et al. reported relapse in 11 of 271 patients with negative ANCA titers (8). However, the pathogenic mechanisms underlying ANCA-negative AAV remain unclear. Chen et al. suggest that neutrophil activation and degranulation play a major role in the pathogenesis of ANCA-negative pauci-immune crescentic glomerulonephritis, which may be due to either autoantibodies other than ANCAs or cell-mediated immune systems (9). Although further studies are necessary to understand how ANCA-negative patients relapse, the intensification of therapy after the consideration of the possibility of relapse of vasculitis can lead to better control of AAV.
At the initial onset of symptoms in our case, we diagnosed her with RLV because there were no surrogate markers for Wegener's granulomatosis and the renal biopsy showed necrotizing glomerulonephritis without granuloma. However, at the relapse event, we diagnosed her with GPA because the pituitary biopsy showed necrotizing granulomatous inflammation with vasculitis. Both diagnoses were based on Watt's classification (3). To our knowledge, there have been no previous reports shifting the diagnosis from RLV to GPA during the follow-up. However, Outh et al. noted that 54% of AAV relapse cases, which is the majority of GPA cases, did not show the same symptoms as at the first episode (10). Therefore, we retrospectively considered that the renal manifestation in the first episode may have also been a feature of GPA.
GPA patients with pituitary manifestation are rare. Kapoor et al. reported that pituitary involvement of vasculitis affects 1.3% of GPA patients (11). A review of 22 cases of reported pituitary GPA from 1966 to 2006 revealed that 74% of patients with pituitary involvement were women (12). The mean age at the time of pituitary involvement was 38.1 years old. In 819 GPA patients, pituitary involvement was present at the onset of GPA in 1 case and occurred later in 8 patients after a median follow up of 58.5 months (range: from 6 to 165 months) (13). Our case presented pituitary involvement after seven years, which was compatible with the previous report. DI is the most frequently reported manifestation of GPA-related pituitary dysfunction (11,13,14). The differential diagnosis of GPA-associated pituitary diseases includes infectious and inflammatory granulomatous disease, such as tuberculosis, sarcoidosis, idiopathic giant cell granulomatosis, Crohn's disease, lymphocytic hypophysitis, and xantomatous hypophysitis (15). In our case, the pathology findings of the pituitary showed evidence of vasculitis. The pituitary gland volume decreased after rituximab therapy, so we excluded the possibility of these diseases. Pituitary abnormalities were able to be detected with MRI or CT in 91% of total patients with GPA-related pituitary involvement, and 80% of the abnormal presentations observed in these patients were an enlarged pituitary gland (12). In cases where pituitary involvement is the only manifestation of the disease, where other diagnoses are suspected, or where the response to treatment is not as expected, it may be necessary to obtain tissue samples for a histopathologic evaluation (11).
For pituitary involvement of GPA, a treatment modality, administration route, dose, or duration of therapy have not yet been established. No randomized controlled clinical trials have been performed to compare the results of different therapeutic strategies due to the low prevalence and the systemic coexistence of manifestations. A case-based review reported that pituitary involvement in GPA has been treated with high-dose corticosteroids, cyclophosphamide, rituximab, infliximab, azathioprine, and methotrexate (16). Parisot et al. reported that 69% of GPA cases with pituitary involvement were treated with a cyclophosphamide-based regimen, with a relapse rate of the systemic disease of 11% and a median follow-up of 58.8 months (13). Several studies also reported an increased risk of malignancy in patients with AAV who were treated with cyclophosphamide compared with the general population, especially for non-melanoma skin cancer, bladder cancer, malignant lymphoma, and leukemia (17-24). Rituximab has emerged as a substitute for cyclophosphamide (25,26). The initial findings from randomized control trials showed a similar treatment efficacy in AAV patients treated with either cyclophosphamide or rituximab (27-29). However, concerns have been raised about a possible increased malignancy rate in patients treated with rituximab (30,31). The trial results regarding malignancy incidence were interpreted as such due to their sample size and the short follow-up of a maximum 24 months. In their retrospective study, Daalen et al. showed that the malignancy risk in patients with AAV was lower in rituximab-treated patients than in cyclophosphamide-treated patients during a mean follow-up of 5.6 years (32). Owing to these results we decided to use rituximab in our case. However, that study also indicated that a cumulative rituximab dose over 6.0 g was related to the development of a malignancy (32). Therefore, a longer follow-up is important to determine both the safety and efficiency of rituximab therapy in AAV.
In conclusion, we herein report a case of the relapse of AAV in the pituitary gland with no ANCA elevation after renal remission. Rituximab treatment led to an improvement in the pituitary involvement with vasculitis relapse. Despite negative test findings for ANCA, the administration of intensified therapy after taking into consideration the possibility of relapse of vasculitis facilitated better control of AAV.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Yuriko Sawa, Norihiko Suzuki, and Naoko Asano for their excellent technical assistance.
References
- 1.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 12: 1680-1691, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 12: 570-579, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Watts R, Lane S, Hanslik T, et al. . Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 66: 222-227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi H, Hagirawa D, Handa T, et al. . Diagnosis of central diabetes insipidus using a vasopressin radioimmunoassay during hypertonic saline infusion. Endocr J 67: 267-274, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Hoffman GS, Leavitt RY, et al. . Lung in Wegener's Granulomatosis. Am J Surg Pathol 15: 315-333, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Kemna MJ, Damoiseaux J, Austen J, et al. . ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol 26: 537-542, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Ando M, Kato S, et al. . Increase of antimyeloperoxidase antineutrophil cytoplasmic antibody (ANCA) in patients with renal ANCA-associated vasculitis: association with risk to relapse. J Rheumatol 42: 1853-1860, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Sada KE, Matsumoto Y, et al. . Association between reappearance of myeloperoxidase-antineutrophil cytoplasmic antibody and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: subgroup analysis of nationwide prospective cohort studies. Arthritis Rheumatol 70: 1626-1633, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol 5: 313-318, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Outh R, Lemaire A, Mania A, Berland P, et al. . Relapse in patients with anti-neutorphil cytoplasmic antibody-associated vasculitis: a retrospective study. Clin Rheumatol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor E, Cartin-Ceba R, Specks U, Leavitt J, Erickson B, Erickson D. Pituitary dysfunction in granulomatosis with polyangiitis: the Mayo Clinic experience. J Clin Endocrinol Metab 99: 3988-3994, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Yong TY, Li JY, Amato L, et al. . Pituitary involvement in Wegener's granulomatosis. Pituitary 11: 77-84, 2008. [DOI] [PubMed] [Google Scholar]
- 13.De Parisot A, Puechal X, Langrand C, et al. . Pituitary involvement in granulomatosis with polyangiitis: report of 9 patients and review of the literature. Medicine (Baltimore) 94: e748, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holle JU, Gross WL. Neurological involvement in Wegener's granulomatosis. Curr Opin Rheumatol 23: 7-11, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Esposito D, Trimpou P, Giugliano D, Dehlin M, Ragnarsson O. Pituitary dysfunction in granulomatosis with polyangiitis. Pituitary 20: 594-601, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vega-Beyhart A, Medina-Rangel IR, Hinojosa-Azaola A, et al. . Pituitary dysfunction in granulomatosis with polyangiitis. Clin Rheumatol 39: 595-606, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman GS, Kerr GS, Leavitt RY, et al. . Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 116: 488-498, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Westman KW, Bygren PG, Olsson H, Ranstam J, Wieslander J. Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol 9: 842-852, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Knight A, Askling J, Ekbom A. Cancer incidence in a population-based cohort of patients with Wegener's granulomatosis. Int J Cancer 100: 82-85, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Faurschou M, Sorensen IJ, Mellemkjaer L, et al. . Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol 35: 100-105, 2008. [PubMed] [Google Scholar]
- 21.Heijl C, Harper L, Flossmann O, et al. . Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European Vasculitis Study Group clinical trials. Ann Rheum Dis 70: 1415-1421, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Silva F, Seo P, Schroeder DR, et al. . Solid malignancies among etanercept-treated patients with granulomatosis with polyangiitis (Wegener's): long-term followup of a multicenter longitudinal cohort. Arthritis Rheum 63: 2495-2503, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmattulla C, Berden AE, Wakker SC, et al. . Incidence of malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis diagnosed between 1991 and 2013. Arthritis Rheumatol 67: 3270-3278, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Faurschou M, Mellemkjaer L, Voss A, Keller KK, Hansen IT, Baslund B. Prolonged risk of specific malignancies following cyclophosphamide therapy among patients with granulomatosis with polyangiitis. Rheumatology (Oxford) 54: 1345-1350, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener's granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 44: 2836-2840, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener's granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med 173: 180-187, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones RB, Furuta S, Tervaert JW, et al. . Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 74: 1178-1182, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Specks U, Merkel PA, Seo P, et al. . Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 369: 417-427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone JH, Merkel PA, Spiera R, et al. . Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221-232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karassa FB. Rituximab or cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 2073, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Falk RJ, Jennette JC. Rituximab in ANCA-associated disease. N Engl J Med 363: 285-286, 2010. [DOI] [PubMed] [Google Scholar]
- 32.van Daalen EE, Rizzo R, Kronbichler A, et al. . Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis 76: 1064-1069, 2017. [DOI] [PubMed] [Google Scholar]