Abstract
A 68-year-old man visited our hospital due to anorexia, weight loss and a fever. We diagnosed the patient with disseminated Mycobacterium avium complex (MAC) and confirmed the presence of interferon (IFN)-γ neutralizing autoantibodies (IFN-γAb). His lesions improved following antibiotic therapy, but chylous ascites (CA) developed seven months after treatment. CA was able to be controlled by subcutaneous octreotide and diet therapy. IFN-γAb is recognized as having a critical role in the pathogenesis of disseminated MAC disease, but its clinical features are not fully understood. CA may be a complication that develops during the treatment of disseminated MAC infection.
Keywords: IFN-γAb, chylous ascites, disseminated nontuberculous mycobacteriosis
Introduction
Disseminated nontuberculous mycobacteriosis (NTM) is a systemic disease that occurs in patients with underlying immunodeficiency (1). Although HIV is the most important background for disseminated NTM, interferon-γ (IFN-γ) has recently been recognized to play another critical role in antibacterial immunity against Mycobacterium avium infection (2-5). Indeed, reports of disseminated NTM disease with IFN-γ neutralizing autoantibodies (IFN-γAb) have increased (6-9). Most recently, the clinical features have been shown to differ between HIV-positive and IFN-γAb-positive disseminated NTM patients (10), but the clinical information is limited.
Chylous ascites (CA) is a possible complication in disseminated NTM (11) and is known to indicate a poor prognosis in HIV-positive disseminated NTM (12). However, CA has not been reported in patients with IFN-γAb-positive disseminated NTM.
We herein report the case of a 68-year-old man with disseminated M. avium infection and IFN-γAb that was further complicated by CA.
Case Report
A 68-year-old man who was an ex-smoker (30 pack-year history) and had no immunosuppressive history visited our hospital complaining of anorexia and 10-kg weight loss over 2 months.
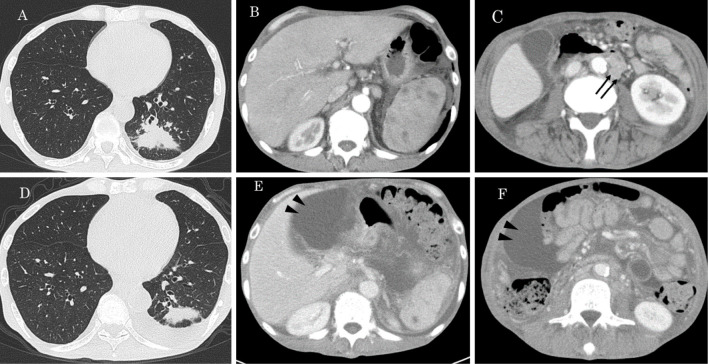
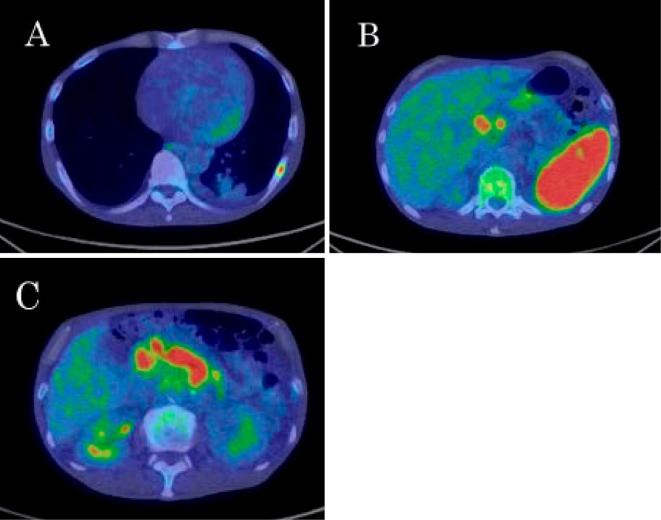
His body temperature was 37.8℃, and oxygenation was within the normal range. Table 1 shows the results of a blood examination on admission. An increased white blood cell (WBC) count and C-reactive protein (CRP) level were seen. Soluble interleukin-2 receptor (sIL-2R) showed marked elevation. The QuantiFERON TB-3 G (QFT-3G) test result was indeterminate due to the negative result for the positive control. On chest X-ray, an infiltrative shadow was observed in left lower lung fields. Contrast-enhanced computed tomography (CT) showed a systemic lesion with an infiltrative shadow in the left lower lobe, osteolytic change in left 8th rib, hepatosplenomegaly, sporadic observation of a poor-contrast area in the spleen, enlargement of multiple intra-abdominal lymph nodes and ascites fluid (Fig. 1A-C). On 18F fluorodeoxyglucose (FDG) positron-emission tomography (PET)/CT, the increased accumulation of FDG was observed in the lesions detected by enhanced CT (Fig. 2).
Table 1.
Laboratory Data on Admission.
| Hematology | Biochemistry | Tumor markers | ||||||
| WBC | 21,720 | /μL | TP | 7.2 | g/dL | CEA | 2.0 | ng/mL |
| Neut | 83 | % | Alb | 2.2 | g/dL | CA19-9 | 8.5 | U/mL |
| Lym | 10 | % | T-Bil | 1.0 | mg/dL | CYFRA | 1.9 | ng/mL |
| Mono | 1 | % | ALP | 1,706 | IU/L | SLX | 28.2 | U/mL |
| Eos | 6 | % | γ-GTP | 320 | IU/L | ProGRP | 29 | pg/mL |
| RBC | 348 | ×104/μL | AST | 47 | IU/L | NSE | 7.9 | ng/mL |
| Hb | 8.1 | g/dL | ALT | 68 | IU/L | sIL2-R | 12,039 | U/mL |
| Plt | 15.8 | ×104/μL | LDH | 217 | IU/L | |||
| CD4/CD8 | CK | 7.0 | IU/L | Infection | ||||
| CD4 | 39.8 | % | BUN | 11.8 | mg/dL | HIV-Ab | negative | |
| CD8 | 16.4 | % | Cre | 0.69 | mg/dL | HTLV-1 Ab | negative | |
| Serology | Na | 136 | mEq/L | MAC Ab* | >10.0 | U/mL | ||
| CRP | 17.8 | mg/dL | K | 4.9 | mEq/L | QFT-3G | indeterminate | |
| IgG | 1,786 | mg/dL | Cl | 99 | mEq/L | T-SPOT | negative | |
| IgA | 984 | mg/dL | Ca | 8.9 | mg/dL | |||
| IgM | 143 | mg/dL | Glu | 105 | mg/dL | |||
*anti-MAC GPL core IgA antibody. WBC: white blood cells, Neut: neutrophils, Lym: lymphocytes, Mono: monocyte, Eos: eosinophils, RBC: red blood cells, Hb: hemoglobin, Plt: platelet, CD4: cluster of differentiation 4, CD8: cluster of differentiation 8, CRP: C-reactive protein, IgG: immunoglobulin G, IgA: immunoglobulin A, IgM: immunoglobulin M, Tp: total protein, Alb: albumin, T-Bil: total bilirubin, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, AST: aspartate amino transferase, ALT: alanine amino transferase, LDH: lactate dehydrogenase, CK: creatinine kinase, BUN: blood urine nitrogen, Cre: creatinine, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, Glu: glucose, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, CYFRA: cytokeratin-19, SLX: sialyl Lewis-x antigen, ProGRP: pro-gastrin releasing peptide, NSE: neuron-specific enolase, sIL2-R: soluble interleukin-2 receptor, HIV-Ab: human immunodeficiency virus antibody, HTLV-1 Ab: human t-cell leukemia virus type 1 antibody, MAC Ab: anti-mycobacterium avium complex glycopeptidolipid core IgA antibody, QFT: quantiferon
Figure 1.
Chest and abdominal contrast-enhanced CT on admission (A-C) and 7 months after treatment (D-F). By treatment, lung lesions, spleen lesions and intra-abdominal lymphadenopathy (arrows) have been improved but ascites increased (arrow heads).
Figure 2.
FDG PET/CT on admission. Increased FDG accumulations were observed in left 8th rib (A), spleen (B) and multiple intra-abdominal lymph nodes (B, C).
Because severe bacterial infection was suspected, antibiotic treatment (meropenem: 1,500 mg/day) was initiated, but the inflammatory reaction and imaging findings did not improve. Because of the negative IFN-γ release response to mitogen, we suspected disseminated NTM disease with IFN-γAb. For a definitive diagnosis, samples were harvested from lesions of the lung, bone marrow and abdominal lymph node. M. avium was detected from bronchial wash and abdominal lymph nodes collected by a CT-guided biopsy, and sporadic granulomas with multinucleated giant cells were found from a bone marrow clot. We therefore diagnosed the patient with disseminated M. avium infection. Furthermore, we assessed the serum IFN-γAb level because he had no immunosuppressive history, and the positive control of QFT-3G showed a negative result and confirmed the presence of IFN-γAb (Table 2).
Table 2.
Results of Serum IFN-γ Neutralizing Autoantibodies.
| STAT1-PI | 17.76 (control: 556.59) |
| IFN-γ antibodies | 1,210.97 E.U. (control: 1.91 E.U.) |
STAT1-PI: signal transducer and activator transcription 1 phosphorylation index, IFN-γ: interferon-γ
For treatment of disseminated M. avium infection, we administered antibiotherapy comprising clarithromycin (600 mg/day), rifampicin (450 mg/day), ethambutol (750 mg/day) and streptomycin (660 mg/day; twice weekly for the first 3 months of treatment). Sitafloxacin (100 mg/day) was added during the second month of treatment.
Five months after treatment initiation, his inflammatory reaction had improved (WBC: 6,500/μL, CRP: 6.5 mg/dL), and sIL2-R had decreased to 2,492 U/mL. In addition, enhanced CT showed the resolution of most of the previously abnormal findings. For ascites, temporary drainage was performed several times. Consequently, one month after the start of treatment, abdominal fullness improved, and temporary drainage became unnecessary. However, seven months after treatment initiation, the patient complained of abdominal fullness again, and increased ascites was found without recurrence of the other lesions (Fig. 1D-F). In addition, the ascites color had changed from a clear-yellowish color to milky white (Fig. 3), and CA was diagnosed based on the markedly high triglyceride level of the ascites fluid.
Figure 3.

Milky white ascites was obtained from the aseptic fluid puncture fluid.
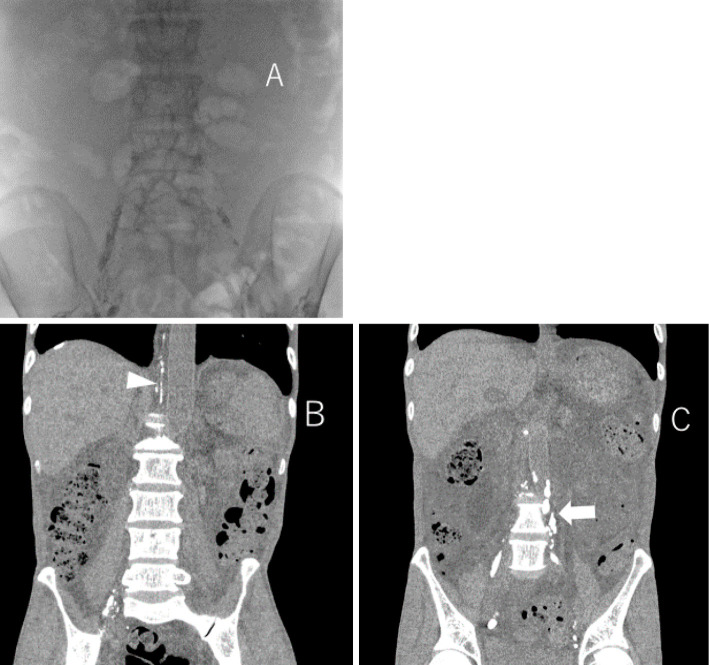
The patient was subsequently hospitalized and started on a fat-restricted diet and subcutaneous octreotide (300 μg/day). On day 5 of hospitalization, lymphangiogram was performed using iodized oil emulsion (LipiodolⓇ, Guerbet Japan, Tokyo, Japan) from the left and right inguinal lymph nodes. Abdominal CT taken the next day showed normal lymph vessels on the right side but disrupted lymph vessels on the left side at the level of the 4th lumbar spine (Fig. 4). His abdominal fullness subsequently improved, and he was discharged on day 9 of hospitalization.
Figure 4.
Lymphangiogram was performed from the left and right inguinal lymph nodes (A) and abdominal CT was taken the day after lymphangiography. Inflow to the thoracic duct can be confirmed by lymphangiography performed from the right inguinal lymph node (arrow head) (B) but, lymphangiography performed from the left inguinal lymph node showed that the lymph vessels were occluded at the level of the 4th lumbar spine (arrow) (C).
At nine months after his first visit, the patient exhibited no increase in ascites or deterioration of each lesion.
Discussion
We encountered a case of disseminated M. avium infection in a patient with IFN-γAb. Recently, it was reported that IFN-γAb was detected in 81% of Asian patients with disseminated NTM infection and normal CD4-positive cell counts (13). The evaluation of IFN-γ Ab has gained importance for the pathophysiological diagnosis of disseminated NTM in patients without obvious immunodeficiency (14).
The primary manifestations of disseminated NTM with IFN-γAb are nonspecific, and lesions are generally seen in multiple organs, such as the lungs, bones, lymph nodes and bone marrow (10). Furthermore, the diagnosis of disseminated NTM with IFN-γAb can be challenging for clinicians, as anamnestic immune deficiency usually has not been detected when clinicians encounter this disease. Indeed, the mean period from the disease onset to the diagnosis is four months (10). Therefore, disseminated NTM with IFN-γAb should be considered when systemic serious infections are suspected in individuals without anamnestic immune deficiency.
A high sIL-2R level is often seen at the diagnosis of disseminated Mycobacterium avium complex (MAC) disease (15), which may give attending physicians a false impression of malignant lymphoma (10). sIL-2R is released into the blood from T cells, B cells and monocytes (16-18). The measured values reflect T or B cell immune activation (19). In patients with pulmonary tuberculosis, sIL-2R levels increase as the lesion expands (20). In this case, the sIL-2R levels appeared to have increased due to the lesion spreading throughout the body. Accordingly, clinicians must recognize disseminated NTM as a possible pathological condition in cases with elevated sIL-2R levels.
The present patient was complicated with CA during the treatment course for disseminated M. avium infection. CA is defined as the accumulation of triglyceride-rich fluid in the peritoneal cavity (21), and its clinical incidence is rare (22,23). However, Mycobacterium infection is the third-most common etiology of atraumatic CA in adults after malignancy and liver cirrhosis (11). Koizumi et al. also reported a case of disseminated MAC with IFN-γ Ab complicated with CA (24). In their case, lymph node swelling and CA occurred during the discontinuation of antimicrobial therapy, and the patient died three months after the disease onset. In contrast, in the present case, CA occurred after lymph node swelling had improved by antibacterial therapy. Phillips et al. reported six HIV-infected patients who were complicated with CA related to intra-abdominal MAC immune reconstitution syndrome (25). MAC was cultured from ascites in only one of these six patients, and only that patient died. These results suggest that disseminated MAC infection control is important and may improve the survival in patients with CA.
Various mechanisms may be responsible for the production of CA in patients with mycobacterial infection, including the obstruction of the lymphatic system due to enlarged lymph nodes or lymph nodal fibrosis (26). In the present case, CA developed after the improvement of lymph node swelling. Therefore, we speculate that obstruction of the lymphatic system due to lymph nodal fibrosis occurred after inflammation, which subsequently led to CA.
Serum IFN-γAb concentration was quantified using an enzyme-linked immunosorbent assay, as reported previously (14). However, the measurement of serum the IFN-γAb concentration requires specialized equipment. In the present case, indeterminate QFT-3G results prompted us to suspect the presence of IFN-γAb in the patient's serum. Ishi et al. reported that the discrepancy between QFT-3G and T SPOT-TB results indicates the presence of IFN-γAb (27). In the present case, the T-SPOT test result was negative because the patient had no history of tuberculosis, and the QFT-3G test result was indeterminate due to the presence of neutralizing antibodies. Even if IFN-γAb is present in the patient's serum, care must be taken in interpreting the results, as there is no marked difference between the T-SPOT and QFT-3G results in patients without tuberculosis infection, as seen in the present case.
In conclusion, we encountered a case of disseminated M. avium infection complicated with CA in a patient with IFN-γAb. When examining a patient who has multiple organ findings and elevated inflammation, it is necessary to consider the possibility of this disease. In addition, CA should be recognized as a possible complication of disseminated MAC induced by IFN-γAb.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Kazutaka Yoshizawa for performing IFN-γ neutralizing autoantibody measurements.
References
- 1.Adjemian J, Olivier KN, Seitz AE, et al. . An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Shiratsuchi H, Johnson JL, Toba H, Ellner JJ. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-gamma. J Infect Dis 162: 932-938, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Frucht DM, Holland SM. Defective monocyte costimulation for IFN-gamma production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol 157: 411-416, 1996. [PubMed] [Google Scholar]
- 4.Newport MJ, Huxley CM, Huston S, et al. . A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 335: 1941-1949, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Holland SM, Eisenstein EM, Kuhns DB, et al. . Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med 330: 1348-1355, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Höflich C, Sabat R, Rosseau S, et al. . Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 103: 673-675, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Smita Y. Patel, Li Ding, Margaret R. Brown, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 175: 4769-4776, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Kampmann B, Hemingway C, Stephens A, et al. . Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 115: 2480-2488, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koya T, Tsubata C, Kagamu H, et al. . Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother 15: 118-122, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Aoki A, Sakagami T, Yoshizawa K, et al. . Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis 66: 1239-1245, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Steinemann DC, Dindo D, Clavien PA, Nocito A. Atraumatic chylous ascites: systematic review on symptoms and causes. J Am Coll Surg 212: 899-905, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Mallick B, Mandavdhare HS, Aggarwal S, Singh H, Dutta U, Sharma V. Mycobacterial chylous ascites: report of three cases and systematic review. Ther Adv Infect Dis 5: 69-75, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne SK, Burbelo PD, Chetchotisakd P, et al. . Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 367: 725-734, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shima K, Sakagami T, Tanabe Y, et al. . Novel assay to detect increased level of neutralizing anti-interferon gamma autoantibodies in non-tuberculous mycobacterial patients. J Infect Chemother 20: 52-56, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Hori T, Ito K, Fujita T, Ishikawa T, Uchiyama T. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-gamma. Intern Med 46: 1005-1009, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Rubin LA, Kurman CC, Fritz ME, et al. . Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135: 3172-3177, 1985. [PubMed] [Google Scholar]
- 17.Sugamura K, Fujii M, Kobayashi N, Sakitani M, Hatanaka M, Hinuma Y. Retrovirus-induced expression of interleukin 2 receptors on cells of human B-cell lineage. Proc Natl Acad Sci USA 81: 7441-7445, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kniep EM, Strelow I, Lohmann-Matthes ML. The monocyte interleukin-2 receptor light chain: production of cell-associated and soluble interleukin-2 receptor by monocytes. Immunology 75: 299-304, 1992. [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin LA, Nelson DL. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med 113: 619-27, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Setoguchi Y, Nukiwa T, Kira S. Soluble interleukin-2 receptor in sera of patients with pulmonary tuberculosis. Chest 2: 310-314, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med 96: 358-364, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Almakdisi T, Massoud S, Makdisi G. Lymphomas and chylous ascites: review of the literature. Oncologist 10: 632-635, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Vasko JS, Tapper RI. The surgical significance of chylous ascites. Arch Surg 95: 355-368, 1967. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi Y, Sakagami T, Minamiguchi H, et al. . Chylous ascites, anti-interferon-gamma autoantibody, and angioimmunoblastic T-cell lymphoma: a rare but intriguing connection over Mycobacterium avium. Med Microbiol Immunol 208: 33-37, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Phillips P, Lee JK, Wang C, Yoshida E, Lima VD, Montaner J. Chylous ascites: a late complication of intra-abdominal Mycobacterium avium complex immune reconstitution syndrome in HIV-infected patients. Int J STD AIDS 20: 285-287, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Mallick B, Mandavdhare HS, Aggarwal S, Singh H, Dutta U, Sharma V. Mycobacterial chylous ascites: report of three cases and systematic review. Ther Adv Infect Dis 5: 69-75, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii T, Tamura A, Matsui H, et al. . Disseminated Mycobacterium avium complex infection in a patient carrying autoantibody to interferon-γ. J Infect Chemother 19: 1152-1157, 2013. [DOI] [PubMed] [Google Scholar]