Abstract
An 84-year-old man was admitted with hypoxemia and ground-glass opacities with traction bronchiectasis in both lungs and mild fibrosis on computed tomography. We first suspected that he had acute exacerbation of interstitial pneumonia and initiated methylprednisolone pulse therapy. On day 4, he was diagnosed with coronavirus disease 2019 (COVID-19) pneumonia. Although the ground-glass opacities were improved with corticosteroid treatment alone, the hypoxemia persisted, and the plasma D-dimer level increased. Anticoagulant therapy was initiated, and the hypoxemia was improved. COVID-19 pneumonia may result in radiological findings similar to those of acute exacerbation of interstitial pneumonia, and corticosteroids and anticoagulant therapy may lead to favorable outcomes.
Keywords: COVID-19 pneumonia, acute exacerbation of interstitial pneumonia, corticosteroid, cytokine storms, microthrombi
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in December 2019 in Wuhan, China, after which it spread rapidly worldwide. Most patients with COVID-19 have a good outcome, whereas some progress to severe illness and death (1).
The hallmarks of COVID-19 pneumonia on computed tomography (CT) include ground-glass opacity (GGO), consolidation, linear opacities, and a crazy-paving pattern, with predominance in the peripheral lung and/or peri-bronchial regions (2). COVID-19 can also cause interstitial lung disease (3). Its characteristics can resemble those of acute exacerbation of interstitial pneumonia (AE-IP), and widespread GGO and consolidation overlying the reticular shadow with traction bronchiectasis are observed (4). In addition, corticosteroids may be effective against COVID-19 pneumonia (5,6).
We herein report a patient with COVID-19 pneumonia that resembled AE-IP in which corticosteroids and anticoagulant therapies were beneficial.
Case Report
An 84-year-old man with hypertension, type 2 diabetes, and hyperuricemia first experienced dyspnea that had gradually worsened while walking 2 months earlier. He experienced a low-grade fever eight days before admission.
On admission, a physical examination revealed normal vital signs and a respiratory rate of 18/min, but his oxygen saturation was 86% on ambient air. Clubbing was not found. The skin findings did not indicate a collagen vascular disease. Laboratory data revealed increased inflammation (C-reactive protein 4.88 mg/dL), abnormal coagulation parameters (D-dimer 6.95 μg/mL), renal dysfunction (creatinine 1.27 mg/dL), and elevated myogenic enzyme (creatine kinase 725 U/L) (Table). Although the ferritin and Krebs von den Lungen-6 levels were within the normal range at 69.3 ng/mL and 454 U/mL, respectively, the surfactant protein-D level was elevated at 418.4 ng/mL.
Table.
Laboratory Data on Admission.
| Hematology | Biochemistry | ||||||||
| WBC | 8,560 | /μL | AST | 55 | IU/L | HDL-C | 28 | mg/dL | |
| Neutrophils | 77.5 | % | ALT | 31 | IU/L | LDL-C | 129 | mg/dL | |
| Lymphocytes | 15.4 | % | LDH | 476 | IU/L | TG | 172 | mg/dL | |
| Monocytes | 6.8 | % | ChE | 210 | IU/L | TSH | 2.17 | μIU/mL | |
| Eosinocytes | 0.1 | % | T-Bil | 0.7 | mg/dL | FT4 | 1.29 | ng/dL | |
| Basocytes | 0.2 | % | ALP | 264 | IU/L | Glu | 121 | mg/dL | |
| Hb | 13.1 | g/dL | γ-GT | 50 | IU/L | HbA1c | 6.9 | % | |
| Plt | 22.3 | ×10,000 /μL | TP | 7.1 | g/dL | KL-6 | 454 | U/mL | |
| Alb | 3 | g/dL | SP-D | 418.4 | ng/mL | ||||
| Coagulation | CK | 725 | IU/L | BNP | 21.9 | pg/mL | |||
| PT | 103.1 | % | CK-MB | 30 | IU/L | Ferritin | 69.3 | ng/mL | |
| APTT | 31.5 | second | UN | 23.8 | mg/dL | Aldolase | 28.3 | IU/L | |
| Fbg | 602 | mg/dL | Cr | 1.27 | mg/dL | ANA | <×40 | ||
| D-dimer | 6.95 | μg/mL | UA | 5.8 | mg/dL | ACPA | 7.0 | U/mL | |
| ESR1h | 83 | mm | Na | 146 | mEq/L | RF | 26 | IU/mL | |
| K | 3.4 | mEq/L | Anti-ARS antibody | negative | |||||
| Urynalysis | Cl | 105 | mEq/L | Anti-MDA5 antibody | negative | ||||
| Proteinuria | negative | Ca | 8.2 | mg/dL | |||||
| Haematuria | negative | CRP | 4.88 | mg/dL | |||||
| melituria | negative | ||||||||
| RBC | 0-1 | /HPF | |||||||
| WBC | 0-1 | /HPF | |||||||
WBC: white blood cell, Hb: hemoglobin, Plt: platelets, PT: prothrombin time, APTT: activated partial thromboplastin time, Fbg: fibrinogen, ESR1h: erythrocyte sedimentation rate 1 hour, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ChE: cholinesterase, T-Bil: total bilirubin, ALP: alkaline phosphatase, γ-GT: gamma-glutamyltranspeptidase, TP: total protein, Alb: albumin, CK: creatine kinase, UN: urea nitrogen, Cr: creatinine, UA: uric acid, Na: sodium, K: potassium, Cl: chlorine, Ca: calcium, CRP: C-reactive protein, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein choledterol, TG: triglyceride, TSH: thyroid stimulating hormone, FT4: free thyroxine 4, Glu: gulcose, HbA1c: hemoglobin A1c, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, BNP: brain natriuretic peptide, ANA: anti nuclear antibody, ACPA: anti-cyclic citrullinated petide antibody, RF: rheumatoid factor, ARS: aminoacyl transfer ribonucleic acid synthetase, MDA: melanoma differentiation-associated gene, RBC: red blood cell, HPF: high power field
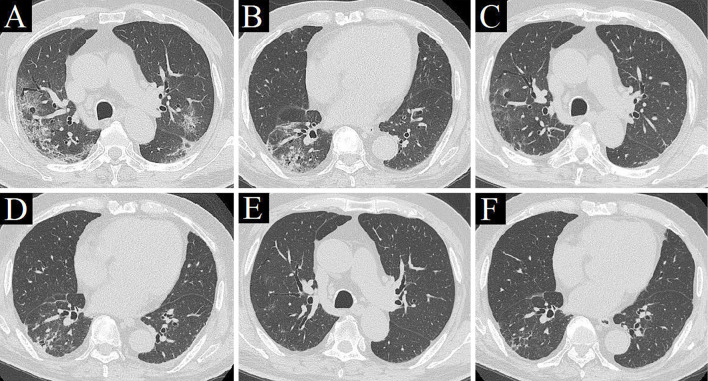
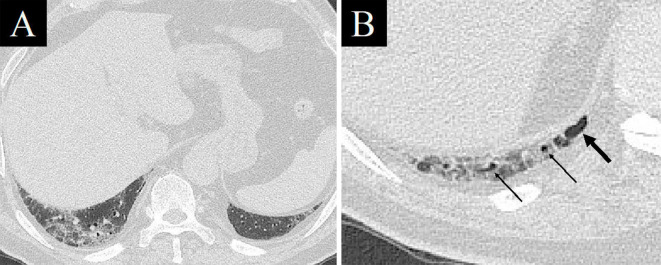
An electrocardiogram showed premature atrial contraction, but no evidence of myocardial damage was found. Contrast-enhanced CT revealed GGO and reticular shadows located predominantly in the peripheral lung with traction bronchiectasis (Fig. 1A, B), and cystic lesions at the bottom of the lung suggesting mild chronic fibrosis (Fig. 2). No clear contrast defect was found in the pulmonary artery or deep veins of the lower extremities. We first considered AE-IP because of his history of illness and image findings. He was prescribed antibiotics (ceftriaxone intravenously and azithromycin orally) in our isolation ward where he awaited the results of the SARS-CoV-2 nucleic acid test.
Figure 1.
Chest computed tomography (CT) images. CT on admission revealed ground-glass opacities (GGO) and reticular shadows with predominance in the peripheral lung, with traction bronchiectasis and volume loss of the right lower lobe (A, B). CT on day 4 revealed improvement in GGO (C, D). CT on day 24 revealed that GGO in both lungs had improved further, and traction bronchiectasis was obscured (E, F).
Figure 2.
Chest computed tomography images at the bottom of the lung on admission. At some distance from the diaphragm, no cystic lesions could be seen just below the pleura, such as a honeycomb lung, although GGO and reticular shadows were found (A). A cystic lesion (thick arrow) and bronchioles close to the pleura (thin arrows), suggestive of mild chronic fibrosis, were found just below the pleura near the diaphragm (B).
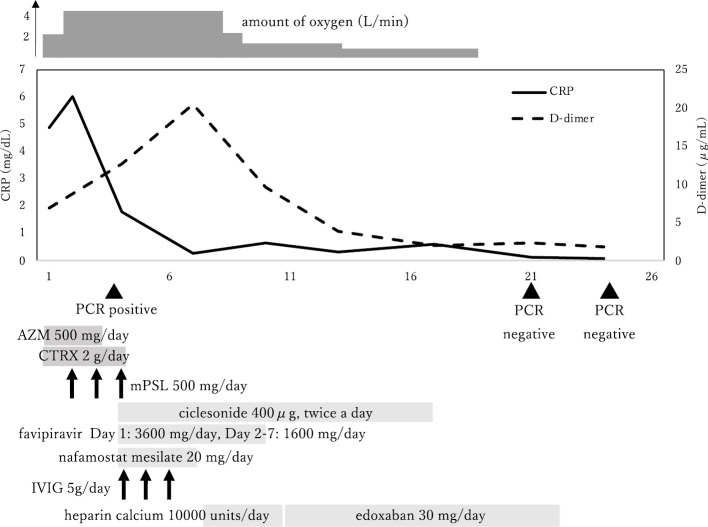
The following day, his respiratory failure worsened, and he was prescribed intravenously steroid pulse therapy with methylprednisolone (mPSL) at a dose of 500 mg/day ×3 days for suspected AE-IP. On day 4 of hospitalization, COVID-19 pneumonia was diagnosed, and he was also administered ciclesonide, favipiravir, intravenous immunoglobulin (5 g/day ×3 days), and nafamostat mesilate (20 mg/day). CT performed on day 4 showed a clear improvement in GGO (Fig. 1C, D), which confirmed the effectiveness of the steroid pulse therapy. We believed that long-term administration of corticosteroids for COVID-19 would not be favorable and terminated the pulse therapy after three days. However, subsequent improvements in his respiratory failure were minimal, and the D-dimer level increased over time (20.44 μg/dL on day 7). Although ultrasonography of the lower extremities revealed no clear deep vein thrombosis, he was prescribed prophylactic anticoagulation therapy with heparin calcium at a dose of 10,000 units/day on day 7. To reduce the healthcare providers' exposure to COVID-19 patients, heparin calcium was changed to edoxaban at a dose of 30 mg/day on day 10. Both his D-dimer level and respiratory condition then gradually began to improve.
On day 20 of hospitalization, he no longer required oxygen therapy. CT performed on day 24 revealed that the GGO in both lungs had improved further and that the traction bronchiectasis was obscured (Fig. 1E, F). SARS-CoV-2 nucleic acid tests performed on days 21 and 24 were both negative, and he was finally discharged on day 28 (Fig. 3).
Figure 3.
The clinical course. AZM: azithromycin, CRP: C-reactive protein, CTRX: ceftriaxone, IVIG: intravenous immunoglobulin, mPSL: methylprednisolone, PCR: nucleic acid test with polymerase chain reaction
Discussion
The present case is valuable because it suggests three important points. First, COVID-19 pneumonia may have a pathogenesis and imaging findings that are similar to those of AE-IP. Second, corticosteroids may have been effective in improving his GGO. Third, anticoagulation therapy may have contributed to the improvement in his respiratory failure, while pulmonary embolism and deep vein thrombosis were not observed.
In this case, volume loss and traction bronchiectasis were first observed, prompting our belief that the background lung exhibited relatively chronic changes, with the addition of GGO and consolidation as part of an acute disease course. However, the subsequent improvement in volume loss and traction bronchiectasis suggested that these findings may not have been associated with the chronicity of any disease but rather with severe COVID-19 pneumonia. The possibility of SARS-CoV-2-triggered AE-IP was also considered, but the lack of elevated Krebs von den Lungen-6 levels and the rapid improvement in his illness did not support this.
The CT findings of AE-IP include widespread GGO and consolidation overlying the reticular shadow with traction bronchiectasis (4). Furthermore, in patients with COVID-19, unilateral or bilateral GGO with peripheral and subpleural distribution are commonly observed. GGO is often accompanied by other features or patterns, including reticular and/or interlobular septal thickening and consolidation (7,8). GGO, together with small areas of consolidation, may suggest an organizing pneumonia pattern of lung injury (9). A report on ultra-high-resolution chest CT in COVID-19 patients at our institution indicated lung volume loss calculated as the ratio to the predicted total lung capacity, and the association between lung volume reduction observed on CT and the severity of COVID-19 (2). Others have reported a decreased normal lung volume in COVID-19 patients with pneumonia and acute respiratory distress syndrome (ARDS) (10). Although the main cause of traction bronchiectasis in COVID-19 pneumonia may be volume loss due to the alveolar collapse, with disease progression, the range of involved alveoli and mucosa increases, and the inflammatory damage to the bronchial wall can cause fibrosis, bronchiectasis, and bronchial wall thickening (11,12). Thus, COVID-19 can be complicated by ARDS and result in CT findings that are similar to those of AE-IP.
We decided to initiate steroid pulse therapy based on the possibility of AE-IP before the diagnosis of COVID-19 was confirmed. As a result, an improvement in GGO was observed with steroid pulse therapy alone. Inhibition of excessive inflammation through the timely administration of corticosteroids in the early stage of infection may have effectively prevented the occurrence of ARDS and also protected the organ function in this patient. Cytokine storm has been observed in patients with severe COVID-19 and is considered a major cause of ARDS and multiple-organ failure (13). Effective suppression of cytokine storm is an important mechanism by which the deterioration in COVID-19 patients can be prevented, which can save patients' lives (5). For patients with progressive deterioration of oxygenation indicators, rapidly expanding GGO on imaging, and an excessive inflammatory response, the short-term use of glucocorticoids (3-5 days) may be appropriate, and the recommended dose is equivalent to mPSL 1-2 mg/kg/day (14). It was reported that the use of dexamethasone in patients hospitalized with COVID-19 resulted in a lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone (6). We recently reported that the combined treatment of mPSL and favipiravir during the early stage of infection in patients with severe COVID-19 pneumonia may prevent aggravation (15), as seen in this case. We also used ciclesonide and nafamostat mesylate to anticipate an anti-viral effect and intravenous immunoglobulin to anticipate dual effects of immune substitution and immunomodulation.
Due to the high and continuously increasing D-dimer level and the poor improvement in oxygenation after improvement in the abnormal lung shadows, we considered the possibility of microthrombi in the lungs in this case despite the lack of thrombosis being detected by contrast CT or lower extremity ultrasonography. After the initiation of anticoagulation therapy, a trend in an improvement in oxygenation along with an improvement in the D-dimer level was noted. Autopsy reports of COVID-19 patients have shown microthrombi in the lungs and in other organs with associated hemorrhagic foci (16). Severe endothelial dysfunction, driven by cytokine storm and associated hypoxemia, leads to disseminated intravascular coagulation, which causes thromboembolic complications (17). One study that specifically examined the abnormal coagulation parameters identified markedly elevated D-dimer levels as a predictor of mortality (18). According to the interim guidance of the International Society of Thrombosis and Hemostasis for the recognition and management of coagulopathy in COVID-19, a prophylactic dose of low-molecular weight heparin may be suitable for consideration in such patients, including those who are not critically ill and who require hospitalization for COVID-19 infection in the absence of any contraindications (19).
In conclusion, we herein report a case of interstitial lung disease due to COVID-19 that resembled AE-IP. COVID-19 may result in CT findings that are similar to those of AE-IP, and the use of corticosteroids and anticoagulant therapies may lead to a favorable clinical outcome.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 55: 105924, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasawa T, Sato M, Yamaya T, et al. Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn J Radiol 38: 394-398, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luca C, Aurelio S, Ahmed N, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis S1473-3099: 30434-30435, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva CI, Muller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 22: 221-229, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect 80: 607-613, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 30: 3306-3309, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song F, Shi N, Shan F, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology 295: 210-217, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology 295: 16-17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albarello F, Pianura E, Di Stefano F, et al. 2019-novel coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis 93: 192-197, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Yun Z, Yi W, Zixiang H, Bin S. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 30: 4381-4389, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying Z, Ling W, Suqin B. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Seminars Immunopathol 39: 517-528, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y-H, Qin Y-Y, Lu Y-Q, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J (Engl) 133: 1080-1086, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murohashi K, Hagiwara E, Kitayama T, et al. Outcome of early-stage combination treatment with favipiravir and methylprednisolone for severe COVID-19 pneumonia: a report of 11 cases. Respir Investig 58: 430-434, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuckier LS, Moadel RM, Haramati LB, Freeman L. Diagnostic evaluation of pulmonary embolism during the COVID-19 pandemic. J Nucl Med 61: 630-631, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Griffin DO, Jensen A, Khan M, et al. Pulmonary embolism and increased levels of D-dimer in patients with coronavirus disease. Emerg Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18: 844-847, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18: 1023-1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]