Abstract
Purpose
Reirradiation for rectal cancer (RC) after prior pelvic radiation therapy (RT) has been shown to be safe and effective. However, limited data exist for proton therapy (PT), including pencil beam scanning proton therapy (PBS-PT). We hypothesize that PT is safe and feasible for re-treatment and may allow for decreased toxicity and treatment escalation.
Methods and materials
A single-institution, retrospective, institutional review board–approved analysis of all patients with RC and prior pelvic RT receiving PBS-PT reirradiation was performed. Data on patient and treatment characteristics and outcomes were collected. Local progression, progression-free survival, overall survival, and late grade >3 toxicity were estimated using the Kaplan-Meier method.
Results
Twenty-eight patients (median follow-up: 28.6 months) received PBS-PT reirradiation between 2016 and 2019, including 18 patients with recurrent RC (median prior dose: 54.0 Gy) and 10 patients with de novo RC and variable prior RT. The median reirradiation dose was 44.4 Gy (range, 16.0-60.0 Gy; 21 of 28 twice daily), and 24 of 28 patients received concurrent chemotherapy. Six underwent surgical resection. Three (10.7%) experienced grade 3 acute toxicities, and 1 did not complete RT owing to toxicity. Four (14.2%) had late grade <3 toxicity, including 1 grade 5 toxicity in a patient with a prior RT-related injury. The 1-year local progression, progression-free survival, and overall survival rates were 33.7% (95% confidence interval [CI], 14.5%-52.9%), 45.0% (95% CI, 26.2%-63.8%), and 81.8% (95% CI, 67.3%-96.3%), respectively.
Conclusions
This is the largest series using PT for reirradiation for RC and the first study using PBS-PT. Low acute toxicity rates and acceptable late toxicity support PBS-PT as an option for this high-risk patient population, with a need for continued follow-up.
Introduction
Rectal cancer (RC) is a common malignancy, with approximately 40,000 new cases per year in the United States and 800,000 worldwide. Therapy for locally advanced disease often consists of neoadjuvant concurrent chemotherapy and radiation, followed by surgical resection with total mesorectal excision—a paradigm that has improved local control, survival, and treatment-related toxicities.1, 2, 3 In the modern era, local relapse affects approximately 7% of patients in the long term and is associated with pain, obstruction, bleeding, discharge, and change in stool habits.4
Reirradiation for RC may be implemented in the treatment of locally recurrent RCs and de novo RC with prior radiation for other pelvic malignancy. It may be administered either as part of a curative or palliative regimen, with patients eligible for and receiving curative resection having the most favorable survival outcomes.5,6 However, it presents risk of late complications given the radiation sensitivity of nearby organs and tissues of the abdomen and pelvis—in particular, the bladder, bowel, bone marrow, and lumbosacral plexus. This affects the approach of reirradiation in multiple ways, including treatment volumes, prescribed dose, fractionation scheme, and technique of delivery.
Numerous experiences have described disease and toxicity outcomes of reirradiation for RC, using these aforementioned modifications in treatment delivery to achieve safe and effective therapy.7 A landmark prospective study by Valentini et al demonstrated the safety and efficacy of hyperfractionated reirradiation with concurrent chemotherapy, often given neoadjuvantly.8 This study has served as a reference for both disease outcomes and treatment toxicities. Multiple retrospective studies have corroborated these findings.9, 10, 11, 12 However, to our knowledge, nearly all published studies have evaluated reirradiation using either 3-dimensional conformal radiation therapy (RT) or intensity modulated RT techniques.13,14 Literature evaluating rectal reirradiation with proton therapy (PT) is emerging and has demonstrated a significantly reduced low dose to the bowel and bone marrow, as well as clinical feasibility, safety, and efficacy—thus far with passive-scatter techniques.15,16 PBS-PT provides true intensity modulated PT; thus, we hypothesize that its use may result in improved short- and long-term toxicity profiles in the setting of reirradiation for RC. We report the disease and toxicity outcomes from a retrospective, single-institution experience using PBS-PT for reirradiation for RC.
Methods and materials
Patient selection and treatment
An institutional review board–approved retrospective chart review was completed of all patients treated with reirradiation using PBS-PT at a single institution between 2016 and 2019 for either recurrent RC or de novo RC with prior pelvic RT for another malignancy. Patient demographic information, disease characteristics, and treatment characteristics were collected. Prior RT plan reports were accessed when possible, and all PBS-PT plans underwent peer review per departmental protocol. All patients were presented at a multidisciplinary tumor board. Concurrent chemotherapy and curative-intent surgical resection were planned whenever feasible and appropriate.
Proton beam reirradiation
Patients underwent computed tomography (CT) simulation in the supine or prone position with a comfortably full bladder and vac-lok immobilization. Contouring of the target and organ-at-risk volumes was performed. Gross tumor volume (GTV) was delineated using physical examination, CT simulation, and diagnostic imaging data (positron emission tomography and/or magnetic resonance imaging). A clinical target volume (CTV) was generated by expanding the GTV by 1.5 to 3.0 cm craniocaudally, extending to the pelvic sidewall laterally, and including the presacral space posteriorly. When a boost was planned, a second smaller isotropic expansion of 0.5 to 1.5 cm was used, or simply the gross tumor without expansion. The choice of CTV expansion is based on the method outlined by Valentini et al,8 with smaller expansions on the GTV used in cases of inoperable recurrences involving the bone. Planning target volumes were generated by dosimetry, accounting for setup and proton beam range uncertainty. Earlier cases used a uniform 5 mm expansion, later transitioning to nonuniform expansions (3.5-5 mm) based on translational uncertainty, and eventually generation of a planning target volume using robust optimization algorithms. Dosing was guided by the method by Valentini et al.8 Hyperfractionation was employed when feasible for the patient.
Plan optimization was performed using Eclipse (Varian Medical Systems, Palo Alto, CA) and Raystation (RaySearch Laboratories, Stockholm, Sweden). All patients were treated with PBS-PT, typically with 2 lateral fields with single-field optimization technique, with some cases using a third field and/or multiple-field optimization. Opposed lateral fields, rather than posterior oblique fields, were used in many cases due to relative biological effectiveness (RBE) uncertainty at the end range, because this configuration limits overlap of end-ranging segments of the beams into previously irradiated bladder as well as bowel anterior to the CTV. Furthermore, using PBS-PT, the anterior edge of the field can be shaped and modulated off of bladder and bowel with opposed lateral fields, in contrast to the same arrangement using photon-based 3-dimensional conformal RT, where this is not possible.
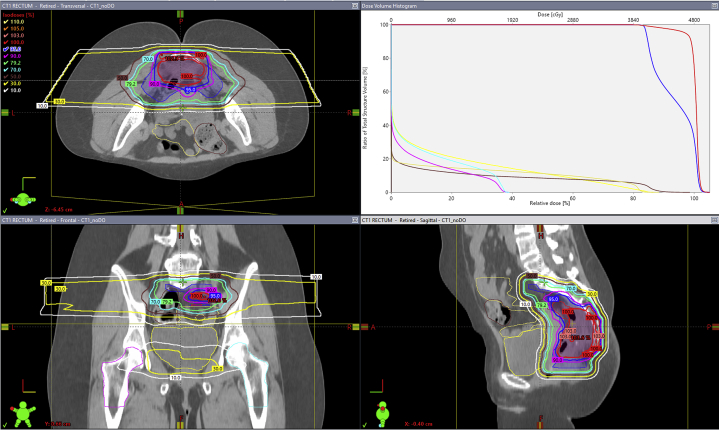
When using a multiple field optimization technique, air in the bowel was accounted for using a density override algorithm. Quality assurance CT images were obtained during the course of therapy, with frequency depending on the particular case and clinical discretion. Dose constraints for the bladder and bowel entailed keeping maximum doses of approximately 90% of the prescription dose, which itself depended on prior radiation dose and time interval. In all cases, these constraints on the bladder and bowel structures were prioritized over full prescription isodose coverage of the anterior target volume. A representative plan is shown in Figure 1.
Figure 1.
Example pencil beam scanning proton therapy plan with axial, coronal, and sagittal views with corresponding dose–volume histogram. The patient was simulated prone, dose was prescribed via a simultaneous integrated boost with clinical target volume 1 (blue; gross target volume with 2-2.5 cm craniocaudal expansion, laterally to pelvic sidewalls and posteriorly to sacrum) receiving 4000 cGy in 100 cGy/fraction and clinical target volume 2 (red; gross target volume + 1.0 cm isotropic expansion) receiving 4800 cGy in 120 cGy/fraction. Proton planning target volumes were generated based on setup and range uncertainty. Two lateral beams using a multiple-field optimization technique were used. Bladder (yellow), large bowel (brown), small bowel (green), right femoral head (turquoise), and left femoral head (fuschia). (A color version of this figure is available at https://doi.org/10.1016/j.adro.2020.10.008.)
Statistics
Efficacy outcomes included local progression (LP), progression-free survival (PFS), and overall survival (OS). LP was calculated from completion of reirradiation to time of local failure by pathologic or radiologic confirmation. Patients who did not experience LP were censored at the time of the last follow-up visit. PFS was calculated from completion of reirradiation to the date of any progression or death, irrespective of the cause. OS was calculated from completion of reirradiation to the date of death, irrespective of the cause. The vital status of patients was checked using medical record/obituary documentation, and OS was censored on the date of the last documentation confirming living status in patients still alive on that date.
Toxicity was assessed using the Common Terminology Criteria for Adverse Events, version 5.0. Acute toxicity was assessed using provider documentation from weekly on-treatment visits. Late toxicity was defined as symptoms attributable to RT persisting or occurring >3 months after completion of reirradiation. All clinical outcomes were estimated using the Kaplan-Meier method. A univariable Cox proportional hazards model was used to model the relationship between GTV and outcome. A P value of <.05 was considered statistically significant. All analyses were completed using SPSS statistical software, version 26.0 (IBM Corp., Armonk, NY).
Patient, disease, and treatment characteristics
Patient, disease, and treatment characteristics are summarized in Table 1. Twenty-eight patients (median follow-up 28.6 months; range, 25.3-31.9 months) received PBS-PT reirradiation from 2016 to 2019. Of these, 18 patients (64.3%) had recurrent RC (median prior dose: 54.0 Gy; range, 43.2-63.0 Gy) and 10 patients had de novo RC and variable prior RT (8 received full-dose external beam RT or brachytherapy for prostate cancer; 1 patient received external beam RT and brachytherapy boost for endometrial cancer; 1 patient received whole abdominal RT for ovarian cancer with a pelvic boost). The median reirradiation dose was 44.4 Gy (range, 16.0-60.0 Gy; 21 of 28 patients twice daily), and 24 of 28 patients (85.7%) received concurrent chemotherapy, with 18 of 28 (64.3%) receiving twice-daily oral capecitabine on days with reirradiation. Overall, 25 of 28 patients (89.3%) completed the planned course of reirradiation.
Table 1.
Patient and treatment characteristics (n = 28)
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 19 (67.9%) |
| Female | 9 (32.1%) |
| Age, y, median (range) | 68 (41-87) |
| Follow-up, mo, median (range) | 28.6 (25.3-31.9) |
| Recurrent rectal cancer | 18 (64.3) |
| Prior RT dose, Gy, median (range) | 54.0 (43.2-63.0) |
| Anatomic location of recurrence | |
| Rectal | 6 (33.3) |
| Presacral | 11 (61.1) |
| Pelvic bone | 1 (5.6) |
| Treatment for initial rectal cancer (n = 18) | |
| Neoadjuvant conformal RT | 8 (44.4) |
| Adjuvant conformal RT | 2 (11.2) |
| Other | 8 (44.4) |
| de novo rectal cancer (n = 10) | 10 (35.7) |
| Prior pelvic RT | Definitive for prostate cancer (equivalent total dose in 2 Gy fractions >70 Gy)8 Definitive for ovarian cancer1 Definitive for endometrial cancer1 |
| Treatment before reirradiation for recurrence | |
| Upfront surgery, n | 2 |
| Systemic therapy, n | 8 |
| Reirradiation dose, Gy, median (range) | 48.0 (16.0-60.0) |
| Reirradiation interval, mo, median (range) | 48.5 (12.7-494.8) |
| Concurrent chemotherapy with reirradiation | 24 (85.7) |
| Hyperfractionated reirradiation | 21 (75.0) |
| Completed reirradiation course | 25 (89.3) |
| Underwent resection after reirradiation | 6 (21.4) |
| R0 | 6 |
| Gross tumor volume, cm3, median (range) | 86.4 (13.6-821.8) |
Abbreviation: RT = radiation therapy.
Disease outcomes and toxicity
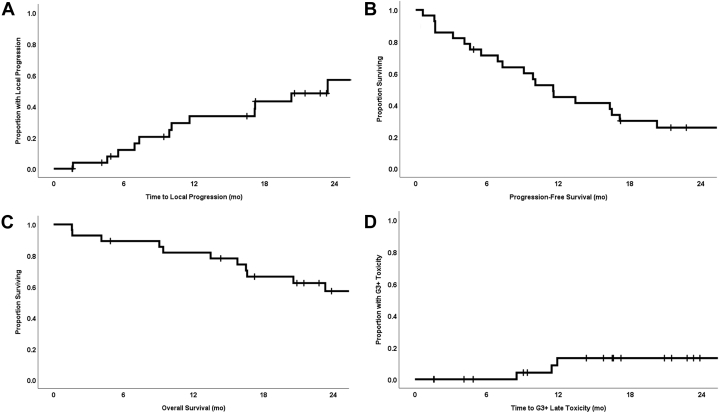
The 1-year LP, PFS, and OS rates were 33.7% (95% confidence interval [CI], 14.5%-52.9%), 45.0% (95% CI, 26.2%-63.8%), and 81.8% (95% CI, 67.3%-96.3%), respectively. The median times to LP, PFS, and OS were 23.4 months (range, 1.6-39.8 months; 95% CI, 12.9-33.9 months), 11.6 months (range, 0.6-39.8 months; 95% CI, 8.6-14.5 months), and 29.2 months (range, 1.6-45.9 months; 95% CI, 19.7-38.8 months), respectively (Fig 2A-C). In a univariable Cox proportional hazards model with GTV (cm3) included as a continuous variable, increasing GTV was highly statistically significantly associated with LP (hazard ratio [HR]: 1.005; 95% CI, 1.002-1.008; P = .003) and PFS (HR: 1.004; 95% CI, 1.002-1.006; P = .001), but not with OS (HR: 1.002; 95% CI, 1.000-1.005; P = .102). The 1-year LP rate for recurrent versus de novo RC was significantly different at 52.3% (95% CI, 23.9%-80.1%) and 0% (P = .002), respectively. The 1-year OS rate for recurrent versus de novo RC was 72.2% (95% CI, 51.4%-93.0%) and 100% (P = .058), respectively.
Figure 2.
Kaplan-Meier curves for (A) local progression, (B) progression-free survival, (C) overall survival, and (D) late grade >3 toxicities for all patients (n = 28).
Six patients (21.4%) underwent surgical resection after reirradiation, with further breakdown by de novo RC and recurrent RC patients being 3 of 10 (30%) and 3 of 18 (16.7%), respectively. All resections resulted in R0 resection. Of these patients, 4 eventually experienced LP (3 with recurrent RC), with a 1-year LP rate of resected patients of 16.7% versus 40% for those who did not receive resection (P = .786). The 1-year OS rate for patients undergoing and not undergoing resection was 100% and 76.8%, respectively; this difference was statistically significant (P = .027).
Acute toxicities are listed in Table 2. The 1-year rate of late grade >3 toxicities (G3+ Tox) was 13.3% in the Kaplan-Meier analysis (Fig 2D). Six late G3+ Tox occurred in 4 separate patients, with no evidence of tumor recurrence at toxicity onset (summarized by patient in Table 3). Patient 1 ultimately developed grade 5 toxicity and had a history of significant late toxicity from prior whole abdominal and pelvic boost radiation for ovarian cancer in the 1970s, including baseline and longstanding radiation colitis, loss of a kidney, and cystitis. This patient developed rectal bleeding at 3 months, a rectovaginal fistula at 9 months, and ultimately a bleeding stage IV decubitus ulcer and died of presacral hemorrhage at 26 months without evidence of tumor recurrence. Patient 2 developed an enterocutaneous fistula passing through the presacral region, as well as pyelonephritis resolving with parenteral antibiotics, approximately 1 year from completion of reirradiation. The treatment course was entirely in a medical ward without stay in either intermediate or intensive care. Of note, this patient was receiving bevacizumab systemic therapy at the time of the fistula diagnosis. This patient also experienced multiple delays and breaks in the planned treatment course related to social and logistic factors. This was the only patient who experienced G3+ Tox in both the acute and late term.
Table 2.
Acute toxicities graded by Common Terminology Criteria for Adverse Events, version 5.0
| Grade | 2, n (%) | 3, n (%) | 4, n (%) | 5, n (%) |
|---|---|---|---|---|
| Gastrointestinal | 4 (14.2) | 2 (7.1) | 0 (0.0) | 0 (0.0) |
| Skin | 4 (14.2) | 1 (3.6) | 0 (0.0) | 0 (0.0) |
| Urologic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hematologic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 8 (28.6) | 3 (10.7) | 0 (0.0) | 0 (0.0) |
Table 3.
Grade ≥3 late toxicities graded by Common Terminology Criteria for Adverse Events, version 5.0
| Patient no. | Grade | Toxicity | Time to toxicity, mo |
|---|---|---|---|
| 1 | 3 | Rectovaginal fistula | 8.5 |
| 1 | 5 | Presacral hemorrhage | 25.6 |
| 2 | 3 | Enterocutaneous fistula | 11 |
| 2 | 3 | Pyelonephritis (resolved) | 11 |
| 3 | 3 | Presacral abscess/rectovaginal fistula | 31 |
| 4 | 3 | Colovaginal fistula (improved) | 12 |
Six grade 3+ events were observed in 4 patients.
Patient 3 developed a presacral abscess and rectovaginal fistula approximately 2.5 years from completion of reirradiation. The site of local recurrence, which was treated with reirradiation, involved the vaginal fornices. Patient 4 had a long history of presacral abscess and transvaginal drainage procedures before reirradiation and developed increased vaginal discharge approximately 1 year from completion of reirradiation. The patient received a diagnosis of colovaginal fistula, which improved with percutaneous drainage.
Discussion
This is the largest report with the longest median follow-up to date of reirradiation for RC using PT. Additionally, all patients in our cohort received PBS-PT, making this the first such report. In contrast with prior studies, this study also included patients receiving prior pelvic RT for other malignancies.
Focusing on disease outcomes and restricting the analysis to recurrent RC only, we noted 1-year LP and OS rates of 52.3% and 71.4% in the present study versus 1-year local control and OS of 76.3% and 87.5% in the historical study by Valentini et al.8 Considerable heterogeneity in this retrospective cohort, particularly with respect to prior therapies (namely surgical resection) may account for the differences in these outcomes. Of the 10 patients who experienced local failure, 2 had multiply recurrent disease before reirradiation, 1 did not receive surgical resection after initial conformal RT, and 3 had received systemic therapy for local recurrence before being referred for reirradiation. All of these circumstances are mutually exclusive. As such, these patients had disease and treatment factors putting them at a high baseline risk and would also not have been eligible for a prospective study such as that by Valentini et al.8 Patients with de novo RC with previously irradiated pelvis show 2 failures as of the present, with 1 of these occurring in a patient who did not receive resection, and appear comparable with what would be expected without a history of pelvic RT. Nevertheless, the majority of patients in this study received hyperfractionated reirradiation with concurrent chemotherapy, reflecting an overall general consistency with reirradiation delivery technique.
Rates of acute grade 3 toxicity were low at 10.7%, and all but 3 patients completed the planned course of reirradiation (only 1 case was toxicity-mediated). These are comparable to the rate reported by Valentini et al (5.1%),8 who only accounted for gastrointestinal toxicity. Furthermore, relative to the study by Valentini et al,8 the median reirradiation dose in the present series was higher (48 vs 40.8 Gy). In addition, all patients in the trial by Valentini et al8 were treated with hyperfractionated RT compared with 75% of patients in our cohort. This supports tolerability of hyperfractionated reirradiation and concurrent chemotherapy with PBS-PT and is corroborated by the existing literature on PT for reirradiation for RC.
Four patients experienced late G3+ Tox, including 1 grade 5 toxicity. Overall, the rate of G3+ Tox at 1 year estimated with the Kaplan-Meier method was low at 13.3% (95% CI, 0.0%-27.7%). Of note, in 2 of these affected patients, a dose of 48 Gy in 1.2 Gy fractions was delivered, and in another patient 54 Gy in 1.5 Gy fractions was used, which represented the upper limit of the total dose in those patients who received a hyperfractionated course in this cohort. Additionally, as noted in the results section, 1 patient who experienced a grade 3 fistula had been undergoing bevacizumab therapy, another had a long history of presacral abscess and transvaginal procedures, and the patient with grade 5 toxicity had a history of significant toxicity with prior RT.
Comparing these late G3+ Tox with those reported by Valentini et al is difficult because late toxicities were not graded (although 1 was noted to require surgical intervention, and no treatment-related death was reported). However, the late G3+ Tox rates in the present report are comparable with prior published experiences of passive scatter PT reirradiation, although with a longer median follow-up of 28.6 months (range, 25.3-31.9 months). In the first known report using PT for reirradiation, 7 patients were treated using a double-scatter proton technique, and the median dose was 6120 cGy (RBE; 4500-6480 cGy) in conventional fractionation, with 6 patients receiving concurrent chemotherapy.15 With a median follow-up of 14 months, there were 3 acute grade 3 toxicities (abdominal pain, diarrhea) probably related to reirradiation, and 3 late grade 4 toxicities (2 bowel obstruction, 1 entero-vaginal fistula) possibly related to reirradiation.
Another experience evaluated 15 patients, also treated with passive-scatter technique, but with a 1.5 Gy accelerated fractionated course to a total dose of 39 to 45 Gy (RBE), and with all patients receiving concurrent chemotherapy.16 With a median follow-up of 14 months, 1 acute grade 3 toxicity (lymphopenia) and 2 late grade 3 toxicities (dysuria, rectal bleeding) were noted. Given the sample sizes of these and the present experiences and differing methods of treatment delivery, it is difficult to elucidate a dose-response relationship with respect to acute or late toxicity. Furthermore, our study solely implemented PBS-PT, thus making it more difficult to directly compare existing data largely gathered using passive-scatter techniques.
Six patients (21.4%) in our series received surgical resection after reirradiation, a lower proportion than in prior experiences (50.8% in the study by Valentini et al8), reflecting the high-risk patient cohort in our report. However, all 6 resulted in an R0 resection. Although the sample sizes are small for comparison, there was a strong trend toward superior OS in patients receiving resection (P = .107). This is consistent with prior reirradiation studies and likely multifactorial.
Other limitations to our study are primarily related to its retrospective nature. First and perhaps most important, there is selection bias inherent with any retrospective report and variable prior treatments. In this cohort, different RT regimens were used, inclusive of both conventionally fractionated and hyperfractionated courses. Second, the assessment of late toxicities was dependent on assessments that were not standardized, with imaging ranging from pelvic magnetic resonance to positron emission tomography/CT to CT.
Maturation and expansion of these data will better establish disease and toxicity outcomes in these patients. Longer follow-up and further accrual may help establish a dose–response relationship with respect to disease outcomes as well as the development of late toxicity. Moreover, such a relationship may be highly dependent on whether disease is recurrent or de novo.
Conclusions
The low acute toxicity rates, rare treatment interruption, and acceptable late toxicity reported here thus far support PBS-PT as an option for this high-risk patient population. Further follow-up and prospective studies, as those completed in the 3-dimensional conformal RT era, evaluating the use of PT for reirradiation for RC will help further clarify disease outcomes and toxicity profiles.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Kaiser reports personal fees from Varian Medical Systems outside of the submitted work.
References
- 1.Swedish Rectal Cancer Trial Group. Cedermark B., Dahlberg M. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 2.Peeters K.C.M.J., Marijnen C.A.M., Nagtegaal I.D. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R., Becker H., Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R., Liersch T., Merkel S. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard P., Efron J. Management of recurrent rectal cancer. Ann Surg Oncol. 2010;17:1343–1356. doi: 10.1245/s10434-009-0861-2. [DOI] [PubMed] [Google Scholar]
- 6.Lingareddy V., Ahmad N.R., Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;38:785–790. doi: 10.1016/s0360-3016(97)00058-8. [DOI] [PubMed] [Google Scholar]
- 7.Guren M.G., Undseth C., Rekstad B.L. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother Oncol. 2014;113:151–157. doi: 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Valentini V., Morganti A.G., Gambacorta M.A. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144–1150. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 10.Mohiuddin M., Lingareddy V., Rakinic J., Marks G. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys. 1993;27:1159–1163. doi: 10.1016/0360-3016(93)90538-7. [DOI] [PubMed] [Google Scholar]
- 11.Mohiuddin M., Marks G.M., Lingareddy V., Marks J. Curative surgical resection following reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;39:643–649. doi: 10.1016/s0360-3016(97)00340-4. [DOI] [PubMed] [Google Scholar]
- 12.Das P., Delclos M.E., Skibber J.M. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys. 2010;77:60–65. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Tao R., Tsai C.J., Jensen G. Hyperfractionated accelerated reirradiation for rectal cancer: An analysis of outcomes and toxicity. Radiother Oncol. 2017;122:146–151. doi: 10.1016/j.radonc.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Youssef F.F., Parikh P.J., DeWees T.A. Efficacy and toxicity of rectal cancer reirradiation using IMRT for patients who have received prior pelvic radiation therapy. Adv Radiat Oncol. 2016;1:94–100. doi: 10.1016/j.adro.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman A.T., Both S., Sharkoski T. Proton reirradiation of recurrent rectal cancer: Dosimetric comparison, toxicities, and preliminary outcomes. Int J Part Ther. 2014;1:2–13. [Google Scholar]
- 16.Moningi S., Ludmir E.B., Polamraju P. Definitive hyperfractionated, accelerated proton reirradiation for patients with pelvic malignancies. Clin Transl Radiat Oncol. 2019;19:59–65. doi: 10.1016/j.ctro.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]