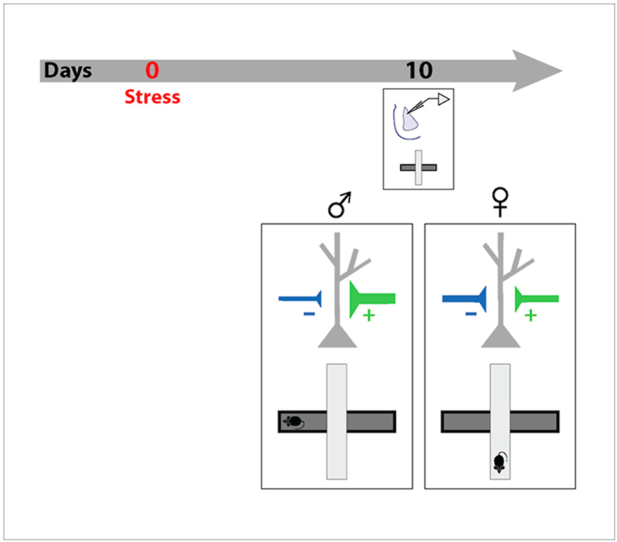
Abstract
There is accumulating evidence that stress triggers specific temporal patterns of morphological plasticity in the amygdala, a brain area that plays a pivotal role in the debilitating emotional symptoms of stress-related psychiatric disorders. Acute immobilization stress is known to cause a delayed increase in the density of dendritic spines on principal neurons in the basolateral amygdala (BLA) of rats. These neuronal changes are also accompanied by a delayed enhancement in anxiety-like behavior. However, these earlier studies used male rats, and the delayed behavioral and synaptic effects of acute stress on the BLA of female rats remain unexplored. Here, using whole-cell recordings in rat brain slices, we find that a single exposure to 2-h immobilization stress leads to an increase, 10 days later, in the frequency of miniature excitatory postsynaptic currents (mEPSCs) recorded from lateral amygdala (LA) principal neurons in male rats. Further, acute stress also causes a reduction in the frequency of miniature inhibitory postsynaptic currents (mIPSCs) in LA neurons 10 days after acute stress. In striking contrast, excitatory and inhibitory synaptic transmission in the LA of female rats does not exhibit any delayed change despite exposure to the same acute stress. Finally, we examined the functional impact of these contrasting synaptic changes at the behavioral level. Male rats exhibit a delayed increase in anxiety-like behavior on the elevated plus-maze 10 days after acute stress. However, the same stress does not lead to a delayed anxiogenic effect in female rats. Together, these results demonstrate that the delayed modulation of the balance of synaptic excitation and inhibition in the amygdala, as well as anxiety-like behavior, differ between males and females. These findings provide a framework, across biological scales, for analyzing how affective symptoms of stress disorders vary between the sexes.
Keywords: Gender, Stress, Anxiety, Synaptic physiology
Graphical abstract
1. Introduction
Accumulating evidence from rodent studies have elucidated various features of stress-induced plasticity in the amygdala (Chattarji et al., 2015; McEwen et al., 2016; Rodrigues et al., 2009). For instance, principal neurons in the basolateral amygdala (BLA) of rats exhibit dendritic hypertrophy and spinogenesis following 10 days (2 h/day) of chronic immobilization stress (Mitra et al., 2005a; Suvrathan et al., 2014; Vyas and Chattarji, 2004; Vyas et al., 2002). These cellular changes are accompanied by enhanced anxiety-like behavior on the elevated plus-maze (Vyas and Chattarji, 2004; Vyas et al., 2002). The same chronic stress enhances conditioned fear by forming new synapses with greater capacity for long-term potentiation in the lateral amygdala (LA) (Suvrathan et al., 2014). However, all of these findings emerged from analyses that were carried out almost exclusively in male rats. The handful of studies that have used female rats suggest sex differences in the impact of chronic stress (Luine et al., 2017). For instance, unlike male rats, chronically stressed female rats do not show enhanced anxiety-like behavior on the elevated plus-maze (Bowman et al., 2009; Gomez and Luine, 2014; Mitra et al., 2005b). Further, neurons in the BLA of female rats undergo dendritic hypotrophy and increased action potential firing following the same chronic stress that triggers hypertrophy, but reduced firing, in male BLA neurons (Blume et al., 2019). While the impact of chronic stress on synaptic physiology in the BLA has been studied in male rats (Padival et al., 2013; Rodriguez Manzanares et al., 2005; Suvrathan et al., 2014; Vouimba et al., 2004, 2006), the female amygdala remains unexplored in this regard.
Interestingly, these alterations in the amygdala are not restricted to chronic stress alone. The temporal profile of structural plasticity in the BLA can also be modulated by the duration of the stressor. If instead of 10 days of chronic immobilization stress, male rats are exposed to just a single episode of the same 2-h immobilization stress, enhanced anxiety-like behavior is observed not 1 day, but 10 days, later (Mitra et al., 2005a). This behavioral effect is accompanied by a delayed increase in spine density in the BLA, also after 10 days (Mitra et al., 2005a). Further, the same acute stress elicits an increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs) in the LA at the same delayed time point (Yasmin et al., 2016). However, it is not known if this acute stress also leads to similar delayed effects in the amygdala of female rats. For instance, does acute stress trigger delayed changes in anxiety-like behavior in female rats? If so, are such behavioral effects paralleled by delayed changes in synaptic physiology in the LA similar to those seen in male rats? And if so, are both excitatory and inhibitory synaptic transmission affected? The goal of the present study is to address these questions by examining if the delayed synaptic and behavioral effects of acute immobilization stress differ in female versus male rats.
2. Materials and methods
2.1. Animals
The foundation colonies of Wistar rats were sourced from Charles River Laboratories and experimental cohorts were bred and maintained in our institute vivarium. The litters were weaned and pair-housed in fresh cages (same sex littermates in a cage) with ad libitum access to food and water and moved to a separate room on postnatal day (PND) 21. A 14 h:10 h light:dark cycle was maintained with lights on from 0600 to 2000 h. Since both control and stress groups were subjected to all the procedures during the light phase of the cycle, any potential effects of the light phase should affect both groups equally. All the procedures were performed strictly complying to the guidelines of the Institutional Animal Ethics Committee, National Centre for Biological Sciences, Bangalore, India.
2.2. Acute stress
All animals were handled for three consecutive days prior to the day of stress. On the day of stress administration (PND 60), littermates were randomly divided into control and stress groups, ensuring that either both cage mates are in control group or both are subjected to stress. Rats to be stressed were moved to another room while the control rats continued to stay in the same room until the day of sacrifice. The housing conditions such as temperature, humidity, light:dark conditions, cage change timing and frequency and availability of food and water were similar for both the control and the stressed groups. Stress was administered by sealing the animals in plastic immobilization cones for 2 h (1000–1200 h) without access to food and water. A hole made at the tip of the plastic cones near the animal's nose allowed air passage for breathing.
2.3. Elevated plus-maze
Animals were tested for anxiety-like behavior on a plus-maze with all four arms 5.5 feet long and at an elevation of 2 feet from the ground. The open-arms were brightly lit (~85 lux) and the closed arms were dark (<0.5 lux) with 0.75 feet high walls. The luminosity at the central square area of the maze was 5–7 lux. All experiments were carried out between 1000 h and 1300 h. The behavior was video-taped for 5 min using an overhead camera and the videos were analyzed remaining blinded to the group assignment. Anxiety Index for each animal was calculated using the following formula (Cohen et al., 2008):
2.4. Preparation of amygdalar slices
Animals were sacrificed under isoflurane anesthesia. Brains were rapidly removed and placed in ice-cold cutting solution composed of (in mM): 204 sucrose, 11 glucose, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 MgSO4, 2 CaCl2; constantly aerated with 95% O2 and 5% CO2, pH 7.3, 305–310 mOsm. 400 μm-thick coronal brain slices containing basolateral amygdala (~Bregma −1.5 mm to −3.5 mm) were obtained in the cutting solution using Leica VT1200S vibratome (Leica, Germany). Slices were immediately moved to artificial cerebrospinal fluid (aCSF) (containing (in mM): 126 NaCl, 10 glucose, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 MgSO4, 2 CaCl2; equilibrated with 95% O2 and 5% CO2, pH 7.3, 305–310 mOsm) to recover at 30 ± 0.5 °C for 1 h followed by incubation at room temperature (22 ± 0.5 °C) until recording.
2.5. Electrophysiology
Slices were placed in submerged-type recording chamber (Warner Instruments, USA). A continuous perfusion of aCSF aerated with 95% O2 and 5% CO2 was maintained at 2.5 ml/min and 30 °C. The LA anatomy and principal neurons therein were visualized using infrared-differential interference contrast camera (Dage MTI, USA) attached to an upright widefield microscope (BX51WI, Olympus, USA). Patch-clamp recording electrodes of 2.5–3.5 MΩ tip resistance were pulled using P1000 micropipette puller (Sutter Instruments, USA). Patchmaster software (HEKA Elektronik, Germany) was used to deliver all stimuli and acquire the data. Data acquisition was done using an EPC-9 amplifier (HEKA Elektronik, Germany). A built-in Bessel filter was used to filter the data at 2.9 kHz and digitization was done at 20 kHz. Throughout all the recordings, access resistance was monitored once every minute, by application of brief 5 mV depolarizing step stimulus. Monitoring the access resistance at this frequency was done to assess whether all of the five 1-min epochs met the criterion for inclusion in the analysis. Testing once every minute helped ensure there was no deviation (beyond 20%) during the course of a 5 min recording. Only the cells where the series resistance (Rs) was always ≦ 25 MΩ and did not vary by more than 20% throughout recording were analyzed.
2.5.1. mEPSC recording
Recording pipettes filled with an internal solution containing (in mM): 135 cesium methanesulfonate, 10 HEPES, 0.2 EGTA, 5 QX314, 5 NaCl, 2 MgATP, 0.3 NaGTP, 5 phosphocreatine; pH 7.3; ~295 mOsm were used to patch onto BLA principal neurons. The cells were held at −70 mV (VHold) and miniature excitatory post-synaptic currents (mEPSCs) were isolated using gabazine (10 μm, HelloBio, UK) and tetrodotoxin (TTX, 1 μm, HelloBio, UK) in the perfusate. Once the whole-cell configuration had been achieved, the cells were allowed to stabilize for 5 min followed by data acquisition. A total of 5 min of mEPSC recordings were analyzed for each cell. Mini Analysis Program (Synaptosoft Inc., USA) was used for the analysis and the threshold amplitude for each event was set at 5 pA. All cells included in the frequency and amplitude bar graph plots were included in the inter-event interval (IEI) cumulative plots. The frequency graph plotted at the cell level while the IEI plots were constructed using individual IEI values from the entire 5 min recording from all cells.
2.5.2. mIPSC recording
Neurons were patched and held at −70 mV (VHold) using pipettes filled with a high chloride internal solution containing (in mM): 135 cesium chloride, 10 HEPES, 10 EGTA, 5 QX314, 2 MgCl2, 4 MgATP, 0.3 NaGTP; pH 7.3; ~295 mOsm. Miniature inhibitory post-synaptic currents (mIPSCs) were isolated using TTX (1 μm), D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 μm, HelloBio, UK) and 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μm, HelloBio, UK) in the perfusate. Similar criteria as described above for mEPSCs were also adopted for mIPSC recording and analysis. The threshold amplitude for each event was set at 10 pA.
2.6. Corticosterone ELISA
Rats were anaesthetized, decapitated and trunk blood was collected into sterile pre-chilled 2 ml centrifuge tubes (Tarsons Products Pvt. Ltd., #500020). The clot formation was allowed to happen for 30–60 min on ice following which the samples were centrifuged at 12,000 g for 20 min at 4 °C. The serum was then aspirated into fresh pre-chilled 2 ml centrifuge tubes and stored at −20 °C for further testing. Corticosterone ELISA was performed using a commercially available kit (Enzo Life Sciences, US) following the manufacturer's instructions. Sensitivity of the assay was 27.0 pg/ml (range 32–20,000 pg/ml). Absolute values of corticosterone concentration were used for statistical analyses.
2.7. Statistical analysis
Statistical testing of the data was performed using GraphPad Prism software (GraphPad software Inc., USA, version 6). All data sets were examined for normality using D'Agostino & Pearson omnibus test. Unpaired t-test was conducted when the sample distributions were normal in the two groups being compared. Mann-Whitney test was performed when non-parametric comparison was required between two groups. Kolmogorov-Smirnov test was used to compare cumulative distributions. To assess the interaction between stress and gender on more than two groups, two-way ANOVA followed by Tukey's multiple comparison was done. All data have been represented in the graphs as Mean ± SEM.
3. Results
3.1. Acute stress causes a delayed hyperexcitability in LA neurons of male rats
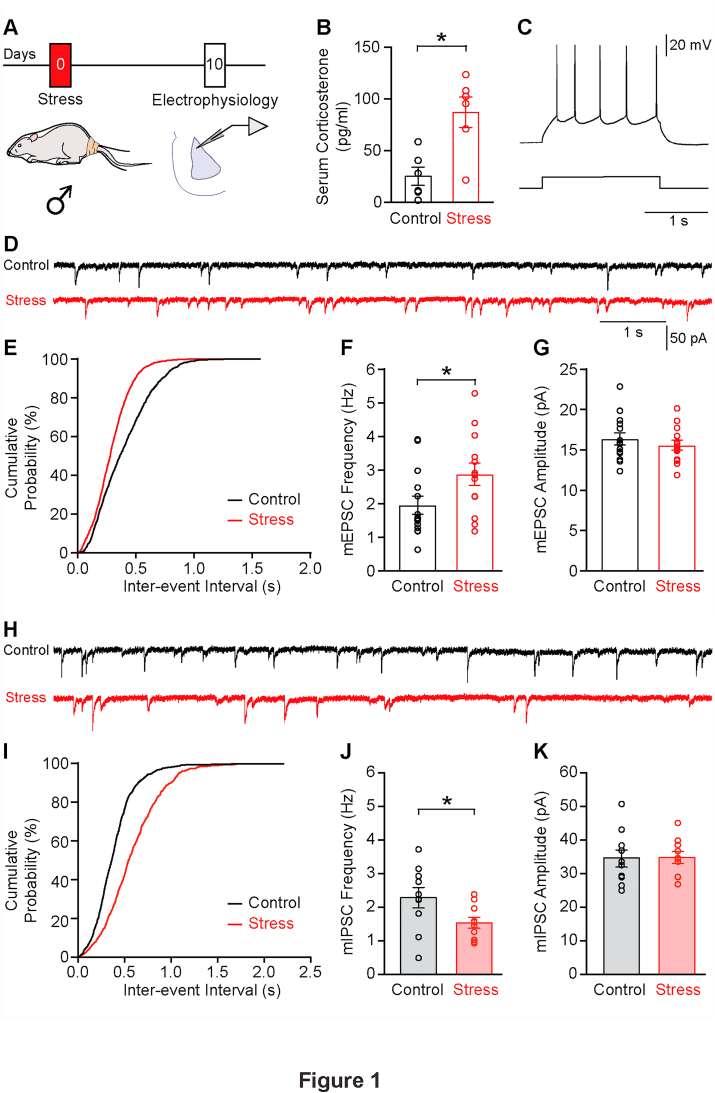
We first verified the efficacy of the 2-h acute immobilization stress paradigm by measuring the level of serum corticosterone immediately after stress. We found a significant increase in the concentration of serum corticosterone levels in stressed male rats compared to their control counterparts (Fig. 1B, Control Male: 25 ± 9 pg/ml; Stress Male: 87 ± 15 pg/ml; n = 6 rats/group; p < 0.05). These levels returned to control levels, 10 days after stress (Fig. S1A, Control: 35 ± 11 pg/ml, n = 8 rats; Stress: 34 ± 6 pg/ml, n = 11 rats; p = 0.69). Acute stress also led to a reduction in weight gain in male rats, weighed 1 day after stress (Fig. S1B, Control: 0.9 ± 0.3%, n = 12 rats; Stress: −1.7 ± 0.3%, n = 12 rats; p < 0.0001). We then investigated if acute stress elicits delayed changes in the balance of synaptic excitation/inhibition (E/I) in the lateral amygdala (LA) of male rats. To this end, we performed whole-cell voltage-clamp recordings from LA principal neurons in brain slices 10 days after acute stress (Fig. 1A). Consistent with earlier reports (Sah et al., 2003), these LA neurons also fired accommodating action potentials in response to depolarizing current injections (Fig. 1C). To assess the impact of acute stress on synaptic excitation, we recorded spontaneous mEPSCs in the presence of TTX (1 μM) and Gabazine (10 μM). We observed a significant increase in the average instantaneous frequency of mEPSCs in LA slices from stressed rats compared to controls (Fig. 1D–F; Control: 1.96 ± 0.27 Hz, n = 14 cells from 6 rats; Stress: 2.88 ± 0.34 Hz, n = 13 cells from 5 rats; p < 0.05). Acute stress, however, did not affect the average amplitude of mEPSCs (Fig. 1G; Control: 16.38 ± 0.75 pA; Stress: 15.60 ± 0.63 pA; p = 0.43).
Fig. 1.
Acute stress causes a delayed increase synaptic excitation, but reduction in synaptic inhibition, in the lateral amygdala of male rats. A. Schematic of experimental protocol. Male rats were subjected to acute stress and 10 days later, sacrificed for electrophysiological recordings from lateral amygdala (LA). B. Stressed rats showed a significant increase in serum corticosterone levels, 1 h after stress. Mann Whitney test. *p < 0.05. C. Representative current-clamp trace from a typical LA principal neuron showing accommodating action potential firing pattern in response to depolarizing current injection. D. Representative voltage-clamp traces of mEPSC recordings from control (black) and stressed (red) male rats. E. Cumulative distribution plot of mEPSC inter-event intervals from control and stressed groups, 10 days after acute stress. KS test. p < 0.0001. F. Summary graph showing significant increase in mEPSC frequency. Unpaired t-test. *p < 0.05. G. No change was observed in average mEPSC amplitude in control vs. stressed male rats. H. Representative mIPSC recordings from control (black) and stressed (red) male rats. I. Cumulative distribution plot of mIPSC inter-event intervals from control and stressed groups, 10 days after acute stress. KS test. p < 0.0001. J. Summary graph showing significant decrease in mIPSC frequency. Unpaired t-test. *p < 0.05. K. No difference was observed in average mIPSC amplitude in control vs. stressed male rats. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Having established the delayed effects of acute stress on excitatory synaptic transmission in LA principal neurons, we examined if this is accompanied by changes in inhibitory synaptic transmission at the same 10-day post-stress time point. We carried out recordings of spontaneous miniature inhibitory postsynaptic currents (mIPSCs) from LA principal neurons in the presence of TTX (1 μM), D-AP5 (50 μM) and CNQX (20 μM). In contrast to the increase in mEPSC frequency, the same acute stress elicited the opposite effect on synaptic inhibition – we found a significant reduction in the average instantaneous frequency of mIPSCs in LA slices obtained from stressed rats relative to controls (Fig. 1H–J; Control: 2.29 ± 0.30 Hz, n = 10 cells from 8 rats; Stress: 1.54 ± 0.16 Hz, n = 10 cells from 6 rats; p < 0.05). Similar to mEPSC amplitude, acute stress had no delayed effect on the mIPSC amplitude (Fig. 1K; Control: 34.37 ± 2.53 pA; Stress: 34.61 ± 1.77 pA; p = 0.94). Taken together, these data show that a single 2-h exposure to immobilization stress elicits delayed shift towards higher glutamatergic excitation and a concurrent decrease in GABAergic synaptic inhibition in the LA of male rats.
3.2. Acute stress does not disrupt E/I balance in LA neurons of female rats
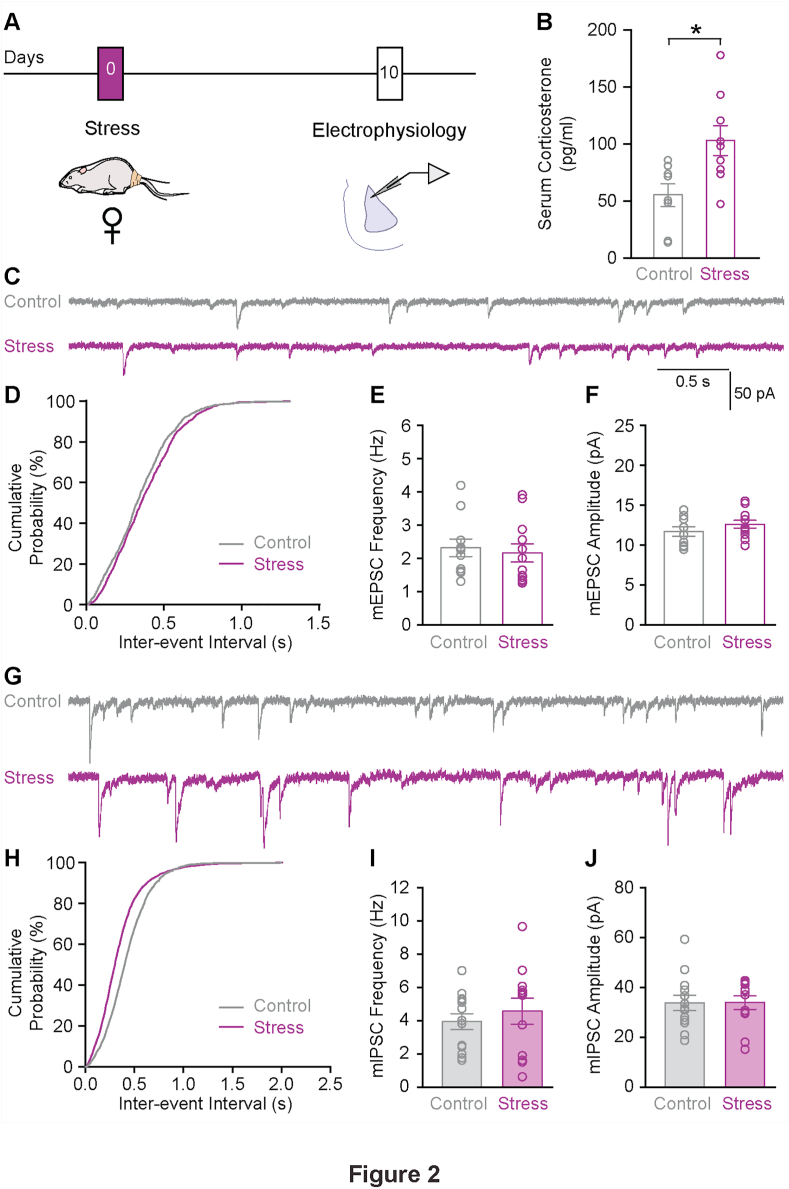
We first checked if, like in male rats, the same stress can lead to enhanced corticosterone levels in female rats. Our results show significantly higher serum corticosterone levels in stressed female rats compared to non-stressed females (Fig. 2B Control: 55 ± 10 pg/ml, n = 8 rats; Stress: 103 ± 13 pg/ml, n = 9 rats; p < 0.05). These levels returned to control levels, 10 days after stress (Fig. S2A, Control: 43 ± 10 pg/ml, n = 10 rats; Stress: 42 ± 7 pg/ml, n = 8 rats; p = 0.62). Further, female rats also weighed significantly lesser than male rats (Fig. S2B, Male: 358 ± 5 g, n = 36 rats; Female: 231 ± 3 g, n = 42 rats; p < 0.0001). Also, acute stress did not affect the change in weight gain in female rats, weighed 1 day after stress (Fig. S2C, Control: 2.2 ± 0.5%, n = 8 rats; Stress: 1.0 ± 0.7%, n = 10 rats; p = 0.21). To test if gender influences the delayed effects of acute stress on synaptic excitation and inhibition, we used the same recording protocols to quantify mEPSCs and mIPSCs in LA principal neurons of female rats, 10 days after stress (Fig. 2A). Our results reveal that the same acute stress that increased the frequency of mEPSCs in male rats, had no effects on either frequency (Fig. 2C–E; Control: 2.31 ± 0.26 Hz, n = 11 cells from 8 rats; Stress: 2.16 ± 0.27 Hz, n = 12 cells from 8 rats; p = 0.69) or amplitude of mEPSCs (Fig. 2F; Control: 11.71 ± 0.59 pA; Stress: 12.60 ± 0.49 pA; p = 0.26) in LA neurons of female rats. Further, mIPSC recordings from the same female rats also showed no differences in frequency (Fig. 2G–I; Control: 3.95 ± 0.48 Hz, n = 13 cells from 9 rats; Stress: 4.58 ± 0.78 Hz, n = 12 cells from 9 rats; p = 0.50) and amplitude (Fig. 2J; Control: 33.82 ± 3.06 pA; Stress: 33.94 ± 2.72 pA; p = 0.98) of spontaneous synaptic events compared to the control group. Taken together, these findings suggest that acute stress triggers a delayed disruption of E/I balance in principal neurons of the LA in male, but not female rats.
Fig. 2.
Acute stress does not affect synaptic excitation and inhibition in the lateral amygdala of female rats. A. Schematic of experimental protocol. Female rats were subjected to acute stress and 10 days later, sacrificed for electrophysiological recordings from lateral amygdala (LA). B. Stressed rats showed a significant increase in serum corticosterone levels, 1 h after stress. Mann Whitney test. *p < 0.05. C. Representative voltage-clamp traces of mEPSC recordings from control (grey) and stressed (maroon) female rats. D. Cumulative distribution plot of mEPSC inter-event intervals from control and stressed groups, 10 days after acute stress. KS test. p = 0.16. E. Summary graph showing absence of delayed effect of acute stress on mEPSC frequency in female rats. F. Summary graph showing no difference in average mEPSC amplitude. G. Representative mIPSC recordings from control (grey) and stressed (maroon) female rats. H. Cumulative distribution plot of mIPSC inter-event intervals from control and stressed groups, 10 days after acute stress. KS test. p < 0.01. I. Summary graph showing no change in mIPSC frequency in female rats. J. No difference was observed in average mIPSC amplitude in control vs. stressed females.
3.3. Acute stress causes a delayed increase in anxiety-like behavior in male rats but not female rats
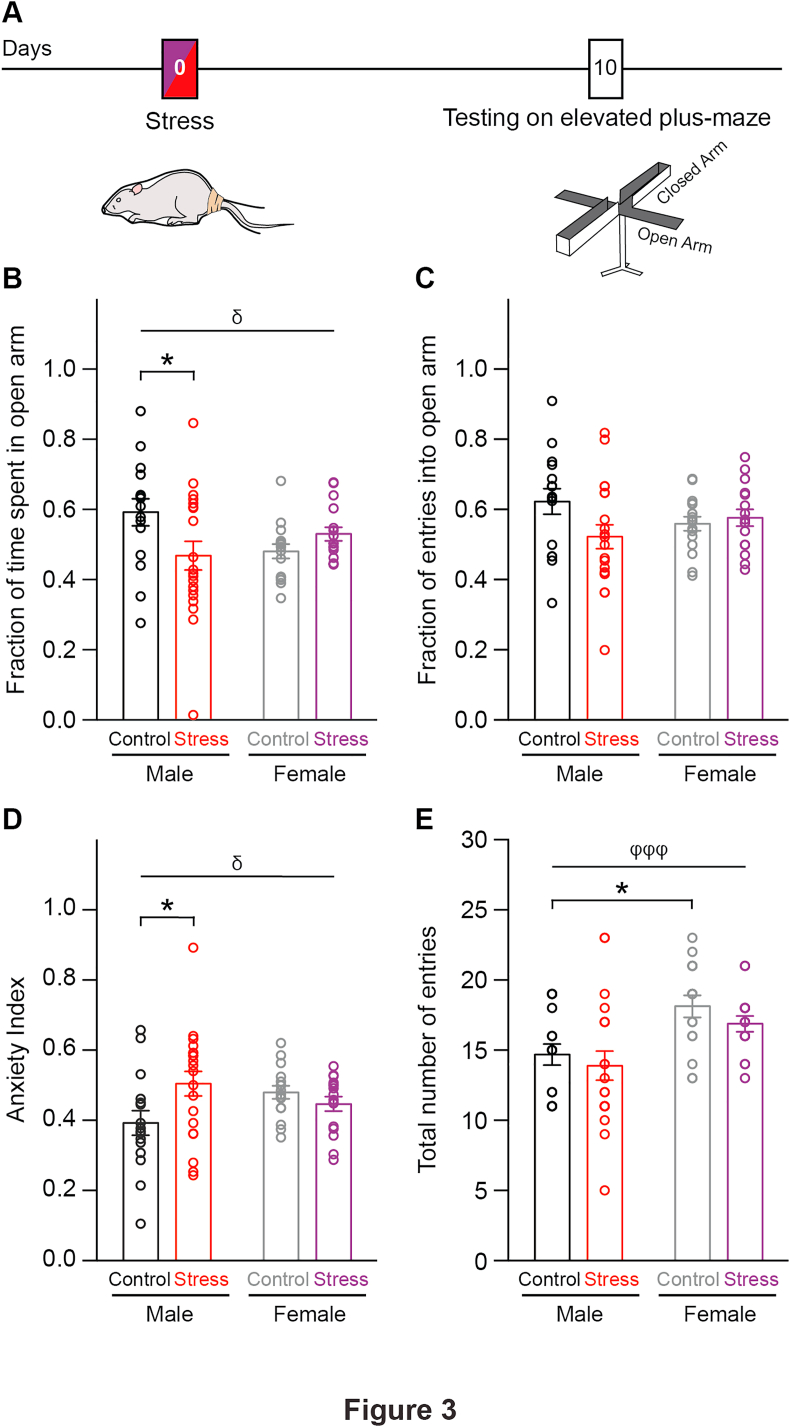
The acute stress paradigm used here has previously been shown to cause a delayed increase in anxiety-like behavior in male rats at the same 10-day time point (Mitra et al., 2005a). The correlation between enhanced anxiety and enhanced excitatory synaptic transmission in the LA is further strengthened by evidence that interventions that prevent the cellular effects also prevent the delayed anxiogenic effects in male rats (Chakraborty and Chattarji, 2019; Chakraborty, 2020). However, similar behavioral analyses using this acute stress paradigm have not been carried out in female rats. This gives rise to the prediction that if acute stress leads to delayed effects on amygdala synaptic physiology that are sexually dimorphic, then this difference should also be evident at the level of anxiety-like behavior. Specifically, we reasoned that acute stress should elicit a delayed increase in anxiety-like behavior in male, but not female rats. To test this prediction, we examined the impact of acute stress on anxiety-like behavior of male and female rats on the elevated plus-maze 10 days later (Fig. 3A). A two-way ANOVA of our results showed that stressed male rats spent significantly less time in open arms of the maze than did the control male rats (Fig. 3B; Control Male: 0.6 ± 0.04, n = 16 rats; Stress Male: 0.5 ± 0.04, n = 20 rats; Control Male vs. Stress Male: p < 0.05). In contrast to this, female rats showed no difference in the fraction of time spent in the open-arms (Control Female: 0.5 ± 0.02; Stress Female: 0.5 ± 0.02; n = 16 rats/group; p = 0.73). Both male and female rats did not show significant differences in open-arm entries (Fig. 3C; Control Male: 0.6 ± 0.04; Stress Male: 0.5 ± 0.03; Control Female: 0.6 ± 0.02; Stress Female: 0.6 ± 0.02). The heightened anxiety-like behavior in stressed male rats is also reflected in their Anxiety Indices that are significantly higher than control male rats (Fig. 3D; Control Male: 0.4 ± 0.04; Stress Male: 0.5 ± 0.04; Control Male vs. Stress Male: p < 0.05). Similarly, Anxiety Indices of control and stressed female rats were not affected by stress (Control Female: 0.5 ± 0.02; Stress Female: 0.5 ± 0.02; p = 0.87). Lastly, we measured the total number of entries into all four arms of the plus-maze to account for any locomotor alterations caused by stress. Stressed rats made a similar number of entries into the four arms of the maze as the control counterparts of the same gender, ruling out stress-induced locomotor deficits (Fig. 3E; Control Male: 15 ± 1; Stress Male: 14 ± 1; Control Female: 18 ± 1; Stress Female: 17 ± 1). Unstressed female rats however explored the maze more than their unstressed male counterparts (Control Male vs. Control Female: p < 0.05; Stress Male vs. Control Female: p < 0.01). In summary, acute stress enhanced anxiety-like behavior in male rats but failed to elicit a similar effect on female rats.
Fig. 3.
Acute stress causes a delayed increase in anxiety-like behavior of male, but not female, rats. A. Schematic of experimental protocol. Male and female rats were subjected to acute stress and 10 days later, tested for anxiety-like behavior using elevated plus-maze (EPM) test. B. Bar graph showing significant reduction in fraction of time spent in open-arms by stressed male rats (red) compared to control male rats (black), whereas stressed female rats (maroon) do not show any difference compared to control females (grey). Two-way ANOVA, δ denotes p < 0.05 for interaction between stress and gender; Tukey's multiple comparisons, Control Male vs. Stress Male: *p < 0.05. C. No significant effects of stress, gender or their interaction were observed in fraction of total entries into the open-arm. D. Summary graph showing significant increase in Anxiety Indices of stressed male rats compared to control male rats. But no such difference was found in female rats. δ denotes p < 0.05 for interaction between stress and gender; Control Male vs. Stress Male: *p < 0.05. E. Total number of entries into the four arms of EPM are similar between control and stressed males as well as between control and stressed females. However, control females made higher total number of entries than control male rats. ΨΨΨ denotes p < 0.001 for main effect of gender; Control Male vs. Control Female: *p < 0.05, Control Male vs. Stress Female: **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
This study explored sex differences in the delayed impact of acute stress on the amygdala at both cellular and behavioral levels. First, the frequency of mEPSCs was elevated in principal neurons of the lateral amygdala in male rats, 10 days after a single exposure to 2-h immobilization stress. This is in agreement with earlier reports using the same stress protocol and thereby confirmed its efficacy in the present study. Notably, we also found a concomitant reduction in the frequency of mIPSCs in these neurons, thereby showing for the first time a long-term effect of acute stress on synaptic inhibition in the amygdala. In female rats, by contrast, the same acute stress failed to elicit either of these effects on synaptic excitation and inhibition in the lateral amygdala 10 days later. The combined impact of these alterations would be to tilt the balance in favor of synaptic excitation over inhibition, thereby leading to a delayed hyperexcitability in the lateral amygdala of male but not female rats. We next investigated if these stress-induced changes in amygdalar excitability are paralleled by sex-specific alterations in amygdala-associated behavior. To this end, both male and female rats were assessed for anxiety-like behavior on the elevated plus-maze, 10 days after exposure to acute stress. We found that acute stress led to heightened anxiety-like behavior in male rats as has been reported earlier. Female rats, on the other hand, did not show delayed increase in anxiety-like behavior. Thus, a single exposure to acute stress that failed to elicit any synaptic changes in the amygdala, also did not lead to any anxiogenic effects in female rats 10 days later. In male rats, by contrast, the same stress elicited changes both at the behavioral and synaptic levels. Together, these results identify sex differences in stress-induced alterations in amygdalar function across levels of neural organization.
Our results showing enhanced excitatory tone in the amygdala of male rats, 10 days after acute stress, are consistent with the earlier reports on increase in mEPSC frequency at the same delayed time point (Yasmin et al., 2016). Here, we report that facilitation of synaptic excitability in the lateral amygdala after acute stress is caused not only by an increase in mEPSC frequency but also a simultaneous decrease in mIPSC frequency. Although similar effects of acute stress on mIPSC frequency have been observed at immediate time points after acute stress (Di et al., 2016; Yasmin et al., 2020), this is the first report of a long-term impact of stress on GABAergic synaptic transmission in the amygdala that is visible as late as 10 days after a 2-h episode of stress. This adds a new dimension to an earlier finding that in vitro bath application of stress-levels of glucocorticoids onto amygdala slices also reduced evoked inhibitory postsynaptic potentials along with increasing intrinsic excitability (Duvarci and Pare, 2007). Such reduction in GABAergic tone due to stress offers insights into how infusion of GABAA receptor antagonists into the amygdala mimic the enhancement of contextual fear memory caused by repeated restraint stress (Rodriguez Manzanares et al., 2005). Further, a recent study also reported that diazepam treatment 1 h after acute stress prevents increase in spine density in the BLA as well as enhanced anxiety-like behavior, 10 days later (Chakraborty and Chattarji, 2019). Since diazepam acts as a positive modulator of GABAA receptor activity, future studies will be needed to test whether the same treatment also prevents the long-term reduction in GABAergic tone in the BLA at the same 10-day time point.
Our observations using an acute stress paradigm add to growing evidence for sexual dimorphism in the effects of stress, much of which was gathered using paradigms of chronic or repeated stress. For example, the increase in synaptic excitability after acute stress is similar to previous reports of enhanced firing rates in LA neurons in male, but not female rats, following chronic stress (Blume et al., 2019). The absence of any effect of stress on excitation-inhibition balance in the LA of female rats may be due to a variety of gender-related factors. These could include baseline morphological and physiological differences between male and female LA neurons, such as higher firing rates and spine density in female compared to male rats (Blume et al., 2017). These neurons also function in a different microenvironment as indicated by lower extracellular levels of serotonin and dopamine in the female basolateral amygdala (Mitsushima et al., 2006). Further, stress induces sex-specific variations in neurotrophin expression in the brain (Bath et al., 2013). For example, brain-derived neurotrophic factor (BDNF) expression in the hippocampus increases in female rats (Lin et al., 2009), but not in male rats after acute stress (Lakshminarasimhan and Chattarji, 2012; Lin et al., 2009). However, it is not yet known how these factors may directly influence neuronal physiology in the amygdala in a sex-specific manner. Earlier studies in the hippocampus have attributed sex differences in the baseline or stressed states to gonadal hormones (Bale and Epperson, 2015; Farrell et al., 2013; McEwen and Alves, 1999). Specifically estrogen has been shown to confer resilience against the adverse effects of stress (Foy et al., 2008; McEwen and Alves, 1999). Moreover, ovariectomized, but not gonadally-intact, female rats showed dendritic atrophy (McLaughlin et al., 2005, 2010) and neuronal death (Takuma et al., 2007) in hippocampal area CA3 following repeated restraint stress. Both these effects were reversed by estrogen administration (McLaughlin et al., 2010; Takuma et al., 2007). Thus, whether some of these factors also contribute to resilience in the amygdala against the delayed impact of acute stress will need further exploration. The current study is a first step towards examining sex-differences in the effects of stress on spontaneous release in excitatory and inhibitory synapses in the absence of action potentials. The hyperexcitability, reflected in the shift in balance in mEPSCs and mIPSCs, may in turn serve as a substrate for disrupting excitation/inhibition balance in evoked responses as well. It will be interesting to investigate this in future studies.
The present study further shows that acute stress enhances anxiety-like behavior in male but not female rats. Although the effects of chronic stress on anxiety-like behavior in females vary across studies, most of the earlier studies concluded that these effects are different from those seen in male rats. Our findings are in agreement with earlier reports of chronic stress induced increase in anxiety-like behavior, in male but not female rats, on the elevated plus-maze (Bowman et al., 2009; Gomez and Luine, 2014; Mitra et al., 2005b). Further, the same stress that lowers anxiety-like behavior in female rats, compared to males, on the plus-maze does not always cause the same effect when assessed in other tests of anxiety, such as open-field test (Bowman and Kelly, 2012).
In the present study we report changes in anxiety-like behavior, amygdalar synaptic physiology and corticosterone following a single episode of stress. This suggests the possibility of a causative relation between these three measures, which could be strengthened through correlative analysis between these different measures in the same animal. However, various potential confounds posed by carrying out these measurements in the same animal led us to monitor these parameters in different animals. For instance, earlier work revealed that testing on the elevated plus-maze is stressful, and can manifest as enhanced anxiety-like behavior (Pellow et al., 1985; Rodgers and Dalvi, 1997; Taukulis and Goggin, 1990; Vyas and Chattarji, 2004). Hence, we did not want this to affect electrophysiological analyses in brain slices collected from the same animal. Moreover, collection of serum for cortisol measurements, immediately after acute stress, would have acted as an additional stressor. Specifically, collecting serum from the tail is likely to be stressful as an intraperitoneal injection, which has been reported to elicit a surge in corticosterone levels (Chakraborty and Chattarji, 2019; Chakraborty, 2020; Rao et al., 2012). Hence, we carried out these measurements in a separate cohort of rats to avoid this problem. Consequently, we ended up using three separate cohorts, thereby missing out on the opportunity to do correlative analysis between these different measures in the same animal.
Anxiety-like behavior is also known to vary with the phases of estrous cycle (Frye et al., 2000; Marcondes et al., 2001). In the current study, we did not examine female rats for estrous cycle phases because unlike in mice, visual inspection does not reliably predict the phases in rats. Further, other methods of monitoring these phases are invasive in nature and can be stressful, and thereby have a confounding effect on our analysis (Sharp et al., 2003). For instance, in earlier studies procedures like oral gavage and intraperitoneal injections have been shown to be stressful, and hence can interfere with the delayed effects of acute stress (Chakraborty and Chattarji, 2019; Chakraborty, 2020; Rao et al., 2012). Therefore, to avoid any such potential complications we did not carry out measurements of estrogen levels in the present study. However, to avoid effects of synchronization of estrous cycles among the females, each experimental group included female rats from at least three separate litters that were staggered over weeks and months. This ensured that the observations were not skewed towards any specific phase of the estrous cycle.
Interestingly, apparent absence of stress effects using common assays of anxiety-like behavior, as seen here and in past reports, may not necessarily imply that female rats are resilient to stress (Shansky, 2015). Instead, studies suggest that female rats may have different modes of expressing anxiety-like states and fear responses (Archer, 1975), such as by darting instead of the conventional ‘freezing’ behavior seen in male rats (Gruene et al., 2015). In fact, we found stressed female rats to exhibit higher locomotor activity on the plus-maze compared to males. This is similar to earlier reports of hyperactivity exhibited by female rats on the plus-maze (Bowman et al., 2009) and Y-maze (Conrad et al., 2003) after chronic stress. Such differences in locomotor activity in unstressed males versus females are suggestive of underlying sex differences in the mechanisms that may contribute to divergent behavioral outcomes after stress exposure. Further, recent studies showed that enhanced anxiety-like behavior was accompanied by social interaction deficits in male rats following acute stress (Chakraborty, 2020; Saxena et al., 2020). In the present study, we did not use specific behavioral tests to examine social interaction or aggression in either males or females. Although no obvious differences in social behavior in male versus female stressed rats were observed during our experiments, we cannot rule out the possibility that sex-differences in social interaction affect the response to stress. Studies by Sharp et al. have shown that responses to stresses due to most of the routine laboratory procedures are similarly buffered by group housing in both males and females (Sharp et al., 2002, 2003). Another study by Graves and Hennessy (2000) showed that presence of an unfamiliar adult female guinea pig reduced the impact of novelty-induced stress on the corticosterone levels and vocalization of pre- and post-weaning guinea pigs. Thus, future studies will be needed to explore these possibilities in greater detail.
In summary, our findings identify sex differences in the delayed effects of acute stress on synaptic physiology in lateral amygdala neurons, as well as anxiety-like behavior. This, in turn, offers a new framework for probing the sex-specific variations in cellular and molecular changes in the amygdala elicited by a brief stressor that gradually leads to amygdalar dysfunction, as well as potential therapeutic interventions against it.
CRediT authorship contribution statement
Kanika Gupta: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft. Sumantra Chattarji: Conceptualization, Resources, Writing - review & editing, Project administration, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgements
The authors acknowledge Dr. Anupam Hazra for helpful advice with slice electrophysiology experiments. This work was supported by funds from the Department of Atomic Energy and Department of Biotechnology, Government of India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100292.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Effect of acute stress on corticosterone levels and body weight of male rats. A. Summary graph showing no difference in serum corticosterone levels between control and stressed groups, 10 days after stress. B. Summary graph showing a reduction in body weight gain in the stressed group compared to the control group, weighed 1 day after stress (Unpaired t-test, ****p < 0.0001).
Effect of acute stress on corticosterone levels and body weight of female rats. A. Summary graph showing no difference in serum corticosterone levels between control and stressed groups, 10 days after stress. B. Summary graph showing difference in baseline body weights on postnatal day 60 in unstressed male versus female rats (Unpaired t-test, ****p < 0.0001). C. Summary graph showing no significant change in body weight gain in the stressed group relative to the control group, weighed 1 day after stress.
References
- Archer J. Rodent sex differences in emotional and related behavior. Behav. Biol. 1975;14:451–479. doi: 10.1016/s0091-6773(75)90636-7. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Epperson C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Blume S.R. Sex- and estrus-dependent differences in rat basolateral amygdala. J. Neurosci. 2017;37:10567–10586. doi: 10.1523/JNEUROSCI.0758-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume S.R. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci. Rep. 2019;9:12292. doi: 10.1038/s41598-019-48683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman R.E., Kelly R. Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: a preliminary examination. Stress. 2012;15:524–532. doi: 10.3109/10253890.2011.645926. [DOI] [PubMed] [Google Scholar]
- Bowman R.E. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol. Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Chakraborty P. Corticosterone after acute stress prevents the delayed effects on the amygdala. Neuropsychopharmacology. 2020;45(13):2139–2146. doi: 10.1038/s41386-020-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Chattarji S. Interventions after acute stress prevent its delayed effects on the amygdala. Neurobiol Stress. 2019;10:100168. doi: 10.1016/j.ynstr.2019.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci. 2015;18:1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Cohen H. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol. Psychiatr. 2008;64:708–717. doi: 10.1016/j.biopsych.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Conrad C.D. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Di S. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J. Neurosci. 2016;36:8461–8470. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S., Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J. Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M.R. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol. Behav. 2013;122:208–215. doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy M.R. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3 alpha,5 alpha-THP. Pharmacol. Biochem. Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gomez J.L., Luine V.N. Female rats exposed to stress and alcohol show impaired memory and increased depressive-like behaviors. Physiol. Behav. 2014;123:47–54. doi: 10.1016/j.physbeh.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves F.C., Hennessy M.B. Comparison of the effects of the mother and an unfamiliar adult female on cortisol and behavioral responses of pre- and postweaning Guinea pigs. Dev. Psychobiol. 2000;36:91–100. [PubMed] [Google Scholar]
- Gruene T.M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan H., Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PloS One. 2012;7 doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebr. Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on cognition in rodents. Pharmacol. Biochem. Behav. 2017;152:13–19. doi: 10.1016/j.pbb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes F.K. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Alves S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.J. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.J. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci. Lett. 2005;383:278–283. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Mitsushima D. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur. J. Neurosci. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Padival M. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rao R.P. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol. Psychiatr. 2012;72:466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Rodriguez Manzanares P.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Saxena K. The same stress has divergent effects on social versus asocial manifestations of anxiety-like behavior over time. Stress. 2020:1–7. doi: 10.1080/10253890.2020.1855421. [DOI] [PubMed] [Google Scholar]
- Shansky R.M. Sex differences in PTSD resilience and susceptibility: challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. Stress-like responses to common procedures in individually and group-housed female rats. Contemp. Top. Lab. Anim. Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- Sharp J.L. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp. Top. Lab. Anim. Sci. 2002;41:8–14. [PubMed] [Google Scholar]
- Suvrathan A. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130151. doi: 10.1098/rstb.2013.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K. 17beta-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience. 2007;146:60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Taukulis H.K., Goggin C.E. Diazepam-stress interactions in the rat: effects on autoanalgesia and a plus-maze model of anxiety. Behav. Neural. Biol. 1990;53:205–216. doi: 10.1016/0163-1047(90)90424-5. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Vouimba R.M. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 2004;19:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Vyas A., Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav. Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin F. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc. Natl. Acad. Sci. U. S. A. 2020;117:650–655. doi: 10.1073/pnas.1910322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin F. The delayed strengthening of synaptic connectivity in the amygdala depends on NMDA receptor activation during acute stress. Phys. Rep. 2016;4 doi: 10.14814/phy2.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of acute stress on corticosterone levels and body weight of male rats. A. Summary graph showing no difference in serum corticosterone levels between control and stressed groups, 10 days after stress. B. Summary graph showing a reduction in body weight gain in the stressed group compared to the control group, weighed 1 day after stress (Unpaired t-test, ****p < 0.0001).
Effect of acute stress on corticosterone levels and body weight of female rats. A. Summary graph showing no difference in serum corticosterone levels between control and stressed groups, 10 days after stress. B. Summary graph showing difference in baseline body weights on postnatal day 60 in unstressed male versus female rats (Unpaired t-test, ****p < 0.0001). C. Summary graph showing no significant change in body weight gain in the stressed group relative to the control group, weighed 1 day after stress.