Abstract
Background
/Objective: This study aimed to evaluate the effectiveness and safety of treating medial malleolar fractures using our patented Mg-Nd-Zn-Zr alloy (abbr. JDBM) screws with Ca–P coating, in order to provide a solid basis for their further clinical translation.
Methods
Nine patients with medial malleolar fractures were treated using coated JDBM screws. All patients had closed injuries, and none had open fractures. Postoperative radiography was performed to evaluate fracture healing and degradation of the JDBM screws. The visual analogue scale (VAS) was used to evaluate the degree of postoperative pain perceived by the patients, and the American Orthopedic Foot and Ankle Society (AOFAS) ankle-hindfoot scoring system was used to evaluate their postoperative ankle function. Postoperative complications, including infection, failure of internal fixation, and malunion, were carefully recorded during follow-up.
Results
The mean follow-up time was 12.2 ± 4.9 months. After the operation, all patients achieved good medial malleolar fracture alignment, and none of them experienced breakage of the JDBM screws before fracture healing. Postoperative radiography indicated JDBM screws gradually degradated with implantation time, and obvious degradation could be observed 12 months, postoperatively. At the final follow-up, the patients’ mean VAS score was 2.3 ± 1.9. The mean AOFAS score was 90.4 ± 8.9, with excellent or good rates of 88.9%. None of the patients experienced infection, failure of internal fixation, malunion, or other complications.
Conclusion
Coated biodegradable JDBM screws are effective for the treatment of medial malleolar fractures, and have good prospects for further clinical translation in the future.
Translational potential statement
The results of this study indicates coated biodegradable JDBM screw is an alternative internal fixation instrument for fracture treatment and has excellent prospects for clinical translation.
Keywords: Biodegradable Mg screws, Medial malleolus fracture, Translational medicine
Introduction
Fractures account for 16% of all musculoskeletal injuries and are quite common in clinical practice [1]. Good fracture fixation is the key to fracture treatment. Therefore, there is a great demand for various internal and external fixation devices for fracture fixation. In 2013, the global market size for such devices was US$ 5.7 billion, which has continued to increase at a compound rate of 7.2% [2]. Currently, the internal fixation devices that are widely used in clinical practice are mainly composed of stainless steel and titanium alloy materials. Such rigid orthopaedic implant materials have excellent mechanical properties and corrosion resistance, but they are still hampered by several shortcomings, such as “stress shielding” effects, poor osteoinductivity, metal artefacts, and requirement of a second surgery for removal [3]. Therefore, it is of great clinical value and social significance to overcome the inherent defects of existing stainless steel and titanium alloy internal orthopaedic implants, as well as to develop a class of internal fixation materials that have adaptable mechanical properties, promising osteoinductivity, and biodegradability.
In recent years, the application of biodegradable magnesium alloy implants in the field of orthopaedic implants has attracted the widespread attention of clinicians. Magnesium ions participate in multiple enzyme-catalysed reactions in the musculoskeletal system, and magnesium is an essential element for maintaining the normal physiological function of the skeleton [4,5]. The mechanical properties of magnesium-based implants are compatible with those of human bones, which could effectively reduce the “stress shielding” effect of metallic implants [6]. Moreover, as a bioactive metal, magnesium alloys also exhibit good osteoinductivity after implantation, and the degradation products of magnesium could promote fracture healing by regulating both bone formation and resorption. Magnesium rods have been shown to effectively increase local bone density in animal model [7], as the magnesium ions produced through the degradation of magnesium nails can induce the osteogenic differentiation of periosteum-derived stem cells by upregulating neuronal calcitonin gene-related polypeptides in the bone cortex and dorsal root ganglia, thereby accelerating fracture healing [8]. Furthermore, the alkaline and high-magnesium microenvironment produced by magnesium degradation can effectively inhibit osteoclast differentiation and bone resorption, as well as wear particle-induced osteolysis [9]. Therefore, magnesium-based implants have broad application prospects in the field of orthopaedic implantation.
Although magnesium alloys have several unique advantages in the field of orthopaedic implants, these have not been completely adopted into clinical practice. In 1900, Payr was the first to use magnesium implants in animal experiments [10]. By 1906, Lambotte had already begun carrying out clinical trials of magnesium alloy implants and was the first to apply magnesium alloys in the treatment of tibial fractures. Subsequently, magnesium-aluminium, magnesium-cadmium, and other magnesium alloy implants were applied in clinical trials and animal experiments [10]. However, these magnesium-based implants shared the common defect of overly rapid degradation. The rapid degradation of magnesium-based implants can produce a large amount of hydrogen, which can disturb the migration of the osteoblasts and isolate the bone tissue, thereby affecting bone repair. More importantly, rapid implant degradation is likely to cause a significant decrease in implant mechanical strength, leading to failure of internal fixation. With the continuous advances in materials science, it is now possible to improve the degradation performance and mechanical properties of magnesium-based implants to meet complex clinical needs.
In recent years, our team has successfully developed a patented biodegradable magnesium alloy Mg-Nd-Zn-Zr (abbr. JDBM) with a nominal composition as follows:, i.e. Nd 3.0, Zn 0.2, Zr 0.5 and Mg balance (mass fraction). The mechanical properties of the JDBM alloy, i.e. ultimate tensile strength (UTS), yield strength (YS) and the tensile elongation of the alloy with extrusion ratio of 8, reached to 312 MPa, 308 MPa and 12%, respectively [11], and are superior to those of existing commercial magnesium alloys WE43 (Mg-4wt%, Y-3.5RE[mixture of Gd and Nd]-0.5wt%Zr) and AZ31 (Mg-3wt%Al-1wt%Zn). More importantly, the JDBM alloy shows uniform degradation in animal models, which can effectively prevent failure of internal fixation caused by local stress concentration [11]. Furthermore, the biocompatibility and corrosion resistance of the JDBM alloy was further improved through the application of a calcium phosphate (Ca–P) coating [12]. In a long-term study up to 18 months in a goat femoral condyle fracture model [13], the JDBM screws with Ca–P coating exhibited excellent osteoinductivity and biocompatibility. In particularly, the degradation rate was reduced greatly by the coating and no obvious gas pocket was observed compared to the JDBM screws without Ca–P coating. Three months after screw implantation, extensive new bone tissue formation could be observed around the coated screw, and excellent osseointegration was also observed in the bone-implant interface. More importantly, the experimental animals did not show any abnormal changes in liver and kidney function during the follow-up period [13]. Our previous animal experiments indicate that the JDBM alloy screws with coating have excellent prospects for clinical translation.
Therefore, in this study, JDBM screws with a Ca–P coating were used in the treatment of medial malleolar fractures in order to determine their therapeutic effectiveness (fracture healing and function recovery) and safety, and provide a basis for their further large-scale clinical translation in the future.
Materials and methods
The study was approved by our ethics committee (No. 2016-123-T72). Patients were selected according to pre-determined inclusion and exclusion criteria. The inclusion criteria were as follows: 1) patients with medial malleolar fractures; 2) Chinese citizens aged between 18 and 80 years; 3) platelet count >50,000/μL, blood glucose <10.0 mmol/L, white blood cell count >4.0 × 109/L, and good nutritional status; 4) the absence of contraindications to implantation; 5) patients who had not participated in clinical trials in the past 3 months; and 6) patients who agreed to participate in this study and provided informed consent in writing. The exclusion criteria were as follows: 1) active infection; 2) poor general physical condition, liver and kidney dysfunction, severe hypertension, or poorly controlled blood glucose; 3) pathologic fracture; and 4) open fracture. Nine patients (3 men, 6 women) were enrolled in this study, and received surgical treatment in our hospital between June 2018 and July 2019. The patients’ mean age was 52.4 ± 15.7 years (range, 26–74). Fractures of included patients were classified based on Lauge-Hansen classification systems for ankle fractures [14]. Informed consent was obtained from all individual participants included in the study. The detailed information of patients was presented in Table 1.
Table 1.
Characteristics of patients.
| Case# | Age | Sex | Fracture site | Associated injury | Lauge-Hansen Classification |
|---|---|---|---|---|---|
| 1 | 56 | Male | Right medial malleolus | Distal tibiofibular syndesmosis injury | PER |
| 2 | 26 | Female | Left medial malleolus | Lateral and posterior malleolar fractures | SER |
| 3 | 49 | Female | Left medial malleolus | Lateral malleolar fracture | SER |
| 4 | 62 | Female | Left medial malleolus | Lateral and posterior malleolar fractures | SER |
| 5 | 34 | Female | Right medial malleolus | / | PA |
| 6 | 64 | Male | Right medial malleolus | Distal tibiofibular syndesmosis injury and fibular fracture | PA |
| 7 | 74 | Female | Right medial malleolus | Lateral malleolar fracture | SER |
| 8 | 43 | Male | Right medial malleolus | Lateral malleolar fracture and dislocation of ankle | PER |
| 9 | 64 | Female | Right medial malleolus | Lateral and posterior malleolar fractures | SER |
All patients were diagnosed based on their medical history, radiological examinations (anteroposterior and lateral ankle radiography, and computed tomography if necessary), and physical examination, followed by manual reduction and external fixation with plaster cast. Once they were admitted, the patients received symptomatic treatment, underwent preoperative examinations, and were scheduled for surgery. The patients then underwent open reduction and internal fixation of the fracture under general anaesthesia. After the patients were successfully anaesthetised, the affected lower limb was routinely disinfected and draped. A longitudinal incision was made on the medial side of the affected ankle joint, then the subcutaneous soft tissue was carefully separated to expose the fracture end, and the soft tissue embedded in the fracture end was debrided. After reduction of the medial malleolar fracture, temporary fixation was performed using Kirschner wires. Once X-ray fluoroscopy confirmed that a good reduction had been achieved, a varying number of magnesium alloy screws were placed slowly according to the clinical requirements. The screw diameter was 3.5 mm, and different screw lengths (18–36 mm in increments of 2 mm) were selected for internal fixation during the operation, also, according to clinical requirements. The number of screws used in operation depended the on size and stability of the fragment. The mean number of screws used in the current study was 1.6 ± 0.7 (range, 1–3). The reduction of the medial malleolus fracture was once again confirmed using fluoroscopy. Once the surgeon was satisfied, the incision was sutured layer by layer. Functional exercise began immediately after the operation, but weightbearing was not allowed for 3 months.
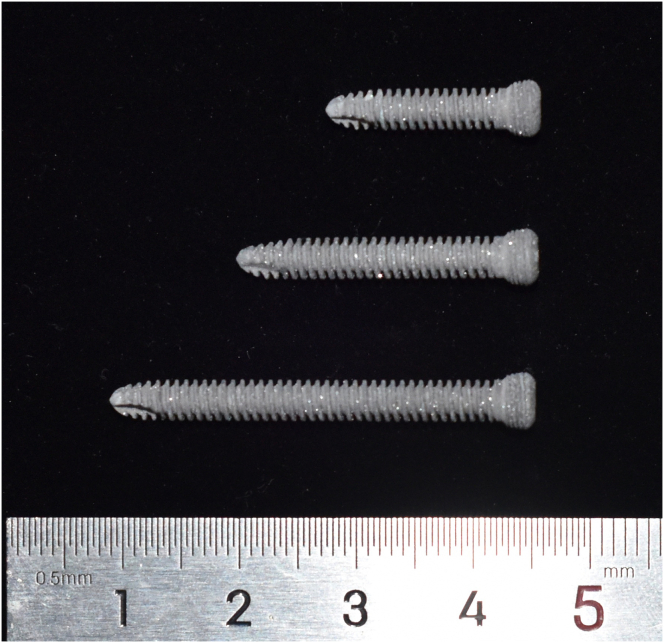
The biodegradable JDBM screws with Ca–P coating (Fig. 1) used in this study were provided by Shanghai Innovation Medical Sci & Tech Co., Ltd, China. The thickness of the biodegradable calcium phosphate coating (brushite, CaHPO4·2H2O) is about 15 μm, and the complete degradation time of the layer of coating in a goat femoral condyle fracture model is about 3–6 months [13]. Due to the protection by the coating, the in vitro degradation rate in Hank’s solution decreased from 0.54 mm/y for uncoated JDBM to 0.39 mm/y for Ca–P coated JDBM, which is a large 28% decrease [12]. The bonding strength between the coating and JDBM screws’ substrate was measured to be over 10 MPa, which ensured that the coating did not peel off during operation [12].
Figure 1.
The biodegradable magnesium screw used in the current study.
Postoperative anteroposterior and lateral ankle radiography examinations were performed to evaluate the patients’ fracture healing and the degradation of the JDBM screws. The visual analogue scale (VAS) was used to evaluate the degree of postoperative pain perceived by the patients [15,16], and the American Orthopedic Foot and Ankle Society (AOFAS) ankle-hindfoot scoring system was used to evaluate the patients’ postoperative ankle function [17]. The VAS and AOFAS score at the final follow-up were used for statistical analysis to determine the functional recovery of included patients. During the follow-up period, postoperative complications (infection, failed internal fixation, malunion etc.) experienced by the patients were carefully recorded. Since the current study was an observation with a single group of patients, inferential analysis was not necessary.
Results
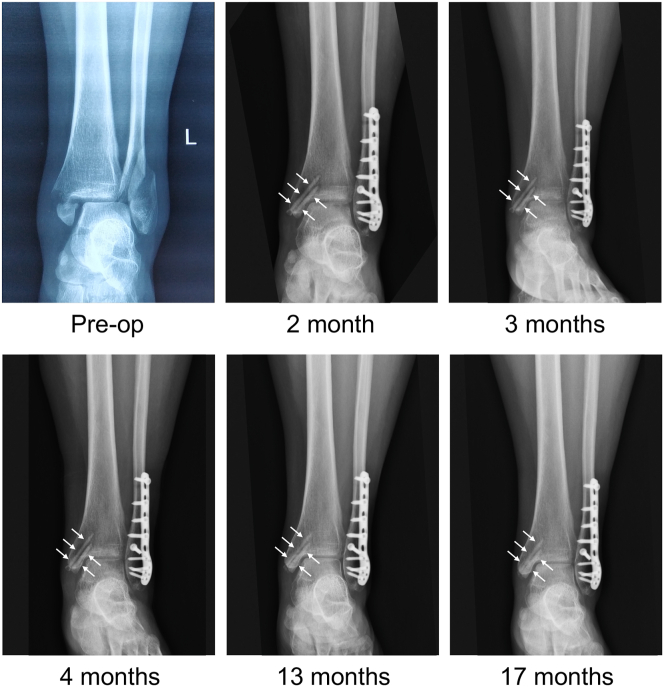
The mean follow-up time was 12.2 ± 4.9 months. None of the patients showed complications such as infection, failure of internal fixation, malunion. At the final follow-up, the mean VAS score was 2.3 ± 1.9. The mean AOFAS score was 90.4 ± 8.9, of which 6, 2, and 1 patient(s) had excellent, good, and fair scores, respectively, giving excellent or good rates of 88.9%. After the operation, the patients’ medial malleolar fracture showed good alignment, and there was no fracture or failure of the JDBM screws before fracture healing had taken place. As shown in Fig. 2, both biodegradable screws (white arrows in Fig. 2) maintained their morphology and did not show signs of failure before fracture healing. Additionally, the radiographs also indicated that both Mg screws gradually degradated with implantation time and obvious degradation could be observed in the radiograph 17 months postimplantation.
Figure 2.
Preoperative and postoperative radiographs of a young female patient with a trimalleolar fracture. Two biodegradable JDBM screws (white arrows) were used to fix the medial malleolar fracture.
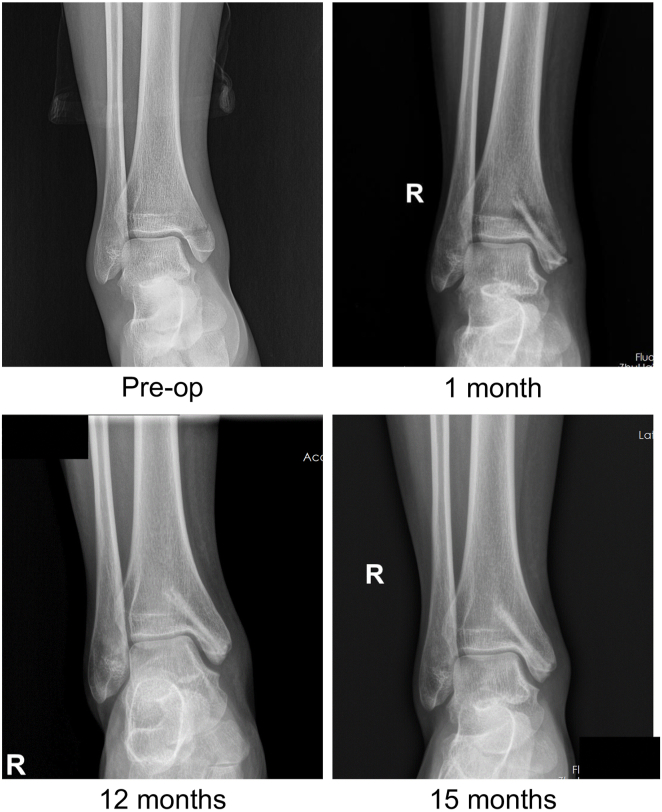
In addition, the ankle radiographs of some patients showed radiolucent zones around the screws. These radiolucent zones could usually be found 1 month postoperatively and gradually disappeared 3 months postoperatively (Fig. 2), which corroborated well with the evolution seen in goat femoral condyle fracture models [13]. As shown in Fig. 3, the patient’s radiograph showed radiolucent zones around screws 1 month postoperatively, which almost disappeared 12 months postoperatively. The radiolucent zones around the JDBM screws did not cause clinical symptoms in the patients.
Figure 3.
Preoperative and postoperative radiographs of a mid-age female patient with a medial malleolar fracture.
Discussion
Through this study, we have taken the initial steps for the clinical translation of the JDBM alloy by using coated JDBM screws in the treatment for medial malleolar fractures. The follow-up results demonstrate that the coated JDBM screws can achieve good fracture reduction and fixation when used to treat medial malleolar fractures, while also providing patients with postoperative pain alleviation and functional recovery. Thus, the coated JDBM screws are an effective method for the treatment of medial malleolar fractures, and the JDBM alloy has good prospects for clinical translation.
At present, the magnesium-based implants that have been studied internationally in clinical translational research mainly include the MAGNEZIX® magnesium alloy implants by Syntellix AG (Germany), as well as the magnesium-calcium alloy by the U&i Corporation (Korea) and high-purity magnesium (99.99%) implants. In 2013, the MAGNEZIX® magnesium alloy screw (with a composition similar to that of WE43) became the world’s first orthopaedic implant to receive the CE marking, and was introduced for clinical application for the internal fixation in osteotomies [18]. In 2015, the magnesium-calcium alloy screw by the U&i Corporation also received Korean certification, and was introduced into the Korean clinical market for fracture treatment [19]. Subsequently, these three types of magnesium alloy implants were gradually applied to the internal fixation of fractures, osteotomies, and the clinical treatment of osteonecrosis [[20], [21], [22]]. The results of previous studies suggest that good therapeutic efficacy can be achieved through the application of magnesium alloy screws in internal fixation for osteotomies and fractures.
The JDBM is a medical Mg-Nd-Zn-Zr alloy implant with good biocompatibility, mechanical properties, and degradation performance. It has a yield strength of 320–380 MPa and elongation of about 10%; hence, its mechanical properties are able to meet the requirements of bone implants [11,23]. Previous studies showed that JDBM screws and bone plates effectively promoted fracture repair in New Zealand rabbits and rats [12,24,25]. Furthermore, our research team verified the effectiveness and safety of JDBM screws in fracture treatment through a large animal experiment, which provides a solid basis for their clinical application [13].
Stable fixation is one of the basic principles of fracture treatment. Once implanted in the body, the magnesium alloy will immediately begin to undergo degradation, which is accompanied by a gradual decline in its mechanical properties. If the magnesium alloy degrades too quickly before fracture healing can take place, then, its local mechanical properties will be insufficient to maintain the stable fixation of the fracture, ultimately leading to a failure of internal fixation. Therefore, compared to the titanium alloy and stainless steel implants, magnesium alloy implants have a higher risk of failed internal fixation. It has been reported that a small number of patients experienced the fracture and failure of magnesium alloy screws before bone healing [19,26,27]. Applying a Ca–P coating on the surface of the magnesium alloy is an effective method for suppressing its degradation. The degradation rate of the JDBM alloy was reduced by about 30% through the application of a Ca–P coating. In a New Zealand rabbit model, the strength of bone plates made from the coated biodegradable JDBM alloy decreased by about 30% at 4 months after implantation, and its mechanical properties were far superior to those of the naked JDBM alloy (decrease in strength of about 48%) [12]. Therefore, the coated biodegradable JDBM alloy was able to provide fixation and support for a longer period of time after implantation, which is expected to reduce the risk of failed internal fixation. To the best of our knowledge, the present clinical cases are the first to be reported for Mg-based implants with coating. The results of this study show that when patients undergo fixation with coated JDBM screws, they demonstrate good postoperative fracture alignment, and do not experience the failure of the magnesium alloy screws. In addition, because of the decrease in degradation rate, we did not observe significant local gas accumulation or inflammatory reaction after the implantation of the coated biodegradable magnesium screws, and the incision showed good healing.
It is worth noting that some patients showed slight radiolucent zones around the magnesium alloy screws under radiography 1–2 months postoperatively, which gradually shrank as time prolonged. In some cases, the radiolucent zones almost disappeared 1 year postoperatively. Fortunately, these slight radiolucent zones did not cause clinical symptoms such as pain or other discomforts. Other research teams have also reported this phenomenon. Kose et al. [22] found in the treatment of medial malleolar fractures using MAGNEZIX® magnesium alloy screws, that radiolucent zones could be observed around the screws in the early stages after implantation, which gradually disappeared 6–12 months postoperatively; the radiolucent zones did not interfere with fracture healing or cause clinical symptoms. In addition, radiolucent zones were also formed locally after the implantation of magnesium-calcium alloy in the human body [19]. From these similar results, we can speculate that these radiolucent zones are a common phenomenon produced by magnesium alloy implants and are unrelated to the specific alloy composition. The underlying cause of these radiolucent zones is unclear. Some researchers think radiolucent areas are caused by hydrogen formation, while some believe that radiolucent zones are non-mineralized bone tissue which cannot be detected by X-ray and may not be problematic for fracture healing. The hypothesis of non-mineralized bone tissue could be verified in animal models by using hard tissue section. Further research is still required to clarify their underlying formation mechanism and their long-term effects on the patients.
We think the formation of radiolucent zones is closely related to the degradation rate of Mg screws. In our previous goat femoral condyle fracture models, radiolucent areas around JDBM screws with Ca–P coating (slower degradation rate) were much smaller than those around JDBM screws without Ca–P coating (faster degradation rate) [13]. Faster degradation rate will cause too much H2 formation because the in vivo degradation of Mg is caused by the following electrochemical reactions [12]:
| Anodic reaction: Mg→Mg2+ + 2e | (1) |
| Cathodic reaction: 2H2O+2e→H2+2OH−. | (2) |
| General reaction: Mg + 2H2O → Mg(OH)2 + H2 | (3) |
According to the above reaction (3), once the formation rate is faster than the diffusion rate of H2, the gas pocket would form. Moreover, the faster degradation rate would not only result in the formation of gas pocket, but also result in a high local pH due to the above reactions. The gas-pocket would isolate the bone tissue and would be unfavourable for the osteogenic process, while the high local pH micro-environment would also have a negative effect on the osteogenic behaviour, which could promote the radiolucent zone formation. Therefore, slowing down the degradation rate via a coating is a promising way to attenuate the radiolucent zone around the Mg implants.
This study has the following limitations. Firstly, it had a relatively small sample size, with only 9 patients enrolled, which may limit its generalisability. Secondly, this study did not have a control group; hence, the efficacy of the magnesium alloy screws for treating medial malleolar fractures could not be compared with that of other existing techniques. Thirdly, we did not set a fixed follow-up time for included patients. However, this study is the first clinical study of the JDBM alloy. Thus, to ensure patient safety, only a small number of patients were included, while the safety and effectiveness of treating medial malleolar fractures with JDBM screws were evaluated over a 12-month follow-up. Based on these existing findings, we will enrol more cases and set up a control group to further evaluate the therapeutic efficacy of JDBM screws in fracture treatment.
Conclusion
The treatment of medial malleolar fractures using coated JDBM Mg alloy screws achieved good fracture reduction and functional recovery. During the follow-up period, none of the patients showed failure of internal fixation or other complications. The results of this study demonstrate that the coated JDBM Mg alloy screws have excellent prospects for clinical translation.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81972058 and 81902194), National Key R&D Program of China (2016YFC1100600, subproject 2016YFC1100604), New Cutting- Edge Technology Project of Shen Kang Hospital Development Center of Shanghai (No. 16CR3025A), Shanghai Municipal Commission of Economy and Information (GYQJ-2019-1-27),the Science and Technology Commission of Shanghai Municipality (17440730700), Multicenter Clinical Research Project of Shanghai Jiao Tong University School of Medicine (DLY201506), and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2019QN2019).
Contributor Information
Weihua Gong, Email: gongwh1446@sh9hospital.org.cn.
Guangying Yuan, Email: gyyuan@sjtu.edu.cn.
Yongqiang Hao, Email: hyq_9hospital@hotmail.com, haoyq1664@sh9hospital.org.cn.
References
- 1.Liu X., McKenzie J.A., Maschhoff C.W., Gardner M.J., Silva M.J. Exogenous hedgehog antagonist delays but does not prevent fracture healing in young mice. Bone. 2017;103:241–251. doi: 10.1016/j.bone.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian L., Tang N., Ngai T., Wu C., Ruan Y., Huang L. Hybrid fracture fixation systems developed for orthopaedic applications: a general review. J Orthop Translat. 2019;16:1–13. doi: 10.1016/j.jot.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes J.S., Richards R.G. The use of titanium and stainless steel in fracture fixation. Expet Rev Med Dev. 2010;7(6):843–853. doi: 10.1586/erd.10.53. [DOI] [PubMed] [Google Scholar]
- 4.Touyz R.M. Magnesium in clinical medicine. Front Biosci. 2004;9:1278–1293. doi: 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- 5.Vormann J. Magnesium: nutrition and metabolism. Mol Aspect Med. 2003;24(1–3):27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 6.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang W., Zhang Y., Yang J., Tan L., Yang K. Potential antiosteoporosis effect of biodegradable magnesium implanted in STZ-induced diabetic rats. J Biomed Mater Res. 2011;99(3):386–394. doi: 10.1002/jbm.a.33201. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O’Laughlin M., Wise H. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai Z., Qu X., Li H., Yang K., Wan P., Tan L. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-kappaB and NFATc1 signaling. Biomaterials. 2014;35(24):6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Yuan G., Niu J., Fu P., Ding W. Microstructure, mechanical properties, biocorrosion behavior, and cytotoxicity of as-extruded Mg-Nd-Zn-Zr alloy with different extrusion ratios. J Mech Behav Biomed Mater. 2012;9:153–162. doi: 10.1016/j.jmbbm.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Niu J., Yuan G., Liao Y., Mao L., Zhang J., Wang Y. Enhanced biocorrosion resistance and biocompatibility of degradable Mg-Nd-Zn-Zr alloy by brushite coating. Mater Sci Eng C Mater Biol Appl. 2013;33(8):4833–4841. doi: 10.1016/j.msec.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Kong X., Wang L., Li G., Qu X., Niu J., Tang T. Mg-based bone implants show promising osteoinductivity and controllable degradation: a long-term study in a goat femoral condyle fracture model. Materials Science & Engineering C-Materials for Biological Applications. 2018;86:42–47. doi: 10.1016/j.msec.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60(5):957–985. [PubMed] [Google Scholar]
- 15.Verhage S.M., Schipper I.B., Hoogendoorn J.M. Long-term functional and radiographic outcomes in 243 operated ankle fractures. J Foot Ankle Res. 2015;8:45. doi: 10.1186/s13047-015-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzzini M., Lanzetti R.M., Lupariello D., Morelli F., Princi G., Perugia D. Comparison between carbon-peek plate and conventional stainless steal plate in ankle fractures. A prospective study of two years follow up. Injury. 2017;48(6):1249–1252. doi: 10.1016/j.injury.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Kitaoka H.B., Alexander I.J., Adelaar R.S., Nunley J.A., Myerson M.S., Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 18.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.W., Han H.S., Han K.J., Park J., Jeon H., Ok M.R. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc Natl Acad Sci U S A. 2016;113(3):716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaass C., von Falck C., Ettinger S., Sonnow L., Calderone F., Weizbauer A. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial. J Orthop Sci. 2018;23(2):321–327. doi: 10.1016/j.jos.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Acar B., Kose O., Unal M., Turan A., Kati Y.A., Guler F. Comparison of magnesium versus titanium screw fixation for biplane chevron medial malleolar osteotomy in the treatment of osteochondral lesions of the talus. Eur J Orthop Surg Traumatol. 2020;30(1):163–173. doi: 10.1007/s00590-019-02524-1. [DOI] [PubMed] [Google Scholar]
- 22.Kose O., Turan A., Unal M., Acar B., Guler F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: short-term clinical and radiological outcomes in eleven patients. Arch Orthop Trauma Surg. 2018;138(8):1069–1075. doi: 10.1007/s00402-018-2941-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Yuan G., Mao L., Niu J., Fu P., Ding W. Effects of extrusion and heat treatment on the mechanical properties and biocorrosion behaviors of a Mg-Nd-Zn-Zr alloy. J Mech Behav Biomed Mater. 2012;7:77–86. doi: 10.1016/j.jmbbm.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Guan X., Xiong M., Zeng F., Xu B., Yang L., Guo H. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg-Nd-Zn-Zr alloy for mandibular bone repair. ACS Appl Mater Interfaces. 2014;6(23):21525–21533. doi: 10.1021/am506543a. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Zhang L., Wang L., Yuan G., Dai K., Pei J. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: an in vitro and in vivo study. Acta Biomater. 2018;65:486–500. doi: 10.1016/j.actbio.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Plaass C., Ettinger S., Sonnow L., Koenneker S., Noll Y., Weizbauer A. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J Orthop Res. 2016;34(12):2207–2214. doi: 10.1002/jor.23241. [DOI] [PubMed] [Google Scholar]
- 27.Klauser H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2019;25(3):398–405. doi: 10.1016/j.fas.2018.02.005. [DOI] [PubMed] [Google Scholar]