Abstract
A fungal metabolite, diatretol, has shown to be a promising antimalarial agent. Diatretol displayed potent in vitro antiparasitic activity against the Plasmodium falciparum K1 strain, with an IC50 value of 378 ng ml−1, as well as in vivo efficacy in a Plasmodium berghei-infected mice model, with ca. 50% inhibition at 30 mg/kg (p.o.).
Subject terms: Phenotypic screening, Infection
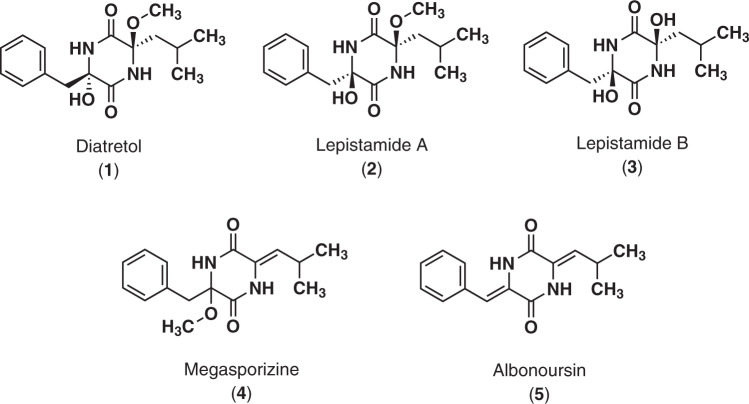
The World Health Organization (WHO) reports that malaria remains endemic in 91 countries, with an estimated 228 million cases and 405,000 deaths in 2018 [1]. The disease shows a global spread in tropical and subtropical regions, with five species of malaria-causing Plasmodium parasites infecting humans. Among them, malaria caused by P. falciparum is the most lethal and is primarily prevalent in sub-Saharan Africa, infection with the other species usually resulting in mild forms of the disease. Antimalarial drugs the main weapon to combat malaria, either for prophylaxis or treatment. WHO now recommends artemisinin-based combination therapies as the preferred treatment option. However, malaria parasites have historically developed resistance to all newly introduced antimalarials extremely quickly, and so drug resistance has remained a continuous problem [2]. Consequently, there has always been a need to find new antimalarial drugs, preferably those that have a unique or different structure or mode of action, to try and offset or delay the emergence of drug resistance. In the course of our screening program on cultured broths of microorganisms, we have discovered various potent antimalarial seed compounds, such as clonocoprogens [3]. A recent screening effort on the “Ōmura Natural Compound (ŌNC) library” allowed us to identify a fungal α,α′-dioxo-diketopiperazine, diatretol (1) [4], as a promising antimalarial agent (Fig. 1). Compound 1 was deposited in the ŌNC library as a result of the chemical investigation of a cultured broth of Metarhizium anisopliae FKI-7223 (Scheme S1). In this paper, we report the in vitro antimalarial activity of 1 as well as lepistamide A (2) [5], lepistamide B (3) [5], megasporizine (4) [6], and albonoursin (5) [7], all of which were derived from 1 (Fig. 1, Schemes S2 and S3). We also detail some structure/activity relationship (SAR) studies. The in vivo efficacy of 1 in a rodent malaria model is also presented.
Fig. 1.
Structures of diatretol (1), lepistamide A (2), lepistamide B (3), megasporizine (4) and albonoursin (5)
Antimalarial activity was assayed as previously reported [8] following approval from the Kitasato Institute Hospital Research Ethics Committee (No 12102), required because of the donation of human erythrocytes from volunteers. In the in vitro evaluation, cultured P. falciparum parasites (multidrug-resistant K1 and drug-sensitive FCR3 strains) were incubated with test compounds (1–5) or clinically used drugs (chloroquine, artemisinin and artesunate) in 96-well culture plates for 72 h. After incubation, parasite lactate dehydrogenase activity was assayed to determine parasite growth and calculate the degree of antimalarial activity. Diatretol (1) displayed potent in vitro antimalarial activity, with IC50 values of 378 and 334 ng ml−1 against K1 and FCR3 strains, respectively (Table 1 and Fig. S2). Lepistamide A (2) showed about 20 times weaker antimalarial activity than 1, with IC50 values of 7884 and 6939 ng ml−1 against K1 and FCR3 strains, respectively. Lepistamide B (3), megasporizine (4) and albonoursin (5) did not show antimalarial activity, even at 12,500 ng ml−1.
Table 1.
In vitro antimalarial activity of 1–5
| Compound | IC50 (ng/ml) | |
|---|---|---|
| K1 straina | FCR3 strainb | |
| Diatretol (1) | 378 | 334 |
| Lepistamide A (2) | 7,884 | 6,939 |
| Lepistamide B (3) | >12,500 | >12,500 |
| Megasporizine (4) | >12,500 | >12,500 |
| Albonoursin (5) | >12,500 | >12,500 |
| Artemisininc | 31 | 27 |
| Artesunatec | 9 | 7 |
| Chloroquinec | 456 | 40 |
aChloroquine-resistant strain
bChloroquine-sensitive strain
cDrugs commonly used to treat malaria (IC50: nM)
In vivo antimalarial evaluation of 1 was conducted using the Peters’ 4-day suppressive test [8]. Drug-sensitive rodent malaria P. berghei N strain parasites were used to infect ICR (CD1) mice (Day 0). Administration of 1 or artesunate was done at 30 mg/kg/day by intraperitoneal injection and orally for 4 consecutive days (Day 0–3). Parasitaemia was determined on day 4, with blood smears to calculate the percentage inhibition. As a result (Table 2), 1 showed 54.4% inhibition when used by the intraperitoneal injection at 30 mg/kg/day. More beneficially for the development of antimalarial drugs, 1 displayed 56.2% inhibitory activity by the oral route at the same dose.
Table 2.
In vivo antimalarial activity on Peters’ 4-day suppressive test of 1 and artesunate (30 mg/kg × 4 days) in a mouse model
| Compound | Route | Inhibition (%) |
|---|---|---|
| Diatretol (1) | i.p. | 54.4 |
| p.o. | 56.2 | |
| Artesunatea | i.p. | 99.1 |
| p.o. | 99.6 |
aDrug commonly used to treat malaria
Diatretol (1) [4] belongs to the very rare α,α′-dioxo-diketopiperazine chemical family. The first α,α′-dioxo-diketopiperazine discovered from a naturally occurring source was picroroccellin (Fig. S1) [9]. Other natural compounds with this molecular skeleton are the lepistamides (2, 3) [5], polarazines [10], and pestaloxazine A [11] (all from fungi), the pelopurins [12] (from the marine bacterium Pelomonas puraquae), and bicyclomycin [13] from a Streptomyces organism (Fig. S1). They were reported to possess antibacterial, phytotoxic, cytotoxic, and immunomodulating properties. Diatretol (1) was initially found as a fungal secondary metabolite from a cultured broth of Clitocybe diatrea. It demonstrated little bioactivity, apart from low antibacterial activity [4] and inhibition of the MLC reaction [14]. To the best of our knowledge, this report is the first to detail the in vitro and in vivo antimalarial profile of α,α′-dioxo-diketopiperazines. The antimalarial activity of 1 was about 20-fold greater than that of 2, which is an epimer of 1 at the α-position methoxy function. This result suggested that trans α,α′-dioxo function groups are more important for antimalarial activity than cis α,α′-dioxo function. According to comparative studies of cyclo(L-Val-L-Orn(Z)) and cyclo(L-Val-D-Orn(Z)), the latter showed about 21-fold greater in vitro antimalarial activity against P. berghei [15], which is in agreement with the relationship between 2 and 1. Compound 3, a demethyl analog of 2, displayed no antimalarial activity, indicating that a methoxy functional group at the α-position is also important to bestow antimalarial characteristics. Compounds 4 and 5, dehydrated analogs of 1, similarly showed no antimalarial activity, suggesting the importance of the oxygenated function at the α-position.
Several naturally occurring diketopiperazines have been investigated for potential as antimalarials [16–19]. However, there have been no reports showing clearly both in vitro and in vivo antimalarial activity. We suggest that the α,α′-dioxo-diketopiperazine scaffold might be a key element for development of a novel antimalarial drug. Total synthesis and determination of the absolute configuration of 1 is reported in an accompanying paper [20]. Further studies of 1, including its efficacy against Plasmodium vivax, mode of action, SARs and the creation of a library of more potent derivatives, are urgently required.
Supplementary information
Acknowledgements
We are grateful to Distinguished Emeritus Professor Satoshi Ōmura (Kitasato University) for his helpful support and valuable suggestions. We thank Dr. K. Nagai and Ms. N. Sato (School of Pharmacy, Kitasato University) for various instrumental analyses. This study was also partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the Japan Agency for Medical Research & Development (AMED) under Grant Number JP19am0101096.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aki Ishiyama, Rei Hokari
Supplementary information
The online version of this article (10.1038/s41429-020-00390-2) contains supplementary material, which is available to authorized users.
References
- 1.World Health Organization. World Malaria Report 2019. 2019. Geneva, Switzerland. https://www.who.int/publications-detail/world-malaria-report-2019.
- 2.World Health Organization, Aretemisinin resistance and artemisinin-based combination therapy efficacy. Global Malaria Programme. 2019. Geneva, Switzerland: WHO. https://www.who.int/docs/default-source/malaria/who-cds-gmp-2019-17-eng.pdf?sfvrsn=df6e57b1_2.
- 3.Ouchi T, Watanabe Y, Nonaka K, Muramatsu R, Noguchi C, Tozawa M, et al. Clonocoprogens A, B and C, new antimalarial coprogens from the Okinawan fungus Clonostachys compactiuscula FKR-0021. J Antibiot. 2020;73:366–71. doi: 10.1038/s41429-020-0292-7. [DOI] [PubMed] [Google Scholar]
- 4.Alberto A, Silvia C, Gianluca N, Stefano VM, Orso VP. Secondary mold metabolites. Part 52. Structure elucidation of diatretol—a new diketopiperazine metabolite from the fungus Clitocybe diatreta. Liebigs Ann. 1996;11:1875–7. [Google Scholar]
- 5.Xiang-Lian C, Wu M, Ti HH, Wei XY, Li TH. Three new 3, 6-dioxygenated diketopiperazines from the Basidiomycete Lepista sordida. Helv Chim Acta. 2011;94:1426–30. doi: 10.1002/hlca.201000455. [DOI] [Google Scholar]
- 6.Nozawa K, Udagawa S, Nakajima S, Kawai K. A dioxopiperazine derivative from Penicillium megasporum. Phytochemistry. 1989;28:929–31. doi: 10.1016/0031-9422(89)80145-1. [DOI] [Google Scholar]
- 7.Shin C, Chigira Y, Masaki M, Ohta M. Synthesis of albonoursin. Chem Soc Jpn. 1969;42:191–3. doi: 10.1246/bcsj.42.191. [DOI] [PubMed] [Google Scholar]
- 8.Otoguro K, Kohana A, Manabe C, Ishiyama A, Ui H, Shiomi K, et al. Potent antimalarial activities of polyether antibiotic, X-206. J Antibiot. 2001;54:658–63. doi: 10.7164/antibiotics.54.658. [DOI] [PubMed] [Google Scholar]
- 9.Sebastian MM, John AE. A structural revision of picroroccellin. Tetrahedron Lett. 1983;24:1445–8. doi: 10.1016/S0040-4039(00)81678-0. [DOI] [Google Scholar]
- 10.Soledade M, Pedras C, Biesenthal CJ. Isolation, structure determination, and phytotoxicity of unusual dioxopiperazines from the phytopathogenic fungus Phoma lingam. Phytochemistry. 2001;58:905–9. doi: 10.1016/S0031-9422(01)00348-X. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y-L, Wei MY, Chen HY, Guan FF, Wang CY, Shao CL. (+)- and (-)-Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro[oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org Lett. 2015;17:4216–9. doi: 10.1021/acs.orglett.5b01995. [DOI] [PubMed] [Google Scholar]
- 12.He X-X, Chen XJ, Peng GT, Guan SY, Lei LF, Yao JH, et al. Pelopuradazole, a new imidazole derivative alkaloid from the marine bacteria Pelomonas puraquae sp. nov. Nat Prod Res. 2014;28:680–2. doi: 10.1080/14786419.2014.891591. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi T, Miyairi N, Aoki H, Kosaka M, Sakai H. Bicyclomycin, a new antibiotic. I. Taxonomy, isolation and characterization. J Antibiot. 1972;25:569–75. doi: 10.7164/antibiotics.25.569. [DOI] [PubMed] [Google Scholar]
- 14.Iijima M, Masuda T, Nakamura H, Naganawa H, Kurasawa S, Okami Y. Correction to: Metacytofilin, a novel immunomodulator produced by Metarhizium sp. TA2759 [Erratum to document cited in CA118:055707] J Antibiot. 2018;71:908–9. doi: 10.1038/s41429-018-0045-z. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Picaso L, Olivo HF, Argotte-Ramos R, Rodríguez-Gutiérrez M, Rios MY. Linear and cyclic dipeptides with antimalarial activity. Bioorg Med Chem Lett. 2012;22:7048–51. doi: 10.1016/j.bmcl.2012.09.094. [DOI] [PubMed] [Google Scholar]
- 16.Isaka M, Palasarn S, Rachtawee P, Vimuttipong S, Kongsaeree P. Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Org Lett. 2005;7:2257–60. doi: 10.1021/ol0507266. [DOI] [PubMed] [Google Scholar]
- 17.Buedenbender L, Robertson LP, Lucantoni L, Avery VM, Kurtböke DÍ, Carroll AR. HSQC-TOCSY fingerprinting-directed discovery of antiplasmodial polyketides from the marine ascidian-derived Streptomyces sp. (USC-16018) Mar Drugs. 2018;16:189–99. doi: 10.3390/md16060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Xu P, Zhang W, Yuan Y, He X, Su D, et al. Three new diketopiperazines from the previously uncultivable marine bacterium Gallaecimonas mangrovi HK-28 cultivated by iChip. Chem Biodiv. 2020;17:e2000221. doi: 10.1002/cbdv.202000221. [DOI] [PubMed] [Google Scholar]
- 19.Mohamad ZN, Baba MS, Zainal-Abidin AH, Latip J, Mazlan NW, Edrada-Ebel R, et al. Gancidin W, a potential low-toxicity antimalarial agent isolated from an endophytic Streptomyces SUK10. Drug Des Dev Ther. 2017;11:351–63. doi: 10.2147/DDDT.S121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi S, Kimishima A, Hirose T, Yamada T, Sugawara A, Shirahata T, et al. Tetrahedron lett. 2021, submitted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.