Abstract
We herein report a case of allergic bronchopulmonary aspergillosis (ABPA) with marked eosinophilia and high attenuation mucus (HAM) on chest computed tomography (CT), which demonstrated a rapid and remarkable improvement with benralizumab treatment. A 67-year-old Japanese woman, who was diagnosed with asthma at the age of 64 years, was admitted with dyspnea. Her blood test results showed marked eosinophilia (peripheral blood eosinophil count 24403/μL) and elevated serum IgE levels. Chest CT also revealed ground-glass opacity. Sputum cytology detected filamentous fungi, suggesting an infection with Aspergillus spp. Based on these findings, ABPA was diagnosed. Following systemic corticosteroid treatment, her respiratory symptoms and chest radiography findings showed improvements. However, with the gradual tapering and eventual discontinuance of the corticosteroid therapy, a concomitant increase in the peripheral blood eosinophils and a recurrence of the clinical symptoms, was observed. In addition, her pulmonary function decreased and chest CT revealed worsened bronchial mucus plugs. To control the asthma with ABPA exacerbation, benralizumab was administered. Following treatment with benralizumab, the patient's asthmatic symptoms improved, together with a decrease in her peripheral eosinophil count. Mucus plugs were no longer visible on chest CT. Pulmonary function test result also showed a remarkable improvement. There was no relapse of dyspnea and no reappearance of the mucus plugs. This case suggests that benralizumab may be a suitable treatment option for patients with ABPA with marked eosinophilia and HAM on chest CT.
Keywords: Allergic bronchopulmonary aspergillosis, Benralizumab, Eosinophilia, High-attenuation mucus
1. Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an eosinophilic pulmonary disorder caused by a hypersensitivity reaction to a fungal species known as Aspergillus fumigatus. It characteristically presents with uncontrolled bronchial asthma, eosinophilia, elevated serum IgE levels, and radiological findings such as central bronchiectasis and mucus plugging [1]. The mainstream and most effective therapeutic options for ABPA include systemic glucocorticoids and antifungal agents; however, patients usually experience exacerbations after these treatments. Furthermore, long-term systemic glucocorticoid use, which is necessary for patients with refractory ABPA, may cause serious adverse effects including infections and osteoporosis and so on [2]. Since a systemic corticosteroid-sparing agent is required, recent case reports and several studies have shown the efficacy of Th2 inflammatory cascade inhibitors, such as omalizumab, mepolizumab, and benralizumab, for the treatment of ABPA. A biological response prediction is necessary to identify a suitable biologic treatment for refractory ABPA. Here, we report a case of ABPA with marked eosinophilia and high attenuation mucus (HAM) on computed tomography (CT), which demonstrated rapid and remarkable improvement with benralizumab treatment.
2. Case report
A 67-year-old Japanese woman diagnosed with asthma at the age of 64 years, was given prednisolone (the initial dose was 0.5 mg/kg) for 6 months to treat eosinophilic pneumonia. The prednisolone dose was reduced and eventually discontinued, while a combination of high-dose inhaled corticosteroids (ICS) and long-acting β-agonists constituted the subsequent treatment. However, she experienced frequent asthma attacks. One year after terminating prednisolone administration, she was admitted to our hospital with productive cough and dyspnea. Upon admission, she was tachypneic with oxygen saturation of 93% with an oxygen nasal cannula delivering 2 L/min. Her blood test results were as follows: white blood cell count of 32,800/μL with marked eosinophilia (peripheral blood eosinophil count 24403/μL), total serum IgE level of 1146 IU/mL and negative MPO-ANCA and PR3-ANCA results. Chest radiography and CT revealed ground-glass opacity in the upper lobes of the bilateral lungs and HAM in the right lower and left upper lobe (right mucus; CT values of 94 Hounsfield units, left mucus; CT values of 88 Hounsfield units) (Fig. 1A and B). Serological test results for Aspergillus-specific IgE and those for antibodies against A. fumigatus were negative and specific IgE antibodies for other fungus were also negative. Although the result of sputum culture obtained by bronchoscopy did not reveal other fungi other than Candida albicans which is not pathogenetic, sputum cytology detected filamentous fungi, suggesting Aspergillus spp. (Fig. 1C). Based on these findings, she was diagnosed with probable ABPA, despite not fulfilling the International Society for Human and Animal Mycology (ISHAM) criteria. Systemic corticosteroids (0.5mg/kg) and leukotriene receptor antagonists were administered. Three months later, the patient's respiratory symptoms and chest radiography findings showed improvements (Table 1). As the corticosteroids were gradually tapered and discontinued, clinical symptoms recurred concomitantly with an increase in peripheral blood eosinophils (peripheral blood eosinophil count 3942/μL) (Table 1). In addition, chest radiography and CT revealed worsened bronchial mucus plugs and atelectasis in the lingular segment of the left lung (Fig. 2A and B). Even though long-acting muscarinic antagonists were added, and the ICS dose was increased, the symptoms did not improve, and she had poor pulmonary function (Table 1). Therefore, to control asthma with ABPA exacerbation, benralizumab (30 mg subcutaneously every 4 weeks for the first 3 doses and then 8 weeks afterward) was administered. After 2 weeks of benralizumab treatment, her asthmatic symptoms immediately improved, concomitantly with a decrease in peripheral eosinophil count (0 cells/μL). After 2 months of benralizumab treatment, mucus plugs were no longer visible on chest radiography and CT (Fig. 2C and D). After 3 months of treatment, pulmonary function test results showed remarkable improvements; there was no dyspnea relapse and no reappearance of mucus plugs for 10 months.
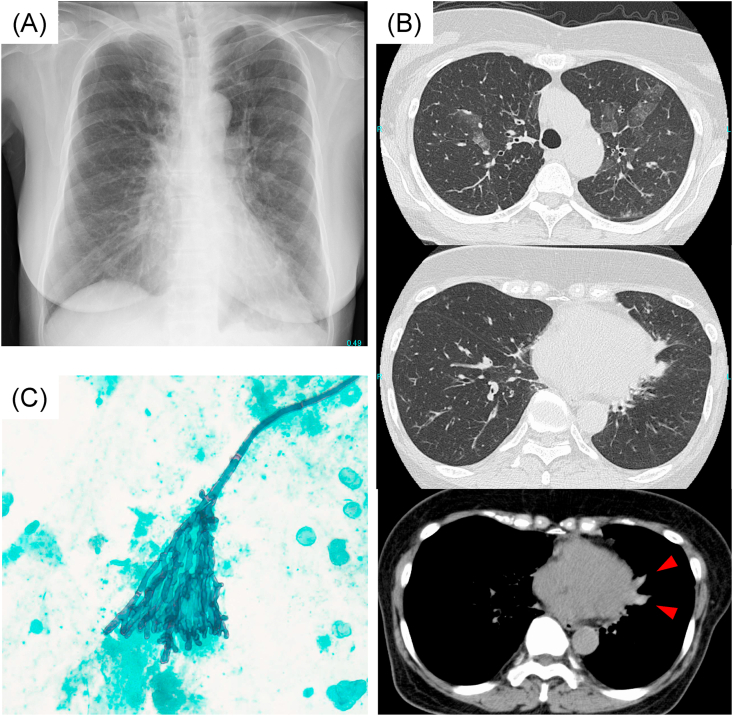
Fig. 1.
Chest imaging appearance and sputum cytology at the time of diagnosis.
(A) Chest radiography on admission showing ground-glass attenuation in bilateral lung fields.
(B) Chest CT revealing ground-glass attenuation in bilateral upper lobe areas and central bronchiectasis with high-attenuation mucus (HAM) (allow) in the left lung.
(C) Sputum cytology showing some filamentous fungi, which were suspected to be Aspergillus spp. (Papanicolaou staining × 200).
Table 1.
The patient's clinical course.
| Pre-treatmentwith prednisolone | Post-treatment with prednisolone (after 3 months) | Pre-treatment with benralizumab | Post-treatment with benralizumab (after 3 months) | |
|---|---|---|---|---|
| WBC (/μl) | 32800 | 14300 | 10800 | 6100 |
| Eosino (/μl) | 24403 | 14.3 | 3942 | 0 |
| IgE (IU/ml) | 1146 | 258 | 469 | 522 |
| FeNO (ppb) | – | – | 28 | 14 |
| FEV1 (ml) (%FEV1 (%)) |
– | – | 1200(66.2) | 1970(108.9) |
| ACT | 13 | 24 | 18 | 24 |
| SpO2 (%) | 93(nasal cannula 2L/min) | 97(Room air) | 94(Room air) | 97(Room air) |
WBC: white blood cell, Eosino: eosinophils cell count, FeNO: fractional exhaled nitric oxide.
FEV1: forced expiratory volume in 1 s, %FEV1: percent predicted forced expiratory volume in 1 s.
ACT: asthma control test.
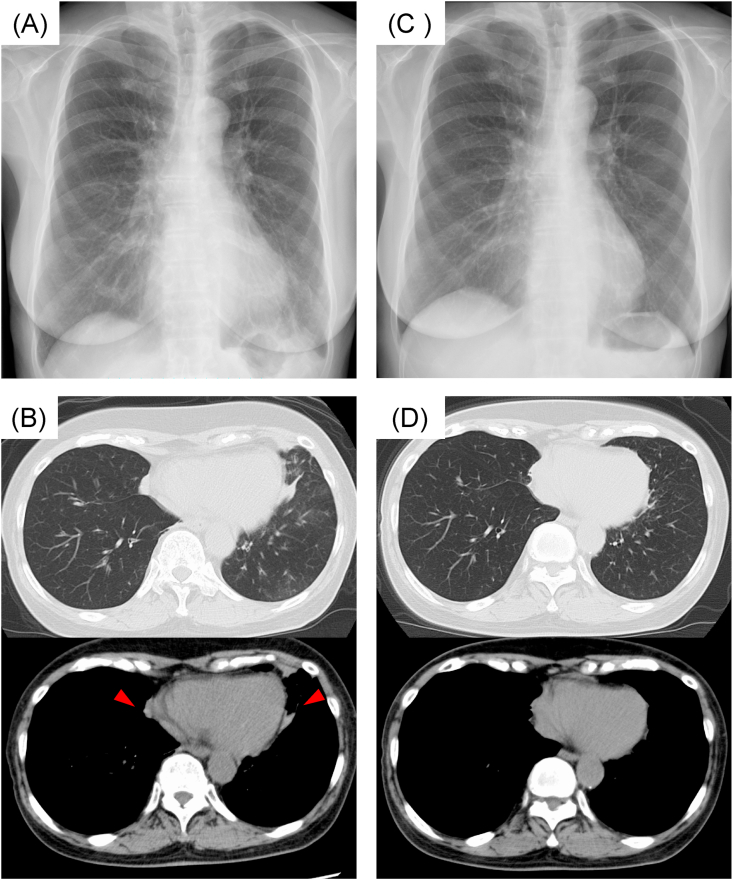
Fig. 2.
Chest imaging appearance before and after treatment with benralizumab.
(A) Chest radiography before treatment with benralizumab showing ground-glass attenuation in the left lower lung field.
(B) Chest CT before treatment with benralizumab showing high-attenuation mucus plugs in bilateral lungs (allow) and atelectasis in the lingular segment of the left lung.
(C) Chest radiography 3 months after treatment with benralizumab, showing improvement in ground-glass attenuation in the left lower lung field.
(D) Chest CT 3 months after treatment with benralizumab, showing high attenuation mucus plugs and atelectasis.
3. Discussion
We describe a case of ABPA with marked eosinophilia that was successfully treated with benralizumab. The patient's asthmatic symptoms and bronchial mucus plugs rapidly improved, followed by a decrease in the peripheral blood eosinophil count.
The Th2 inflammatory response along with IgE and eosinophils is considered as the pathogenesis of ABPA. Interleukin-5 (IL-5) is a cytokine mediator that induces eosinophil development, activation, and proliferation. Eosinophils are activated by IL-5 and form mucoid impactions that damage the bronchial wall. However, recent studies have shown the efficacy of Th2 inflammatory cascade inhibitors. Specifically, omalizumab, (a monoclonal antibody against IgE), mepolizumab, (a monoclonal antibody against IL-5), and benralizumab, (an anti-IL-5 receptor alpha monoclonal antibody), are used for the treatment of ABPA as systemic glucocorticoid-sparing agents. A systematic review reported that omalizumab treatment used in ABPA patients resulted in a significant reduction in serum IgE levels, exacerbation rates, and steroid requirement, while it also improved pulmonary function [3]. Another review presented eight ABPA cases, treated successfully with mepolizumab. Mepolizumab improved pulmonary function, radiological findings, and quality of life [4]. Excluding our patient, only four cases have been described for the use of benralizumab [[5], [6], [7]]. Similar to our case, all patients who were treated with benralizumab showed a rapid improvement in respiratory symptoms, pulmonary function, and radiological findings within 4 months.
Bronchial asthma shows marked heterogeneity in clinical aspects. Asthma can be divided into two different endotypes based on the degree of Th2 inflammation [8]. IgE and eosinophils play a pivotal role in Th2 inflammation. Specifically, IgE is involved early in the inflammatory cascade, leading to allergic airway inflammation, while eosinophilia can be considered as a consequence of the whole process [9]. An anti-IL-5 strategy is often used for bronchial asthma patients with marked eosinophilia. As with bronchial asthma, the biological background should be considered for the treatment of individual ABPA patients as this could predict the patient's likely response to treatment. A systematic review revealed baseline characteristics of ABPA patients treated with omalizumab. According to the review, the average of total IgE level was 1901 IU/mL and the mean specific IgE concentration for A. fumigatus infection was 31.72 IU/mL, while the average eosinophil count was 676/μL [3]. A recent case report described a patient with ABPA whose initial treatment with omalizumab was replaced with benralizumab. The prior oral corticosteroids affected omalizumab dosing according to the true IgE [6]. Similar to this case report, in our patient, the prior long-term oral corticosteroid treatment might have affected the test results of Aspergillus-specific IgE and precipitating antibodies against A. fumigatus. However, peripheral blood eosinophil count was incomparably elevated (24403/μL), suggesting that eosinophils, responsible for bronchial wall damage and the final effector cells in Th2 inflammation, were definitely involved in the pathogenesis in our case. Therefore, we administered benralizumab to treat eosinophilia in a patient with recurrent ABPA; her clinical symptoms, pulmonary function, and radiological findings were rapidly and remarkably improved.
High-resolution chest CT in patients with ABPA often demonstrates bronchiectasis, mucoid impaction, tree-in-bud opacities, centrilobular nodules, mosaic attenuation, and pleuropulmonary fibrosis [10]. Patients with central bronchiectasis sometimes present HAM. The occurrence of HAM has been shown to affect approximately 20–41% of patients with ABPA [11,12]. Several studies have reported that the presence of HAM is associated with the severity and recurrence of ABPA [11,13]. Moreover, patients with HAM present higher peripheral blood eosinophil cell counts and higher rates of Aspergillus detection isolated in sputum, similarly to our case [13]. These findings indicate that the degree of eosinophilic inflammation might be correlated with mucus plug formation and the risk of relapse. Recent evidence highlights the eosinophil-derived cytolytic extracellular trap cell death (ETosis) in the formation of eosinophilic mucoid impaction, especially in ABPA. Activated eosinophils can release extracellular chromatin to form DNA traps through ETosis [14]. Benralizumab is an IgG1κ mAb that targets the epitope of the α subunit of the IL-5 receptor on the eosinophil surface and inhibits IL-5-mediated cell proliferation [15]. In addition, benralizumab can activate NK cells and macrophages to induce antibody-dependent cytotoxicity (ADCC) against both eosinophils and basophils [16,17]. As a result, within 24 hours of the first dose of benralizumab, the blood eosinophils were completely depleted, and sputum and tissues were almost completely depleted [18]. In fact, all ABPA patients (including our case) treated with benralizumab showed a rapid improvement of symptoms, and one patient demonstrated rapid clearance of mepolizumab-resistant bronchial mucus plugs. For the prevention of central bronchiectasis, it is important to control HAM as a point of treatment for recurrent ABPA. Benralizumab may be more effective through the regulation of eosinophilic inflammation in ABPA patients with marked eosinophilia and HAM.
In this report, we described a case of an ABPA patient with recurrent exacerbation of asthmatic symptoms that were successfully treated with benralizumab. The degree of eosinophilia and the presence or absence of HAM might indicate which biologic drug is a suitable option for patients with ABPA.
Patient consent for publication
Written informed consent was obtained from the patient.
Funding
None.
Declaration of competing interest
All the authors declare no relevant conflict of interest.
Acknowledgements
None.
References
- 1.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135:805–826. doi: 10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 2.Alastruey-Izquierdo A., Cadranel J., Flick H., Godet C., Hennequin C., Hoenigl M., Kosmidis C., Lange C., Munteanu O., Page I. Treatment of chronic pulmonary aspergillosis: current standards and future perspectives. Respiration. 2018;96:159–170. doi: 10.1159/000489474. [DOI] [PubMed] [Google Scholar]
- 3.Li J.X., Fan L.C., Li M.H., Cao W.J., Xu J.F. Beneficial effects of Omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literature. Respir. Med. 2017;122:33–42. doi: 10.1016/j.rmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Tolebeyan A., Mohammadi O., Vaezi Z., Amini A. Mepolizumab as possible treatment for allergic bronchopulmonary aspergillosis: a review of eight cases. Cureus. 2020;12 doi: 10.7759/cureus.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soeda S., Kono Y., Tsuzuki R., Yamawaki S., Katsube O., To M., To Y. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J. Allergy Clin. Immunol. Pract. 2019;7:1633–1635. doi: 10.1016/j.jaip.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Bernal-Rubio L., de-la-Hoz Caballer B., Almonacid-Sánchez C., González-de-Olano D. Successful treatment of allergic bronchopulmonary aspergillosis with benralizumab in a patient who did not respond to omalizumab. J Investig. Allergol. Clin. Immunol. 2020;30:378–379. doi: 10.18176/jiaci.0564. [DOI] [PubMed] [Google Scholar]
- 7.Tomomatsu K., Sugino Y., Okada N., Tanaka J., Oguma T., Asano K. Rapid clearance of mepolizumab-resistant bronchial mucus plugs in allergic bronchopulmonary aspergillosis with benralizumab treatment. Allergol. Int. 2020;69:636–638. doi: 10.1016/j.alit.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff P.G., Modrek B., Choy D.F., Jia G., Abbas A.R., Ellwanger A., Koth L.L., Arron J.R., Fahy J.V. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matucci A., Vultaggio A., Maggi E. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018;19:113. doi: 10.1186/s12931-018-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur M., Sudan D.S. Allergic bronchopulmonary aspergillosis (ABPA)-The high resolution computed tomography (HRCT) chest imaging scenario. J. Clin. Diagn. Res. 2014;8:RC05–7. doi: 10.7860/JCDR/2014/8255.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R., Gupta D., Aggarwal A.N., Saxena A.K., Chakrabarti A., Jindal S.K. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007;132:1183–1190. doi: 10.1378/chest.07-0808. [DOI] [PubMed] [Google Scholar]
- 12.Oguma T., Taniguchi M., Shimoda T. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol. Int. 2018;67:79–84. doi: 10.1016/j.alit.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Lu H.W., Mao B., Wei P. The clinical characteristics and prognosis of ABPA are closely related to the mucus plugs in central bronchiectasis. Clin. Res. J. 2020;14:140–147. doi: 10.1111/crj.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muniz V.S., Silva J.C., Braga Y.A.V., Melo R.C.N., Ueki S., Takeda M., Hebisawa A., Asano K., Figueiredo R.T., Neves J.S. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J. Allergy Clin. Immunol. 2018;141:571–585. doi: 10.1016/j.jaci.2017.07.048. e7. [DOI] [PubMed] [Google Scholar]
- 15.Menzella F., Bertolini F., Biava M., Galeone C., Scelfo C., Caminati M. Severe refractory asthma: current treatment options and ongoing research. Drugs Context. 2018;7:212561. doi: 10.7573/dic.212561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolbeck R., Kozhich A., Koike M., Peng L., Andersson C.K., Damschroder M.M., Reed J.L., Woods R., Dall'acqua W.W., Stephens G.L. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010;125:1344–1353. doi: 10.1016/j.jaci.2010.04.004. e2. [DOI] [PubMed] [Google Scholar]
- 17.Caminati M., Menzella F., Guidolin L., Senna G. Targeting eosinophils: severe asthma and beyond. Drugs Context. 2019;8:212587. doi: 10.7573/dic.212587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazi A., Trikha A., Calhoun W.J. Benralizumab--a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity--a novel approach for the treatment of asthma. Expet Opin. Biol. Ther. 2012;12:113–118. doi: 10.1517/14712598.2012.642359. [DOI] [PMC free article] [PubMed] [Google Scholar]