Abstract
Diabetes mellitus affects the heart through various mechanisms such as microvascular defects, metabolic abnormalities, autonomic dysfunction and incompatible immune response. Furthermore, it can also cause functional and structural changes in the myocardium by a disease known as diabetic cardiomyopathy (DCM) in the absence of coronary artery disease. As DCM progresses it causes electrical remodeling of the heart, left ventricular dysfunction and heart failure. Electrophysiological changes in the diabetic heart contribute significantly to the incidence of arrhythmias and sudden cardiac death in diabetes mellitus patients. In recent studies, significant changes in repolarizing K+ currents, Na+ currents and L-type Ca2+ currents along with impaired Ca2+ homeostasis and defective contractile function have been identified in the diabetic heart. In addition, insulin levels and other trophic factors change significantly to maintain the ionic channel expression in diabetic patients. There are many diagnostic tools and management options for DCM, but it is difficult to detect its development and to effectively prevent its progress. In this review, diabetes-associated alterations in voltage-sensitive cardiac ion channels are comprehensively assessed to understand their potential role in the pathophysiology and pathogenesis of DCM.
Keywords: Diabetes, Action potential, Cardiac ion channels, L-type Ca2+ channels, Potassium channels, Sodium channels
Core Tip: Diabetes mellitus is a multisystemic disease that affects many organs. It causes diabetic cardiomyopathy (DCM) in the heart which is a distinctive pathology that occurs independent of vascular complications. In DCM, altered action potential morphology and contractile dysfunction are mostly associated with defective cardiac ion channels such as voltage-gated K+, Na+ and Ca2+ channels. Therefore, with therapeutic agents specific to cardiac ion channels, both arrhythmogenic events and other functional problems can be mitigated in the diabetic heart.
INTRODUCTION
Diabetes mellitus (DM) is a complex and heterogeneous chronic metabolic disease caused by high blood sugar levels. Diabetic heart disease is a growing and serious public health risk which affects more than 350 million people worldwide[1]. Considering the fact that these figures refer to the year 2011, it is unfortunate to note that the numbers will increase much more in the coming years. DM is divided into four different etiological categories: Type 1, type 2, gestational DM and other specific types. Type 1 DM results from T cell-mediated autoimmune destruction of pancreatic cells which leads to insulin deficiency[2] and it mostly occurs in young people, usually up to the age of 30. Type 2 DM is characterized by both insulin resistance and the failure of pancreatic cells. Other specific types of DM are caused either by other pathological diseases of the pancreas due probably to single genetic mutations or drugs. Gestational diabetes, on the other hand, develops during pregnancy.
The mortality rate associated with DM due to cardiovascular disease is 65%. It is, therefore, considered a risk equivalent to coronary heart disease and generally affects the heart in three ways: Cardiac autonomic neuropathy, coronary artery disease (CAD) and diabetic cardiomyopathy (DCM)[3]. DCM is characterized by abnormal myocardial structure and reduced contractile performance even in the absence of other risk factors such as CAD, hypertension and significant valvular heart disease in individuals with DM. It was first described in the postmortem pathological findings of 4 diabetic patients who showed heart failure (HF) symptoms without coronary artery or valvular heart disease in 1972, and it was later confirmed in diabetic women with a 5-fold higher incidence of HF in the Framingham Heart Study in 1974[4,5]. DCM was described in 2013 as a clinical condition of ventricular dysfunction in patients with DM in the absence of coronary atherosclerosis and hypertension, in collaboration with the American College of Cardiology Foundation, the American Heart Association, the European Society of Cardiology and the European Association for Diabetes Research[6,7].
In the early stages of DM, significant changes occur in myocardial function and structure due to DCM, and these changes include left ventricular hypertrophy, increased fibrous tissue and cell signal abnormalities. These pathological changes cause cardiac contractile and diastolic dysfunction associated with ventricular fibrosis and hypertrophy which are the earliest pathophysiological complications in DCM[8,9]. Mechanisms underlying these changes include hyperglycemia, systemic and cardiac insulin resistance, increased free fatty acid levels, systemic and tissue inflammation, oxidative stress, renin-angiotensin-aldosterone system and activation of the sympathetic nervous system[10]. On the other hand, systolic dysfunction develops in the later stages of the disease and may be caused by diastolic dysfunction and decreased cardiac compliance resulting from the progression of DCM[8-10]. Fur-thermore, when systolic dysfunction occurs, the cardiac output gradually decreases with the severity of the disease and thus leads to HF. Consistent with this, The Framingham Heart Study showed that the frequency of HF was five times higher in diabetic women and two times higher in diabetic men than in age-matched control subjects[11]. HF leads to a low quality of life in individuals and makes it quite difficult to treat DM by simply changing the pharmacokinetics of anti-diabetic drugs. Therefore, diagnosing these patients faster and treating them earlier is extremely important. This review focuses on the role of voltage-sensitive ion channels in the electrophysiological disturbance of the diabetic heart and thus provides refined evidence that enables the understanding of the molecular mechanisms underlying the pathogenesis of DCM which may help to develop diagnostic methods and treatment strategies.
ELECTROPHYSIOLOGICAL CHANGES IN THE DIABETIC HEART
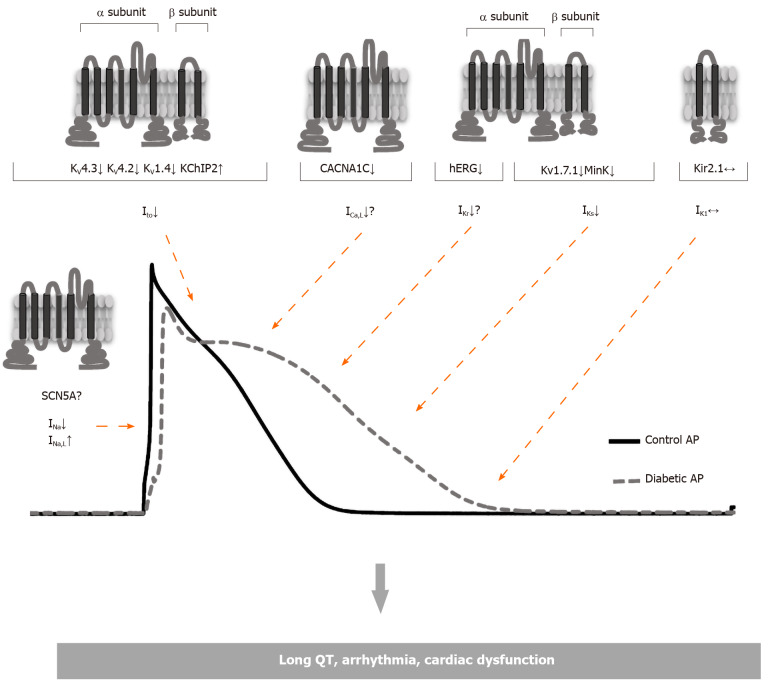
In diabetic patients, the incidence of cardiac arrhythmia is higher, as well as ventricular fibrillation and sudden death, and significant changes mostly associated with the repolarization of ventricles are observed in the electrocardiogram (ECG). Diabetic patients have higher heart rates, lower ECG potential amplitudes and more T-wave inversions than normal individuals. In addition, DM leads to sudden cardiac deaths that may be associated with an increase in the QT interval[12,13]. In type 1 DM, prolonged QTc interval and increased QTc dispersion have been observed[14,15]. In follow-up using Holter ECG monitoring, the occurrence of ventricular late potentials in patients with type 1 DM is observed more frequently than in healthy people and is more common in patients with type 2 DM[4,5]. This increased QTc interval is thought to be associated with an increased risk of mortality, like non-diabetic subjects with QTc prolongation[16,17]. Studies performed in twins have shown that QTc is longer in type 1 diabetic than non-diabetic subjects which implies that QTc prolongation is caused by diabetes rather than genetic factors[18]. The changes in the ECG are mostly associated with the prolonged cardiac action potential (AP) which is mostly ascribed to diabetes-induced alterations in repolarizing potassium currents (Figure 1)[19-21]. On the other hand, experimental studies have shown that these changes in repolarizing currents of cardiac myocytes can be different depending on the species of animal used, the type and duration of diabetes (Table 1)[22-25].
Figure 1.
Pathological alterations in voltage-gated cardiac ion channels that contribute to the action potential of the ventricular myocytes due to diabetes mellitus.
Table 1.
Overview of diabetes mellitus-induced alterations in cardiac K+ currents
DIABETES-INDUCED ALTERATIONS IN CARDIAC ACTION POTENTIAL
DCM is often associated with impaired contraction and ECG abnormalities. The changes in the ECG that have been attributed to prolonged cardiac AP duration arise due to a decrease in repolarizing potassium currents caused by diabetes (Figure 1)[19-21].
The set off and regular spread of cardiac electrical stimulation depends on the formation of a normal cardiac AP throughout the myocardium. Depolarization and repolarization of AP are mediated by multiple inward and outward currents passing through specific membrane ion channels. The initial depolarization phase is generated by the inward Na+ current (INa), mainly through voltage-sensitive sodium channels (Nav1.5), in the form of a rapid upstroke. In the subsequent early repolarization and plateau phases, the transient outward K+ current and the inward L-type Ca2+ current (ICaL) are prevalent, respectively. During this process, the Ca2+ ions entering through L-type Ca2+ channels (LTCC) induce a large amount of Ca2+ release from the sarcoplasmic reticulum (SR), thereby activating the excitation-contraction coupling. The repolarization, which ultimately returns the membrane to its resting potential, is mainly driven by the outward current through the voltage-gated K+ channels (Kv)[26]. K+ channel activity is the main determinant of AP duration as it limits depolarization time and the refractory period as well as the time period of Ca2+ -mediated contraction. There are numerous and diverse types of K+ channels, each with specific kinetic and voltage dependent properties. They have specific roles in different repolarization stages of cardiac AP such that they determine the repolarization time and repolarization reserve as well as maintaining the resting membrane potential. The repolarization reserve refers to the partially overlapping function of these currents, namely rapid delayed rectifier K+ currents (IKr), slow delayed rectifier K+ currents (IKs) and inward rectifier K+ currents (IK1)[27]. Repolarization kinetics of these K+ currents is highly variable depending on the region of the heart and the species studied. This reflects the difference in the expression and density of different K+ channel subtypes. Experimental studies have shown that different repolarizing currents decrease depending on the type of animal used to induce type 1 DM[28-30]. In the human heart ventricle, the main repolarizing currents are fast transient-outward K+ current (Ito,f), slow transient-outward K+ current (Ito,s), IKr, IKs and steady-state K+ current (Iss), while they are Ito,f, ultra-rapid delayed rectifier K+ current (IKur) and IKs current in the atrium. All these features suggest that the investigation of the K+ channels is important for understanding the mechanisms underlying cardiac dysfunction and arrhythmias caused by DCM and this can be a useful pharmacological target for the development of therapeutic agents.
POTASSIUM CURRENTS IN DIABETIC CARDIOMYOCYTES
K+ channels represent the most functional and diverse types of cardiac ion channels[31-34]. They tightly regulate the cardiac repolarization, thereby providing a stable and consistent AP signal. Different K+ channel types may have overlapping functions that provide some degree of functional redundancy and thereby contribute to the repolarization reserve[27,35]. All of the α-subunits of different K+ channel types have a pore-forming region that has a selective permeability to the K+ ion. This can be associated with a particular structural motif and allows K+ movement from the plasma membrane under the effect of an electrochemical gradient. In addition, there are ligand binding sites that can change the channel conformation and gating mechanisms in response to membrane depolarization.
Transient outward potassium current (Ito)
In diabetic patients, the incidence of cardiac arrhythmia, ventricular fibrillation and sudden cardiac death is higher, and most of them have significant changes in ECG recordings due most probably to abnormal AP repolarization. Accordingly, in myocytes isolated from diabetic hearts, Ito is the mainly affected repolarizing current.
Ito is basically responsible for the early repolarization phase of the AP. Two subtypes of Ito are defined; one is blocked by 4-aminopyridine (4-AP) and not dependent on Ca2+ (Ito1), while the other is not blocked by 4-AP but modulated by Ca2+ (Ito2)[34]. Cardiac regions with shorter AP duration, such as the epicardium, right ventricle, and septum, have higher transient outward K+ channel expression. Due to their discrete characteristics, Ito1 currents are subdivided into Ito,f and Ito,s components. Ito,f and Ito,s currents are both present in the ventricles, however, Ito,f is the dominant current expressed in the atrium[36]. Although the Ito,s currents have not so long inactivation time, their classification as "slow" is only relative to Ito,f. Nevertheless, both Ito,f and Ito,s channels are activated and inactivated rapidly compared to other K+ channels.
Many studies have been conducted in rats to elucidate the cellular mechanisms of diabetes-induced repolarization abnormalities[19,37-43]. In these studies, it has been shown that the Ito amplitude reduction which is responsible for the prolongation of AP repolarization in diabetic rats is associated with downregulation of the expression of Kv4.3 and Kv4.2 channel proteins[39-42,44]. However, an increase in the protein expression of Kv1.4, which is responsible for the regulation of Ito,s currents has been reported[29,44,45]. In the case of a depressed Ito channel, protein expression may change in the opposite direction and thus the upregulation of Kv1.4 and KChIP2 may be associated with a decrease in Kv4.3 expression (Figure 1). Consistently, in the Kv4.3 gating model supported by Patel et al[46], KChIP2 isoforms have suggested an acceleration in the recovery from inactivation and promotion of the open-state inactivation with slower closed-state inactivation. As a result, the upregulated KChIP2 causes slower inactivation in depolarized potentials and enhances the re-opening of the Ito channels during membrane repolarization. This eventually increases the repolarizing force in the plateau phase which may contribute to late repolarization[46]. However, in rat myocardium, ventricular repolarization includes different mechanisms to dogs, humans, and other mammals as it lacks a pronounced plateau phase and has a short AP duration. Therefore, these results obtained in rats have relatively limited value in understanding the repolarization abnormalities observed in diabetic myocardium. Expression of ion channel proteins has also been widely investigated to elicit the molecular mechanisms underlying electrophysiological changes by generating an experimental type 1 DM model in animal species with a pattern of cardiac repolarization and ionic currents that are more similar to those in the human heart. In dog cardiomyocytes, both Ito reduction and downregulation of Kv4.3 protein (the dominant subunit forming the pore in dog and human ventricular myocytes) are consistent with the data obtained in rats[29]. As a result, Ito currents and expression of those proteins involved in channel regulation are consistent in rats and dogs. Contrary to these findings obtained in other studies, no significant change in Ito current was observed in rabbits. The reason for this discrepancy can be explained by the fact that the rabbit Ito current has a different molecular basis. In rabbits, Ito is mediated mostly by Kv1.4 channels, but not Kv4.3 channels as in rats, dogs and humans[47]. Nevertheless, different results have also been obtained in animal models in which the experimental type 2 DM model was induced by different methods. In myocytes isolated from db/db mice, a leptin receptor mutant showing type 2 DM symptoms, K+ currents have been shown to decrease and AP duration is prolonged[48]. On the other hand, there was no change in K+ currents and AP duration measured in the type 2 DM rat model induced by feeding on a diet enriched with fructose for 6-10 wk[49]. Thus, it can be concluded that ionic currents and the expression of protein channel domains that precipitate the prolongation of AP duration observed in diabetes vary according to the animal species studied, the diabetes model created and duration of the diabetic condition.
Although studies have demonstrated that DM causes a significant decrease in Ito,f and Ito,s current amplitudes, neither the voltage dependence of the inactivation nor the time dependency of the reactivation has changed[20,30,50,51]. However, contrary to the results generally obtained, in long-term diabetes (24-30 wk) significant changes in inactivation and reactivation kinetics of Ito have also been reported in rat cardiac myocytes[52]. Therefore, it seems likely that different results in channel kinetics will be seen depending on the duration of the diabetic state.
Two hypotheses have been proposed regarding the effects of type 1 DM on potassium currents in the heart muscle. The first hypothesis involves insulin deficiency as it affects the gene expression of a large number of proteins, including potassium channel proteins[53]. Incubation of diabetic cardiac myocytes with insulin for 6 h restored the Ito,f to control values and this effect was prevented by protein synthase inhibitors[54]. In a study using a cardiomyocyte-restricted insulin receptor knockout (CIRKO, cardiac-specific insulin receptor knockout) mouse model, impaired insulin signaling resulted in a decrease in mRNA and protein expression of K+ channels prominent in ventricular repolarization. Specifically, in isolated left ventricular CIRKO myocytes, Kv4.2 and KChiP2 expression decreased, consistent with a decrease in Ito,f amplitude. The alleviated Ito,f in turn resulted in a prolonged ventricular AP and prolonged QT interval in surface ECG[30]. These results support the idea that the lack of insulin signal in the heart is sufficient to cause repolarization abnormalities described in other diabetic animal models. The second hypothesis assumes that the cause of decreased cardiac Ito,f in DM is defective glucose metabolism. This hypothesis was supported by the reversal of potassium currents in diabetic cardiomyocytes to normal levels after 6 h of incubation with metabolic enhancers such as L-carnitine, glutathione, or pyruvate[38,51,55].
Activation of the renin-angiotensin system has also been demonstrated in insulin-dependent diabetic rats, and Ito has been reduced with increased angiotensin II (Ang II) levels[56]. Inhibition of the production or action of Ang II has been found to reverse the decreased Ito in both type 1 and type 2 DM. In ventricular myocytes of streptozotocin (STZ)-induced type 1 DM rats, decreased Ito and Iss currents have been shown to be significantly increased after incubation with the Ang II receptor blockers saralasin or valsartan[48]. Incubation of ventricular myocytes isolated from the mutant db/db mice with valsartan (> 6 h) has been shown to reverse the reduced Ito and Iss currents[48]. These results confirm that cardiac myocytes contain a local renin-angiotensin system that is activated in diabetes. These effects of Ang II can be explained by the fact that it has a large number of various cellular effects mediated by protein kinase A, protein kinase C and tyrosine kinases that may lead to inhibition of some ionic channels. It is suggested that the changes caused by the chronic release of Ang II on ionic currents and AP can be eliminated by blocking the formation or effect of Ang II. Consistently this implies that Ang II receptor blockage or angiotensin-converting enzyme inhibition can protect against cardiac arrhythmias that may occur in DCM. However, more studies are needed to explain these elaborate findings unequivocally.
Delayed rectifier potassium current
Delayed rectifiers, along with other ion channels, mainly determine the waveform as well as the AP duration and thus play critical roles in heart physiology and pathophysiology (Figure 1). Disruption of the normal functions of the delayed rectifier channels makes the heart more sensitive to abnormal electrical activity and prone to arrhythmia. This class of K+ channels includes IKs, IKr and atrial specific IKur channels.
Like Ito, IKur is also effective in the early repolarization phase of the AP. This current quickly activates in less than 10 milliseconds at plateau voltage ranges and slowly disappears through the AP repolarization period[57-59]. IKur current is the dominant delayed rectifier current for atria, and therefore shorter AP duration is seen in the atrial myocytes compared to ventricles[36,57,59,60]. In regions where IKur currents are observed, ion channels are not evenly distributed on the myocyte membrane and are mostly localized in the intercalated discs[32]. This specific localization of IKur in the atrium makes it an interesting target for atrial selective treatment, so that inhibition of IKur prolongs the AP duration of atrial myocytes, this prolongation is not observed in the ventricles[32].
On the other hand, IKr currents are critical for phase 3 repolarization of AP. It shows a relatively rapid activation with depolarization, however, the rate of inactivation is about 10 times higher than the rate of activation. This ensures that these channels are relatively non-conductive during the 1st and 2nd stages of cardiac AP[61-63]. So even though this current is called delayed rectifier, it also shows an inward rectification property at positive potentials[24,62,63]. However, as the membrane potential reaches 0 mV with the end of phases 1 and 2, IKr is activated once again, but deactivation is much slower during this phase. This causes a large outward flow of K+ ions during phase 3 repolarization[27,64]. IKr is found in both the atrium and ventricles of humans but is expressed at higher levels in the left atrium and ventricular endocardium[36].
Cardiac repolarization is also affected by the IKs current which is slowly activated at potentials around -20 mV. Unlike IKr, IKs is almost completely inactive in phase 2 repolarization and significantly affects phase 3 repolarization of cardiac AP[27,65,66]. This feature of IKs is especially important in relatively longer atrial and ventricular APs. It is also important for the reactive shortening of AP duration during a physiological increase in heart rate. Namely, a significant increase in the heart rate leads to a decrease in the inactivation time of IKs current which results in higher IKs and a steeper decrease in the repolarization phase of AP[67,68]. Blocking IKs current causes a prolongation in AP duration at particularly increased heart rates[68]. Inhibition of IKs current can also increase the reactivation of voltage-sensitive Ca2+ channels, thereby increasing the risk of arrhythmic events[27]. All cardiac cell types have IKs, but their expression is significantly reduced in the middle of the myocardial wall; this explains the longer AP duration in this region[36].
Studies performed using various animal models have reported a decreased IKs current in diabetic dog and rabbit hearts[28,29]. However, there are controversial findings regarding the effects of diabetes on IKr current because it has been reported to decrease in diabetic rabbits[21] or not to change in diabetic rabbits, dogs, and mice[28,29,51]. A significant reduction in IKr current and hERG expression along with a prolonged QTc interval have been demonstrated in alloxan-induced diabetic rabbits[21,69,70]. In these reports, the DM-induced changes are apparent after an 11-wk period, whereas QTc prolongation is less pronounced in rabbits at the end of the 3-wk diabetes period, and no changes were observed in the IKr current[28]. These results show that DM-induced changes in different ionic currents of cardiac myocytes may develop at different time points of the disease. However, neither activation nor deactivation kinetics of IKr current have been changed in diabetic cardiac myocytes[28]. In addition, it has been shown that the suppression of IKs current along with a moderate extension of the QTc interval occurs at an early stage, such as the third week of DM. However, after the eighth week of alloxan-induced diabetes, no change in IKr current has been observed, whereas IKs current was suppressed in dog myocytes[29]. In addition to the fact that the regional differences in AP duration and left ventricular ionic currents are important in the reduction of the repolarization reserve in diabetes, the severity of diabetes is also prominent in the extent of these changes[37,71-73]. Therefore, more experimental and clinical data are needed to clarify this issue.
It is surprising that the decrease in the density of the IKs and the expression of the regulatory β-subunit channel protein, MinK, is associated with increased expression of the pore-forming α-subunit Kv1.7.1[74]. On the other hand, there may be more direct interactions between Kv1.7.1 and hERG, which are α subunits of IKs and IKr currents, respectively, and Kv1.7.1 can modulate not only the distribution but also the biophysical properties of hERG. Indeed, Kv1.7.1 overexpression has been shown to elicit a dramatic increase in hERG current density[74]. Therefore, it can be considered that the downregulation of MinK is the primary result of diabetes, and the concomitant upregulation of Kv1.7.1 may be a secondary compensatory process that can partially oppose the downregulation of MinK arising due to diabetes. Besides, it has been demonstrated that hERG is negatively modulated by hyperglycemia, tumor necrosis factor, ceramide and reactive oxygen species which are cellular metabolites accumulated in diabetic tissues[75,76]. It has also been reported that insulin metabolism affects the hERG expression as well as IKr/hERG function and it is quite possible for insulin to modulate different ion channels through separate mechanisms[21]. These results in total suggest that IKr/hERG is a potential target for the treatment of cardiac arrhythmias in diabetic patients.
Deschênes et al[77] reported that when Kv4.3 was co-expressed with MinK, the current density was five times higher than that of Kv4.3 expressed alone and that the inactivation and reactivation kinetics of Kv4.3 slowed down through MinK. Therefore, modifying MinK by diabetes can at least partially explain the diminution of Ito density in the dog myocytes. It is also worth noting that insulin administration can completely prevent the diabetes-induced reduction of IKs current (and associated changes in the expression of channel proteins), but only a limited protective effect on Ito. The reason for this discrepancy remains uncertain, but it should be noted that patients with type 1 DM may have an increased proarrhythmic risk even when they are treated regularly with insulin.
Inward rectifier K+ current (IK1)
Inward rectifier K+ current is active in a narrow membrane potential range. The rectifying property results in a marked decrease in IK1 conductivity in positive depolarized membrane potentials and an increase in IK1 current in negative membrane potentials. As a result, this has the effect of stabilizing the resting membrane potential close to the K+ equilibrium potential[64]. The channel mediating the IK1 current does not show voltage-dependent activation and does not have a voltage sensor. However, the IK1 current modulation associated with the movement of Mg2+ and polyamines provides indirect sensitivity of the channel to voltage[78-81]. Since the channel is inhibited by Mg2+ and polyamines at membrane potentials more positive than 20 mV, IK1 channel has no conductivity between phase 0 and phase 2 of the AP. When the potential returns to more negative values (typically around -40 mV), the blockade mediated by Mg2+ and polyamines on IK1 channel conductivity is relieved, and this contributes to the phase 3 repolarization of cardiac AP[80]. IK1 current is present in both atria and ventricles and therefore plays an important role in determining resting membrane potentials. Channels that transmit IK1 current are more expressed in the ventricles, making the ventricles less sensitive to the pacemaker effect[36].
As mentioned, IK1 current has been one of the most widely studied K+ currents in DCM due to its importance in stabilizing the membrane potential and its contribution to AP duration (Figure 1). In these studies where different animal species (mouse, rat, rabbit, and dog) were used and different diabetes periods were applied (3 wk, 4 wk, 8 wk, 10 wk) changes in IK1 were extensively examined. However, neither the IK1 current amplitude[19,20,38,49,50] nor the expression of Kir2.1, the main component of the IK1 channel, changed in the diabetic heart[69,82]. Therefore, these findings confirming the absence of a shift in resting membrane potential of diabetic cardiomyocytes imply that IK1 current is less likely to contribute to DM-induced AP prolongation as well.
VOLTAGE-GATED Ca2+ CHANNELS IN DIABETIC CARDIOMYOCYTES
Intracellular Ca2+ dysregulation is a well-defined complication in DCM and it has been demonstrated in both type 1 and type 2 DM (Figure 1 and Table 2)[83-86]. Although the cellular mechanisms underlying this impaired Ca2+ handling have not been fully explained, a significant decrease in SR Ca2+ content associated with reduced SERCA2 expression/activity, decreased phospholamban phosphorylation and increased ryanodine receptor (RyR) Ca2+-leak have been widely reported in type 1 and type 2 diabetic heart myocytes, and as a result the diastolic Ca2+ concentration increased and the amplitude and decay rate of the Ca2+ transients significantly decreased[40,84,87-91]. Also, there was a decrease in Na+-Ca2+ exchanger (NCX) expression in type 1 diabetic myocardium[92]. In addition to these findings, the role of LTCC in the impaired Ca+2 handling in DCM has not been fully clarified.
Table 2.
Changes in L-type Ca2+ and Na+ currents in diabetic heart myocytes
Basically, bulk Ca2+ release from SR into the cytosol is mediated by activation of RyR which is triggered by inward Ca2+ current through LTCC. This mechanism is described as Ca2+-induced Ca2+ release and it is a critical event for excitation-contraction coupling in cardiac myocytes[93,94]. LTCC which acts as a trigger for excitation-contraction coupling is an ion channel family with four different members. Of these, CaV1.2 is the main Ca2+ channel expressed in cardiomyocytes which has four homologous domains and expressed by CACNA1C or α1C[95]. Each of these domains is characterized by six transmembrane α-helix structures. This channel has a current-voltage relationship that activates at the potential value of -40 mV, gives the maximum amplitude at potentials between 0-10 mV, and reverses at +60 to +70 mV[96,97].
Many studies have examined LTCC in DCM. However, contradictory findings have been reported about the activity of LTCC in these studies (Table 2). Some of them have demonstrated an unchanged current-voltage relationship of LTCC in DCM[29,50,98-102]. In these studies, diabetes duration is generally less than 10 wk (4-8 wk)[50,99,101,102]. The lack of significant change in L-type Ca2+ current (ICaL) despite the reduced Ca2+ transient amplitude and slowed rate of removal indicates that the coupling between the LTCC and RyR is impaired in DCM[99,101]. Accordingly, Lacombe et al[101] showed a reduced gain in the Ca2+-induced Ca2+ release mechanism of diabetic myocytes due most probably to altered LTCC-RyR coupling. Dysregulation of intracellular Ca2+ handling in the diabetic heart might also be mediated by alterations in NCX, SERCA2, PLB and RyR expression or phosphorylation[98].
However, some other studies have reported significant changes in the amplitude of ICaL current[52,89,103-108]. This discrepancy regarding the activation of ICaL current might have arisen due to two main factors: the diabetes model used in the study and the duration of the diabetic state. For type 2 DM, transgenic animal models have been mostly used and, in these studies generally, ICaL current has been found to decrease in ventricular myocytes[89,105-107]. However, in the type 1 DM models induced by chemical agents, the experimental period varies between 4 and 12 wk and this may at least partially explain the difference observed in ICaL current densities[109]. Nevertheless, some studies using a similar duration of diabetes have also demonstrated different ICaL current amplitudes in ventricular myocytes. In general, it is most likely that there is a decrease in the amplitude of ICaL current of the ventricular myocytes in the STZ-induced DM model after ten or more weeks of diabetes duration[52,103]. Similar to that of the type 2 DM model, the density of ICaL current decreased in cardiac myocytes of transgenic animals with type 1 DM[104].
In the studies where ICaL current amplitude was found to be decreased in diabetic heart myocytes compared to that of control, it was approximately 15%-30% lower in the negative membrane potentials range, and this difference maintains up to +20 mV[89,104,106,108]. This reduction in ICaL current may be due to the activation/inactivation kinetics of the channels, the expression of the channel proteins, or the change in the single-channel conductance. Pereira et al[89] measured the single-channel current in diabetic myocytes to test whether it is the likely explanation for the reduced ICaL current and they did not find a significant difference compared to control myocytes. Thus, it was concluded that the activity of the single-channel current is not responsible for the decreased macroscopic ICaL currents in the diabetic heart. Instead, it can be ascribed to the altered channel kinetics or reduced expression of channel proteins due to diabetes.
Considering that ICaL current reaches its maximum value between 0-10 mV membrane potentials, the DCM-related decrease in current amplitude may have occurred due to the altered channel kinetics. As a matter of fact, the potential value required for half of the channels to be open (Vh) has shifted to more positive values in diabetic myocytes. This may explain why fewer LTCC channels are opened at lower potentials and why the measured current is lower. There was no significant difference observed in the half-inactivation potential (V1/2) where half of the channels are closed and recovered from inactivation in diabetic myocytes[89,104,106,110].
Another explanation for the reduced ICaL current in DM is the change in channel protein expression. As mentioned earlier, CaV1.2 is the main Ca2+ channel type expressed in the heart, and studies have shown that expression of the a1C subunit of CaV1.2 decreases in type 1 and type 2 diabetic hearts[89,104]. Therefore, it is likely that the decrease in ICaL is due to both the change in LTCC activation and the change in channel expression[111].
The physiological mechanisms underlying this decrease in ICaL current in diabetic cardiac myocytes could be the phosphatidylinositol 3-kinase (PI3K)/Akt pathway[112-114]. Consistently, activation of the PI3K/Akt pathway, which is a potent modulator of ICaL currents, is downregulated due to diabetes and this decrease triggered the reduction of ICaL in diabetic myocytes[115]. It is known that insulin or insulin growth factor (IGF-1) mediated activation of PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to form phosphatidylinositol 3,4,5-trisphosphate (PIP3), and thus PIP3 synthesis stimulates Akt (protein kinase B)[112]. Recent studies have shown that the PI3K/PIP3/Akt pathway performs its mediator effect by providing phosphorylation of the CaVβ2 subunit of LTCC[116,117]. However, PI3Ks are a large molecular family in which PI3Kα is in the class I group and it is one of the prominent mediators in the activation of LTCC in cardiac myocytes. Consistently, ICaL current density has been shown to decrease in PI3Kα-null cells due to the downregulation of LTCC[118]. It has also been demonstrated that the activation of Akt with PIP3 infusion can reverse the decrease in ICaL current and that CaVβ2 phosphorylation protects the CaV1.2 pore-forming subunit from proteolytic degradation[116].
Another important point is that PI3Kα and PI3Kβ both take part in the T-tubule network. Since LTCC is primarily located in the T-tubule, ICaL current density decreases due to T-tubule disorganization in PI3Kα and PI3Kβ deficiency[119]. As mentioned earlier, the subunits of PI3Ks is an important point that needs to be considered during intervention since ICaL density may decrease in treatments that are not specific to PI3Kα[112,120-122]. PI3Ks can also reduce the response to β-AR stimulation through associated kinases. Particularly, PI3-δ, which is in the class I B PI3K group, shows its effect through the G proteins. Accordingly, in a study of transgenic PI3K-/- animals, isoproterenol application to ventricular myocytes increased ICaL and intracellular Ca2+ transients more than control myocytes, and this was claimed to lead to HF[121,123].
In conclusion, conflicting findings in ICaL current densities and CaV1.2 expression in DCM may be related to the type of diabetes model and its duration. In studies using transgenic animals for both type 1 and type 2 DM models, have been shown to cause a marked reduction in ICaL current density and CaV1.2 expression. The difference observed in the diabetes model induced with STZ injection may be related to the duration of diabetes and the amount of STZ administered. As previously emphasized, ICaL current has been shown to decrease significantly in diabetes periods of more than 10 wk, while findings varied in studies with shorter diabetes duration. This may be due to multiple physiological mechanisms acting on ICaL current, whose activity is changed by the duration of the diabetic condition. Currently, the most likely mechanism suggested to be responsible for the reduced ICaL current in DCM is the altered PI3K/PIP3/Akt pathway due to insulin or IGF-1 decrease. However, current findings need to be supported using comparable models for both types of diabetes, and it is also important to clearly determine whether cellular signaling mechanisms underlying the pathogenesis of these types of disease are similar and how changes in these molecular pathways affect ICaL current depending on the duration of the disease.
VOLTAGE-GATED Na+ CHANNELS IN DIABETIC CARDIOMYOCYTES
Na+ ion plays a vital role in many cellular mechanisms such as the upstroke phase of AP (voltage-dependent Na channels, NaV), Ca2+ cycling (NCX), metabolic processes (Na+-glucose cotransporter) and regulation of the intracellular pH (Na+-HCO3- cotransporter, Na+/H+ exchanger) in cardiomyocytes[93,124,125]. However, the main scope of this review is the NaV channels that ensure the fast depolarization phase of cardiac AP. So far, nine different NaV types have been identified (NaV1.1 to NaV1.9, respectively)[126], and the major NaV type expressed in cardiomyocytes is NaV1.5 encoded by the SCN5A gene[127]. This channel has four homologous domains (D1-D4) and each domain consists of six transmembrane segments (S1-S6)[128,129].
Although the intracellular Na+ concentration has been shown to increase dramatically in diabetic cardiomyocytes, few studies have examined the changes in the structure and activation of NaV channels[125]. Earlier studies have suggested that there is no significant change in NaV channels associated with DCM, while recent studies have shown altered INa current in diabetic cardiac myocytes (Table 2)[69,130-132]. These conflicting results regarding the amplitude of INa current may be related to the duration of diabetes, as in Ca2+ channels. As a matter of fact, Bilginoglu et al[130] reported that there was no change in INa current of ventricular myocytes at the end of the 4-wk diabetes period, whereas there was a significant decrease at 7-8 wk. In addition, a leftward shift has been observed in both activation and inactivation curves of NaV channels in diabetic myocytes[130]. The observation of similar findings in metabolic syndrome, in which insulin resistance is increased, suggests that these effects may be mediated directly or indirectly by insulin signaling[133,134]. Stabler et al[131] have also observed a significant decrease in the amplitude of INa current in diabetic rabbit ventricles, while the channel kinetics did not change.
There is also a significant change in late Na+ currents (INa,L), which have recently been reported to be responsible for many cardiologic pathologies including DCM[135-137]. Although the amplitudes of these currents are up to only 1% of con-ventional voltage-dependent fast Na+ currents, it is thought that the long-term activation of Na+ channels can trigger pathological changes in AP[136,138].
INa,L current has been shown to increase significantly in DCM and is therefore suggested to increase the likelihood of arrhythmia by causing prolongation of AP[135]. Although many different treatments and interventions (ranolazine, mexiletine, PIP3, etc) to inhibit INa,L current have been shown to reverse the prolonged AP duration, it is difficult to attribute the prolongation of AP solely to INa,L current due to the prominent role of K+ and Ca2+ currents in AP morphology[113,132,135,139]. Most importantly the role of K+ currents in AP prolongation has been extensively investigated in diabetic hearts for a long time even though the number of studies showing the effect of INa,L current on AP duration is relatively limited. Therefore, this finding should be carefully examined and confirmed by new studies in both type 1 and type 2 DM models.
CONCLUSION
DM is one of the most common chronic diseases worldwide and is mostly associated with serious cardiovascular complications that significantly increase the risk of mortality in diabetic patients. The abnormalities observed in the ECG and cardiac function of diabetic patients are mostly related to alterations in the voltage-gated ion channels that are critical determinants of the duration and morphology of cardiac AP. At the cellular level, prolongation of AP and defective contractile function typically arise due to a combination of reduced K+ currents, irregularities in Na+ currents and changes in Ca2+ currents along with impaired intracellular Ca2+ handling in diabetic cardiomyocytes. DM can affect not only the amplitude but also kinetics of the cardiac ion channels by modifying the biophysical behavior and/or the expression levels of the channel-forming proteins. Disruption of protein expression or alteration of biophysical properties of ion channels or both can contribute to the reduced currents caused by DM in diabetic cardiomyocytes. However, observing that there is often no change in the inactivation or reactivation kinetics of the cardiac ion channels in diabetic cardiomyocytes suggests that an abnormality in protein expression is more likely.
As a result, under pathological conditions such as DM, depressed K+ currents may cause abnormal prolongation in AP duration due to insufficient repolarization and therefore lead to the development of early and late afterdepolarizations[140-142]. Therefore, it is likely that decreased K+ currents in diabetic myocardium will reduce the repolarization reserve and increase the risk of arrhythmias. Nevertheless, as stated earlier, cardiac Na+ and Ca2+ channels also have important effects that cannot be neglected in diabetic cardiac pathologies and should be taken into account in order to understand the pathogenesis of DCM.
Future perspectives
The effect of DM on the electrical conduction of the myocardium and the development of cardiac arrhythmias is becoming more evident. Due to its complex and multifactorial nature, the relationship between diabetes and cardiac arrhythmias is not yet fully understood. Hence, understanding the precise ionic mechanisms of APD/QT prolongation in DM is of great importance to develop more distinctive approaches for the prevention and treatment of electrical disturbance in diabetic patients.
Remodeling of the expression of K+, Na+ and Ca2+ channels in various physiological and pathological conditions is a complex phenomenon that can alter both the morphology of cardiac AP and contractile function of the heart. In diabetic patients, voltage-gated ion channels play a vital role in cardiac AP repolarization, and expectedly they are potential targets for the development of specific treatments to prevent cardiac arrhythmia and DCM-associated ventricular dysfunction. Using drugs particularly effective on ion channels and optimizing the effectiveness of their therapeutic action on the arrhythmogenic trend will minimize the potential cardiac and extracardiac toxicity problems. However, due to their complex mechanisms, more experimental and clinical research is needed to fully elucidate the relationship between diabetes and arrhythmias and to develop new therapeutic strategies.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Manuscript source: Invited manuscript
Peer-review started: June 25, 2020
First decision: October 23, 2020
Article in press: November 13, 2020
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, Papazafiropoulou A, Sahoo J S-Editor: Zhang H L-Editor: Webster JR P-Editor: Ma YJ
Contributor Information
Nihal Ozturk, Department of Biophysics, Akdeniz University Faculty of Medicine, Antalya 07058, Turkey.
Serkan Uslu, Department of Biophysics, Akdeniz University Faculty of Medicine, Antalya 07058, Turkey.
Semir Ozdemir, Department of Biophysics, Akdeniz University Faculty of Medicine, Antalya 07058, Turkey. osemir@akdeniz.edu.tr.
References
- 1.Wang ZV, Hill JA. Diabetic cardiomyopathy: catabolism driving metabolism. Circulation. 2015;131:771–773. doi: 10.1161/CIRCULATIONAHA.115.015357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Report on Diabetes. WHO Publications. Available from: URL: https://www.who.int/publications/i/item/9789241565257. [Google Scholar]
- 3.Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J Diabetes. 2013;4:177–189. doi: 10.4239/wjd.v4.i5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 6.WRITING COMMITTEE MEMBERS. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Authors/Task Force Members. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schächinger V, Scheen A, Schirmer H, Strömberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 8.Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag. 2010;6:883–903. doi: 10.2147/VHRM.S11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes S, Soares E, Fernandes J, Viana S, Carvalho E, Pereira FC, Reis F. Early cardiac changes in a rat model of prediabetes: brain natriuretic peptide overexpression seems to be the best marker. Cardiovasc Diabetol. 2013;12:44. doi: 10.1186/1475-2840-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham Study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 12.Mahgoub MA, Abd-Elfattah AS. Diabetes mellitus and cardiac function. Mol Cell Biochem. 1998;180:59–64. [PubMed] [Google Scholar]
- 13.Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, Kass D, Feldman AM, Marban E. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 14.Suys BE, Huybrechts SJ, De Wolf D, Op De Beeck L, Matthys D, Van Overmeire B, Du Caju MV, Rooman RP. QTc interval prolongation and QTc dispersion in children and adolescents with type 1 diabetes. J Pediatr. 2002;141:59–63. doi: 10.1067/mpd.2002.125175. [DOI] [PubMed] [Google Scholar]
- 15.Veglio M, Giunti S, Stevens LK, Fuller JH, Perin PC EURODIAB IDDM Complications Study Group. Prevalence of Q-T interval dispersion in type 1 diabetes and its relation with cardiac ischemia: the EURODIAB IDDM Complications Study Group. Diabetes Care. 2002;25:702–707. doi: 10.2337/diacare.25.4.702. [DOI] [PubMed] [Google Scholar]
- 16.Veglio M, Sivieri R, Chinaglia A, Scaglione L, Cavallo-Perin P. QT interval prolongation and mortality in type 1 diabetic patients: a 5-year cohort prospective study. Neuropathy Study Group of the Italian Society of the Study of Diabetes, Piemonte Affiliate. Diabetes Care. 2000;23:1381–1383. doi: 10.2337/diacare.23.9.1381. [DOI] [PubMed] [Google Scholar]
- 17.Rossing P, Breum L, Major-Pedersen A, Sato A, Winding H, Pietersen A, Kastrup J, Parving HH. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med. 2001;18:199–205. doi: 10.1046/j.1464-5491.2001.00446.x. [DOI] [PubMed] [Google Scholar]
- 18.Lo SS, Sutton MS, Leslie RD. Information on type 1 diabetes mellitus and QT interval from identical twins. Am J Cardiol. 1993;72:305–309. doi: 10.1016/0002-9149(93)90677-5. [DOI] [PubMed] [Google Scholar]
- 19.Magyar J, Rusznák Z, Szentesi P, Szûcs G, Kovács L. Action potentials and potassium currents in rat ventricular muscle during experimental diabetes. J Mol Cell Cardiol. 1992;24:841–853. doi: 10.1016/0022-2828(92)91098-p. [DOI] [PubMed] [Google Scholar]
- 20.Casis O, Gallego M, Iriarte M, Sánchez-Chapula JA. Effects of diabetic cardiomyopathy on regional electrophysiologic characteristics of rat ventricle. Diabetologia. 2000;43:101–109. doi: 10.1007/s001250050013. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Xiao J, Wang H, Luo X, Wang J, Villeneuve LR, Zhang H, Bai Y, Yang B, Wang Z. Restoring depressed HERG K+ channel function as a mechanism for insulin treatment of abnormal QT prolongation and associated arrhythmias in diabetic rabbits. Am J Physiol Heart Circ Physiol. 2006;291:H1446–H1455. doi: 10.1152/ajpheart.01356.2005. [DOI] [PubMed] [Google Scholar]
- 22.Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 23.Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- 24.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 26.Huang CL. Murine Electrophysiological Models of Cardiac Arrhythmogenesis. Physiol Rev. 2017;97:283–409. doi: 10.1152/physrev.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 28.Lengyel C, Virág L, Kovács PP, Kristóf A, Pacher P, Kocsis E, Koltay ZM, Nánási PP, Tóth M, Kecskeméti V, Papp JG, Varró A, Jost N. Role of slow delayed rectifier K+-current in QT prolongation in the alloxan-induced diabetic rabbit heart. Acta Physiol (Oxf) 2008;192:359–368. doi: 10.1111/j.1748-1716.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 29.Lengyel C, Virág L, Bíró T, Jost N, Magyar J, Biliczki P, Kocsis E, Skoumal R, Nánási PP, Tóth M, Kecskeméti V, Papp JG, Varró A. Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc Res. 2007;73:512–520. doi: 10.1016/j.cardiores.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Izquierdo A, Pereira RO, Wende AR, Punske BB, Abel ED, Tristani-Firouzi M. The absence of insulin signaling in the heart induces changes in potassium channel expression and ventricular repolarization. Am J Physiol Heart Circ Physiol. 2014;306:H747–H754. doi: 10.1152/ajpheart.00849.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giudicessi JR, Ackerman MJ. Potassium-channel mutations and cardiac arrhythmias--diagnosis and therapy. Nat Rev Cardiol. 2012;9:319–332. doi: 10.1038/nrcardio.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 34.Snyders DJ. Structure and function of cardiac potassium channels. Cardiovasc Res. 1999;42:377–390. doi: 10.1016/s0008-6363(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 35.Roden DM. Taking the "idio" out of "idiosyncratic": predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 36.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 37.Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620–628. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- 38.Xu Z, Patel KP, Rozanski GJ. Metabolic basis of decreased transient outward K+ current in ventricular myocytes from diabetic rats. Am J Physiol. 1996;271:H2190–H2196. doi: 10.1152/ajpheart.1996.271.5.H2190. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchida K, Watajima H. Potassium currents in ventricular myocytes from genetically diabetic rats. Am J Physiol. 1997;273:E695–E700. doi: 10.1152/ajpendo.1997.273.4.E695. [DOI] [PubMed] [Google Scholar]
- 40.Ozdemir S, Ugur M, Gürdal H, Turan B. Treatment with AT(1) receptor blocker restores diabetes-induced alterations in intracellular Ca(2+) transients and contractile function of rat myocardium. Arch Biochem Biophys. 2005;435:166–174. doi: 10.1016/j.abb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Ozturk N, Yaras N, Ozmen A, Ozdemir S. Long-term administration of rosuvastatin prevents contractile and electrical remodelling of diabetic rat heart. J Bioenerg Biomembr. 2013;45:343–352. doi: 10.1007/s10863-013-9514-z. [DOI] [PubMed] [Google Scholar]
- 42.Aydemir M, Ozturk N, Dogan S, Aslan M, Olgar Y, Ozdemir S. Sodium tungstate administration ameliorated diabetes-induced electrical and contractile remodeling of rat heart without normalization of hyperglycemia. Biol Trace Elem Res. 2012;148:216–223. doi: 10.1007/s12011-012-9350-8. [DOI] [PubMed] [Google Scholar]
- 43.Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan B. Effects of selenium on altered mechanical and electrical cardiac activities of diabetic rat. Arch Biochem Biophys. 2004;426:83–90. doi: 10.1016/j.abb.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM. Altered K(+) channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol Heart Circ Physiol. 2001;281:H1800–H1807. doi: 10.1152/ajpheart.2001.281.4.H1800. [DOI] [PubMed] [Google Scholar]
- 45.Qin D, Huang B, Deng L, El-Adawi H, Ganguly K, Sowers JR, El-Sherif N. Downregulation of K(+) channel genes expression in type I diabetic cardiomyopathy. Biochem Biophys Res Commun. 2001;283:549–553. doi: 10.1006/bbrc.2001.4825. [DOI] [PubMed] [Google Scholar]
- 46.Patel SP, Parai R, Parai R, Campbell DL. Regulation of Kv4.3 voltage-dependent gating kinetics by KChIP2 isoforms. J Physiol. 2004;557:19–41. doi: 10.1113/jphysiol.2003.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKinnon D. Molecular identity of Ito: Kv1.4 redux. Circ Res. 1999;84:620–622. doi: 10.1161/01.res.84.5.620. [DOI] [PubMed] [Google Scholar]
- 48.Shimoni Y. Inhibition of the formation or action of angiotensin II reverses attenuated K+ currents in type 1 and type 2 diabetes. J Physiol. 2001;537:83–92. doi: 10.1111/j.1469-7793.2001.0083k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimoni Y, Ewart HS, Severson D. Type I and II models of diabetes produce different modifications of K+ currents in rat heart: role of insulin. J Physiol. 1998;507 (Pt 2):485–496. doi: 10.1111/j.1469-7793.1998.485bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jourdon P, Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993;470:411–429. doi: 10.1113/jphysiol.1993.sp019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres-Jacome J, Gallego M, Rodríguez-Robledo JM, Sanchez-Chapula JA, Casis O. Improvement of the metabolic status recovers cardiac potassium channel synthesis in experimental diabetes. Acta Physiol (Oxf) 2013;207:447–459. doi: 10.1111/apha.12043. [DOI] [PubMed] [Google Scholar]
- 52.Wang DW, Kiyosue T, Shigematsu S, Arita M. Abnormalities of K+ and Ca2+ currents in ventricular myocytes from rats with chronic diabetes. Am J Physiol. 1995;269:H1288–H1296. doi: 10.1152/ajpheart.1995.269.4.H1288. [DOI] [PubMed] [Google Scholar]
- 53.O'Brien RM, Granner DK. Regulation of gene expression by insulin. Physiol Rev. 1996;76:1109–1161. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 54.Shimoni Y, Ewart HS, Severson D. Insulin stimulation of rat ventricular K+ currents depends on the integrity of the cytoskeleton. J Physiol. 1999;514 (Pt 3):735–745. doi: 10.1111/j.1469-7793.1999.735ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 56.Shimoni Y, Hunt D, Chuang M, Chen KY, Kargacin G, Severson DL. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J Physiol. 2005;567:177–190. doi: 10.1113/jphysiol.2005.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nattel S, Yue L, Wang Z. Cardiac ultrarapid delayed rectifiers: a novel potassium current family of functional similarity and molecular diversity. Cell Physiol Biochem. 1999;9:217–226. doi: 10.1159/000016318. [DOI] [PubMed] [Google Scholar]
- 58.Snyders DJ, Tamkun MM, Bennett PB. A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol. 1993;101:513–543. doi: 10.1085/jgp.101.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 60.Uebele VN, England SK, Gallagher DJ, Snyders DJ, Bennett PB, Tamkun MM. Distinct domains of the voltage-gated K+ channel Kv beta 1.3 beta-subunit affect voltage-dependent gating. Am J Physiol. 1998;274:C1485–C1495. doi: 10.1152/ajpcell.1998.274.6.C1485. [DOI] [PubMed] [Google Scholar]
- 61.Piper DR, Hinz WA, Tallurri CK, Sanguinetti MC, Tristani-Firouzi M. Regional specificity of human ether-a'-go-go-related gene channel activation and inactivation gating. J Biol Chem. 2005;280:7206–7217. doi: 10.1074/jbc.M411042200. [DOI] [PubMed] [Google Scholar]
- 62.Tseng GN. I(Kr): the hERG channel. J Mol Cell Cardiol. 2001;33:835–849. doi: 10.1006/jmcc.2000.1317. [DOI] [PubMed] [Google Scholar]
- 63.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 64.Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 2005;20:408–416. doi: 10.1152/physiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 66.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 67.Delpón E, Valenzuela C, Pérez O, Casis O, Tamargo J. Propafenone preferentially blocks the rapidly activating component of delayed rectifier K+ current in guinea pig ventricular myocytes. Voltage-independent and time-dependent block of the slowly activating component. Circ Res. 1995;76:223–235. doi: 10.1161/01.res.76.2.223. [DOI] [PubMed] [Google Scholar]
- 68.Jurkiewicz NK, Sanguinetti MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993;72:75–83. doi: 10.1161/01.res.72.1.75. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Xiao J, Lin H, Luo X, Wang H, Bai Y, Wang J, Zhang H, Yang B, Wang Z. Ionic mechanisms underlying abnormal QT prolongation and the associated arrhythmias in diabetic rabbits: a role of rapid delayed rectifier K+ current. Cell Physiol Biochem. 2007;19:225–238. doi: 10.1159/000100642. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res. 1999;84:551–561. doi: 10.1161/01.res.84.5.551. [DOI] [PubMed] [Google Scholar]
- 71.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 72.Thorneloe KS, Liu XF, Walsh MP, Shimoni Y. Transmural differences in rat ventricular protein kinase C epsilon correlate with its functional regulation of a transient cardiac K+ current. J Physiol. 2001;533:145–154. doi: 10.1111/j.1469-7793.2001.0145b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrer T, Gallego M, Madrigal-Quiñónez R, Torres-Jácome J, Navarro-Polanco R, Cásis O, Sánchez-Chapula JA. DITPA restores the repolarizing potassium currents Itof and Iss in cardiac ventricular myocytes of diabetic rats. Life Sci. 2006;79:883–889. doi: 10.1016/j.lfs.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 74.Ehrlich JR, Pourrier M, Weerapura M, Ethier N, Marmabachi AM, Hébert TE, Nattel S. KvLQT1 modulates the distribution and biophysical properties of HERG. A novel alpha-subunit interaction between delayed rectifier currents. J Biol Chem. 2004;279:1233–1241. doi: 10.1074/jbc.M309087200. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem. 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Han H, Wang J, Wang H, Yang B, Wang Z. Impairment of human ether-à-go-go-related gene (HERG) K+ channel function by hypoglycemia and hyperglycemia. Similar phenotypes but different mechanisms. J Biol Chem. 2003;278:10417–10426. doi: 10.1074/jbc.M211044200. [DOI] [PubMed] [Google Scholar]
- 77.Deschênes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 78.Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 79.Guo D, Lu Z. Mechanism of IRK1 channel block by intracellular polyamines. J Gen Physiol. 2000;115:799–814. doi: 10.1085/jgp.115.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on I(K1) J Mol Cell Cardiol. 2001;33:625–638. doi: 10.1006/jmcc.2001.1344. [DOI] [PubMed] [Google Scholar]
- 81.Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–2428. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 83.Dillmann WH. Diabetic Cardiomyopathy. Circ Res. 2019;124:1160–1162. doi: 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cesario DA, Brar R, Shivkumar K. Alterations in ion channel physiology in diabetic cardiomyopathy. Endocrinol Metab Clin North Am. 2006;35:601–610, ix. doi: 10.1016/j.ecl.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 86.Ozturk N, Olgar Y, Ozdemir S. Trace elements in diabetic cardiomyopathy: An electrophysiological overview. World J Diabetes. 2013;4:92–100. doi: 10.4239/wjd.v4.i4.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fauconnier J, Lanner JT, Zhang SJ, Tavi P, Bruton JD, Katz A, Westerblad H. Insulin and inositol 1,4,5-trisphosphate trigger abnormal cytosolic Ca2+ transients and reveal mitochondrial Ca2+ handling defects in cardiomyocytes of ob/ob mice. Diabetes. 2005;54:2375–2381. doi: 10.2337/diabetes.54.8.2375. [DOI] [PubMed] [Google Scholar]
- 88.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53:3201–3208. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 89.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gómez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 90.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, Wuytack F, Holvoet P, Flameng W, Balligand JL, Herijgers P. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–379. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 91.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 92.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci. 2006;100:315–322. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- 94.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benitah JP, Alvarez JL, Gómez AM. L-type Ca(2+) current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 96.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 97.Roden DM, Balser JR, George AL Jr, Anderson ME. Cardiac ion channels. Annu Rev Physiol. 2002;64:431–475. doi: 10.1146/annurev.physiol.64.083101.145105. [DOI] [PubMed] [Google Scholar]
- 98.Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–H1408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- 99.Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes. 2005;54:3082–3088. doi: 10.2337/diabetes.54.11.3082. [DOI] [PubMed] [Google Scholar]
- 100.Ricci E, Smallwood S, Chouabe C, Mertani HC, Raccurt M, Morel G, Bonvallet R. Electrophysiological characterization of left ventricular myocytes from obese Sprague-Dawley rat. Obesity (Silver Spring) 2006;14:778–786. doi: 10.1038/oby.2006.90. [DOI] [PubMed] [Google Scholar]
- 101.Lacombe VA, Viatchenko-Karpinski S, Terentyev D, Sridhar A, Emani S, Bonagura JD, Feldman DS, Györke S, Carnes CA. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1787–R1797. doi: 10.1152/ajpregu.00059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao CH, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol. 2007;42:234–246. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 103.Bracken N, Howarth FC, Singh J. Effects of streptozotocin-induced diabetes on contraction and calcium transport in rat ventricular cardiomyocytes. Ann N Y Acad Sci. 2006;1084:208–222. doi: 10.1196/annals.1372.018. [DOI] [PubMed] [Google Scholar]
- 104.Lu Z, Jiang YP, Xu XH, Ballou LM, Cohen IS, Lin RZ. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes. 2007;56:2780–2789. doi: 10.2337/db06-1629. [DOI] [PubMed] [Google Scholar]
- 105.Howarth FC, Qureshi MA, Hassan Z, Al Kury LT, Isaev D, Parekh K, Yammahi SR, Oz M, Adrian TE, Adeghate E. Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp Physiol. 2011;96:325–337. doi: 10.1113/expphysiol.2010.055574. [DOI] [PubMed] [Google Scholar]
- 106.Lu Z, Ballou LM, Jiang YP, Cohen IS, Lin RZ. Restoration of defective L-type Ca2+ current in cardiac myocytes of type 2 diabetic db/db mice by Akt and PKC-ι. J Cardiovasc Pharmacol. 2011;58:439–445. doi: 10.1097/FJC.0b013e318228e68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Howarth FC, Qureshi MA, Hassan Z, Isaev D, Parekh K, John A, Oz M, Raza H, Adeghate E, Adrian TE. Contractility of ventricular myocytes is well preserved despite altered mechanisms of Ca2+ transport and a changing pattern of mRNA in aged type 2 Zucker diabetic fatty rat heart. Mol Cell Biochem. 2012;361:267–280. doi: 10.1007/s11010-011-1112-y. [DOI] [PubMed] [Google Scholar]
- 108.Lee TI, Chen YC, Lin YK, Chung CC, Lu YY, Kao YH, Chen YJ. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 110.Fu L, Rao F, Lian F, Yang H, Kuang S, Wu S, Deng C, Xue Y. Mechanism of electrical remodeling of atrial myocytes and its influence on susceptibility to atrial fibrillation in diabetic rats. Life Sci. 2019;239:116903. doi: 10.1016/j.lfs.2019.116903. [DOI] [PubMed] [Google Scholar]
- 111.Pereira L, Ruiz-Hurtado G, Rueda A, Mercadier JJ, Benitah JP, Gómez AM. Calcium signaling in diabetic cardiomyocytes. Cell Calcium. 2014;56:372–380. doi: 10.1016/j.ceca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 112.Ghigo A, Laffargue M, Li M, Hirsch E. PI3K and Calcium Signaling in Cardiovascular Disease. Circ Res. 2017;121:282–292. doi: 10.1161/CIRCRESAHA.117.310183. [DOI] [PubMed] [Google Scholar]
- 113.Ballou LM, Lin RZ, Cohen IS. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ Res. 2015;116:127–137. doi: 10.1161/CIRCRESAHA.116.303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viard P, Butcher AJ, Halet G, Davies A, Nürnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 115.Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- 116.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rusconi F, Ceriotti P, Miragoli M, Carullo P, Salvarani N, Rocchetti M, Di Pasquale E, Rossi S, Tessari M, Caprari S, Cazade M, Kunderfranco P, Chemin J, Bang ML, Polticelli F, Zaza A, Faggian G, Condorelli G, Catalucci D. Peptidomimetic Targeting of Cavβ2 Overcomes Dysregulation of the L-Type Calcium Channel Density and Recovers Cardiac Function. Circulation. 2016;134:534–546. doi: 10.1161/CIRCULATIONAHA.116.021347. [DOI] [PubMed] [Google Scholar]
- 118.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009;120:318–325. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, Mathias RT, Cohen IS, Song LS, Entcheva E, Lin RZ. PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PLoS One. 2011;6:e24404. doi: 10.1371/journal.pone.0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ghigo A, Perino A, Mehel H, Zahradníková A Jr, Morello F, Leroy J, Nikolaev VO, Damilano F, Cimino J, De Luca E, Richter W, Westenbroek R, Catterall WA, Zhang J, Yan C, Conti M, Gomez AM, Vandecasteele G, Hirsch E, Fischmeister R. Phosphoinositide 3-kinase γ protects against catecholamine-induced ventricular arrhythmia through protein kinase A-mediated regulation of distinct phosphodiesterases. Circulation. 2012;126:2073–2083. doi: 10.1161/CIRCULATIONAHA.112.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 122.Lu Z, Jiang YP, Ballou LM, Cohen IS, Lin RZ. Galpha q inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:40347–40354. doi: 10.1074/jbc.M508441200. [DOI] [PubMed] [Google Scholar]
- 123.Marcantoni A, Levi RC, Gallo MP, Hirsch E, Alloatti G. Phosphoinositide 3-kinasegamma (PI3Kgamma) controls L-type calcium current (ICa,L) through its positive modulation of type-3 phosphodiesterase (PDE3) J Cell Physiol. 2006;206:329–336. doi: 10.1002/jcp.20467. [DOI] [PubMed] [Google Scholar]
- 124.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 125.Doliba NM, Babsky AM, Osbakken MD. The Role of Sodium in Diabetic Cardiomyopathy. Front Physiol. 2018;9:1473. doi: 10.3389/fphys.2018.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DeMarco KR, Clancy CE. Cardiac Na Channels: Structure to Function. Curr Top Membr. 2016;78:287–311. doi: 10.1016/bs.ctm.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang D, Shi H, Tonggu L, Gamal El-Din TM, Lenaeus MJ, Zhao Y, Yoshioka C, Zheng N, Catterall WA. Structure of the Cardiac Sodium Channel. Cell 2020; 180: 122-134. :e10. doi: 10.1016/j.cell.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rook MB, Evers MM, Vos MA, Bierhuizen MF. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res. 2012;93:12–23. doi: 10.1093/cvr/cvr252. [DOI] [PubMed] [Google Scholar]
- 130.Bilginoglu A, Kandilci HB, Turan B. Intracellular levels of Na(+) and TTX-sensitive Na(+) channel current in diabetic rat ventricular cardiomyocytes. Cardiovasc Toxicol. 2013;13:138–147. doi: 10.1007/s12012-012-9192-9. [DOI] [PubMed] [Google Scholar]
- 131.Stables CL, Musa H, Mitra A, Bhushal S, Deo M, Guerrero-Serna G, Mironov S, Zarzoso M, Vikstrom KL, Cawthorn W, Pandit SV. Reduced Na⁺ current density underlies impaired propagation in the diabetic rabbit ventricle. J Mol Cell Cardiol. 2014;69:24–31. doi: 10.1016/j.yjmcc.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khazraei H, Mirkhani H, Shabbir W. Electrocardiological effects of ranolazine and lidocaine on normal and diabetic rat atrium. J Interv Card Electrophysiol. 2020:Epub ahead of print. doi: 10.1007/s10840-020-00742-w. [DOI] [PubMed] [Google Scholar]
- 133.Bilginoglu A, Selcuk MFT, Nakkas H, Turan B. Pioglitazone provides beneficial effect in metabolic syndrome rats via affecting intracellular Na+ Dyshomeostasis. J Bioenerg Biomembr. 2018;50:437–445. doi: 10.1007/s10863-018-9776-6. [DOI] [PubMed] [Google Scholar]
- 134.Durak A, Bitirim CV, Turan B. Titin and CK2α are New Intracellular Targets in Acute Insulin Application-Associated Benefits on Electrophysiological Parameters of Left Ventricular Cardiomyocytes From Insulin-Resistant Metabolic Syndrome Rats. Cardiovasc Drugs Ther. 2020;34:487–501. doi: 10.1007/s10557-020-06974-2. [DOI] [PubMed] [Google Scholar]
- 135.Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, Rosen MR, Cohen IS, Lin RZ. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62:4257–4265. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 Suppl 4:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moreno JD, Clancy CE. Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol. 2012;52:608–619. doi: 10.1016/j.yjmcc.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. J Physiol. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sossalla S, Maier LS. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacol Ther. 2012;133:311–323. doi: 10.1016/j.pharmthera.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 140.Drici MD, Barhanin J. Cardiac K+ channels and drug-acquired long QT syndrome. Therapie. 2000;55:185–193. [PubMed] [Google Scholar]
- 141.Priori SG, Napolitano C. Genetics of cardiac arrhythmias and sudden cardiac death. Ann N Y Acad Sci. 2004;1015:96–110. doi: 10.1196/annals.1302.008. [DOI] [PubMed] [Google Scholar]
- 142.Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005;115:2025–2032. doi: 10.1172/JCI25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Varro A, Baláti B, Iost N, Takács J, Virág L, Lathrop DA, Csaba L, Tálosi L, Papp JG. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol. 2000;523 Pt 1:67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57:2924–2932. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu Z, Patel KP, Rozanski GJ. Intracellular protons inhibit transient outward K+ current in ventricular myocytes from diabetic rats. Am J Physiol. 1996;271:H2154–H2161. doi: 10.1152/ajpheart.1996.271.5.H2154. [DOI] [PubMed] [Google Scholar]