Abstract
BACKGROUND
Some patients with hepatocellular carcinoma (HCC) are more likely to experience disease progression despite continuous transarterial chemoembolization (TACE), which is called TACE refractoriness. At present, it is still difficult to predict TACE refractoriness, although some models/scoring systems have been developed. At present, radiological-based radiomics models have been successfully applied to predict cancer patient prognosis.
AIM
To develop and validate a computed tomography (CT)-based radiomics nomogram for the pre-treatment prediction of TACE refractoriness.
METHODS
This retrospective study consisted of a training dataset (n = 137) and an external validation dataset (n = 81) of patients with clinically/pathologically confirmed HCC who underwent repeated TACE from March 2009 to March 2016. Radiomics features were retrospectively extracted from preoperative CT images of the arterial phase. The pre-treatment radiomics signature was generated using least absolute shrinkage and selection operator Cox regression analysis. A CT-based radiomics nomogram incorporating clinical risk factors and the radiomics signature was built and verified by calibration curve and decision curve analyses. The usefulness of the CT-based radiomics nomogram was assessed by Kaplan-Meier curve analysis. We used the concordance index to conduct head-to-head comparisons of the radiomics nomogram with the other four models (Assessment for Retreatment with Transarterial Chemoembolization score; α-fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response score; CT-based radiomics signature; and clinical model). All analyses were conducted according to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis statement.
RESULTS
The median duration of follow-up was 61.3 mo (interquartile range, 25.5-69.3 mo) for the training cohort and 67.1 mo (interquartile range, 32.4-71.3 mo) for the validation cohort. The median number of TACE sessions was 4 (range, 3-7) in both cohorts. Eight radiomics features were chosen from 869 candidate features to build a radiomics signature. The CT-based radiomics nomogram included the radiomics score (hazard ratio = 3.9, 95% confidence interval: 3.1-8.8, P < 0.001) and four clinical factors and classified patients into high-risk (score > 3.5) and low-risk (score ≤ 3.5) groups with markedly different prognoses (overall survival: 12.3 mo vs 23.6 mo, P < 0.001). The accuracy of the nomogram was considerably higher than that of the other four models. The calibration curve and decision curve analyses verified the usefulness of the CT-based radiomics nomogram for clinical practice.
CONCLUSION
The newly constructed CT-based radiomics nomogram can be used for the pre-treatment prediction of TACE refractoriness, which may provide better guidance for decision making regarding further TACE treatment.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Refractoriness, Radiomics, Nomogram, Computed tomography
Core Tip: At present, it is still difficult to predict transarterial chemoembolization (TACE) refractoriness. The main finding of this study is that our computed tomography (CT)-based radiomics nomogram can be used to individually predict patients’ refractory state before the first session of TACE. The predictive calibration curves of the training and validation datasets demonstrated agreement with the ideal curve. This CT-based radiomics nomogram may provide an unprecedented opportunity to improve clinical decision making for the patients who are repeatedly treated by TACE and, eventually, improve the overall survival of these patients.
INTRODUCTION
Transarterial chemoembolization (TACE) has been established as the mainstay treatment for intermediate-stage hepatocellular carcinoma (HCC)[1]. Depending on the heterogeneity of the tumour, including the size, number, growth pattern, and anatomical location, a treatment response is not always easy to obtain from a single session of TACE[2,3]. Thus, repeated TACE is often performed to achieve a sufficient outcome. Nevertheless, in some cases, despite an initial response induced by TACE, repetitive TACE treatments could gradually impair liver function and, even worse, have adverse effects on patient survival[4]. This state was initially termed TACE refractoriness and was first recommended by the Japan Society of Hepatology (JSH) in 2010[5]. Furthermore, intermediate HCC patients whose disease was refractory to TACE who received sorafenib experienced an increase in survival compared with those who continued TACE[6,7]. Therefore, it is very important to identify patients who could benefit from consecutive TACE sessions early to improve their outcome.
To predict TACE refractoriness early, some scoring systems have been developed. Sieghart et al[8] developed a scoring system known as the Assessment for Retreatment with Transarterial Chemoembolization (ART) score. The ART score is composed of radiologic tumour response, Child-Pugh increase, and aspartate aminotransferase increase by 25%. Adhoute et al[9] contructed the ABCR score, which included α-fetoprotein (AFP) level, Barcelona Clinic Liver Cancer (BCLC) stage, Child–Pugh score, and Response score. Subsequent studies in Italian and Japanese cohorts have failed to demonstrate the usefulness of the ART score[10,11]. The Milan group criticized the ABCR score for its “overfitting”[12]. Recently, Kloeckner et al[13] also demonstrated that both scores were not sufficient to support clear-cut clinical decisions, and further efforts are needed to determine TACE refractoriness.
Computed tomography (CT) is the most frequently used imaging technique due to its excellent spatial resolution, tissue contrast, and availability. Compared with conventional CT-based imaging features, radiomics signatures reveal the tumour phenotype and tumour biological heterogeneity and therefore can quantitatively estimate patient survival pre-operatively by transforming traditional medical images into high-throughput quantitative imaging features[14,15]. Radiological-based radiomics models have been successfully applied to predict cancer patient survival[16,17]. Unfortunately, Park et al[18] found that the overall scientific quality of radiomics studies is currently insufficient. To improve the reporting of studies on clinical prediction models, a strict guideline called the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) was released in January 2015[19]. Therefore, following the TRIPOD statement, our study aimed to develop and validate a novel CT-based radiomics nomogram to select patients who could benefit from consecutive TACE cycles.
MATERIALS AND METHODS
This study was performed retrospectively in compliance with the Health Insurance Portability and Accountability Act, and a waiver for the requirement of informed patient consent was obtained from the institutional review board.
Study population
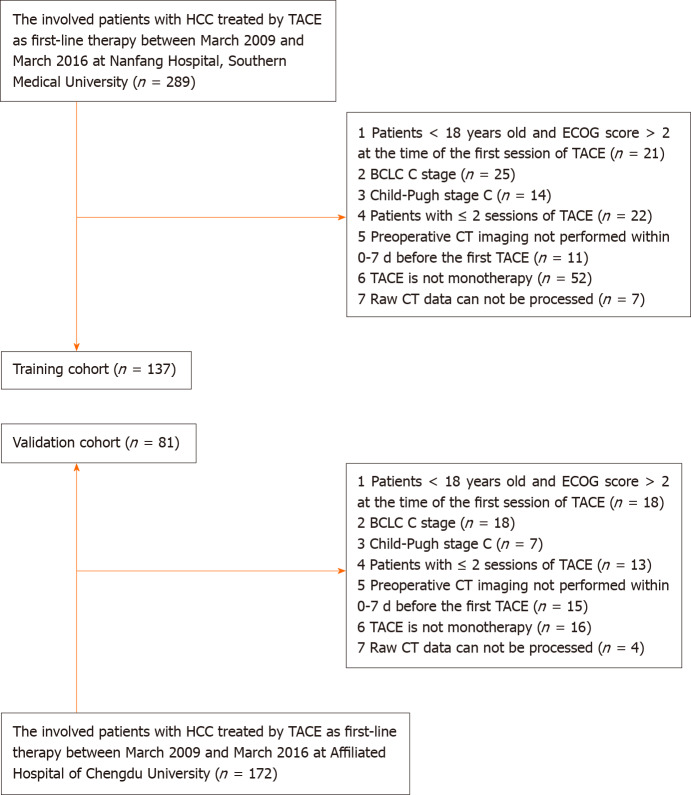
Training cohort: Patients with a diagnosis of HCC who were treated by TACE at the Southern Medical University Nanfang Hospital from March 2009 to March 2016 were included in this study. The inclusion criteria of this retrospective study were as follows: (1) Patients underwent more than two TACE sessions during their therapeutic course (the first two sessions were performed within 3 mo. Then, the rest of the TACE procedures were performed “on-demand”); (2) HCC was diagnosed according to the European Association for the Study of the Liver criteria or histological examination; (3) TACE was performed as monotherapy; (4) patients were ≥ 18 years old and had an ECOG performance status score of ≤ 2 at the time of the first TACE session; (5) patients had HCC at BCLC stage A or B (TACE was chosen by the patient voluntarily after the treatment options had been presented) and well-preserved liver function (Child-Pugh class A or B); and (6) preoperative CT imaging was performed within 0-7 d before the first session of TACE. The exclusion criteria were as follows: (1) Patients had BCLC stage C or Child-Pugh class C disease; (2) Patients had another percutaneous treatment combined with TACE (including radiofrequency/microwave ablation, etc.); and (3) The raw CT data could not be processed (Figure 1). Finally, 137 patients (median age, 54.3 years; range, 19-79 years) were included in the training cohort.
Figure 1.
Flowchart for inclusion and exclusion of patients within the training and validation cohort. TACE: Transarterial chemoembolization; ECOG: Eastern Cooperative Oncology Group; BCLC: Barcelona Clinic Liver Cancer; CT: Computed tomography; HCC: Hepatocellular carcinoma.
Validation cohort: An independent external validation cohort comprised patients who underwent TACE procedures at the Affiliated Hospital of Chengdu University. Consecutive patients who underwent TACE between March 2009 and March 2016 were identified from the local electronic patient recording system. The selection and exclusion criteria for the validation cohort were the same as those for the training cohort. Overall, 81 patients (median age, 57.1 years; range, 21-78 years) were included.
TACE procedure: All TACE procedures were conducted under local anaesthesia via a traditional femoral artery approach. Our method of TACE was consistent with the practical standard method of TACE in Asian countries, which was previously reported[20]. After the first two TACE sessions, both institutions used treatment “on demand”, and TACE was scheduled upon the detection of viable tumours (local or distant intra-/extra-hepatic recurrences) in patients with Child-Pugh A/B, which can still be treated by TACE.
Factors analysed
Primary outcome: Overall survival (OS) was defined as the period from 1 d before the first TACE procedure to death or the last follow-up (March 2020). Survival data were obtained by one researcher who was blinded to the study purpose. There was no loss to follow-up in the present study.
TACE refractoriness: In the present study, TACE refractoriness was defined according to the JSH and the Liver Cancer Study Group of Japan (JSH-LCSGJ) criteria[21] as follows: Two or more consecutive insufficient responses of the treated tumour (viable lesion > 50%) or an increase in tumour number after changing the chemotherapeutic agents and/or reanalysis of the feeding artery seen on the response evaluation CT/MRI at 1-3 mo after having adequately performed selective TACE; the appearance of vascular invasion; the appearance of extrahepatic spread; or a continuous elevation in tumour markers immediately after TACE even though a slight transient decrease was observed.
Candidate clinical variables: Based on the ART and ABCR scores, we collected as much clinical data as possible. Before the first TACE session, the presence or absence of a tumour capsule was evaluated according to Liver Imaging Reporting and Data System version 2018 (LI-RADS v2018) ancillary features[22]. Irregular tumour margins were defined as non-smooth margins, presenting as the multinodular confluent type, and nodular type with extranodular extension and/or with an infiltrative appearance[23]. Those radiologic imaging evaluations were assessed by two senior radiologists (more than 10 years in interpreting abdominal imaging) with consensus.
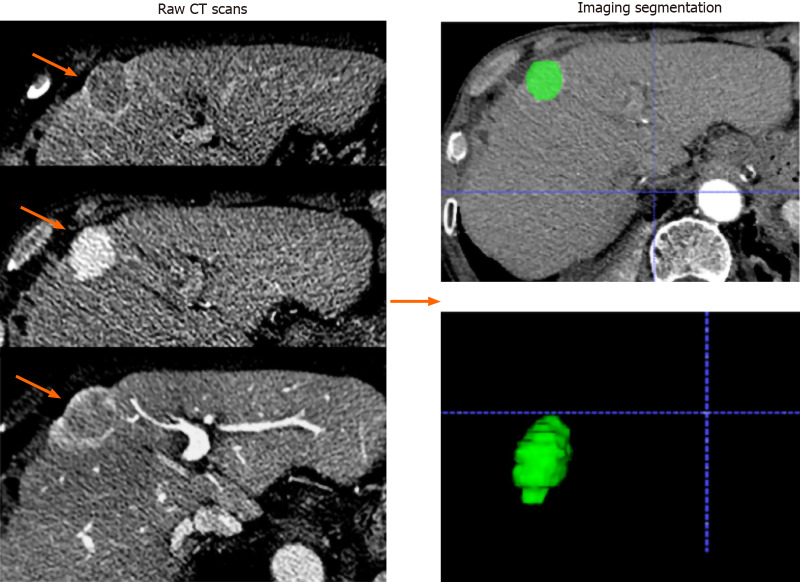
Radiomics feature selection: All patients underwent multidetector CT examination (Siemens Somatom Definition 64; VCT 64; GE Medical Systems). A 1.5 mL/kg body weight bolus of iohexol (Omnipaque, GE Healthcare Co., Ltd.) was injected intravenously at a flow rate of 2.5 mL/s, followed by a 20-mL saline flush. Images of the arterial phase, portal venous phase, and delay phase were obtained at 35 s, 65 s, and 120 s, respectively, after intravenous contrast material injection. The scanning parameters used in this study were as follows: Tube voltage, 120 kVp; detector collimation, 128 mm × 0.625 mm; field of view, 250-400 mm; pixel size, 512 × 512; rotation time, 0.5 s; slice interval, 0 mm; slice thickness, 5 mm; and reconstructed section thicknesses, 1 mm. The processing flow of the radiomics analysis included the following four steps: Tumour segmentation, feature extraction, model building, and model evaluation. Radiomics features were extracted from CT images of the pre-treatment arterial phase. The largest tumours were manually delineated on each transverse image by using ITK-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php) (Figure 2). Two researchers (with 5 and 8 years of experience in abdominal imaging) who were blinded to the research purpose independently performed tumour delineation and feature extraction with the open-source PyRadiomics package (version 2.2.0). Images were standardized to a voxel size of 1 mm × 1 mm × 1 mm, and voxel intensity values were discretized by using a fixed bin width of 10 HU to reduce image noise and normalize intensities, allowing for a constant intensity resolution across all tumour images[24]. The interobserver reproducibility of the radiomics features was evaluated by the intraclass correlation coefficient (ICC).
Figure 2.
Imaging segmentation. The images were acquired form a 65-year-old man with hepatocellular carcinoma (orange arrows). Serial raw computed tomography scans obtained before transarterial chemoembolization treatment; regions of interest were manually depicted along with the tumor outline on each axial slice and automatically merged into a volume of interest (green area).
Statistical analysis
The image features and clinical data were evaluated by applying the Student’s t-test, the chi-squared test, or the Mann-Whitney U-test, where appropriate. Missing data were considered at random and handled using multiple imputations by the iterative Markov chain Monte Carlo method (10 iterations)[25]. All statistical analyses were conducted by using R software (http://www.Rproject.org). A two-tailed P < 0.05 was regarded as statistically significant.
Step 1 - construct radiomics score: Radiomics features that had ICCs greater than 0.80 were used for subsequent analysis. We used the least absolute shrinkage and selection operator (LASSO) Cox regression method to determine radiomics features that predicted OS. The radiomics score (Rad-score) cut-off value was determined with the “surv_cutpoint” function of the “survminer” R package.
Step 2 - construct the clinical model/CT-based radiomics nomogram: Univariate and multivariate Cox proportional hazards analyses were used to identify clinical factors related to patient survival. The B regression coefficients of the Cox regression model were multiplied by 2 and rounded to obtain the risk score calculation. A CT-based radiomics nomogram was constructed by incorporating the clinical risk factors and radiomics signature by the use of a multivariable Cox regression model. The radiomics model used the median value of the total score to stratify patients into high- and low-risk groups, and ultimately, a nomogram was built.
Step 3 - model evaluation: The OS of different groups was estimated by using the Kaplan-Meier method and compared by the log-rank test. The performance of each model was evaluated by the concordance index (C-index). The C-index is the area under the curve for continuous time-to-event survival data used to measure the discrimination of a prognostic model. The calibration curve was established, and the Hosmer-Lemeshow test was used to analyse the prognostic performance of the CT-based radiomics nomogram in both cohorts. Decision curve analysis (DCA) was conducted to determine the clinical usefulness of the nomogram in the validation cohort.
RESULTS
Patient characteristics
Between March 2009 and March 2016, a total of 461 HCC patients underwent TACE as first-line therapy. A total of 137 patients were included in the training cohort, and 81 were included in the validation cohort. The median duration of follow-up was 61.3 mo (interquartile range, 25.5-69.3 mo) for the training cohort and 67.1 mo (interquartile range, 32.4-71.3 mo) for the validation cohort. The median number of TACE sessions was 4 (range, 3-7) in both cohorts. The median survival time for the training cohort was 21.6 mo (95% confidence interval [CI]: 13.6-28.1), while that of the validation cohort was 19.7 mo (95%CI: 12.3-25.2) (training vs validation, P = 0.324).
In the training cohort, 67% of patients were male, and most (80.0%) patients tested positive for HBsAg. The majority of patients were graded as having Child-Pugh A (61%) and BCLC stage B (85%). The proportion of patients with a tumour size of 5 cm or greater was 34%. The included variables were not significantly different between the two cohorts (Table 1).
Table 1.
Patient characteristics
| Characteristic |
Training cohort
|
Validation cohort
|
P value | ||
|
n
|
%
|
n
|
%
|
||
| Before treatment | 137 | 81 | |||
| Age (yr) | 0.778 | ||||
| < 60 | 87 | 63 | 49 | 60 | |
| ≥ 60 | 50 | 37 | 32 | 40 | |
| Sex | 0.159 | ||||
| Male | 91 | 67 | 45 | 55 | |
| Female | 46 | 33 | 36 | 45 | |
| HBsAg status | 0.950 | ||||
| Positive | 110 | 80 | 65 | 80 | |
| Negative | 27 | 20 | 16 | 20 | |
| Child-Pugh class | 0.879 | ||||
| A | 84 | 61 | 51 | 62 | |
| B | 53 | 39 | 30 | 38 | |
| Largest tumor size (mean ± SD, cm) | 0.321 | ||||
| < 5 | 91 | 66 | 47 | 58 | |
| ≥ 5 | 46 | 34 | 34 | 42 | |
| Tumor distribution | 0.360 | ||||
| Solitary | 89 | 64 | 45 | 56 | |
| Multiple | 48 | 36 | 36 | 44 | |
| BCLC stage | 0.249 | ||||
| A | 20 | 15 | 16 | 19 | |
| B | 117 | 85 | 65 | 81 | |
| AFP (IU/mL) | 0.410 | ||||
| < 200 | 29 | 21 | 21 | 26 | |
| ≥ 200 | 108 | 79 | 60 | 74 | |
| ECOG performance status score | 0.321 | ||||
| 0 | 42 | 30 | 31 | 38 | |
| 1 | 85 | 62 | 43 | 53 | |
| 2 | 10 | 8 | 7 | 9 | |
| AST (U/L) | 0.106 | ||||
| < 40 | 50 | 36 | 37 | 46 | |
| ≥ 40 | 87 | 64 | 44 | 54 | |
| Irregular tumor margin | 0.579 | ||||
| Absent | 62 | 45 | 33 | 41 | |
| Present | 75 | 55 | 48 | 59 | |
| Capsule | 0.165 | ||||
| Absent | 67 | 48 | 31 | 38 | |
| Present | 70 | 52 | 50 | 62 | |
BCLC: Barcelona Clinic Liver Cancer; AST: Aspartate aminotransferase; AFP: Alpha fetoprotein; ECOG: Eastern Cooperative Oncology Group.
Radiomics score building
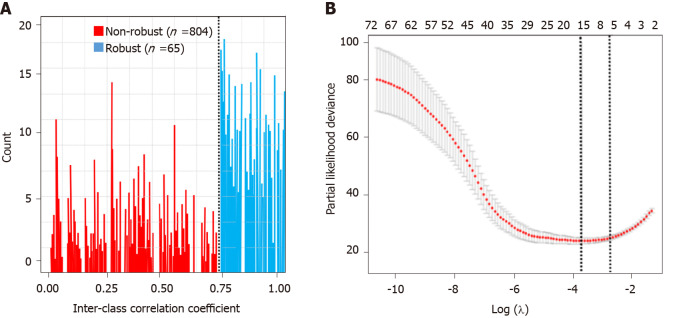
A total of 869 radiomics features were extracted. Sixty-five radiomics features remained in the arterial phase after the reproducibility test (Figure 3A). All the selected features were entered into the LASSO Cox regression analysis. Eight features were selected to build the radiomics signature using the LASSO regression model (Figure 3B): Two volumetric texture features and six wavelet texture features, respectively. The CT-based radiomics signature can be calculated with the following formula: Radiomics score = 0.174 + 0.432 × Shape_Maximum 3D Diameter - 0.432 × Shape_Elongation + 0.017 × wavelet-HHH_GLSZM_Gray Level Variance - 0.263 × wavelet-HHL_GLSZM_Size Zone Non-Uniformity Normalized + 0.046 × wavelet-HLH_GLSZM_Gray Level Non-Uniformity Normalized - 0.367 × wavelet-HLH_GLSZM_Zone Variance + 0.562 × wavelet-HHH_GLRLM_Low Gray Level Zone Emphasis - 0.214 × wavelet- LHL_GLDM_Dependence Non-Uniformity Normalized. The median Rad-score was 0.75 (range, -1.3-1.7). The cut-off value of the Rad-score was 0.1.
Figure 3.
Feature selection. A: Histogram of the inter-class correlation coefficient. The intraclass correlation coefficient (ICC) was used to determine the stability of the features. Features with an ICC < 0.8 were excluded from the analysis. After robustness test, 65 of the initial 869 computed tomography image features in the arterial phase were retained; B: Partial likelihood deviance was drawn vs log (λ) in the arterial phase.
Development of a clinical model/CT-based radiomics nomogram
Clinical model: A multivariate model based on Cox analyses revealed four clinical factors affecting OS: BCLC stage, irregular tumour margin, largest tumour size, and tumour number. These factors were combined to create a clinical score (Table 2).
Table 2.
Univariate and multivariate-Cox regression analyses of prognostic factors in hepatocellular carcinoma patients treated by transarterial chemoembolization in the training cohort
|
Variable
|
Univariate analysis
|
Multivariate analysis
|
Score
|
|||||
|
Before first TACE
|
Hazard ratio
|
95%CI
|
P
value
|
Hazard ratio
|
95%CI
|
P
value
|
B
|
|
| Age (yr), < 60/≥ 60 | 0.781 | 0.324-1.012 | 0.124 | |||||
| Sex, male/female | 0.891 | 0.456-1.213 | 0.211 | |||||
| HBsAg status, positive/negative | 0.671 | 0.319-0.987 | 0.121 | |||||
| Child-Pugh class, A/B | 1.178 | 0.614-1.418 | 0.256 | |||||
| Largest tumor size (cm), < 5/≥ 5 | 1.619 | 1.671-2.341 | 0.005 | 1.312 | 0.981-1.992 | < 0.001 | 0.4168 | 1 |
| Tumor number, solitary/multiple | 1.987 | 1.561-2.354 | 0.007 | 1.289 | 1.481-2.002 | < 0.001 | 0.3178 | 1 |
| BCLC stage, A/B | 1.671 | 1.319-1.987 | <0.001 | 1.789 | 1.289-2.112 | < 0.001 | 0.4288 | 1 |
| AFP (IU/mL), < 200/≥ 200 | 0.678 | 0.214-1.018 | 0.128 | |||||
| ECOG performance status score, 0/1/2 | 1.102 | 0.781-1.456 | 0.199 | |||||
| AST (U/L), < 40/≥ 40 | 0.543 | 0.178-0.967 | 0.089 | |||||
| Irregular tumor margin, absent/present | 1.562 | 1.211-1.897 | < 0.001 | 1.457 | 1.090-2.089 | < 0.001 | 0.404 | 1 |
| Capsule, absent/present | 1.432 | 1.121-1.976 | 0.032 | 1.321 | 1.007-1.764 | 0.082 | ||
The regression coefficients (B) in multivariate-Cox regression analysis were multiplied by 2 and rounded to calculate the clinical score. The clinical model used the median value (2) of total score (4) to stratify patients into high and low-risk group. HCC: Hepatocellular carcinoma; TACE, Transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; AST: Aspartate aminotransferase; AFP: Alpha fetoprotein; ECOG: Eastern Cooperative Oncology Group; CI: Confidence interval.
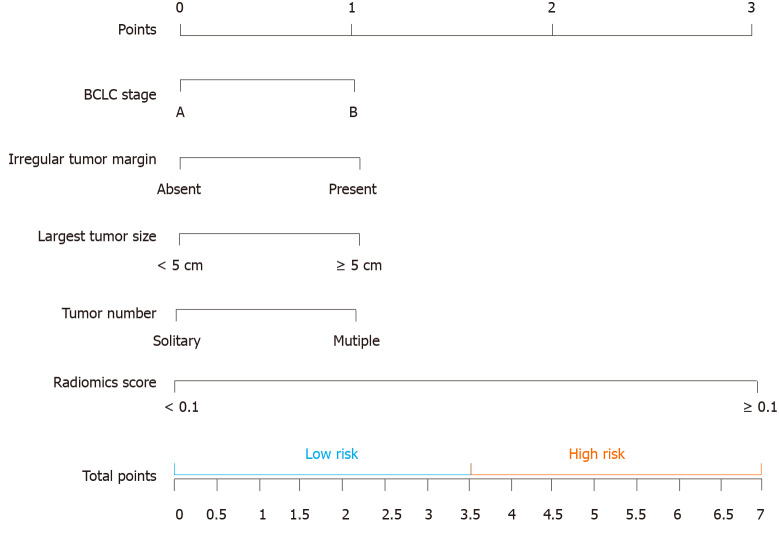
CT-based radiomics nomogram: The results of the univariate analysis showed that Rad-score, BCLC stage, irregular tumour margin, largest tumour size, tumour number, irregular tumour margin, and AFP level had significant effects on OS (Table 3). Multivariate Cox regression analysis showed that the following five factors affected OS: Rad-score, BCLC stage, irregular tumour margin, largest tumour size, and tumour number (Table 4). The median value of the CT-based radiomics nomogram was 3.5 (Figure 4). We used the median value to classify our patients into high- and low-risk groups.
Table 3.
Univariate analysis of prognostic factors in hepatocellular carcinoma patients treated by transarterial chemoembolization in the training cohort
| Variable | n |
Overall survival (mo)
|
P value | |
|
Median
|
95%CI
|
|||
| Before treatment | ||||
| Age (yr) | 0.213 | |||
| < 60 | 87 | 19.8 | 16.8-23.4 | |
| ≥ 60 | 50 | 17.6 | 14.3-21.1 | |
| Sex | 0.178 | |||
| Male | 91 | 15.4 | 12.5-19.6 | |
| Female | 46 | 19.6 | 14.3-23.1 | |
| HBsAg status | 0.121 | |||
| Positive | 110 | 14.9 | 12.3-19.2 | |
| Negative | 27 | 18.1 | 14.1-22.8 | |
| Child-Pugh stage | ||||
| Child-Pugh class | 0.301 | |||
| A | 84 | 23.5 | 19.1-32.4 | |
| B | 53 | 19.8 | 13.7-23.8 | |
| Largest tumor size (cm) | 0.032 | |||
| < 5 | 91 | 19.6 | 19.7-24.2 | |
| ≥ 5 | 46 | 14.1 | 14.1-18.8 | |
| Tumor number | 0.029 | |||
| Solitary | 89 | 19.1 | 17.4-22.7 | |
| Multiple | 48 | 14.7 | 13.8-18.1 | |
| BCLC stage | 0.033 | |||
| A | 20 | 26.7 | 22.3-31.2 | |
| B | 117 | 16.1 | 13.7-24.2 | |
| AFP (IU/mL) | 0.041 | |||
| < 200 | 29 | 20.7 | 17.2-29.4 | |
| ≥ 200 | 108 | 16.8 | 9.5-18.2 | |
| ECOG performance status score | 0.195 | |||
| 0 | 42 | 21.6 | 13.2-26.7 | |
| 1 | 85 | 19.3 | 10.3-24.4 | |
| 2 | 10 | 17.1 | 9.1-22.3 | |
| AST (U/L) | 0.261 | |||
| < 40 | 50 | 18.3 | 15.2-23.7 | |
| ≥ 40 | 87 | 16.9 | 12.3-22.9 | |
| Irregular tumor margin | 0.018 | |||
| Absent | 62 | 20.5 | 14.7-27.2 | |
| Present | 75 | 12.6 | 8.7-16.4 | |
| Capsule | 0.087 | |||
| Absent | 67 | 16.7 | 12.1-22.4 | |
| Present | 70 | 19.3 | 13.5-25.1 | |
| Radiomics score | 0.003 | |||
| < 0.1 | 102 | 23.3 | 19.8-28.6 | |
| ≥ 0.1 | 35 | 10.3 | 6.5-14.3 | |
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; BCLC: Barcelona Clinic Liver Cancer; AST: Aspartate aminotransferase; AFP: Alpha fetoprotein; ECOG: Eastern Cooperative Oncology Group; CI: Confidence interval.
Table 4.
Multivariate stepwise backward Cox regression analysis of prognostic factors in hepatocellular carcinoma patients treated by transarterial chemoembolization in the training cohort
|
Variable
|
Hazard ratio ratio
|
95%CI
|
B
|
Score
|
P
value (Cox regression)
|
| Before treatment | |||||
| BCLC stage | |||||
| A | 1 | 0 | 0 | ||
| B | 2.3 | 1.2-3.1 | 0.39 | 1 | 0.032 |
| Irregular tumor margin | |||||
| Absent | 1 | 0 | 0 | ||
| Present | 1.9 | 0.7-3.3 | 0.42 | 1 | 0.028 |
| Largest tumor size (cm) | |||||
| < 5 | 1 | 0 | 0 | ||
| ≥ 5 | 1.7 | 0.6-2.9 | 0.47 | 1 | 0.017 |
| Tumor number | |||||
| Solitary | 1 | 0 | 0 | ||
| Multiple | 2.1 | 1.1-3.1 | 0.33 | 1 | 0.021 |
| Radiomics score | |||||
| < 0.1 | 1 | 0 | 0 | ||
| ≥ 0.1 | 3.9 | 3.1-8.8 | 1.72 | 3 | < 0.001 |
The regression coefficients (B) in multivariate-Cox regression analysis were multiplied by 2 and rounded to calculate the radiomics-clinical score. The radiomics-clinical model used the median value (3.5) of total score (7) to stratify patients into high and low-risk group. HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; BCLC: Barcelona Clinic Liver Cancer; B: B regression coefficient; CI: Confidence interval.
Figure 4.
The computed tomography-based nomogram was built. The computed tomography (CT)-based nomogram was obtained by combining the effective clinical factors and radiomics signature of artery phase contrast-enhanced CT images. We choose the median value (3.5) to classify our patients into high and low-risk groups. BCLC: Barcelona Clinic Liver Cancer.
Model evaluation
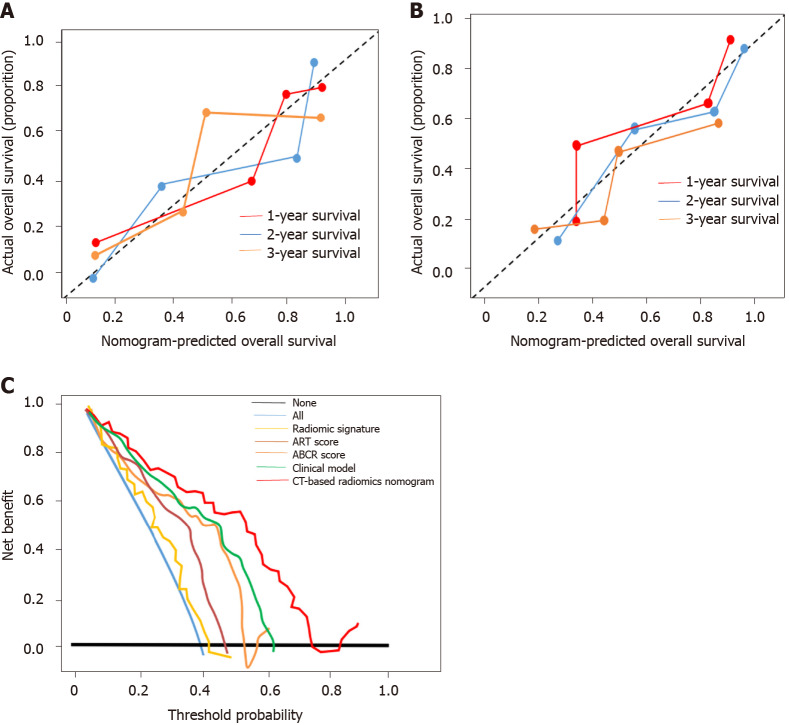
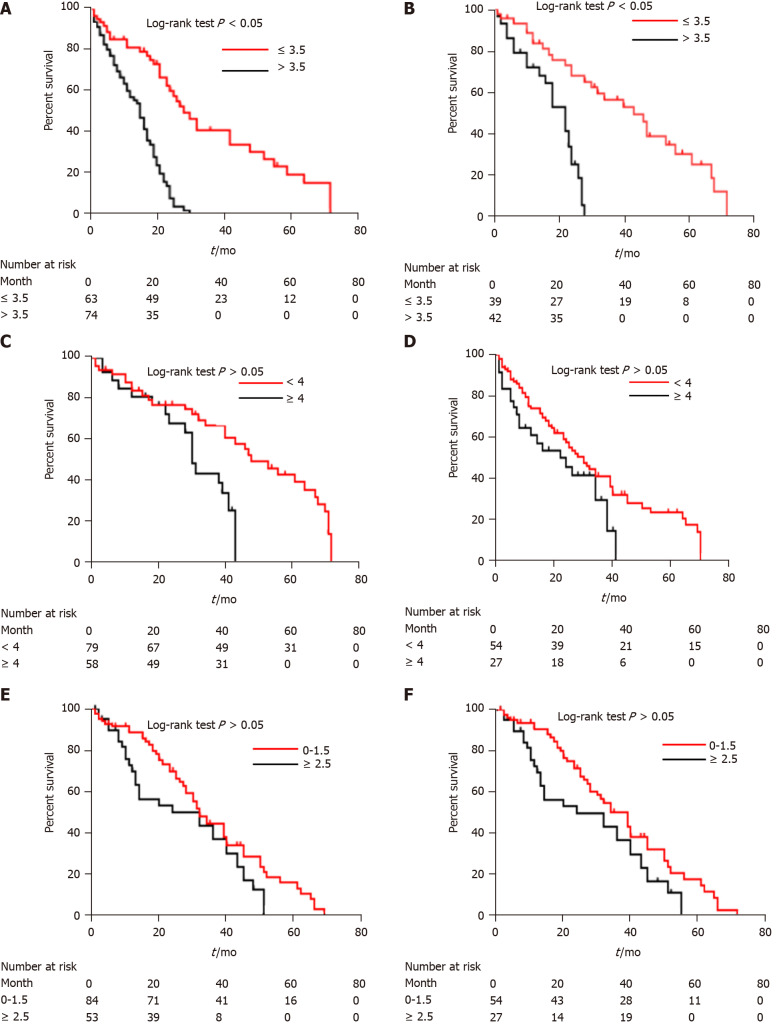
The CT-based radiomics nomogram stratified the patients in the training cohort into two subgroups with distinct prognoses. Patients with a score ≤ 3.5 (low-risk group) had a median OS of 23.6 mo (95%CI: 16.3-28.7), whereas patients with a score > 3.5 (high-risk group) had a median OS of 12.3 mo (95%CI: 7.9-16.4; P < 0.001). In the validation cohort, the same trend was observed. The Hosmer-Lemeshow test demonstrated that there was no significant difference between the predictive curve (CT-based radiomics nomogram) and the ideal curve in the training and validation cohorts (P = 0.137 and 0.165, respectively) (Figure 5A and B). DCA indicated that the CT-based radiomics nomogram adds more net benefit than the other four models/scoring systems in the validation cohort (Figure 5C). We used the ART and ABCR scores to identify TACE refractoriness in our cohorts. In both the ART and ABCR groups, the median OS duration did not show a significant difference in either the training or validation cohorts (Figure 6). The C-indexes of the CT-based radiomics nomogram were 0.844 (0.762-0.901) and 0.831 (0.742-0.881) in the training and validation cohorts, respectively, and the nomogram significantly better predicted OS than the other models (the ART score, the ABCR score, our newly constructed clinical model, and the radiomics score) (Table 5).
Figure 5.
Calibration curve and clinical utility analyses. A: Calibration curves of the nomogram on the training dataset. The Hosmer-Lemeshow test yielded a P value of 0.137 in the training dataset; B: Calibration curves of the nomogram on the validation dataset. The Hosmer-Lemeshow test yielded a P value of 0.165 in the validation dataset; C: Decision curve analysis for each model in the validation dataset. The Y-axis measures the net benefit, which is calculated by summing the benefits (true-positive findings) and subtracting the harms (false-positive findings). The decision curve showed that if the threshold probability is over 10%, the application of computed tomography-based radiomics nomogram to predict transarterial chemoembolization-refractoriness adds more benefit compared with the other scores/models. ART: Assessment for Retreatment with Transarterial Chemoembolization; ABCR: α-Fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response; CT: Computed tomography.
Figure 6.
Kaplan-Meier curves of survival outcomes in different patient groups. A: Kaplan-Meier analysis of overall survival of high-risk (> 3.5) and low-risk (≤ 3.5) patients according to computed tomography (CT)-based radiomics nomogram in the training cohort; B: Kaplan-Meier analysis of overall survival of high-risk (> 3.5) and low-risk (≤ 3.5) patients according to CT-based radiomics nomogram in the validation cohort; C: Kaplan-Meier analysis of overall survival of high-risk (≥ 4) and low-risk (< 4) patients according to α-fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response (ABCR) score in the training cohort; D: Kaplan-Meier analysis of overall survival of high-risk (≥ 4) and low-risk (< 4) patients according to ABCR score in the validation cohort; E: Kaplan-Meier analysis of overall survival of high-risk (≥ 2.5) and low-risk (0-1.5) patients according to Assessment for Retreatment with Transarterial Chemoembolization (ART) score in the training cohort; F: Kaplan-Meier analysis of overall survival of high-risk (≥ 2.5) and low-risk (0-1.5) patients according to ART score in the validation cohort.
Table 5.
Predictive performance of each model/score
| Prediction model | Training cohort C-index (95%CI) | Validation cohortC-index (95%CI) |
P
value
|
|||
|
3 vs 1
|
3 vs 2
|
3 vs 4
|
vs
5
|
|||
| 1 Clinical model | 0.643 (0.613-0.712) | 0.629 (0.601-0.678) | 0.025a/0.023b | 0.003a/0.002b | 0.007a/0.006b | 0.009a/0.004b |
| 2 Radiomics score | 0.723 (0.634-0.778) | 0.734 (0.641-0.793) | ||||
| 3 CT-based radiomics nomogram | 0.844 (0.762-0.901) | 0.831 (0.742-0.881) | ||||
| 4 ART score | 0.714 (0.632-0.771) | 0.690 (0.601-0.761) | ||||
| 5 ABCR score | 0.732 (0.646-0.801) | 0.701 (0.632-0.789) | ||||
Significant difference of C-index between computed tomography (CT)-based radiomics nomogram vs other models/scores in the training cohort.
Significant difference of C-index between CT-based radiomics nomogram vs other models/scores in the validation cohort. CI: Confidence interval; CT: Computed tomography; ART: Assessment for Retreatment with Transarterial Chemoembolization; ABCR: α-Fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response.
DISCUSSION
The main finding of this study is that our CT-based radiomics nomogram can be used to individually predict a patient’s refractory state before the first session of TACE. The predictive calibration curves of the training and validation datasets demonstrated agreement with the ideal curve. Compared with the existing ART and ABCR scores, our new model showed the best discrimination for selecting patients who could benefit from consecutive TACE sessions, and DCA proved that our newly constructed nomogram was clinically useful in the current study.
Our newly developed CT-based radiomics nomogram includes four clinical parameters: BCLC stage, irregular tumour margin, largest tumour size, and tumour number. These four clinical parameters mainly emphasize tumour burden and tumour aggressiveness before initial treatment. Currently, BCLC staging is the most commonly used method worldwide for HCC treatment allocation. Despite the limitations of the BCLC staging system, e.g., it does not include tumour characteristics (including tumour distribution and heterogeneity), it remains the most validated and reliable system for HCC prognostication. Tumour size has been broadly recognized as a major predictive factor for response to TACE[26-28]. The blood supply of HCC is mainly from the hepatic artery. Large tumours always have a more abundant blood supply, grow faster, break through the capsule more easily, and readily infiltrate the surrounding liver tissue. Large tumours also more easily cause invasion of the portal vein, increasing the probability of post-TACE intrahepatic recurrence. Katayama et al[29] proved that pre-treatment tumour number is a useful factor for predicting TACE response. Multiple tumours in the liver are mostly due to intrahepatic metastasis, and an increase in tumour number usually indicates more rapid metastasis. For these patients, even if the visible tumours are totally embolized, small metastases may also be present in the liver, which is consistent with the finding of this study that patients with multiple tumours had a higher rate of TACE treatment failure. Irregular tumour margin was confirmed as a reliable predictor of microvascular invasion (MVI)[30]. MVI has been regarded as an important risk factor affecting the prognosis of HCC patients after treatment[31]. In our study, irregular tumour margin had a significant effect on patient survival, indicating that it also had an increasing trend in the development of TACE refractoriness.
CT imaging is commonly used in clinical procedures for HCC patients, such as tumour detection and staging. However, since conventional imaging evaluation relies on semantic features and provides relatively few metrics, the large amount of additional useful information about tumour heterogeneity has been underutilized[32]. Radiomics is a rapidly advancing form of medical image analysis that enables the quantification of tumour phenotypic characteristics to provide prognostic information[33]. To facilitate the clinical use of a radiomics model, we constructed a nomogram to visualize and quantify the results of the complex radiomics analysis. Considering the weaknesses of the preceding radiomics models and the doubts about their reproducibility and robustness, we took effective measures to guarantee the objectivity and reproducibility of our radiomics model. First, we applied voxel intensity discretization and voxel size resampling to reduce the dependency of differences in image specifications, in accordance with recent CT radiomics studies[34,35]. Second, two radiologists carried out the tumour segmentation step, and ICCs were used to minimize subjectivity and operator error. Both the segmentation software and feature extraction software that we used were commonly adopted in earlier investigations and had been verified by those studies[36,37]. With all the above measures, a relatively evidence-based radiomics-clinical model was constructed for the pre-treatment evaluation of patients who are likely to develop TACE refractoriness.
The LASSO method is a powerful method for the regression of high-dimensional predictors[38]. In our study, 65 potential features were chosen from 869 candidate radiomics features of the arterial phase via the LASSO test method to build a radiomics signature. The majority of previous studies[34,35,39] applied morphological features and grey-level texture features [such as histograms and grey-level co-occurrence matrices (GLCM)] extracted from regions of interest. Morphological features have limited value in predicting tumour treatment response, and GLCM features mainly represent the heterogeneity of peripheral tumour regions. Alternatively, wavelet transformation can provide comprehensive spatial and frequency distributions for characterizing intratumoural and peritumoural regions in terms of low- and high-frequency signals. These properties may improve the performance of the radiomics model[40,41]. Not surprisingly, in the present study, the CT-based radiomics signature was composed primarily of wavelet-transformed textures. The wavelet transformation decomposes images into high frequency signals (heterogeneity) and low frequency signals (homogeneity) for both intratumoural and peritumoural regions, which is a strong suggestion of the tumour microenvironment heterogeneity. Recently, Chun et al[42] and Zhou et al[43] also proved that wavelet-based features can be used for disease diagnosis and the prediction of therapy response.
There are several limitations to our study. First, because this was a retrospective study, the selection bias may exist. Further large external validation cohort should be used to testify the robustness and reproducibility of the model. Second, we cannot obtain genomic data of each tumor, which may hinder better interpretation of radiomics features. Last, other clinical features may also be valuable for the construction of the predictive model, but we excluded them from the present study for reasons of data integrity and only selected the most reasonable features. In spite of these limitations, we believe that our approach offers advantages for the following reasons: (1) We performed the study in accordance with the TRIPOD statement, which may improve clinical utility of our results; and (2) In addition to the clinical data, the combination of radiomics and traditional imaging characteristics may improve the clinical acceptability of our newly constructed model.
CONCLUSION
In conclusion, based on CT-radiomics, we developed a novel and externally validated, noninvasive, objective nomogram for the pre-treatment prediction of patients who are likely to have TACE refractoriness. This may provide an unprecedented opportunity to improve clinical decision making for patients who are repeatedly treated by TACE and, therefore, improve the OS of these patients.
ARTICLE HIGHLIGHTS
Research background
The point at which transarterial chemoembolization (TACE) should be continued or stopped is currently not addressed by any guideline to our knowledge. Repeated TACE cycles are, however, associated with an increase of related side effects and liver damage, potentially preventing an even greater survival advantage. In the era of personalized oncology, radiomics has allowed digitally encrypted medical images to be transformed into high-throughput quantitative features that provide information on patient prognosis.
Research motivation
In previous studies, patients with high Assessment for Retreatment with Transarterial Chemoembolization (ART) or α-fetoprotein, Barcelona Clinic Liver Cancer, Child-Pugh, and Response score (ABCR) scores tended to have a poor prognosis. Nonetheless, in terms of predictive ability, neither score was reliable enough to allow for clinical decision-making. Although previous studies have shown the prognostic value of computed tomography (CT) radiomic features for different cancer sites, there is scarcity of multi-centre radiomics research on TACE refractoriness.
Research objectives
The purpose of this study was to develop and validate a CT-based radiomics nomogram for the pre-treatment prediction of TACE refractoriness.
Research methods
Our study consisted of a training dataset (n = 137) and an external validation dataset (n = 81) of patients with clinically/pathologically confirmed hepatocellular carcinoma who underwent repeated TACE from March 2009 to March 2016. The radiomics features were retrospectively extracted from preoperative CT images of the arterial phase. The radiomics signature was built by least absolute shrinkage and selection operator (LASSO) regression. The CT-based radiomics nomogram incorporating clinical risk factors was built by multivariable logistic regression analysis. The performance of the nomogram was assessed with respect to its calibration, discrimination, and clinical usefulness. We used the concordance index to conduct head-to-head comparisons of the radiomics nomogram with the other four models (ART score, ABCR score, CT-based radiomics signature, and clinical model).
Research results
Eight features were selected to build the radiomics signature using the LASSO regression model. The CT-based radiomics nomogram included the radiomics score (HR = 3.9, 95% confidence interval: 3.1-8.8, P < 0.001) and four clinical factors and classified patients into high-risk (score > 3.5) and low-risk (score ≤ 3.5) groups with markedly different prognoses (overall survival: 12.3 mo vs 23.6 mo, P < 0.001). The accuracy of the nomogram was considerably higher than that of the other four models (ART score, ABCR score, CT-based radiomics signature, and clinical model). The calibration curve and decision curve analyses verified the usefulness of the CT-based radiomics nomogram for clinical practice.
Research conclusions
The CT-based radiomics nomogram is valuable in preoperatively predicting TACE refractoriness, which may aid interventional radiologist in determining the optimal treatment approach.
Research perspectives
First, additional information, such as gene sequence data or the molecular pathway, might be necessary for better interpretation of radiomics features, and this issue is left for future research. Second, larger prospective multicenter studies are needed to externally validate our newly constructed model in the future.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Affiliated Hospital of Chengdu University, approval No. PJ2019-019-01.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Manuscript source: Unsolicited manuscript
Peer-review started: November 2, 2020
First decision: December 3, 2020
Article in press: December 16, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Donadon M S-Editor: Huang P L-Editor: Wang TQ P-Editor: Ma YJ
Contributor Information
Xiang-Ke Niu, Department of Interventional Radiology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province, China; Department of Interventional Radiology, Affiliated Hospital of Chengdu University, Chengdu 610081, Sichuan Province, China.
Xiao-Feng He, Department of Interventional Radiology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province, China. ozonetherapy@163.com.
Data sharing statement
No additional data are available.
References
- 1.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 2.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed "on demand". J Hepatol. 2012;57:1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 4.Hu K, Lu S, Li M, Zhang F, Tang B, Yuan J, Shan Y, Xu P, Chen R, Ren Z, Yin X. A Novel Pre-treatment Model Predicting Risk of Developing Refractoriness to Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma. J Cancer. 2020;11:4589–4596. doi: 10.7150/jca.44847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 6.Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, Sakurai T, Nishida N, Kudo M. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer. 2015;4:253–262. doi: 10.1159/000367743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M, Yokosuka O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330–341. doi: 10.1159/000365993. [DOI] [PubMed] [Google Scholar]
- 8.Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 9.Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, Folgoc GL, Castellani P, Bronowicki JP, Bourlière M. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855–862. doi: 10.1016/j.jhep.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Terzi E, Terenzi L, Venerandi L, Croci L, Renzulli M, Mosconi C, Allegretti G, Granito A, Golfieri R, Bolondi L, Piscaglia F. The ART score is not effective to select patients for transarterial chemoembolization retreatment in an Italian series. Dig Dis. 2014;32:711–716. doi: 10.1159/000368007. [DOI] [PubMed] [Google Scholar]
- 11.Arizumi T, Ueshima K, Iwanishi M, Minami T, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Ida H, Minami Y, Sakurai T, Nishida N, Kitano M, Kudo M. Evaluation of ART Scores for Repeated Transarterial Chemoembolization in Japanese Patients with Hepatocellular Carcinoma. Oncology. 2015;89 Suppl 2:4–10. doi: 10.1159/000440625. [DOI] [PubMed] [Google Scholar]
- 12.Facciorusso A, Bhoori S, Sposito C, Mazzaferro V. Repeated transarterial chemoembolization: An overfitting effort? J Hepatol. 2015;62:1440–1442. doi: 10.1016/j.jhep.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Kloeckner R, Pitton MB, Dueber C, Schmidtmann I, Galle PR, Koch S, Wörns MA, Weinmann A. Validation of Clinical Scoring Systems ART and ABCR after Transarterial Chemoembolization of Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:94–102. doi: 10.1016/j.jvir.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng BH, Liu LZ, Zhang ZZ, Shi JY, Dong LQ, Tian LY, Ding ZB, Ji Y, Rao SX, Zhou J, Fan J, Wang XY, Gao Q. Radiomics score: a potential prognostic imaging feature for postoperative survival of solitary HCC patients. BMC Cancer. 2018;18:1148. doi: 10.1186/s12885-018-5024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K, Xia YX, Zhang YD, Jiang WJ, Li XC, Wang XH. Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes. Radiology. 2019;290:90–98. doi: 10.1148/radiol.2018181408. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Lim Y, Ko ES, Cho HH, Lee JE, Han BK, Ko EY, Choi JS, Park KW. Radiomics Signature on Magnetic Resonance Imaging: Association with Disease-Free Survival in Patients with Invasive Breast Cancer. Clin Cancer Res. 2018;24:4705–4714. doi: 10.1158/1078-0432.CCR-17-3783. [DOI] [PubMed] [Google Scholar]
- 18.Park JE, Kim D, Kim HS, Park SY, Kim JY, Cho SJ, Shin JH, Kim JH. Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol. 2020;30:523–536. doi: 10.1007/s00330-019-06360-z. [DOI] [PubMed] [Google Scholar]
- 19.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S, Okusaka T Japan Interventional Radiology in Oncology Study Group (JIVROSG); Korea Interventional Radiology in Oncology Study Group (KIVROSG) Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490–500. doi: 10.1016/j.jvir.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87 Suppl 1:22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 22.Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816–830. doi: 10.1148/radiol.2018181494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, Choi JS, Han KH, Kim E, Kim KW. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol. 2009;19:1744–1751. doi: 10.1007/s00330-009-1331-8. [DOI] [PubMed] [Google Scholar]
- 24.Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology. 2020;294:568–579. doi: 10.1148/radiol.2020191470. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, Ingrand P, Tasu JP. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26:1640–1648. doi: 10.1007/s00330-015-3982-y. [DOI] [PubMed] [Google Scholar]
- 27.Sun S, Wang X, Chen J. Using Pre-Treatment Neutrophil-to-Lymphocyte Ratio to Predict the Prognosis of Young Patients with Hepatocellular Carcinoma Implemented Minimally Invasive Treatment. J Adolesc Young Adult Oncol. 2020;9:85–89. doi: 10.1089/jayao.2019.0046. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:250–258. doi: 10.3350/cmh.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama K, Imai T, Abe Y, Nawa T, Maeda N, Nakanishi K, Wada H, Fukui K, Ito Y, Yokota I, Ohkawa K. Number of Nodules but not Size of Hepatocellular Carcinoma Can Predict Refractoriness to Transarterial Chemoembolization and Poor Prognosis. J Clin Med Res. 2018;10:765–771. doi: 10.14740/jocmr3559w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reginelli A, Vanzulli A, Sgrazzutti C, Caschera L, Serra N, Raucci A, Urraro F, Cappabianca S. Vascular microinvasion from hepatocellular carcinoma: CT findings and pathologic correlation for the best therapeutic strategies. Med Oncol. 2017;34:93. doi: 10.1007/s12032-017-0949-7. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Li R, Liu F. Novel Prognostic Nomograms for Predicting Early and Late Recurrence of Hepatocellular Carcinoma After Curative Hepatectomy. Cancer Manag Res. 2020;12:1693–1712. doi: 10.2147/CMAR.S241959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127–157. doi: 10.3322/caac.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi T, Ouhmich F, Gonzalez-Cabrera C, Felli E, Saviano A, Agnus V, Savadjiev P, Baumert TF, Pessaux P, Marescaux J, Gallix B. Radiomics in hepatocellular carcinoma: a quantitative review. Hepatol Int. 2019;13:546–559. doi: 10.1007/s12072-019-09973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, Hollebecque A, Scoazec JY, Marabelle A, Massard C, Soria JC, Robert C, Paragios N, Deutsch E, Ferté C. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19:1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 35.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts HJWL. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017;77:e104–e107. doi: 10.1158/0008-5472.CAN-17-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY) 2017;42:1695–1704. doi: 10.1007/s00261-017-1072-0. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Han F, Jin Y, Wang X, Zhang J. A Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma. Front Oncol. 2020;10:1654. doi: 10.3389/fonc.2020.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, Zhao HW, Chen W, He YL, Wang HY, Xie D, Luo JH. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14:1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 39.Mosconi C, Cucchetti A, Bruno A, Cappelli A, Bargellini I, De Benedittis C, Lorenzoni G, Gramenzi A, Tarantino FP, Parini L, Pettinato V, Modestino F, Peta G, Cioni R, Golfieri R. Radiomics of cholangiocarcinoma on pretreatment CT can identify patients who would best respond to radioembolisation. Eur Radiol. 2020;30:4534–4544. doi: 10.1007/s00330-020-06795-9. [DOI] [PubMed] [Google Scholar]
- 40.Majeed Alneamy JS, A Hameed Alnaish Z, Mohd Hashim SZ, Hamed Alnaish RA. Utilizing hybrid functional fuzzy wavelet neural networks with a teaching learning-based optimization algorithm for medical disease diagnosis. Comput Biol Med. 2019;112:103348. doi: 10.1016/j.compbiomed.2019.103348. [DOI] [PubMed] [Google Scholar]
- 41.Das DK, Dutta PK. Efficient automated detection of mitotic cells from breast histological images using deep convolution neutral network with wavelet decomposed patches. Comput Biol Med. 2019;104:29–42. doi: 10.1016/j.compbiomed.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Chun SH, Suh YJ, Han K, Park SJ, Shim CY, Hong GR, Lee S, Lee SH, Kim YJ, Choi BW. Differentiation of left atrial appendage thrombus from circulatory stasis using cardiac CT radiomics in patients with valvular heart disease. Eur Radiol. 2020 doi: 10.1007/s00330-020-07173-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Lu J, Gao C, Zeng J, Zhou C, Lai X, Cai W, Xu M. Predicting the response to neoadjuvant chemotherapy for breast cancer: wavelet transforming radiomics in MRI. BMC Cancer. 2020;20:100. doi: 10.1186/s12885-020-6523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.