Abstract
This cohort study evaluates the association of etanercept treatment with outcomes of reactive infectious mucocutaneous eruption in hospitalized children and adolescents.
Reactive infectious mucocutaneous eruption (RIME) is a recently proposed entity that encompasses what was previously known as oral erythema multiforme secondary to Mycoplasma pneumoniae, Mycoplasma-induced rash and mucositis, and Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) secondary to infection with Mycoplasma species or other respiratory pathogens.1,2,3 Reactive infectious mucocutaneous eruption is distinct from drug-related SJS and TEN and is a cause of serious mucocutaneous eruptions in the pediatric population.3 In addition, RIME often requires inpatient hospitalization and has no standardized treatment. In previous studies, patients were treated with topical corticosteroids, cyclosporine, intravenous immunoglobulin, and oral corticosteroids with varying outcomes.2,4 Little is known about the pathogenesis of RIME. In this cohort study, we analyzed patients with RIME who were treated successfully with etanercept, a known medication for drug-related adult SJS and TEN and a recently suggested treatment for drug-related pediatric SJS and TEN.5
Methods
This retrospective cohort study used medical record review for 6 patients who were admitted to Children’s Hospital Los Angeles for RIME between June 1, 2018, and May 31, 2020. Patients were excluded if they had any medication exposure known to cause SJS and TEN. The study received approval from the institutional review board of Children’s Hospital Los Angeles, which waived informed consent given the retrospective nature of the review.
According to the site protocol, treatment with etanercept was considered only in patients who displayed evidence of disease progression within the 24 hours before drug administration. A single dose of subcutaneous etanercept, 0.6 to 0.8 mg/kg, was administered to those who weighed less than 60 kg, or a dose of 50 mg was administered to those who weighed more than 60 kg. A second dose was considered in the event of continued worsening after 48 hours of the first dose.
Results
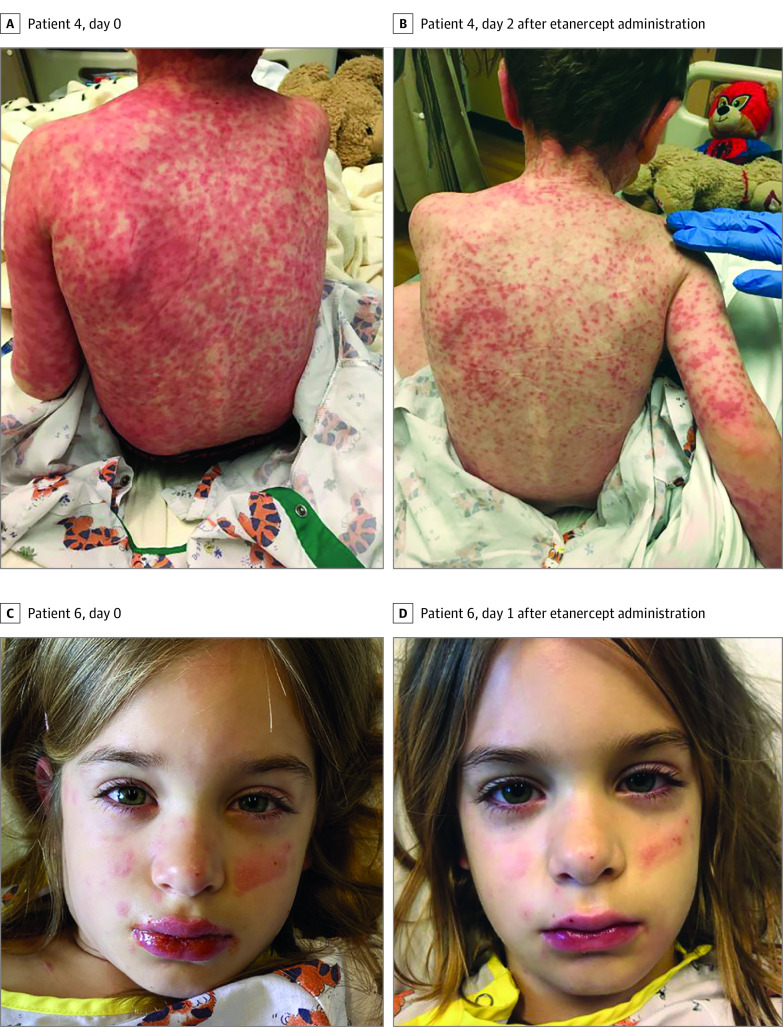
The disease courses and responses of patients are summarized in the Table, and photographic evidence is shown in the Figure. Patient ages ranged from 5 to 13 years, and the sample comprised 3 boys and 3 girls. Five of the 6 patients had more than 1 mucosal surface involved. Cutaneous involvement was universally described as erythematous to dusky papules and plaques, some of which had central vesicles and bullae. All patients had clinical history and laboratory evidence of infection before the onset of the rash. No patients required supplemental oxygen. Five of the 6 patients were treated with antibiotics for presumed atypical bacterial pneumonia. Most patients did not receive any other treatment modalities, such as intravenous immunoglobulin or systemic corticosteroids, except for patient 3, who received a dose of dexamethasone before transfer. No adverse events associated with the administration of etanercept were observed.
Table. Patient Disease Courses and Responses.
| Patient No./age group | Prodromal symptoms | Mucocutaneous involvementa | Infectious workupb | Length of hospital stay (days to discharge after etanercept dose), d | Other interventionsc | Improvement on physical examination after etanercept treatment, d | Advancement of diet after etanercept treatment, d | Discontinuation of as-needed pain medications after etanercept treatment, dd | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1/Child | 5-d History of fever, productive cough, progressive rash, and mucositis | Oral and ocular; 5% BSA affecting trunk and extremities | CXR with perihilar infiltrates; positive for Mycoplasma pneumoniae by PCR; negative for RVPA by PCR; negative for HSV; negative for HSV 1 and 2 IgG and IgM by PCR | 12 (12) | Azithromycin ×5 d; amniotic ocular membrane; nasogastric tube placement | 3 | 5 | 5 | No ophthalmological consequences; no permanent cutaneous consequences |
| 2/Adolescent | 6-d History of fever; productive cough; and generalized weakness, progressive rash, and mucositis | Oral, ocular and genital; 25% BSA affecting face, trunk, and extremities | No CXR; positive for M pneumoniae IgM and IgG; positive for rhinovirus; negative for HSV 1 and 2 and VZV by PCR | 4 (4) | Azithromycin ×5 d; topical mupirocin 2% ointment; lidocaine-diphenhydramine hydrochloride-Maalox mouthwash; dexamethasone-neomycin sulfate-polymyxin B sulfate eye drops | 1 | No change | Did not require any as-needed pain medications during hospital stay | No ophthalmological consequences; no permanent cutaneous consequences |
| 3/Adolescent | 5-d History of fever, dysphagia, productive cough, and mucositis | Oral and ocular; <1% BSA | CXR with bibasilar, bilateral infiltrates, positive for M pneumoniae by PCR; negative RVP result; negative for HSV 1 and 2 by PCR | 3 (3) | 1 Oral dose of dexamethasone at outside hospital ED; clindamycin hydrochloride ×2 d; azithromycin ×5 d | 2 | No change | 2 | No follow-up |
| 4/Child | 2-wk History of cough and generalized malaise with 3 d of progressive rash | Oral, ocular, and genital; 60% BSA affecting face, back, trunk, palms, soles, and extremities | CXR with perihilar opacities; negative for M pneumoniae by PCR; positive for M pneumoniae IgM and IgG; negative RVP result | 5 (5) | Azithromycin ×5 d; erythromycin ocular ointment; prednisolone sodium phosphate eye drops | 2 | 1 | 2 | No follow-up |
| 5/Child | 9-d History of fevers and joint pains and mucositis | Oral; <1% BSA | No CXR; negative for M pneumoniae by PCR; positive for M pneumoniae IgM, negative for M pneumoniae IgG; negative RVP result; negative for HSV 1 and 2 by PCR, negative for HSV 1 and 2 IgG and IgM | 9 (9) | Acyclovir ×2 d; azithromycin ×5 d; clindamycin ×5 d; nasogastric tube placement | 2 | 2 | 4 | No ophthalmological consequences; no permanent cutaneous consequences |
| 6/Child | 2-d History of fevers, malaise, dysphagia, and progressive rash | Oral, ocular and anogenital; 10% BSA affecting abdomen, forearms, dorsal surface of hands, and proximal thighs | No CXR; negative for M pneumoniae by PCR; negative for M pneumoniae IgM and IgG and influenza A and B; positive for Coxsacksie B3 IgG; negative RVP result | 4 (4) | No antibiotics, lidocaine-diphenhydramine-Maalox mouthwash; artificial tears | 1 | 1 | 1 | No ophthalmological consequences; no permanent cutaneous consequences |
Abbreviations: BSA, body surface area; CXR, chest x-ray; ED, emergency department; HSV, herpes simplex virus; IgG/IgM, immunoglobulin G or M; PCR, polymerase chain reaction; RVPA, right ventricle pulmonary artery; RVP, respiratory virus panel; VZV, varicella zoster virus.
BSA was identified retrospectively by photographs.
Respiratory virus panel nasal PCR at our institution included adenovirus, coronavirus, metapneumovirus, rhinovirus, enterovirus, influenza A and B, parainfluenza, and respiratory syncytial virus.
Oral or by mouth intake was based on volume of liquid consumed in a day or the advancement of diet from liquids to soft solids to solid.
As-needed pain medications included oral acetaminophen, toradol, and morphine sulfate.
Figure. Clinical Photographs of Patients With Reactive Infectious Mucocutaneous Eruption Before and After Etanercept Treatment.
Every patient had evidence of disease progression in the 24 hours preceding etanercept administration, and 5 of the 6 patients had objective improvement in physical examination within 2 days of administration. Conjunctival injection and eyelid swelling were the first reported areas of improvement. In general, patients had advancement of diet and discontinuation of as-needed pain medications within 3 days of treatment. Although a direct comparison was not possible, the mean (SD) length of hospital stay was 6 (3.5) days, which was a better outcome than that for other treatment options, including supportive care, cyclosporine, intravenous immunoglobulin, and systemic corticosteroids.2,4
Discussion
This study was limited by its small number of patients, retrospective nature, and lack of a control group. As such, it was difficult to ascertain whether etanercept was superior to supportive care alone.
The proposed mechanisms of mucocutaneous disease in RIME include immune complex deposition, molecular mimicry, and direct respiratory pathogen cutaneous infection.1 To our knowledge, no studies have been conducted on the Fas ligand pathway and granulysin and their association with RIME, although these are known as the key mediators of drug-related adult SJS and TEN.6 The rapid response to etanercept in the patients suggests that Fas ligand pathway and granulysin may be associated with RIME.
Findings from this study support the potential use of etanercept in the management of RIME to halt progression, decrease pain, expedite the advancement of diet, and decrease the length of hospital stay. The results also suggest that granulysin may be associated with RIME. However, larger prospective studies are needed to better characterize the efficacy and safety of etanercept for treatment of this disease.
References
- 1.Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72(2):239-245. doi: 10.1016/j.jaad.2014.06.026 [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia J, Wan J, Lee DH, Treat J, Yan AC. Mycoplasma-associated Stevens-Johnson syndrome in children: retrospective review of patients managed with or without intravenous immunoglobulin, systemic corticosteroids, or a combination of therapies. Pediatr Dermatol. 2014;31(6):664-669. doi: 10.1111/pde.12481 [DOI] [PubMed] [Google Scholar]
- 3.Ramien ML, Bruckner AL. Mucocutaneous eruptions in acutely ill pediatric patients-think of Mycoplasma pneumoniae (and other infections) first. JAMA Dermatol. 2020;156(2):124-125. doi: 10.1001/jamadermatol.2019.3589 [DOI] [PubMed] [Google Scholar]
- 4.Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23(6):608-612. doi: 10.1177/1203475419874444 [DOI] [PubMed] [Google Scholar]
- 5.Eliades P, Fonseca M, Harp J. Use of etanercept in a series of pediatric patients with Stevens-Johnson syndrome-toxic epidermal necrolysis spectrum disease. JAMA Dermatol. 2020;156(8):921-922. doi: 10.1001/jamadermatol.2019.3731 [DOI] [PubMed] [Google Scholar]
- 6.Chung W-H, Hung S-I, Yang J-Y, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343-1350. doi: 10.1038/nm.1884 [DOI] [PubMed] [Google Scholar]