This multimodality study reports a detailed cardiovascular phenotyping and genotyping in young adults from an Amish family with a frameshift variant (c.699_700dupTA) in SERPINE1, the gene that codes for plasminogen activator inhibitor-1.
Key Points
Question
What is the genetic cause of cardiac fibrosis observed in young adults from 3 related Amish families?
Findings
In this study, cardiovascular phenotyping and genotyping of 17 participants (mean age, 24 years) was performed, including cardiovascular imaging, whole-exome sequencing, and examination of stem cell–derived cardiomyocytes. An autosomal recessive phenotype of cardiac fibrosis in individuals homozygous for a frameshift variant in SERPINE1, the gene that codes for plasminogen activator inhibitor-1 (PAI-1), was identified.
Meaning
In this study, genetic deficiency of PAI-1 was associated with early-onset cardiac fibrosis, suggesting an optimal range of PAI-1 is needed for cardiac homeostasis in humans.
Abstract
Importance
Cardiac fibrosis is exceedingly rare in young adults. Identification of genetic variants that cause early-onset cardiomyopathy may inform novel biological pathways. Experimental models and a single case report have linked genetic deficiency of plasminogen activator inhibitor-1 (PAI-1), a downstream target of cardiac transforming growth factor β, with cardiac fibrosis.
Objective
To perform detailed cardiovascular phenotyping and genotyping in young adults from an Amish family with a frameshift variant (c.699_700dupTA) in SERPINE1, the gene that codes for PAI-1.
Design, Setting, and Participants
This observational study included participants from 3 related nuclear families from an Amish community in the primary analysis and participants from the extended family in the secondary analysis. Participants were recruited from May 2015 to December 2016, and analysis took place from June 2015 to June 2020.
Main Outcomes and Measures
(1) Multimodality cardiovascular imaging (transthoracic echocardiography and cardiac magnetic resonance imaging), (2) whole-exome sequencing, and (3) induced pluripotent stem cell–derived cardiomyocytes.
Results
Among 17 participants included in the primary analysis, the mean (interquartile range) age was 23.7 (20.9-29.9) years and 9 individuals (52.9%) were confirmed to be homozygous for the SERPINE1 c.699_700dupTA variant. Late gadolinium enhancement was present in 6 of 9 homozygous participants (67%) with absolute PAI-1 deficiency vs 0 of 8 in the control group (P = .001). Late gadolinium enhancement patterns tended to be dense and linear, usually subepicardial but also midmyocardial and transmural with noncoronary distributions. Targeted whole-exome sequencing analysis identified that homozygosity for c.699_700dupTA SERPINE1 was the only shared pathogenic variant or variant of uncertain significance after examination of cardiomyopathy genes among those with late gadolinium enhancement. Induced pluripotent stem cell–derived cardiomyocytes from participants homozygous for the SERPINE1 c.699_700dupTA variant exhibited susceptibility to cardiomyocyte injury in response to angiotensin II (increased transforming growth factor β1 secretion and release of lactate dehydrogenase) compared with control induced pluripotent stem cell–derived cardiomyocytes. In a secondary analysis based on echocardiography in 155 individuals across 3 generations in the extended family, no difference in global longitudinal strain was observed in carriers for the SERPINE1 c.699_700dupTA variant compared with wild-type participants, supporting an autosomal recessive inheritance pattern.
Conclusions and Relevance
In this study, a highly penetrant, autosomal recessive, cardiac fibrosis phenotype among young adults with homozygous frameshift variant for SERPINE1 was identified, suggesting an optimal range of PAI-1 levels are needed for cardiac homeostasis.
Introduction
Plasminogen activator inhibitor-1 (PAI-1) plays a key role in regulating fibrinolysis through inhibition of urokinase-type plasminogen activator and tissue-type plasminogen activator.1 Beyond this well-established role, PAI-1 regulates cell migration, senescence, and extracellular matrix deposition, each of which contribute to fibrosis.2 In experimental models, increased PAI-1 levels correlate with fibrosis in vitro and in vivo.1 Counterintuitively, the absence of PAI-12,3 has been reported to cause spontaneous cardiac fibrosis in murine models, suggesting the need of an optimal range of PAI-1 for cardiac homeostasis.
Loss-of-function variants in SERPINE1, the gene that codes for PAI-1, have the potential to inform biological relevance of PAI-1 in cardiac fibrosis among humans. We recently reported the incidental finding of cardiac fibrosis in 2 members of an Amish community who are homozygous for a frameshift variant (c.699_700dupTA) in the SERPINE1 gene resulting in a premature stop codon and nonfunctional truncated protein.4,5 To follow up on these preliminary findings and better define the link between PAI-1 and cardiac fibrosis, we undertook a systematic study of detailed cardiovascular phenotyping (multimodality cardiovascular imaging [transthoracic echocardiography and cardiac magnetic resonance imaging (CMR)]), genetic sequencing (whole-exome sequencing [WES]), and induced pluripotent stem cell–derived cardiomyocytes (iPSC-CM).
Methods
Amish Study Participants
We enrolled 17 participants from 3 related Amish families in the primary study between May 2015 and December 2016. Participants underwent detailed cardiac phenotyping, WES, and urine collection to generate iPSC-CM. In a secondary analysis, we performed speckle-tracking analysis on echocardiograms in 155 participants of the extended kindred. The institutional review boards at the Indiana Hemophilia and Thrombosis Center Inc and Northwestern University approved the study protocols. All participants provided written informed consent.
Multimodality Cardiovascular Imaging
All study participants in the primary and secondary studies underwent comprehensive 2-dimensional transthoracic echocardiogram with Doppler and tissue Doppler imaging using a commercially available ultrasound system with harmonic imaging (Vivid 7 or Vivid I; GE Healthcare) and a 3.5-MHz transducer. Deidentified images were analyzed offline using GE EchoPAC software version 7.0.0 (GE Healthcare). All measurements were made by a single experienced sonographer and verified by an experienced investigator (S.J.S.) (both blinded to clinical data and genotype).6 Cardiac magnetic resonance imaging was performed on a clinical 1.5-T scanner (MAGNETOM Aera; Siemens Healthcare) with analysis in Medis QMass MR 7.6 (Medis Medical Imaging Systems) by a single experienced researcher (B.C.B.) and verified by an experienced investigator (D.C.L.) (both blinded to clinical data and genotype).
Whole-Exome Sequencing
Exome sequencing was performed at the NUSeq Core Facility at the Northwestern University Feinberg School of Medicine. Sequencing and bioinformatics analyses are detailed in the eMethods in the Supplement.
Statistical Analyses
We compared baseline demographics between participants homozygous for the c.699_700dupTA SERPINE1 variant and controls using models adjusted for age, sex, and family structure. For the primary analysis, our exposure defined a priori was homozygosity for the c.699_700dupTA SERPINE1 variant and the end point of interest was late gadolinium enhancement (LGE) on CMR based on the experimental literature. We also investigated the association of homozygosity for the c.699_700dupTA SERPINE1 variant with other cardiovascular parameters. Analyses were conducted using Sequential Oligogenic Linkage Analysis Routines, version 8.1.1 (Southwest Foundation for Biomedical Research) given the high degree of relatedness between participants in this closed founder population. Two-sided P values less than .05 for Sequential Oligogenic Linkage Analysis Routines analyses were considered to indicate statistical significance. We also calculated the estimated logarithm of the odds score according to the formula recommended by the Clinical Genome Resource Consortium (eMethods in the Supplement). Analysis began June 2015 and ended June 2020.
Stem Cell–Derived Cardiomyocytes
As a proof-of-concept study, we used previously characterized iPSC lines to pursue preliminary mechanistic in vitro studies.7,8,9 Cardiomyocyte differentiation of iPSCs was achieved by following published protocols10,11 with details outlined in the eMethods in the Supplement from 1 participant homozygous for the c.699_700dupTA SERPINE1 variant and 1 wild-type control participant. Experimental results examining cardiomyocyte response to angiotensin II and metabolic stress are presented as mean (SEM). Given that the multiple cardiomyocyte differentiations (n = 5) were derived from a single participant homozygous for the c.699_700dupTA SERPINE1 variant and a single wild-type participant, no formal statistical analyses were performed to conclude significant differences.
Results
Of 17 participants enrolled in the primary study, the mean (interquartile range) age was 23.7 (20.9-29.9) years (Table). Participants homozygous for the SERPINE1 (c.699_700dupTA) had no evidence of detectable PAI-1 antigen or activity in plasma. No participants reported symptoms or a clinical diagnosis of heart failure. During follow-up, 1 SERPINE1−/− participant experienced sudden death, but autopsy was declined by the family. In 3 years of follow-up, none of the other SERPINE1−/− participants have reported signs or symptoms of heart failure or sustained arrhythmias with annual in-person contact.
Table. Baseline Characteristics by SERPINE1 Genotype Status.
| Characteristic | Median (IQR) | P valueb | |
|---|---|---|---|
| SERPINE1−/− (n = 9) | SERPINE1+/+ or +/− (n = 8)a | ||
| Age, y | 24 (22-32) | 23 (19-27) | .31 |
| Female, No. (%) | 6 (67) | 2 (25) | .10 |
| Blood pressure, mm Hg | |||
| Systolic | 116 (110-119) | 125 (117-134) | .05 |
| Diastolic | 72 (70-76) | 79 (77-83) | .12 |
| BMI | 23 (21-26) | 24 (23-26) | .40 |
| Fasting glucose, mg/dL | 84 (79-85) | 85 (79-92) | .40 |
| Total cholesterol, mg/dL | 158 (136-175) | 152 (128-186) | .61 |
| HDL cholesterol, mg/dL | 59 (56-66) | 52 (44-60) | .07 |
| Triglyceride, mg/dL | 60 (59-72) | 78 (63-97) | .41 |
| LDL cholesterol, mg/dL | 81 (67-97) | 87 (64-113) | .61 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factor: To convert cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; and triglycerides to millimoles per liter, multiply by 0.0113.
Includes 5 individuals who were wild-type and 3 heterozygotes for the mutant SERPINE1 variant (c.699_700dupTA).
Model adjusted for age, sex, and family structure in Sequential Oligogenic Linkage Analysis Routines.
Cardiovascular phenotyping parameters are shown in the eTable in the Supplement. Of 9 SERPINE1−/− participants, 6 had LGE (Figure 1) compared with 0 of 8 in the control group, including no heterozygous participants (median [interquartile range] % of fibrosis for the 2 groups: 7.9% [0%-13.4%] vs 0%; Wilcoxon 2-sample P = .001). Late gadolinium enhancement patterns tended to be dense and linear, usually subepicardial but also midmyocardial and transmural with noncoronary distributions, supporting a nonischemic etiology.
Figure 1. Late Gadolinium Enhancement From 2 Representative Participants Who Are Homozygous for the Null SERPINE1 Allele (c.699_700dupTA) From the Old-Order Amish Kindred.
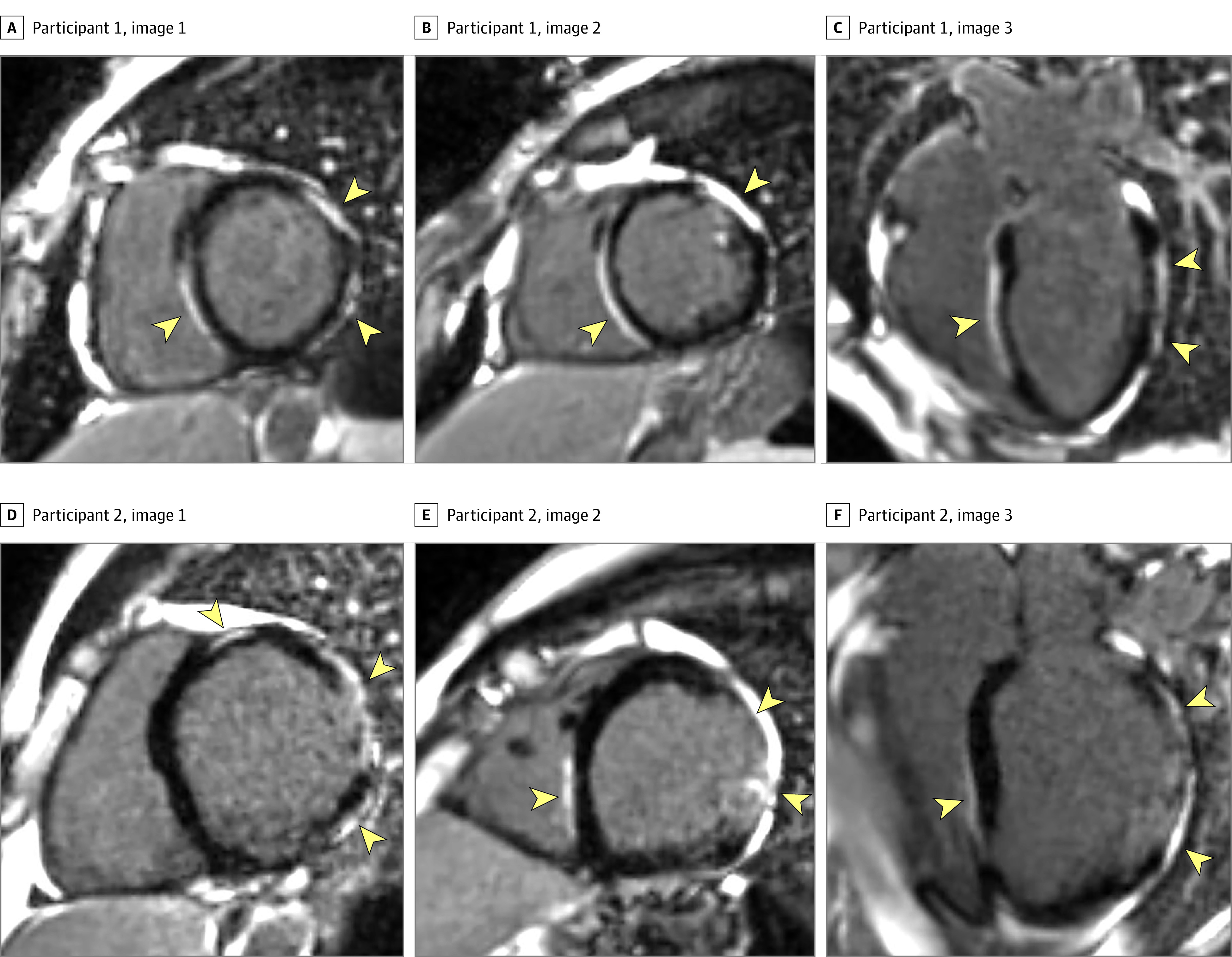
A pattern of dense late gadolinium enhancement is shown (multiple bright foci denoted by arrowheads) in 2 representative individuals who are homozygous for the loss-of-function variant in SERPINE1. In contrast, areas with normal myocardium are black.
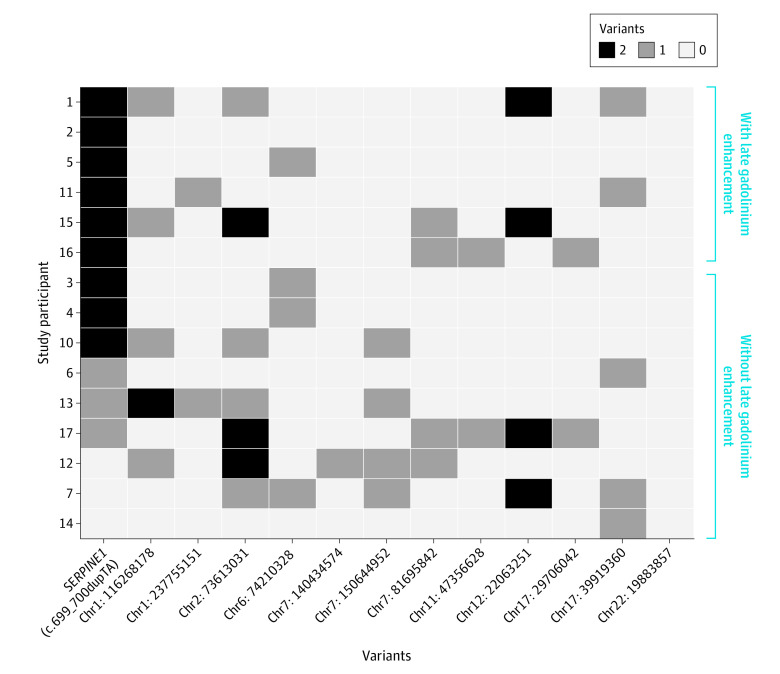
Targeted WES analysis performed focused only on known cardiomyopathy genes did not identify any pathogenic or likely pathogenic variants in cardiomyopathy genes in any study participant. While we did identify several distinct genetic variants of uncertain significance in cardiomyopathy genes, none of these variants visually segregated with the LGE phenotype among the participants (Figure 2). Homozygosity for c.699_700dupTA SERPINE1 was the only shared variant of uncertain significance with biologic plausibility associated with cardiac fibrosis among all participants with LGE. The estimated logarithm of the odds score generated for homozygosity for the c.699_700dupTA SERPINE1 variant was 4.01 (based on the Clinical Genome Resource Consortium formula).
Figure 2. Whole-Exome Sequencing Heat Map Among Old-Order Amish Participants Stratified by Presence or Absence of Late Gadolinium Enhancement or Early-Onset Cardiac Fibrosis.
Whole-exome sequencing heat map demonstrates that homozygosity in the loss-of-function variant in SERPINE1 is the only shared variant among all individuals with late gadolinium enhancement. None of the other variants of uncertain significance identified in cardiomyopathy genes cosegregate with the late gadolinium enhancement phenotype. Chr indicates chromosome.
Preliminary studies in iPSC-CM (eFigure 1 in the Supplement) from a participant homozygous for the c.699_700dupTA SERPINE1 variant exhibited increased transforming growth factor β1 secretion in response to angiotensin II compared with control iPSC-CMs (eFigure 2 in the Supplement). Gene expression levels for connective tissue growth factor and Smad7 were increased among SERPINE1-null iPSC-CM compared with controls (eFigure 3 in the Supplement). This profibrotic transcriptional pattern was also present in response to stress and exposure to angiotensin II. SERPINE1-null iPSC-CMs were associated with an increased stress response to H2O2 and angiotensin II as indicated by increased release of lactate dehydrogenase when compared with control iPSC-CMs, suggesting increased susceptibility to cardiomyocyte injury with PAI-1 deficiency.
A secondary analysis to examine global longitudinal strain from echocardiograms was undertaken in 155 previously recruited participants from the same Amish community. No differences in mean (SD) global longitudinal strain was observed between carriers for the c.699_700dupTA SERPINE1 variant (n = 36; 20.6% [2.5%]) and wild-type participants (n = 119; 20.6% [3.0%]).
Discussion
In this analysis, we identified a highly penetrant, autosomal recessive pattern of inheritance of a cardiac fibrosis phenotype in young adults with a frameshift variant in SERPINE1 (c.699_700dupTA) based on rigorous assessment with multimodality imaging, targeted WES analysis, and preliminary iPSC-CM experimental studies. Further supporting the clinical validity of this gene-disease association is the fact that this rare variant has not been identified in any frequency in the general population (Genome Aggregation Database that includes data from more than 140 000 exomes and genomes). These findings build on extensive experimental evidence (in vivo and in vitro) from our group and others supporting the findings that complete PAI-1 deficiency predicates and is sufficient to promote spontaneous cardiac-specific fibrosis in mammals.2
The use of CMR in this study enabled us to characterize and quantify the pattern and extent of LGE among asymptomatic young adults with absolute PAI-1 deficiency who did not harbor any other known pathogenic variants in cardiomyopathy genes. While LGE is found in several distinct forms of genetic cardiomyopathies (eg, desmoplakin and lamin A/C cardiomyopathy),12,13,14 the presence of LGE in young adults is uncommon, especially in the absence of significant left ventricular dilation or hypertrophy. The closest pattern of LGE to what we observed has been previously described in Duchenne muscular dystrophy.15 Myocarditis can also result in epicardial LGE; however, the enhancement pattern is typically more localized (eg, inferolateral wall) and less dense. The presence of dense LGE reflects in vivo replacement fibrosis of the myocardium and was only identified among individuals with complete PAI-1 deficiency, supporting the role of PAI-1 as an important regulator of cardiac fibrosis.
Limitations
Limitations of this study include the small sample size of our analysis, but we successfully enrolled all 9 known individuals who are homozygous for this SERPINE1 variant to inform the cosegregation and penetrance analyses. Second, preliminary experiments from iPSC-CM are presented as hypothesis generating to provide mechanistic insights but only included a single participant who was homozygous for the c.699_700dupTA SERPINE1 variant and a single wild-type control. However, the proof-of-concept nature of these in vitro studies provides additional support of the biologic plausibility of the imaging and genetic analyses. Third, we did not have adequate power to examine effect modification by sex or other rare variants in noncardiomyopathy genes.
Conclusions
In conclusion, we identified a highly penetrant, autosomal recessive, cardiac fibrosis phenotype among young adults with homozygous frameshift variant for SERPINE1, suggesting an optimal range of PAI-1 levels are needed for cardiac homeostasis. Further studies are needed to inform the mechanistic pathways supporting the role of PAI-1 as a broader molecular driver of human myocardial fibrosis.
eMethods
eTable. Cardiovascular Phenotype by SERPINE1 Genotype Status
eFigure 1. Characterization of induced pluripotent stem cell-derived cardiomyocytes
eFigure 2. Functional characterization of WT and PAI-1 deficient iPSC-derived cardiomyocytes
eFigure 3. Expression levels of specific fibrotic genes
References
- 1.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227(2):493-507. doi: 10.1002/jcp.22783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh AK, Bradham WS, Gleaves LA, et al. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122(12):1200-1209. doi: 10.1161/CIRCULATIONAHA.110.955245 [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Castellino FJ, Ploplis VA. Plasminogen activator inhibitor-1 (PAI-1) is cardioprotective in mice by maintaining microvascular integrity and cardiac architecture. Blood. 2010;115(10):2038-2047. doi: 10.1182/blood-2009-09-244962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flevaris P, Khan SS, Eren M, et al. Plasminogen activator inhibitor type i controls cardiomyocyte transforming growth factor-β and cardiac fibrosis. Circulation. 2017;136(7):664-679. doi: 10.1161/CIRCULATIONAHA.117.028145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SS, Shah SJ, Klyachko E, et al. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv. 2017;3(11):eaao1617. doi: 10.1126/sciadv.aao1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Afzal MZ, Gartz M, Klyachko EA, et al. Generation of human iPSCs from urine derived cells of a patient with a novel homozygous PAI-1 mutation. Stem Cell Res. 2016;17(3):657-660. doi: 10.1016/j.scr.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzal MZ, Gartz M, Klyachko EA, et al. Generation of human iPSCs from urine derived cells of a non-affected control subject. Stem Cell Res. 2017;18:33-36. doi: 10.1016/j.scr.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afzal MZ, Gartz M, Klyachko EA, et al. Generation of human iPSCs from urine derived cells of patient with a novel heterozygous PAI-1 mutation. Stem Cell Res. 2017;18:41-44. doi: 10.1016/j.scr.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848-E1857. doi: 10.1073/pnas.1200250109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8(1):162-175. doi: 10.1038/nprot.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho CY, López B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363(6):552-563. doi: 10.1056/NEJMoa1002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmström M, Kivistö S, Heliö T, et al. Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson. 2011;13:30. doi: 10.1186/1532-429X-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith ED, Lakdawala NK, Papoutsidakis N, et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141(23):1872-1884. doi: 10.1161/CIRCULATIONAHA.119.044934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giglio V, Puddu PE, Camastra G, et al. Patterns of late gadolinium enhancement in Duchenne muscular dystrophy carriers. J Cardiovasc Magn Reson. 2014;16:45. doi: 10.1186/1532-429X-16-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable. Cardiovascular Phenotype by SERPINE1 Genotype Status
eFigure 1. Characterization of induced pluripotent stem cell-derived cardiomyocytes
eFigure 2. Functional characterization of WT and PAI-1 deficient iPSC-derived cardiomyocytes
eFigure 3. Expression levels of specific fibrotic genes