Key Points
Question
How well does the depression polygenic risk score (PRS) in combination with parental psychosocial factors determine the absolute risk of depression before the age of 30 years?
Findings
In this case-cohort study of 17 098 patients with depression, the absolute risk of depression by the age of 30 years differed substantially depending on an individual’s combination of risk factors, ranging from 1.0% among male individuals with high socioeconomic status in the bottom 2% of the PRS distribution to 23.7% among female individuals in the top 2% of PRS with a parental history of psychiatric disorders.
Meaning
The PRS was not superior to other factors but was useful in conjunction with other risk factors.
Abstract
Importance
Combining information on polygenic risk scores (PRSs) with other known risk factors could potentially improve the identification of risk of depression in the general population. However, to our knowledge, no study has estimated the association of PRS with the absolute risk of depression, and few have examined combinations of the PRS and other important risk factors, including parental history of psychiatric disorders and socioeconomic status (SES), in the identification of depression risk.
Objective
To assess the individual and joint associations of PRS, parental history, and SES with relative and absolute risk of early-onset depression.
Design, Setting, and Participants
This case-cohort study included participants from the iPSYCH2012 sample, a case-cohort sample of all singletons born in Denmark between May 1, 1981, and December 31, 2005. Hazard ratios (HRs) and absolute risks were estimated using Cox proportional hazards regression for case-cohort designs.
Exposures
The PRS for depression; SES measured using maternal educational level, maternal marital status, and paternal employment; and parental history of psychiatric disorders (major depression, bipolar disorder, other mood or psychotic disorders, and other psychiatric diagnoses).
Main Outcomes and Measures
Hospital-based diagnosis of depression from inpatient, outpatient, or emergency settings.
Results
Participants included 17 098 patients with depression (11 748 [68.7%] female) and 18 582 (9429 [50.7%] male) individuals randomly selected from the base population. The PRS, parental history, and lower SES were all significantly associated with increased risk of depression, with HRs ranging from 1.32 (95% CI, 1.29-1.35) per 1-SD increase in PRS to 2.23 (95% CI, 1.81-2.64) for maternal history of mood or psychotic disorders. Fully adjusted models had similar effect sizes, suggesting that these risk factors do not confound one another. Absolute risk of depression by the age of 30 years differed substantially, depending on an individual’s combination of risk factors, ranging from 1.0% (95% CI, 0.1%-2.0%) among men with high SES in the bottom 2% of the PRS distribution to 23.7% (95% CI, 16.6%-30.2%) among women in the top 2% of PRS distribution with a parental history of psychiatric disorders.
Conclusions and Relevance
This study suggests that current PRSs for depression are not more likely to be associated with major depressive disorder than are other known risk factors; however, they may be useful for the identification of risk in conjunction with other risk factors.
This case-cohort study assesses the individual and joint associations of polygenic risk scores, parental history, and socioeconomic status with relative and absolute risks of early-onset depression.
Introduction
Major depression is a debilitating and complex disorder,1,2 with an estimated lifetime prevalence of approximately 7% to 21% in high-income countries.3,4,5 Family, adoption, and twin studies6,7 have consistently found that up to 40% of the variation in liability for major depression can be attributed to genetic factors. A previous study8 found substantial comorbidity between major depression and other psychiatric disorders. The underlying liability to major depression is polygenic, implying that this heritable component is distributed across numerous genetic variants. Estimates of the genome-wide single-nucleotide polymorphism–based heritability3 (ie, the proportion of liability variance in the population attributable to common variants) range from approximately 8% to 32%, depending on phenotype definition,9,10,11,12 and polygenic risk scores (PRSs; ie, a weighted sum of total polygenic burden) explain approximately 1.9% of the variance.12 Furthermore, evidence for specific variants is emerging because 102 broad depression-associated loci have now been identified.10,12
Evidence suggests that parental history of depression or other severe mental disorders is associated with childhood depression.13 Similarly, epidemiologic investigations have found that parental socioeconomic status (SES) during the child’s upbringing is associated with risk of major depression14 and that major depression is associated with poor socioeconomic outcomes across the entire life span.15 However, the impact of these risk factors may partly be mediated by the offspring’s genetic predisposition to depression.16 The association between parental mental illness and risk of depression in the proband could also be confounded by socioeconomic background; that is, dynastic effects (ie, genetic factors in the parent that are not transmitted yet nonetheless influence the child’s environment) associated with parental assortative mating on genetic architectures of depression may lead to an overstatement of the genetic contribution to risk.17,18,19 Few studies20,21 have attempted to quantify the interplay between parental risk factors and the genetic liability for depression or evaluated PRSs for depression alongside known epidemiologic factors. Such quantification is essential if clinical decisions should be guided by risk models, especially those that include PRSs.
Most research studies7,9,10,11,12 report only relative risk estimates; however absolute estimates of risk are more clinically useful in identifying risk20,21 and more useful for communicating risk to lay populations22 Thus, estimating absolute and relative risks is crucial for assessing the potential clinical relevance of PRSs.23 Recently, studies24,25,26,27 in cancer and coronary heart disease have reported absolute risks associated with PRSs, but we are not aware of any study that has estimated such absolute risk of depression. One reason is the paucity of relevant genetic data. Odds ratios are available in genome-wide association studies (GWASs), but these studies often lack the population sampling frame needed to obtain absolute risks. Another reason is that GWASs of depression have only recently become large enough to make PRS-based risk identification tenable.10,12
We had the opportunity to use Denmark’s population-based registers,28 the Danish population-based genetic case-cohort sample iPSYCH2012,29 and separate metadata from the largest published depression GWAS.10,12 Our aims were to (1) quantify the association between the PRS for depression and absolute risk of early-onset, major depression treated in secondary care in a population-based sample; (2) compare the association of the PRS with the associations of several known nongenetic risk factors, including sex, parental SES, and parental history of mental disorders; or (3) estimate the joint association of PRS and nongenetic risk factors with absolute risk of depression.
Methods
Data were obtained using the Danish Civil Registration System, which was established in 1968.30 It is possible to link data among registers using a unique identification number assigned to all Danish residents. It is also possible to link parents with children and to retrieve dates of death and emigration. Information on mental diseases was obtained from the Danish Psychiatric Central Research Register,31 which contains information on all admissions to psychiatric inpatient facilities since 1969 and visits to outpatient wards since 1995.31 All diagnoses are clinically assigned by physicians and have been validated with good results.32,33 Socioeconomic data were obtained from the Integrated Database for Longitudinal Labour Market Research, which covers the entire population and contains yearly information from 1980 on labor market affiliation, educational attainment, and marital status.34 iPSYCH is approved by the Danish Scientific Ethics Committee, the Danish Health Data Authority, the Danish Data Protection Agency, Statistics Denmark, and the Danish Neonatal Screening Biobank Steering Committee.29,35,36 The Danish Scientific Ethics Committee, in accordance with Danish legislation, has, for this study, waived the need for informed consent in biomedical research based on existing biobanks.36 All data were pseudonymized. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
iPSYCH is a population-based, case-cohort sample (N = 88 764) selected from all singletons born between May 1, 1981, and December 31, 2005, who were living in Denmark at their first birthday (N = 1 472 762).29 From May 1981 onward, frozen blood spots for almost 100% of neonates were stored in the Danish Neonatal Screening Biobank.35 The iPSYCH sample consists of a uniform randomly selected subcohort with 30 000 individuals, so that the population-based sampling fraction is 2.0% = 30 000/1 472 762. The remaining iPSYCH cohort consists of all additional individuals who were registered with certain psychiatric disorders in the Danish Psychiatric Central Research Register between January 1, 1994, and December 31, 2012.29
For this study, we included all subcohort members and all individuals who were diagnosed with major depression (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes F32-F33).5 The sample was further restricted to individuals (1) with Danish-born parents to reduce population stratification, (2) who were living in Denmark on their 10th birthday (start of follow-up) before December 31, 2012 (end of follow-up), and (3) who were successfully genotyped and passed quality control. To further mitigate confounding by population stratification, we computed the orthogonalized Gnanadesikan-Kettenring robust Mahalanobis distance of the 10 leading principal components and excluded individuals with a logarithm distance larger than 3.37,38 A detailed flowchart of the sample selection process is shown in eFigure 1 in the Supplement. Sample, genotyping, and quality-control details have been published previously.39,40,41
The PRSs were derived using the LDpred method42 from summary statistics based on the recent depressive disorder GWAS by Howard et al12 (discovery sample of 116 829 cases and 327 060 controls excluding iPSYCH). The procedure for generating PRSs in iPSYCH has been published previously.39,40,41,43 The PRS was standardized in the subcohort to make it population based. Analyses with PRSs are adjusted for the first 10 principal components.43
Using the Danish Psychiatric Central Research Register, we added mutually exclusive and hierarchical explanatory variables that indicated whether parents had had a psychiatric contact before their offspring’s 10th birthday with (1) major depression, (2) bipolar or psychotic disorders, or (3) other mental disorders. Diagnostic details were previously published.5
Variables for SES included educational attainment, labor market affiliation, and marital status recorded in the year of the study participants’ 10th birthdays. Educational attainment was grouped into (1) postgraduate education, (2) bachelor’s degree (high school plus 3-4 years of education), (3) high school or vocational training, or (4) primary school (9-10 years). Labor market affiliation was categorized into (1) white collar (eg, managerial employee, clerical worker, or public employee), (2) self-employed (entrepreneur, businessperson, or farmer), or (3) blue collar (skilled, manual, and unskilled workers) or unemployed or otherwise not working (recipient of social welfare or disability benefits, students, or pensioners). Marital status was classified as married or cohabitating vs living alone. The main analyses included variables for maternal educational attainment, paternal labor market affiliation, and maternal marital status because these variables capture the association of SES with risk of severe mental disorders.44,45,46 Hazard ratios (HRs) for the remaining parental SES variables (parental income, paternal educational level, and maternal labor market affiliation) are included in eTable 1 in the Supplement.
The HRs and absolute risks were estimated using Cox proportional hazard models for case-cohort designs.47 Using age as the time axis, individuals were followed up from their 10th birthday until the date when they were first diagnosed with depression in a hospital-based setting or until death, emigration, or December 31, 2012. Because the iPSYCH case cohort consists of individuals who were born after May 1981, all patients were younger than 32 years at the time of diagnosis. We used robust SEs, the subcohort sampling fraction, and Barlow weighting to account for the undersampling of controls.48 Because the PRS in 50 parts did not have a superior association over the continuous PRS, we used the continuous PRS in all subsequent models. Statistical theory has established that HRs from a case-cohort study are unbiased and approximate the analogous HRs estimated from full population data.49 All analyses were conducted in R, version 3.5.1 (R Foundation for Statistical Computing) and SAS, version 9.4 (SAS Institute Inc). Lastly, we challenged the necessity for excluding ancestral outliers in the iPSYCH design by repeating all analyses without exclusion of individuals based on principal components.50
Results
Participants included 17 098 patients with depression (11 748 [68.7%] female) and 18 582 (9429 [50.7%] male) individuals randomly selected from the base population. Because the subcohort is a random sample of the Danish population, by chance it included 362 individuals with depression. Table 1 gives the sample characteristics and crude HRs of depression in association with sex, PRS, parental psychiatric history, paternal labor market affiliation, maternal educational attainment, and marital status. The HRs were in keeping with previously published results.44,45,46,51 For instance, women were twice as likely to be diagnosed with depression as men (HR, 2.25; 95% CI, 2.14-2.36), and the hazard increased by 31% per 1-SD increase in the PRS (95% CI, 1.28-1.34). The hazard of depression was elevated among individuals with a maternal or paternal history of depression and psychiatric disorders and among those with lower parental SES.
Table 1. Sample Characteristics of the Study Population and Associated Major Depression HRsa.
| Characteristic | Major depression cohort | Subcohortb | Crude HR (95% CI)c |
|---|---|---|---|
| Sex | |||
| Female | 11 748 (68.7) | 9153 (49.3) | 2.25 (2.14-2.36) |
| Male | 5350 (31.3) | 9429 (50.7) | 1 [Reference] |
| Polygenic risk score, mean (SD)d | 0.22 (0.98) | 0.0 (1.00)d | 1.31 (1.28-1.34) |
| Maternal psychiatric history | |||
| Major depression | 386 (2.3) | 305 (1.6) | 2.25 (1.87-2.71) |
| Bipolar, mood, or psychotic disorder | 181 (1.1) | 98 (0.5) | 2.25 (1.70-2.97) |
| Other mental disorder | 898 (5.3) | 642 (3.5) | 2.02 (1.78-2.28) |
| No mental disorder | 15 633 (91.4) | 17 537 (94.4) | 1 [Reference] |
| Paternal psychiatric history | |||
| Major depression | 251 (1.5) | 190 (1.0) | 2.13 (1.68-2.69) |
| Bipolar, mood, or psychotic disorder | 179 (1.1) | 105 (0.6) | 1.89 (1.44-2.49) |
| Other mental disorder | 850 (5.0) | 635 (3.4) | 1.65 (1.47-1.87) |
| No mental disorder | 15 818 (92.5) | 17 652 (95.0) | 1 [Reference] |
| Maternal marital status | |||
| Living alone | 4079 (23.9) | 3169 (17.1) | 1.61 (1.52-1.71) |
| Married or cohabitating | 13 019 (76.1) | 15 413 (83.0) | 1 [Reference] |
| Maternal educational attainment | |||
| Primary school | 6457 (37.8) | 4727 (25.4) | 1.65 (1.45-1.87) |
| High school or vocational training | 6495 (38.0) | 7927 (42.7) | 1.29 (1.14-1.45) |
| Bachelor or equivalent degree | 3627 (21.2) | 4852 (26.1) | 1.20 (1.06-1.37) |
| Postgraduate education | 519 (3.0) | 1076 (5.8) | 1 [Reference] |
| Paternal labor market affiliation | |||
| Unemployed | 2583 (15.1) | 1855 (10.0) | 1.75 (1.61-1.89) |
| Blue collar | 8131 (47.6) | 8542 (46.0) | 1.22 (1.16-1.29) |
| Self-employed | 1434 (8.4) | 1838 (9.9) | 0.92 (0.85-1.03) |
| White collar | 4950 (29.0) | 6347 (34.2) | 1 [Reference] |
Abbreviation: HR, hazard ratio.
Data are presented as number (percentage) of patients unless otherwise indicated.
A total of 362 individuals with major depression were by chance also in the subcohort because the subcohort is a uniform randomly selected subset of the entire population.
Crude HRs are adjusted for year of birth by stratification.
The polygenic risk score is standardized in the population-based cohort.
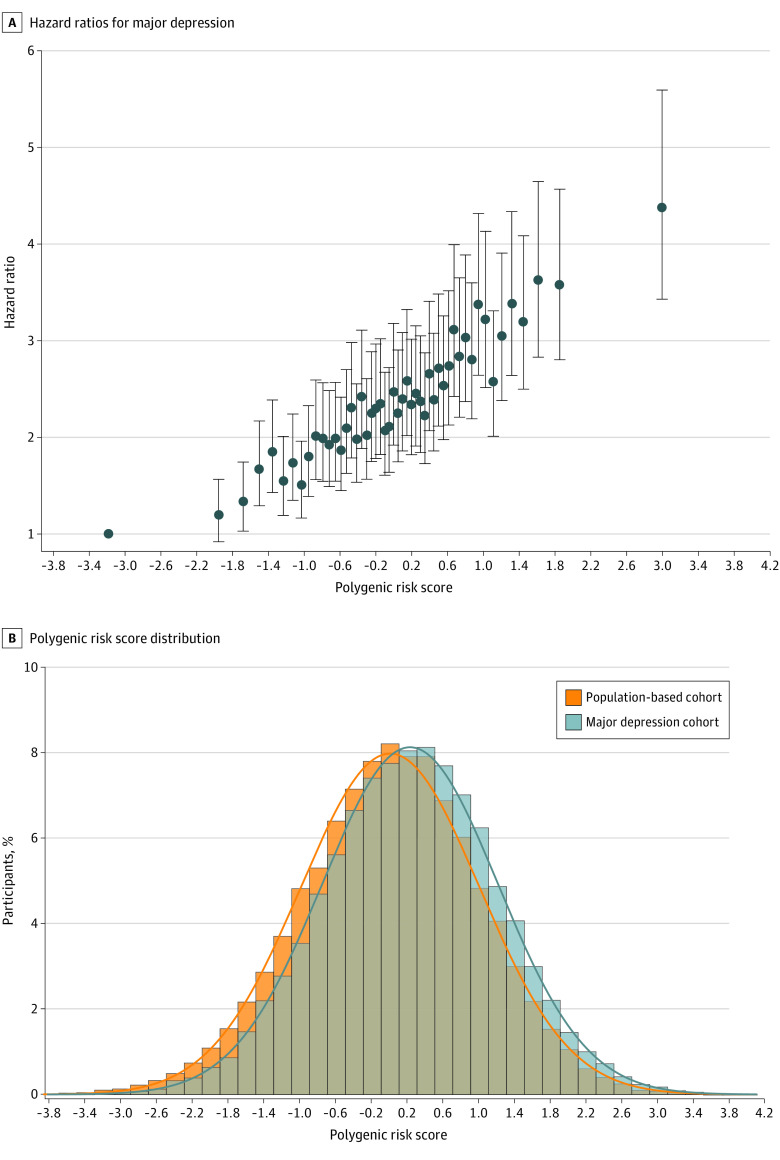
Figure 1 shows the HRs for depression for every second PRS percentile and the PRS distribution for the depression cases and subcohort. Although the distribution for depression cases is shifted only slightly compared with the subcohort of individuals, a strong and consistent dose-response association was found between the HRs and the PRS. There was little evidence of interaction between the PRS and the parental risk factors (eg, maternal major depression: HR, 1.26; 95% CI, 1.05-1.52; paternal major depression: HR, 1.17; 95% CI, 0.92-1.50) (eTable 2 in the Supplement). Table 2 gives the HRs for depression in relation to parental risk factors in various adjustment scenarios to investigate whether particular associations were mediated or confounded. For instance, the HRs for parental factors were only slightly attenuated after adjusting for the PRS (model 2 vs model 1), which indicates that the association of parental risk factors was only somewhat mediated through the polygenic liability for depression (eg, maternal major depression: HR, 2.19; 95% CI, 1.81-2.64 in model 1 vs HR, 2.15; 95% CI, 1.78-2.60; paternal major depression: HR, 1.81; 95% CI, 1.42-2.31 in model 1 vs HR, 1.68; 95% CI, 1.31-2.17). The adjusted analysis (model 5) suggests that the association of paternal psychiatric history and SES was mutually confounded or mediated. The HR associated with the PRS score was largely unaltered (model 1: HR, 1.32; 95% CI, 1.29-1.35; model 3: HR, 1.30; 95% CI, 1.27-1.34; model 4: HR, 1.31; 95% CI, 1.28-1.34; model 5: HR, 1.30; 95% CI, 1.26-1.33), which suggests that the association was not mediated or confounded by parental psychiatric or socioeconomic circumstances. The corresponding area under the receiver operating characteristic curve (AUC) from a logistic regression analysis, including these covariates, is 0.757. Polychoric and tetrachoric correlations52 between PRS and parental risk factors are given in eTable 3 in the Supplement.
Figure 1. Hazard Ratios for Major Depression and Polygenic Risk Score Distribution in Major Depression Cases and the Population-Based Cohort .
A, Hazard ratios for major depression for every second percentile of the polygenic risk score adjusted for ancestry using the first 10 genomic principal components and year of birth by stratification. The reference is the lowest 50th. Error bars represent 95% CIs. B, Frequency distribution and histogram plot for depression cases (n = 17 098) and the population-based subcohort (n = 18 582).
Table 2. HRs for Major Depression Associated With Conditional Parental Risk Factors and the Polygenic Risk Score on Various Adjustments Scenarios.
| Variable | HR (95% CI)a | ||||
|---|---|---|---|---|---|
| Model 1b | Model 2c | Model 3d | Model 4e | Model 5f | |
| Maternal psychiatric history | |||||
| Major depression | 2.19 (1.81-2.64) | 2.15 (1.78-2.60) | 2.02 (1.67-2.43) | NA | 1.99 (1.64-2.40) |
| Bipolar, mood or psychotic disorder | 2.23 (1.67-2.98) | 2.13 (1.57-2.90) | 1.93 (1.44-2.60) | NA | 1.86 (1.35-2.55) |
| Other mental disorder | 1.86 (1.64-2.11) | 1.80 (1.58-2.05) | 1.58 (1.39-1.80) | NA | 1.54 (1.34-1.76) |
| No mental disorder | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] |
| Paternal psychiatric history | |||||
| Major depression | 1.81 (1.42-2.31) | 1.68 (1.31-2.17) | 1.56 (1.22-2.01) | NA | 1.46 (1.12-1.90) |
| Bipolar, mood or psychotic disorder | 2.01 (1.52-2.67) | 1.89 (1.41-2.53) | 1.64 (1.23-2.20) | NA | 1.59 (1.18-2.13) |
| Other mental disorder | 1.61 (1.42-1.82) | 1.54 (1.36-1.75) | 1.33 (1.17-1.51) | NA | 1.29 (1.13-1.48) |
| No mental disorder | 1 [Reference] | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] |
| Maternal educational attainment | |||||
| Primary school | 1.42 (1.25-1.62) | 1.37 (1.21-1.57) | NA | 1.36 (1.19-1.55) | 1.31 (1.14-1.49) |
| High school or vocational training | 1.18 (1.04-1.33) | 1.16 (1.02-1.31) | NA | 1.16 (1.02-1.32) | 1.16 (1.02-1.32) |
| Bachelor or equivalent degree | 1.13 (0.99-1.28) | 1.11 (0.98-1.27) | NA | 1.11 (0.98-1.26) | 1.11 (0.98-1.27) |
| Postgraduate education | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] |
| Paternal labor market affiliation | |||||
| Unemployed | 1.68 (1.54-1.82) | 1.63 (1.50-1.77) | NA | 1.53 (1.40-1.66) | 1.39 (1.27-1.52) |
| Blue collar | 1.13 (1.07-1.20) | 1.12 (1.06-1.19) | NA | 1.13 (1.07-1.20) | 1.11 (1.05-1.18) |
| Self-employed | 0.92 (0.84-1.00) | 0.92 (0.84-1.00) | NA | 0.93 (0.85-1.01) | 0.93 (0.85-1.02) |
| White collar | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] |
| Marital status | |||||
| Living alone | 1.44 (1.35-1.54) | 1.41 (1.32-1.51) | NA | 1.35 (1.26-1.44) | 1.32 (1.23-141) |
| Married or cohabitating | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] |
| Polygenic risk score | 1.32 (1.29-1.35) | NA | 1.30 (1.27-1.34) | 1.31 (1.28-1.34) | 1.30 (1.26-1.33) |
Abbreviations: HR, hazard ratio; NA, not applicable.
All HRs are adjusted for sex and year of birth by stratification.
Model 1: maternal and paternal psychiatric history are mutually adjusted. Analogously, maternal educational level, paternal labor market affiliation, and marital status are mutually adjusted.
Model 2: HRs in basic model (model 1) are further adjusted for the polygenic risk score and ancestry using the first 10 genomic principal components.
Model 3: maternal and paternal psychiatric history adjusted for maternal educational level, paternal labor market affiliation, and parental marital status.
Model 4: maternal education level and paternal labor market affiliation adjusted for their psychiatric history.
Model 5: all parental factors are mutually adjusted and should be interpreted with caution. The model is further adjusted for the polygenic risk score (per 1-SD increase) and ancestry using the first 10 genomic principal components. The corresponding area under the receiver operating characteristic curve from the logistic regression analysis is 0.757.
Figure 2 shows the absolute risks of depression as a function of age in association with sex, PRS, parental psychiatric history, and SES. Risk of depression diagnosis by the age of 30 years ranged from a low of 2.7% (95% CI, 2.4%-2.9%) among individuals in the bottom 2% of the PRS distribution to a high of 8.1% (95% CI, 7.3%-8.9%) in the top 2% of the PRS distribution. For comparison, risk of depression diagnosis by the age of 30 years was 14.6% (95% CI, 7.3%-21.3%) among individuals with a history of psychiatric disorders in both parents and 7.1% (95% CI, 6.3%-7.8%) among individuals with unemployed fathers and mothers with primary education only. Estimates of absolute risk were 6.5% (95% CI, 5.9%-7.0%) in women and 3.0% (95% CI, 2.5%-3.4%) in men, which were similar to prior estimates based on the entire population.5
Figure 2. Absolute Risk of Major Depression as a Function of Age in Association With Sex, Polygenic Risk Score (PRS), Maternal and Paternal Psychiatric History, and Socioeconomic Status (SES).
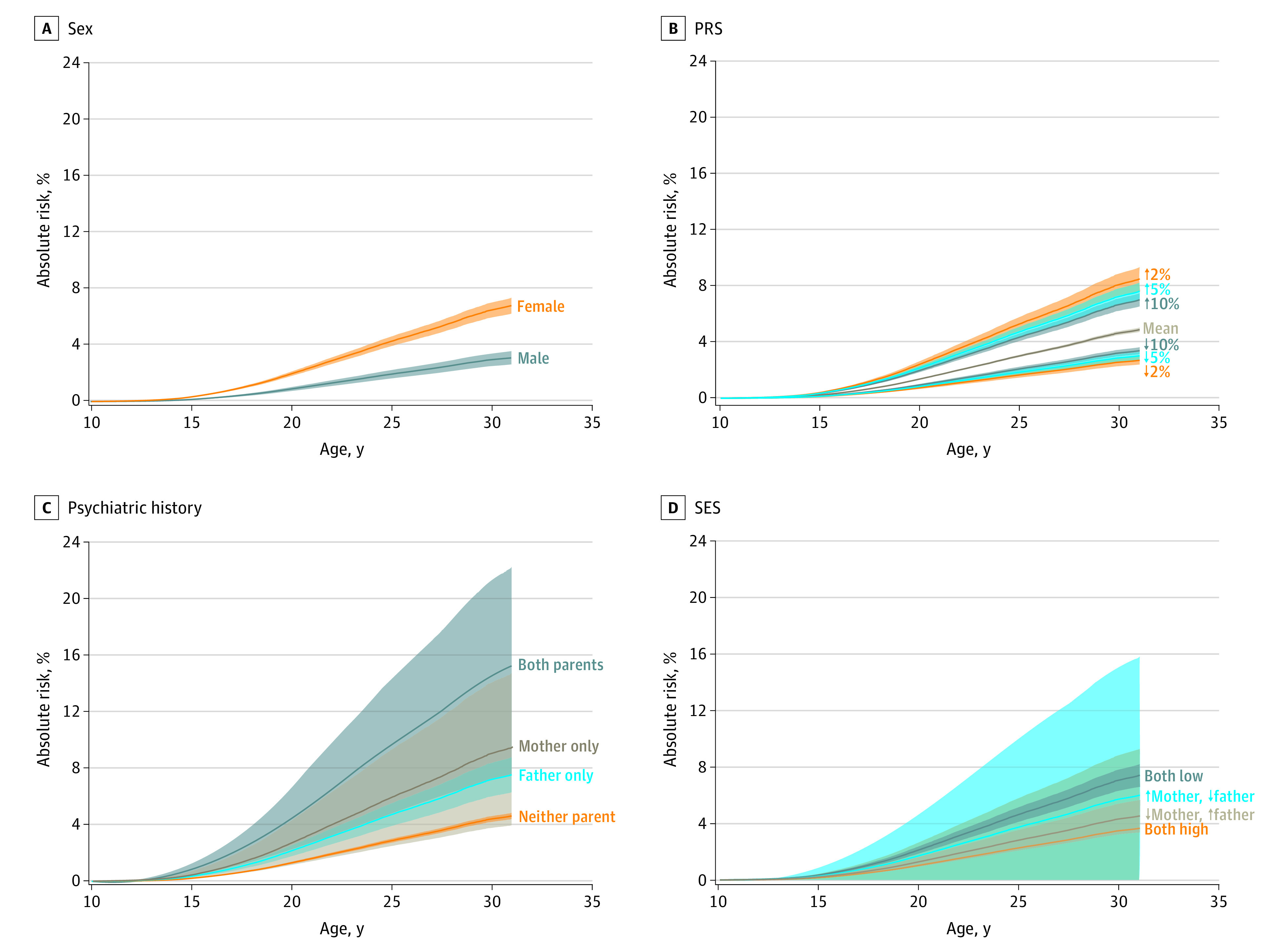
A, Crude absolute risk of major depression related to sex. For example, the absolute risk for women 30 years of age is 6.5% (95% CI, 5.9%-7.0%). The analogous risk for men is 3.0% (95% CI, 2.5%-3.4%). B, The upper curve is the risk for individuals with the highest 2% population-based polygenic liability for depression, whereas the lower curve is the absolute risk for individuals with the lowest 2% liability. For instance, at 30 years of age, the risks are 8.1% (95% CI, 7.3%-8.9%) for individuals with the highest 2% population-based polygenic liability for depression and 2.7% (95% CI, 2.4%-2.9%) for individuals with the lowest 2% liability. C, Risk of depression as a function of age according to parental psychiatric history. The upper curve shows the risk for individuals when both parents have a psychiatric disorder. The lower curve is the risk associated with no history of psychiatric disorder in parents. For these 2 groups of individuals, the risks at 30 years of age are 14.6% (95% CI, 7.3%-21.3%) when both parents have a psychiatric disorder and 4.4% (95% CI, 4.2%-4.6%) when neither parent has a history of psychiatric disorder. The 2 curves in between correspond to the cases in which only the mother or only the father have been diagnosed with a psychiatric disorder. D, Upper risk curve applies to individuals whose mother has completed only primary school and whose father is unemployed (risk at 30 years of age: 7.1% [95% CI, 6.3%-7.8%]). Lower risk curve applies to individuals whose mother has a postgraduate degree and whose father is a white-collar worker (risk at 30 years of age: 3.5% [95% CI, 0.0%-8.8%]). The 2 risk curves in between correspond to the scenarios in which mothers have completed only primary school and fathers are managers and where fathers are unemployed and mothers have postgraduate degrees. Upward arrow indicates increased risk; downward arrow, decreased risk.
Figure 3 shows the absolute risks for men and women associated with PRS, parental psychiatric history, and SES. Combining information on sex, PRS, and parental history yielded a small group of individuals with a high absolute risk of depression diagnosis by the age of 30 years. As shown in Figure 3A, 23.7% (95% CI, 16.6%-30.2%) of women in the highest 2% of the PRS distribution and whose parents (both mother and father) had a psychiatric disorder will be diagnosed with depression before the age of 30 years. This risk increased to 39.1% (95% CI, 26.0%-49.8%) among those for whom both parents had been diagnosed with depression (eFigure 2 in the Supplement). The corresponding percentage for men with the lowest PRS and without parental psychiatric history was 1.3% (95% CI, 1.1%-1.6%). In addition, combining information on PRS with parental SES also yielded groups with increased absolute risk of depression diagnosis. Among women in the top 2% of the PRS distribution with low parental SES, absolute risk of a depression diagnosis by the age of 30 years was 14.4% (95% CI, 12.0%-16.7%) compared with only 1.0% (95% CI, 0.1%-2.0%) among men with high SES and low PRS.
Figure 3. Absolute Risk of Major Depression as a Function of Age in Association With the Combination of Polygenic Risk Score and Parental Psychiatric History and Socioeconomic Status (SES).

A, The top left panel depicts risk curves in females associated with high or low genetic liability combined with present or absent parental psychiatric history. The upper curve shows the risk for females with the highest 2% polygenic liability and whose parents were both diagnosed with a psychiatric disorder (risk at 30 years of age: 23.7% [95% CI, 16.6%-30.2%]). The bottom risk curve applies to females without a parental psychiatric history and with the lowest 2% genetic liability (risk at 30 years of age: 3.0% [95% CI, 2.4%-3.6%]). The 2 risk curves in between correspond to the scenarios (1) no parental psychiatric history but high genetic liability and (2) both parents have had psychiatric history but lowest 2% genetic liability. B, The top right panel shows risk curves for males corresponding to the curves depicted for females in the top left panel. For example, the risk at 30 years of age is 11.25% (95% CI, 7.64%-14.72%) for males with high polygenic liability and parental psychiatric history, whereas the analogous risk is 1.33% (95% CI, 1.08%-1.59%) for males without parental history and low polygenetic liability for depression. C, The lower left panel shows risk curves for females associated with high or low polygenic liability combined with high (mother has a postgraduate degree and father is a manager) or low (mother has completed primary school and father is unemployed) parental SES. The upper curve depicts the risk curve associated with a high polygenic risk and a low parental SES (risk at 30 years of age: 14.4% [95% CI, 12.0%-16.7%]). The bottom curve shows the risk associated with low polygenic liability and high parental socioeconomic status (risk at 30 years of age: 2.4% [95% CI, 0.2%-4.5%]). D, The lower right panel shows risk curves for males corresponding to the curves depicted for females in the bottom left panel. For example, the risk at 30 years of age is 6.6% (95% CI, 5.4%-7.7%) for males with high polygenic liability and a low parental SES, whereas the analogous risk is 1.0% (95% CI, 0.1%-2.0%) for males a high parental SES and low polygenetic liability for depression. Upward arrow indicates increased risk; downward arrow, decreased risk.
The analyses depicted in Figure 3 were also repeated for PRS liabilities of 5% and 10% (eFigures 3 and 4 in the Supplement). As expected, estimates for individuals in the top 5% and 10% of the PRS distribution were less extreme than for the top 2%. In keeping with previous studies,53,54 excluding ancestral outliers in the iPSYCH2012 had no impact (eTables 4 and 5 and eFigures 5, 6, and 7 in the Supplement).
Discussion
This cohort study investigated the risk of depression in association with PRS for depression, parental SES, and parental histories of psychiatric disorders. The absolute risk of depression before the age of 30 years was 8.1% among individuals within the highest 2% of PRS distribution and 2.7% among those with lowest PRSs. Among women 30 years or older with the highest 2% genetic liability, estimated absolute risk of depression was 23.7% for those who also had a history of mental illness in both parents and 14.4% among those who also had low parental SES.
High-risk individuals who are suitable for clinical trials and potentially preventive interventions need to be identified.16,55,56,57 The recent success of the PRS has generated a pronounced enthusiasm, with some studies58,59,60,61 anticipating that it will be possible to mitigate risk years or decades in advance. Polygenic theory shows that most individuals with polygenic disease are expected to have no family history of the disease.62 Family history captures contributions from all genetic factors and factors shared by close family members. In contrast, the PRS only captures variance attributable to identified common variant risk factors; hence, currently for depression, family history is expected to be a more accurate population marker of depression.63 Nonetheless, high PRS could identify individuals at high genetic risk who have no reported family history. It is, however, widely recognized that polygenic predictions are not particularly informative for an individual64 and that PRSs are not yet clinically useful in psychiatry.56 The results of the current study indicate that individuals with the highest 2% polygenic liability have an absolute risk of depression before the age of 30 years that is only slightly higher than the absolute risk among individuals with low parental SES (7.1%) and nearly half that of individuals with a history of severe mental illness in both parents (14.6%). Thus, these findings suggest that current PRSs by themselves do not have a greater association with depression treated in secondary care than nongenetic risk factors. However, these findings also illustrate that incorporating information on the PRS along with other risk factors can identify groups of individuals with substantially elevated risk. It is clear that, if risk identification is a goal, psychiatry needs to build multicomponent risk predictor models based on known factors, such as those already widely accepted in coronary artery disease24 and breast cancer.27 In addition to nongenetic risk factors, we envision that PRSs for comorbid disorders are candidates for inclusion in multicomponent risk prediction models.
Recent methodologic developments in the PRS have been considerable, and GWAS samples steadily increase in size. However, the actual clinical or societal approaches for alleviating the excess risk among individuals exposed to highly indicative risk factors, including high polygenic risk, are unidentified. Work on the development of approaches for preventing depression in high-risk groups must proceed in tandem with the development of PRS variables themselves for their inclusion in clinical practice to be beneficial.
To our knowledge, this is the first study to estimate the association of polygenic liability with absolute risk of developing depression in the general population. Genome-wide association studies tend to use the AUC to assess model accuracy.23 The AUC is a population-level metric that separates cases from controls by evaluating the true-positive rate (sensitivity) vs the false-positive rate (specificity),65 but it provides no information regarding the absolute risk.23 However, one relevant precision medicine use case for genetic risk information is prognosis, an estimation of the likelihood that a certain disease will occur in any single or subgroup of individuals.55 Absolute risk estimates are therefore necessary for precision medicine in psychiatry to advance.
Limitations
This study has 6 important limitations. First, cases were identified through clinical records of individuals who were treated in secondary care for depression. Thus, these results may not generalize to individuals with depression who are untreated or only treated by general practitioners.66 However, cases were diagnosed according to World Health Organization classifications, and clinical register–based diagnoses of depression are of high validity.32,33 Denmark has free, universal health care, making it likely that severe mental disorders are captured in the registry. Second, depression may have an insidious onset, so in some patients depression is diagnosed with delay. As a result, some members of the subcohort may in fact have had depression, which would bias the estimates toward the null.67 Third, the oldest members of the cohort were only 32 years of age at the end of follow-up, which is around the median age of onset for depression; thus, the entire cohort can be considered to have early-onset disease.41 Fourth, the PRS represents a mixture of true and false common risk alleles, and little is known about the biological pathways to depression.68 Biases may arise from ascertained discovery GWAS cohorts69 because they consist of depression defined by minimal phenotyping and strictly defined major depressive disorder.9 Although such composite discovery cohorts may generate highly predictive PRSs, they may also identify loci that are not specific to major depressive disorder.9 Furthermore, the selection of controls is rarely described. It is well known that current PRSs are blunt instruments for individual risk prediction.64 However, the utility of PRSs for risk prediction will increase with increasing discovery sample size and methodologic improvements.70,71 Fifth, the measures of parental SES are crude and may only be weakly associated with the circumstances present during an individual’s upbringing. On the other hand, each factor is simple to ascertain and highly indicative of depression. Sixth, family history and diagnostic information relied on clinical diagnosis assigned by the attending psychiatrist at discharge. However, the risk varied little across different parental psychiatric exposures, which may suggest that phenotypic misclassification and precision are of less concern.
Conclusions
These results suggest that, although the PRS alone does not identify risk of depression better than known risk factors, it contributes independently of known major risk factors. Incorporating the depression PRS with other risk factors should improve risk prediction in the general population, especially as GWAS sample sizes increase.
eFigure 1. Flowchart of the Study Population
eTable 1. Paternal and Maternal Income, Paternal Educational Attainment and Maternal Labour Market Affiliation for the Study Population and Associated Major Depression Hazard Ratios
eTable 2. Interaction Between the Polygenic Risk Score and Parental Risk Factors and Associated Major Depression Hazard Ratios (Confidence Intervals)
eTable 3. Polychoric/Tetrachonic Correlation Matrix
eFigure 2. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score and Parental Psychiatric History of Depression
eFigure 3. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score (top/bottom 5%) and Parental Psychiatric History and Socio-Economic Status
eFigure 4. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score (top/bottom 10%) and Parental Psychiatric History and Socio-Economic Status
eTable 4. Sample Characteristics of the Study Population and Associated Major Depression Hazard Ratios, When Not Excluding Ancestral Outliers
eTable 5. Hazard Ratio for Major Depression in Relation to Parental Risk Factors Conditional on Various Adjustments Scenarios, When Not Excluding Ancestral Outliers
eFigure 5. Hazard Ratios for Major Depression (A) and Polygenic Risk Score Distribution in Major Depression Cases and the Population-Based Cohort (B), When Not Excluding Ancestral Outliers
eFigure 6. Absolute Risk of Major Depression as Functions of Age in Relation to Sex (A), the Polygenic Risk Score (B), Maternal and Paternal Psychiatric History (C) and Maternal Education and Paternal Labour Market Affiliation (D), When Not Excluding Ancestral Outliers
eFigure 7. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score and Parental Psychiatric History and Socio-Economic Status, When Not Excluding Ancestral Outliers
References
- 1.Hammen C. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol. 2018;14:1-28. doi: 10.1146/annurev-clinpsy-050817-084811 [DOI] [PubMed] [Google Scholar]
- 2.Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299-2312. doi: 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336-346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138. doi: 10.1146/annurev-publhealth-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen CB, Mors O, Bertelsen A, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71(5):573-581. doi: 10.1001/jamapsychiatry.2014.16 [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Sources of parent-offspring resemblance for major depression in a national Swedish extended adoption study. JAMA Psychiatry. 2018;75(2):194-200. doi: 10.1001/jamapsychiatry.2017.3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552-1562. doi: 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- 8.Plana-Ripoll O, Pedersen CB, Holtz Y, et al. Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry. 2019;76(3):259-270. doi: 10.1001/jamapsychiatry.2018.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai N, Revez JA, Adams MJ, et al. ; MDD Working Group of the Psychiatric Genomics Consortium . Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet. 2020;52(4):437-447. doi: 10.1038/s41588-020-0594-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard DM, Adams MJ, Clarke TK, et al. ; 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343-352. doi: 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schork AJ, Won H, Appadurai V, et al. A genome-wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nat Neurosci. 2019;22(3):353-361. doi: 10.1038/s41593-018-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wray NR, Ripke S, Mattheisen M, et al. ; eQTLGen; 23andMe; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668-681. doi: 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67(8):822-829. doi: 10.1001/archgenpsychiatry.2010.86 [DOI] [PubMed] [Google Scholar]
- 14.Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367. doi: 10.1093/ije/31.2.359 [DOI] [PubMed] [Google Scholar]
- 15.Hakulinen C, Musliner KL, Agerbo E. Bipolar disorder and depression in early adulthood and long-term employment, income, and educational attainment: a nationwide cohort study of 2,390,127 individuals. Depress Anxiety. 2019;36(11):1080-1088. doi: 10.1002/da.22956 [DOI] [PubMed] [Google Scholar]
- 16.McIntosh AM, Sullivan PF, Lewis CM. Uncovering the genetic architecture of major depression. Neuron. 2019;102(1):91-103. doi: 10.1016/j.neuron.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong A, Thorleifsson G, Frigge ML, et al. The nature of nurture: effects of parental genotypes. Science. 2018;359(6374):424-428. doi: 10.1126/science.aan6877 [DOI] [PubMed] [Google Scholar]
- 18.Davey Smith G, Davies NM. Can genetic evidence help us understand why height and weight relate to social position? BMJ. 2016;352:i1224. doi: 10.1136/bmj.i1224 [DOI] [PubMed] [Google Scholar]
- 19.Morris TT, Davies NM, Davey Smith G. Can education be personalised using pupils’ genetic data? Elife. 2020;9:9. doi: 10.7554/eLife.49962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28(R2):R133-R142. doi: 10.1093/hmg/ddz187 [DOI] [PubMed] [Google Scholar]
- 21.Sugrue LP, Desikan RS. What are polygenic scores and why are they important? JAMA. 2019;321(18):1820-1821. doi: 10.1001/jama.2019.3893 [DOI] [PubMed] [Google Scholar]
- 22.Spiegelhalter D. Risk and uncertainty communication. Annu Rev Stat Appl. 2017;4(1):31-60. doi: 10.1146/annurev-statistics-010814-020148 [DOI] [Google Scholar]
- 23.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19(9):581-590. doi: 10.1038/s41576-018-0018-x [DOI] [PubMed] [Google Scholar]
- 24.Inouye M, Abraham G, Nelson CP, et al. ; UK Biobank CardioMetabolic Consortium CHD Working Group . Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72(16):1883-1893. doi: 10.1016/j.jacc.2018.07.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchenbaecker KB, McGuffog L, Barrowdale D, et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109(7). doi: 10.1093/jnci/djw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Läll K, Lepamets M, Palover M, et al. Polygenic prediction of breast cancer: comparison of genetic predictors and implications for risk stratification. BMC Cancer. 2019;19(1):557. doi: 10.1186/s12885-019-5783-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A, Mavaddat N, Wilcox AN, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708-1718. doi: 10.1038/s41436-018-0406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7)(suppl):12-16. doi: 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 29.Pedersen CB, Bybjerg-Grauholm J, Pedersen MG, et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol Psychiatry. 2018;23(1):6-14. doi: 10.1038/mp.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 31.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7)(suppl):54-57. doi: 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- 32.Kessing L. Validity of diagnoses and other clinical register data in patients with affective disorder. Eur Psychiatry. 1998;13(8):392-398. doi: 10.1016/S0924-9338(99)80685-3 [DOI] [PubMed] [Google Scholar]
- 33.Bock C, Bukh JD, Vinberg M, Gether U, Kessing LV. Validity of the diagnosis of a single depressive episode in a case register. Clin Pract Epidemiol Ment Health. 2009;5:4. doi: 10.1186/1745-0179-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7)(suppl):95-98. doi: 10.1177/1403494811408483 [DOI] [PubMed] [Google Scholar]
- 35.Nørgaard-Pedersen B, Hougaard DM. Storage policies and use of the Danish Newborn Screening Biobank. J Inherit Metab Dis. 2007;30(4):530-536. doi: 10.1007/s10545-007-0631-x [DOI] [PubMed] [Google Scholar]
- 36.Mortensen PB. Response to “Ethical concerns regarding Danish genetic research”. Mol Psychiatry. 2019;24(11):1574-1575. doi: 10.1038/s41380-018-0296-x [DOI] [PubMed] [Google Scholar]
- 37.Maronna RA, Zamar RH. Robust estimates of location and dispersion for high-dimensional datasets. Technometrics. 2002;44(4):307-317. doi: 10.1198/004017002188618509 [DOI] [Google Scholar]
- 38.Privé F, Aschard H, Ziyatdinov A, Blum MGB. Efficient analysis of large-scale genome-wide data with two R packages: bigstatsr and bigsnpr. Bioinformatics. 2018;34(16):2781-2787. doi: 10.1093/bioinformatics/bty185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demontis D, Walters RK, Martin J, et al. ; ADHD Working Group of the Psychiatric Genomics Consortium (PGC); Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium; 23andMe Research Team . Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63-75. doi: 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grove J, Ripke S, Als TD, et al. ; Autism Spectrum Disorder Working Group of the Psychiatric Genomics Consortium; BUPGEN; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; 23andMe Research Team . Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431-444. doi: 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musliner KL, Mortensen PB, McGrath JJ, et al. ; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium . Association of polygenic liabilities for major depression, bipolar disorder, and schizophrenia with risk for depression in the Danish population. JAMA Psychiatry. 2019;76(5):516-525. doi: 10.1001/jamapsychiatry.2018.4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilhjálmsson BJ, Yang J, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium, Discovery, Biology, and Risk of Inherited Variants in Breast Cancer (DRIVE) Study . Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am J Hum Genet. 2015;97(4):576-592. doi: 10.1016/j.ajhg.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agerbo E, Sullivan PF, Vilhjálmsson BJ, et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a danish population-based study and meta-analysis. JAMA Psychiatry. 2015;72(7):635-641. doi: 10.1001/jamapsychiatry.2015.0346 [DOI] [PubMed] [Google Scholar]
- 44.Agerbo E. Effect of psychiatric illness and labour market status on suicide: a healthy worker effect? J Epidemiol Community Health. 2005;59(7):598-602. doi: 10.1136/jech.2004.025288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agerbo E, Byrne M, Eaton WW, Mortensen PB. Marital and labor market status in the long run in schizophrenia. Arch Gen Psychiatry. 2004;61(1):28-33. doi: 10.1001/archpsyc.61.1.28 [DOI] [PubMed] [Google Scholar]
- 46.Eriksson T, Agerbo E, Mortensen PB, Westergaard-Nielsen N. Unemployment and mental disorders. International Journal of Mental Health. 2014;39(2):56-73. doi: 10.2753/IMH0020-7411390203 [DOI] [Google Scholar]
- 47.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data Anal. 2007;51(8):3737-3748. doi: 10.1016/j.csda.2006.12.028 [DOI] [Google Scholar]
- 48.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6(1):39-58. doi: 10.1023/A:1009661900674 [DOI] [PubMed] [Google Scholar]
- 49.Borgan O, Goldstein L, Langholz B. Methods for the analysis of sampled cohort data in the Cox proportional hazards model. Ann Stat. 1995;23(5):1749-1778. doi: 10.1214/aos/1176324322 [DOI] [Google Scholar]
- 50.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51(4):584-591. doi: 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musliner KL, Trabjerg BB, Waltoft BL, et al. Parental history of psychiatric diagnoses and unipolar depression: a Danish National Register-based cohort study. Psychol Med. 2015;45(13):2781-2791. doi: 10.1017/S0033291715000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards JH, Edwards AWF. Approximating the tetrachoric correlation coefficient. Biometrics. 1984;40(2):563-563. [Google Scholar]
- 53.Horsdal HT, Agerbo E, McGrath JJ, et al. Association of childhood exposure to nitrogen dioxide and polygenic risk score for schizophrenia with the risk of developing schizophrenia. JAMA Netw Open. 2019;2(11):e1914401. doi: 10.1001/jamanetworkopen.2019.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wimberley T, Agerbo E, Horsdal HT, et al. Genetic liability to ADHD and substance use disorders in individuals with ADHD. Addiction. 2020;115(7):1368-1377. doi: 10.1111/add.14910 [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee N, Shi J, García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17(7):392-406. doi: 10.1038/nrg.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin AR, Daly MJ, Robinson EB, Hyman SE, Neale BM. Predicting polygenic risk of psychiatric disorders. Biol Psychiatry. 2019;86(2):97-109. doi: 10.1016/j.biopsych.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray GK, Lin T, Austin J, McGrath JJ, Hickie IB, Wray NR. Could polygenic risk scores be useful in psychiatry? a review. JAMA Psychiatry. 2020. doi: 10.1001/jamapsychiatry.2020.3042 [DOI] [PubMed] [Google Scholar]
- 58.Zheutlin AB, Ross DA. Polygenic risk scores: what are they good for? Biol Psychiatry. 2018;83(11):e51-e53. doi: 10.1016/j.biopsych.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palk AC, Dalvie S, de Vries J, Martin AR, Stein DJ. Potential use of clinical polygenic risk scores in psychiatry - ethical implications and communicating high polygenic risk. Philos Ethics Humanit Med. 2019;14(1):4. doi: 10.1186/s13010-019-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson JS, Shade J, DiBlasi E, Shabalin AA, Docherty AR. Polygenic risk scoring and prediction of mental health outcomes. Curr Opin Psychol. 2019;27:77-81. doi: 10.1016/j.copsyc.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wray NR, Lin T, Austin J, et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry. 2020. doi: 10.1001/jamapsychiatry.2020.3049 [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Visscher PM, Wray NR. Sporadic cases are the norm for complex disease. Eur J Hum Genet. 2010;18(9):1039-1043. doi: 10.1038/ejhg.2009.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wray NR, Yang J, Goddard ME, Visscher PM. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet. 2010;6(2):e1000864. doi: 10.1371/journal.pgen.1000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visscher PM, Wray NR, Zhang Q, et al. 10 Years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101(1):5-22. doi: 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janssens ACJW, Martens FK. Reflection on modern methods: revisiting the area under the ROC Curve. Int J Epidemiol. 2020;dyz274. doi: 10.1093/ije/dyz274 [DOI] [PubMed] [Google Scholar]
- 66.Musliner KL, Liu X, Gasse C, Christensen KS, Wimberley T, Munk-Olsen T. Incidence of medically treated depression in Denmark among individuals 15-44 years old: a comprehensive overview based on population registers. Acta Psychiatr Scand. 2019;139(6):548-557. doi: 10.1111/acps.13028 [DOI] [PubMed] [Google Scholar]
- 67.Meier SM, Agerbo E, Maier R, et al. ; MooDS SCZ Consortium . High loading of polygenic risk in cases with chronic schizophrenia. Mol Psychiatry. 2016;21(7):969-974. doi: 10.1038/mp.2015.130 [DOI] [PubMed] [Google Scholar]
- 68.Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM. Research review: polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55(10):1068-1087. doi: 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- 69.Schwartz S, Susser E. Genome-wide association studies: does only size matter? Am J Psychiatry. 2010;167(7):741-744. doi: 10.1176/appi.ajp.2010.10030465 [DOI] [PubMed] [Google Scholar]
- 70.Schork AJ, Schork MA, Schork NJ. Genetic risks and clinical rewards. Nat Genet. 2018;50(9):1210-1211. doi: 10.1038/s41588-018-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wray NR, Kemper KE, Hayes BJ, Goddard ME, Visscher PM. Complex trait prediction from genome data: contrasting EBV in livestock to PRS in humans: genomic prediction. Genetics. 2019;211(4):1131-1141. doi: 10.1534/genetics.119.301859 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of the Study Population
eTable 1. Paternal and Maternal Income, Paternal Educational Attainment and Maternal Labour Market Affiliation for the Study Population and Associated Major Depression Hazard Ratios
eTable 2. Interaction Between the Polygenic Risk Score and Parental Risk Factors and Associated Major Depression Hazard Ratios (Confidence Intervals)
eTable 3. Polychoric/Tetrachonic Correlation Matrix
eFigure 2. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score and Parental Psychiatric History of Depression
eFigure 3. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score (top/bottom 5%) and Parental Psychiatric History and Socio-Economic Status
eFigure 4. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score (top/bottom 10%) and Parental Psychiatric History and Socio-Economic Status
eTable 4. Sample Characteristics of the Study Population and Associated Major Depression Hazard Ratios, When Not Excluding Ancestral Outliers
eTable 5. Hazard Ratio for Major Depression in Relation to Parental Risk Factors Conditional on Various Adjustments Scenarios, When Not Excluding Ancestral Outliers
eFigure 5. Hazard Ratios for Major Depression (A) and Polygenic Risk Score Distribution in Major Depression Cases and the Population-Based Cohort (B), When Not Excluding Ancestral Outliers
eFigure 6. Absolute Risk of Major Depression as Functions of Age in Relation to Sex (A), the Polygenic Risk Score (B), Maternal and Paternal Psychiatric History (C) and Maternal Education and Paternal Labour Market Affiliation (D), When Not Excluding Ancestral Outliers
eFigure 7. Absolute Risk of Major Depression as Functions of Age in Relation to Joint Influences of the Polygenic Risk Score and Parental Psychiatric History and Socio-Economic Status, When Not Excluding Ancestral Outliers