Abstract
Absorption is one of the most important treatment technologies for the removal of volatile organic compounds (VOCs) from tail gases, yet the separation of the absorbents and VOCs remains challenging because of concerns related to environmental impact and large energy requirements. Herein, we explored an absorption and desorption process using N,N-dimethylcyclohexylamine (CyNMe2) as a representative switchable-hydrophilicity solvent (SHS) and toluene as a representative VOC. The results showed that in comparison to common absorbents, CyNMe2 exhibits excellent toluene absorption performance. Desorption efficiencies of toluene from CyNMe2 of up to 94% were achieved by bubbling CO2 at 25 °C, and separation efficiencies of CyNMe2 from water up to 90% were achieved by bubbling N2 at 60 °C. Even after five absorption–desorption cycles, the toluene absorption capacity of CyNMe2 was comparable with that of the fresh absorbent, suggesting that CyNMe2 retains its absorption capacity. We demonstrate an innovative and reversible remediation strategy of VOCs based on SHSs, and the results indicate that SHSs can be used as an alternative to common absorbents for the removal of VOCs to reduce environmental pollution and energy consumption.
1. Introduction
Volatile organic compounds (VOCs) are important precursors of atmospheric secondary organic aerosols.1,2 These compounds are emitted into the atmosphere from a wide range of industries, such as chemical, petrochemical, pharmaceutical, food processing, pulp and paper, color printing, and painting industries.3 It was estimated that in China alone, approximately 10–20 Tg per year of VOCs are released into the atmosphere from anthropogenic sources, with 80% of the emission sources associated with coke and crude oil production and refining.4 Generally, these industrial waste gases contain a high concentration of VOCs. For example, coke oven gas contains about 38 g/m3 of benzoles (benzene 55–80%, toluene 11–22%, and xylene 2.5–6%; data from Kunming Coke Maker Factory, China). VOCs can enter the human body through the skin and respiratory system and cause temporary and permanent damage to the lungs, blood, liver, and other organ systems.5 Therefore, advanced treatment technologies and methods to control atmospheric VOC pollution are urgently needed.
There are many available techniques to control the emission of VOCs, such as activated carbon adsorption, condensation, liquid absorption, membrane separation, incineration or thermal oxidation, catalytic oxidation, and biological treatments, each of which has disadvantages and limitations under different conditions.6,7 Biological treatments, catalytic oxidation, incineration, and thermal oxidation techniques can easily destroy the structures of VOCs. In nondestructive technology, condensation is usually used in combination with compression, adsorption, and absorption. For membrane separation technology, membranes are costly. For adsorption, adsorbent regeneration is complex and can cause secondary pollution. Absorption, in which a contaminated gas is brought in contact with a liquid solvent, is one of the most frequently used technologies for the removal of VOCs from industrial waste gases because it is simple, easy to operate, and has reported removal efficiencies as high as 98%, especially for high-concentration VOCs,8 and the absorbents can be reused.9,10 A crucial aspect of this treatment method is the selection of a suitable absorbent: several common solvents have been used as absorbents for the removal of volatile contaminants, for example, washing oil (WO), vegetable oil,11 mineral oil,12 silicon oil,13−15 diesel oil,16 dimethyl sulfoxide,17 polyglycols,18 alkyl-phthalates, and alkyl-adipates.19,20 Distillation is commonly used to regenerate these absorbents.21 However, this process yields relatively low recovery rates, consumes considerable amounts of energy, and can cause environmental damage.
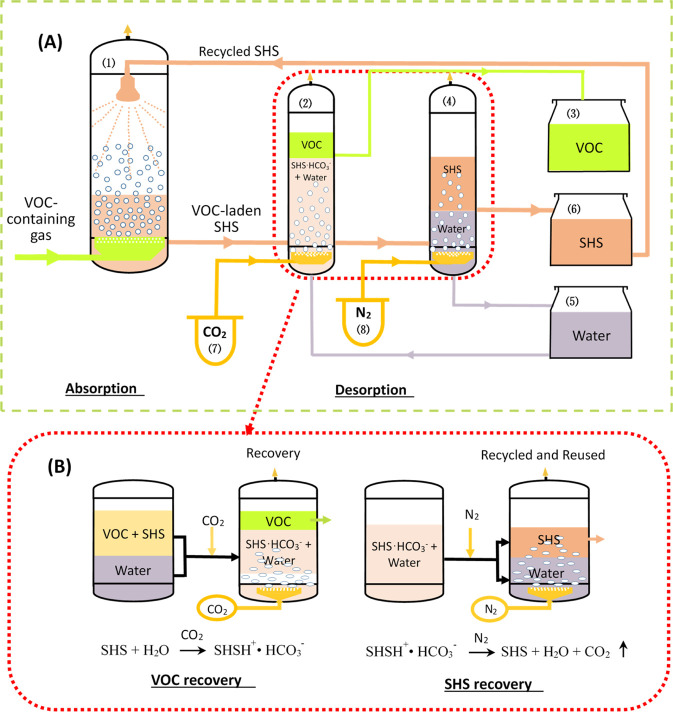
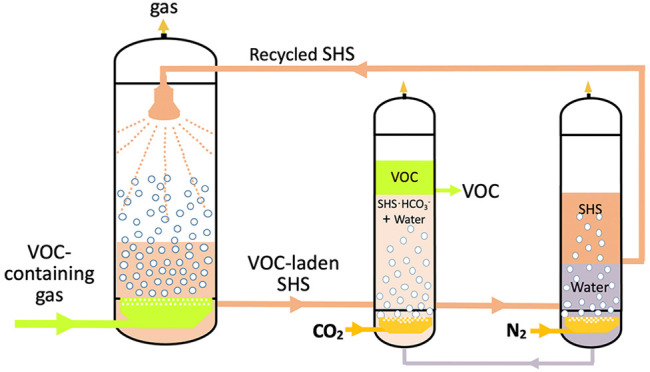
In 2010, Jessop and co-workers first reported a solvent that could be removed from hydrophobic matter without distillation, which was possible because the solvent could be reversibly switched from being hydrophobic to hydrophilic.21 They named these types of solvents switchable-hydrophilicity solvents (SHSs). An SHS is a solvent that can be made, upon application of a trigger, to reversibly switch back and forth between two states, one of which is poorly miscible with water and the other that is completely miscible with water.21−23 Because CO2 is nontoxic, benign, inexpensive, and easily removable, it is preferred as the trigger for the switching processes. We therefore hypothesized that SHSs could be used as absorbents to treat industrial gases containing VOCs and could be regenerated, with contaminant removal, without distillation. However, to our knowledge, use of SHS as absorbents for the removal of organic waste gases has not been reported so far. Consequently, a new method of removing VOCs was proposed based on the characteristics of SHSs. A schematic of the reversible absorption process based on SHSs is shown in Figure 1.
Figure 1.
(A) Overall process for the absorption and desorption of VOCs using SHS as the absorbent; (B) details of the switchable desorption and regeneration steps in the process. VOC-containing gas is fed into the absorber (1) where the VOCs are absorbed by the SHS. The liquid from the absorber (1) is then sent into the desorber (2) and mixed with recovered water from tank (5). CO2 from vessel (7) is sparged into the liquid mixture in the desorber (2) to achieve the separation of VOCs from the SHSs; then the aqueous phase solution containing SHSs and water is transported into separator (4) and is bubbled with N2 to separate the SHS from the water. The red circle in Figure 1A is the process of desorption, the details of which are shown in Figure 1B.
To test the feasibility of this new method for the removal of VOCs by SHSs, a series of experiments was conducted to examine the absorption, desorption, and recycling of SHSs for the removal of VOCs. N,N-Dimethylcyclohexylamine (CyNMe2) and benzyldimethylamine (BDMA) were used as representative examples of the many known SHSs23,25 to verify the feasibility of switchable absorption because of their commercial availability, low toxicity, and high boiling point.22 These two SHS are water-miscible in the presence of CO2 but largely water-immiscible in the absence of CO2. Moreover, WO was used as a representative traditional absorbent11 to compare with the two SHSs. Toluene was chosen as a representative VOC because it is a common toxic compound24 with low solubility in water.25 Although toluene might not be representative of the alkane portion of VOCs, it may be suitable as a model to test the absorption/catalytic performances of many new environmental materials.10,26 The main goals of this study were (i) to determine the VOC absorption capacities of typical SHSs and compare them with those of more traditional absorbents; (ii) to determine Henry’s law constants (E) and activity coefficients (γ) of the VOCs in the presence of SHSs, compare them with those of more traditional absorbents, and investigate the absorption kinetics of VOCs by typical SHSs; (iii) to study the desorption of VOCs from mixtures of VOCs and SHSs; and (iv) to determine the VOC absorption capacities of the regenerated SHSs.
2. Materials and Methods
2.1. Chemicals
Chemicals, suppliers, and purities are listed in Text S1 of the Supporting Information.
2.2. Absorption
The absorption experiment equipment contained three sections: a gas mixing section to prepare the simulated waste gas, an absorption section, and an analysis section (Figure S1). The simulated gas was prepared using a bubbling method28−30 because of its simplicity and ability to obtain a broad range of concentrations. VOC gases were prepared with N2 as the bubbling and diluting gas in the VOC generator (100 mL), and the desired concentrations (2.6–42 g/m3) were obtained by altering the N2 flow rate, which was controlled by an intelligent flowmeter (DSN100CD, Dongguan De Xin Electronic Technology, Ltd. China). The experiments were performed with a total gas flow rate of 430 mL/min. Gas flow lines were constructed from Teflon tubing (3 mm OD). The VOC generator and absorber were placed in a temperature-controlled water bath to control the experimental temperature in the range of 20–55 °C. Within this temperature range, no obvious changes in the volumes of the absorption solutions were observed. Prior to the absorption experiments, the VOC gas from the generator was prepared for over 1 h until its concentration was constant.
The absorption of VOCs was performed in a glass absorber (31 mm OD × 250 mm height) with a porous glass distribution plate (8 mm OD × 250 mm length) that was immersed in the liquid absorbent to produce an even distribution of bubbles in the absorber. The absorbent (20 mL) was added to the absorber in a temperature-controlled water bath. The desired VOC/N2 mixtures from the VOC generator were fed into the absorber at a flow rate of 430 mL/min. The VOC gas concentrations at the inlet and outlet were determined by in-line gas chromatography (GC, automatic six-way valve). The concentration of VOCs in the liquid phase,8CL(t), can be determined by
| 1 |
where QG (m3/s) is the gas flow rate, VL (mL) is the absorbent volume, CG,in (g/m3) and CG,out (g/m3) are the inlet and outlet concentrations of the VOC in gas flow, respectively, and t (min) is the absorption time.
2.3. Determination of Henry’s Law Constants and Viscosities
In this study, headspace GC was employed to determine the Henry’s law constants of toluene in different absorbents, which is an effective method for acquiring such constants according to previous studies.26 The experiments were carried out in 40 mL headspace vials. Initially, to achieve various ratios of gas and liquid volumes, different volume standard solutions of toluene absorbents (5.1 g/L) were added into vials. The vials were placed into a shaker for 10 h to reach vapor/liquid equilibrium at a series of temperatures (three times each, in parallel). The equilibrium gas concentration of toluene was determined by GC (GC522, Shanghai Wu Feng) equipped with a flame ionization detector (FID). The GC column was a 3 m × 3 mm 10% OV-101 (Ouni Instrument Technology Co. Ltd., Shanghai). The GC operating conditions are as follows: the column, injector, and detector temperatures were set at 180, 200, and 230 °C, respectively, and the flow rates of carrier gas (N2), hydrogen, and air were 35, 405 and 40 mL/min, respectively. The dimensionless Henry’s law constants were obtained by averaging the concentrations of toluene at different ratios in the gas and liquid phases. The liquid equilibrium concentration of toluene was calculated from the conservation of mass equation
| 2 |
where CL* (g/m3) and CG (g/m3) are the equilibrium concentrations of toluene in the liquid and gas in the vial, respectively, CS (g/m3) is the concentration of the standard solution of toluene, and VL (mL) and VG (mL) are the liquid and gas phase volumes, respectively.
The viscosities were determined using an NDJ Series digital viscometer (Bonsi Instrument Technology (Shanghai) Co. Ltd.). The speed was 60 rpm, and the temperature was set to 20 °C.
2.4. Determination of Mass Transfer Coefficients
The gas/liquid mass transfer of VOCs in the absorbents was carried out in a double-stirred reactor (60 mm in diameter) with four vertical baffles and two stirring blades, and the area of gas/liquid mass transfer was 2.64 × 10–3 m2 (Figure S2 in the Supporting Information). The double-stirred reactor had a gas/liquid interface that was planar to a close approximation, which is an effective method for the determination of mass transfer coefficients for pollutants in absorbents at a gas/liquid interface.33,34 The experimental setup included three sections, an organic gas generation system to prepare the simulated waste gas, a double-stirred absorption section, and an analysis system. The preparation method of the simulated gas was similar to the absorption experiments. Prior to each run, an external reactor jacket was used to maintain the desired temperature by circulating a constant temperature fluid from a temperature-controlled water bath. The liquid and gas stirring speeds were 130 and 250 rpm, respectively. The VOC vapor (15.73 g/m3) was carried by 150 mL/min of N2 through the reactor at different temperatures. The total pressure was set to 1 atm, and the inlet and outlet gas pressures were measured using a U-type pressure gauge. After 30 min, the absorbent was added to the rector, and the volume of the absorbent was maintained at 230 mL. The VOC concentrations in the inlet and outlet gas were analyzed using a GC–FID analyzer (automatic six-way valve).
2.5. Desorption
2.5.1. Desorption of VOC from SHS by Bubbling CO2
Pure water (10 mL) and standard solutions of toluene in CyNMe2 (10 mL) were placed in a graduated cylinder, which was capped with a septum. A porous glass distribution plate (3 mm OD × 300 mm length) was inserted into the bottom of the aqueous phase. Additionally, a small needle was inserted into the septum to allow the gases to escape. Various constant CO2 flow rates were used. CO2 was bubbled into the mixture until the volume of the upper phase was approximately constant. The desired temperature (20–55 °C) was maintained during the experiments, and the effects of temperatures on the volume changes of desorption systems can be neglected. A 1 μL sample of the toluene that separated from the mixtures was inserted into a headspace vial (40 mL, three times in parallel). After 20 min, a 0.5 mL headspace sample was removed from the vial and injected into the GC–FID with a gas-tight syringe.
The desorption efficiency (Dtoluene) of toluene was calculated as the amount of toluene collected in the upper layer divided by the total amount of toluene, which was calculated using the concentration and volume of the standard solution. In the desorption studies, the recovery of toluene was measured until the upper mixture volumes were nearly constant. The equation of the desorption efficiency of toluene in the mixture was defined as follows
| 3 |
where CG (g/m3) and CS (g/m3) are the concentrations of the headspace sample and standard solution of toluene, respectively; VGS (mL), VHV (mL), and VLS (mL) are the volumes of the headspace sample (0.5 mL), headspace vial (40 mL), and toluene that separated from the mixtures and was inserted into a headspace vial (1 μL), respectively.
Molecular dynamics (MD) simulation was used to study the desorption process of toluene from SHSs. The simulated model was constructed by placing CyNMe2H+/toluene mixture on a water slab to form oil/water interfaces, which represents the state of CyNMe2/toluene system after CO2 was introduced. The simulations were performed using Gromacs package35 with the GROMOS force field.36 The configurations from the MD results were calculated by quantum mechanics (QM), and the intermolecular configurations were optimized using density functional theory at the B3LYP/6-31g* level with Gaussian 16 package.37 Weak interaction analysis for the optimized configurations was performed using Multiwfn software.38 The reduced density gradient was plotted against the electron density ρ(r), and the gradient isosurfaces were visualized using VMD software.39
2.5.2. Recovery of SHS from Mixtures by Bubbling N2
We also performed experiments to explore the separation of CyNMe2 from the water phase. Mixtures (20 mL) of CyNMe2 (10 mL) and pure water (10 mL) were placed in a graduated burette, which was capped with a septum. A porous glass gas distribution tube (3 mm OD × 300 mm length) was inserted into the bottom of the aqueous phase. Additionally, a small needle was inserted into the reaction tubes to allow the gases to escape. Prior to each run, a cooling jacket was fit to the top of the burette to condense steam and decrease the volume losses. Initially, a single-phase mixture of CyNMe2 and pure water was obtained by bubbling CO2 (30 mL/min, 60 min), as previously reported by Jessop et al.21 To achieve the separation of CyNMe2 from the mixture, various constant N2 flow rates were used for the experiments (measured with a DSN500CD intelligent flow meter). N2 was bubbled through the mixture until the volume of the upper phase was approximately constant. The temperatures (25–60 °C) were controlled for all experiments. No obvious effect of temperature was observed on the volume of the carbonated CyNMe2 solutions. The equation for the recovery efficiency of CyNMe2 from the water phase was defined as follows
| 4 |
where Vupper (mL) and VCyNMe2 (mL) are the volume of CyNMe2 in the upper phase and the initial volume of CyNMe2 (10 mL).
2.6. Recycling of SHS
CyNMe2 recovered as described in Section 2.5 was used to conduct recycling experiments. In the recycling experiments, the preparation method of the simulated gas and the experimental processes were the same as described in Sections 2.3 and 2.5. Because some CyNMe2 was lost during each absorption and desorption process, fresh makeup CyNMe2 was added to the absorber to maintain a constant volume (20 mL) of CyNMe2 after the first cyclic absorption experiment. The experiments were carried out in a 25 °C water bath, and the inlet concentration of toluene was 27 mg/L.
3. Results and Discussion
3.1. Absorption Performance of SHSs toward Toluene
Figure 2 shows the gas concentration of toluene at the outlet of the absorber in the presence of different absorbents. Under a relatively low inlet concentration of toluene (2.7 g/m3), the saturated absorption capacities of toluene in the presence of CyNMe2, BDMA, and WO were determined to be 12.3, 13.3, and 14.6 g/L, respectively. The absorption capacities of toluene in the SHSs were comparable to that in WO. When nitrogen containing a high concentration of toluene was bubbled through CyNMe2, BDMA, and WO, the saturated absorption capacities of toluene in CyNMe2, BDMA, and WO were determined to be 71.7, 74.9, and 93.7 g/L, respectively, indicating that the absorption capacities of toluene in SHSs are slightly lower than those in WO. These results also indicate that at a relatively high toluene concentration (27 g/m3), WO displayed a good toluene absorption capacity, 25.1 and 30.7% greater than those of BDMA and CyNMe2, respectively. Nevertheless, the recycling of WO may be an issue because it does not possess switching properties like SHSs that can be separated from toluene and recycled without distillation by a simple process of bubbling CO2/air to alter the hydrophilicity/hydrophobicity of SHSs.21 Considering that advantage, the SHSs may be a better choice for the absorption of toluene at its typical initial concentrations.
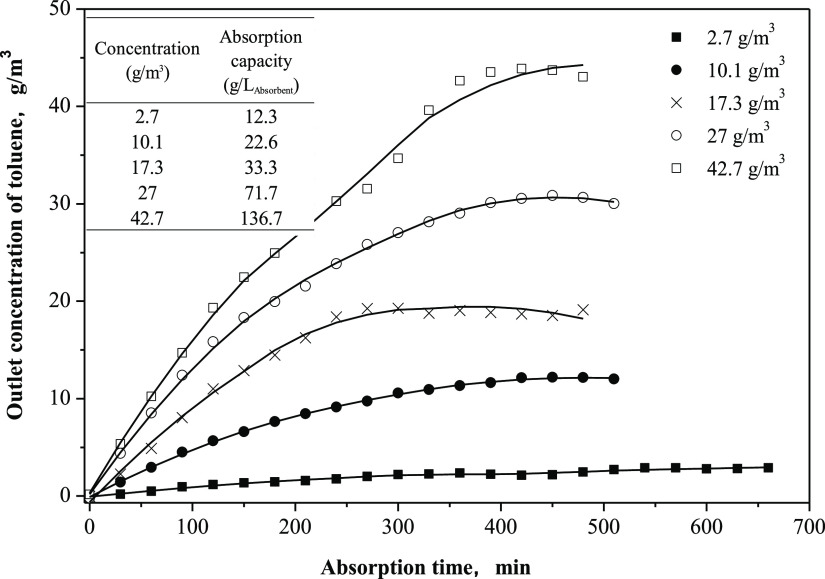
Figure 2.
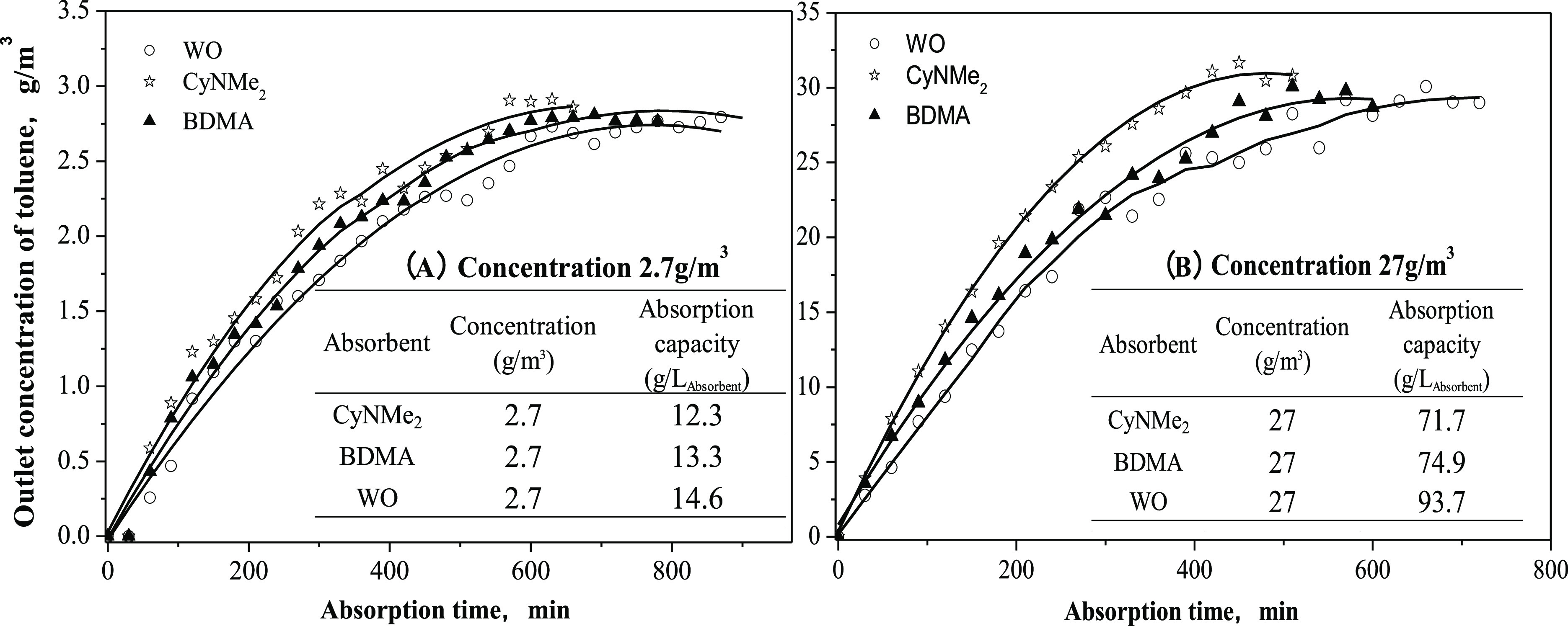
Comparison of toluene absorption of SHSs with common absorbents. 430 mL/min N2 carried 2.7 g/m3 (A) and 27 g/m3 (B) of toluene into 20 mL of absorbent at 25 °C.
To explore the effects of operating conditions on the toluene absorption capacity, CyNMe2 was used as a representative SHS because of its comparable absorption capacity with BDMA (Figure 2) and lower toxicity than BDMA.24 As the inlet gas toluene concentration was increased from 2.7 to 42.7 g/m3, the absorption capacity of CyNMe2 for toluene increased dramatically, ranging from 12.3 to 136.7 g/Labsorbent, respectively (Figure 3). Thus, it can be inferred that the absorption capacity of CyNMe2 was enhanced by the relatively high inlet toluene gas concentrations.
Figure 3.
Effects of inlet toluene concentration on absorption capacities. 430 mL/min N2 carried various concentrations of toluene into 20 mL of CyNMe2 at 25 °C.
As shown in Figure S3, the outlet gas concentration of toluene increased as the temperature was increased from 25 to 45 °C, indicating that the absorption capacity of CyNMe2 decreased at a high absorption temperature. For example, at a low absorption temperature (25 °C), the time required to reach absorption saturation was more than 550 min at Cin = 2.7 g/m3 and the absorption capacity of CyNMe2 was 12.3 g/L, while at a higher temperature (45 °C), the time to saturation was less than 150 min and the absorption capacity significantly decreased to 2.4 g/L. The thermodynamic parameter Gibbs free-energy change (ΔG) was calculated to explore the absorption process of CyNMe2 by the following equations
| 5 |
| 6 |
where Kd (CL/CG) is the distribution coefficient of toluene in liquid phase and gas phase, ΔH and ΔS are the enthalpy and entropy changes of absorption process, respectively, and R is the universal gas constant (8.314 J/mol/K). As indicated by Table S1, the values of ΔG in the temperature range 25–45 °C are negative, indicating that the adsorption is thermodynamically spontaneous. However, lower temperatures are beneficial for absorption because of the more negative ΔG values. These results indicate that the absorption capacity of CyNMe2 was suppressed at higher absorption temperatures, suggesting that higher temperatures are unfavorable for absorption.
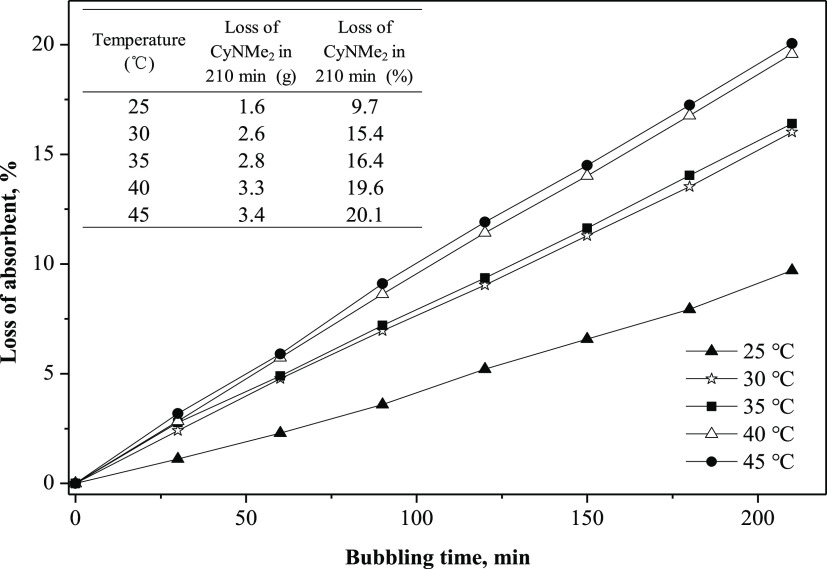
Loss of some of CyNMe2 in the absorption process may occur due to evaporation and bubbling at various temperatures. Thus, the loss of CyNMe2 in the absorption process of toluene was investigated. As shown in Figure 4, as the temperature was increased from 25 to 45 °C, the loss of CyNMe2 increased from 1.6 to 3.4 g, accounting for 9.7 and 20.1%, respectively. These results indicated that a relatively high temperature condition facilitated the loss of CyNMe2 in the absorption process. Thus, to maintain the absorption efficiency and reduce the loss of CyNMe2, some measures should be taken, such as condensation, increasing the height of the absorption device, or selecting a less volatile SHS.
Figure 4.
Effect of temperature on the loss of absorbent. 430 mL/min N2 was bubbled into 20 mL CyNMe2.
3.2. Henry’s Law Constants of Toluene in SHSs and a Conventional Absorbent
According to previous studies, the values of the Henry’s law constant reflect the relative VOC absorption performances of the absorbents.31,32 Therefore, to explore the specific absorption processes, vapor/liquid equilibrium experiments (the measurement of Henry’s law constant) of the absorption system were performed. Table 1 lists the values of the Henry’s law constants determined at different temperatures. As indicated by Table 1, the Henry’s law constants for toluene in the three absorbents were significantly influenced by the temperature. The temperature dependence of the Henry’s law constant can be described by the van’t Hoff equation40
| 7 |
where the constants A and B are determined by regression analysis. The linear relationships between ln H and 1/T for toluene in various absorbents are shown in Figure S4. Linear relationships between ln H and 1/T were observed (R2 > 0.98, p < 0.05) for the different absorption systems, suggesting that the van’t Hoff equation fit the data well, and the values of A and B obtained from the regression are summarized in Table S2.
Table 1. Henry’s Law Constants for Different Solute/Absorbent Systems.
| absorbent | temperature (°C) | H × 10–4 (dimensionless) | HC × 10–3 (atm·L/mol) | E × 10–2 (atm) |
|---|---|---|---|---|
| CyNMe2 | 25 | 2.12 ± 0.06 | 5.18 ± 0.14 | 2.86 ± 0.10 |
| 30 | 3.10 ± 0.02 | 7.70 ± 0.51 | 4.22 ± 0.03 | |
| 35 | 5.27 ± 0.22 | 13.3 ± 0.54 | 7.28 ± 0.30 | |
| 40 | 7.46 ± 0.18 | 19.1 ± 0.47 | 10.0 ± 0.27 | |
| 45 | 10.1 ± 0.08 | 26.3 ± 0.21 | 13.7 ± 0.01 | |
| BDMA | 25 | 2.67 ± 0.14 | 6.52 ± 0.34 | 3.64 ± 0.20 |
| 30 | 5.61 ± 0.36 | 13.9 ± 0.89 | 7.53 ± 0.53 | |
| 35 | 8.47 ± 0.12 | 21.4 ± 0.31 | 11.3 ± 0.20 | |
| 40 | 13.3 ± 0.35 | 34.1 ± 0.89 | 17.7 ± 0.59 | |
| 45 | 20.7 ± 0.22 | 54.0 ± 0.58 | 28.3 ± 0.28 | |
| WO | 25 | 2.43 ± 0.10 | 5.94 ± 0.25 | 3.02 ± 0.14 |
| 30 | 4.01 ± 0.01 | 9.96 ± 0.02 | 5.09 ± 0.03 | |
| 35 | 7.00 ± 0.15 | 17.7 ± 0.38 | 8.92 ± 0.19 | |
| 40 | 10.7 ± 0.29 | 27.5 ± 0.76 | 13.5 ± 0.37 | |
| 45 | 16.0 ± 0.29 | 41.7 ± 0.75 | 22.2 ± 0.61 |
The data in Table 1 show that under different temperatures, the values of Henry’s law constants for toluene in these two SHSs are slightly lower than those for toluene in WO. Moreover, the Henry’s law constants in the SHSs at 25 °C were also low compared to most traditional absorbents (phthalates, polyethylene, silicon oil, and polyethylene glycol) as indicated by Table S3. This may partly explain the high absorption capacity of the SHSs relative to these many common absorbents (Table S3). To further understand the high absorption capacity of the two SHSs, we compared the values of the activity coefficient (γ) with those of common absorbents (Table S3), where the values of γ for the absorption systems were obtained by eq S7 (Text S2.1). According to previous studies, activity coefficient (γ) is a characteristic thermodynamic equilibrium parameter that reflects the interaction between toluene and the absorbent. As listed in Table S3, a γ value of an absorbent lower than unity corresponds to a more favorable thermodynamic equilibrium,8 which indicates that the absorbents have greater trapping capacities (Text S2.2), and thus the SHSs have greater absorption capacities. In addition, the lower viscosities (η) of the SHSs compared to other absorbents promote absorption in the SHSs by enhancing the gas/liquid mass transfer rate. From these observations, it can be concluded that the values of Henry’s law constants, activity coefficients, and viscosities determine the absorption capacity of SHSs for toluene.
3.3. Mass Transfer Characteristics of Absorption Process
A double-stirred reactor was used to determine the liquid phase (KL) and gas phase (KG) mass transfer coefficients of the SHS absorption systems for toluene. The values of KL and KG at 30 °C were determined to be 3.51 × 10–6 m/s and 1.85 × 10–4 mol/(m2·s·atm) using eqs S15 and S20 (Text S2.3), respectively. Therefore, combining these with the Henry’s law constant, the liquid film resistance, and the gas film resistance, one can calculate HC/KL (2.19 m2·s·atm/mol) and 1/KG (5.41 × 103 m2·s·atm/mol), revealing that 1/KG ≫ HC/KL. Thus, the gas film resistance controls the absorption process of toluene in SHSs. For physical absorption, the control of the gas and/or liquid film can be estimated by an empirical formula.41 When ρsHC/MsP > 0.2, the process is controlled by the liquid film, and when ρsHC/MsP < 5 × 10–4, the process is controlled by the gas film. In these equations, ρs (kg/m3) is the density of soluble gases at the actual operating temperature, Ms (g/mol) is the toluene gas molecular weight, and P (kPa) is the total pressure of the gas phase. The calculated results, ρsHC/MsP = 3.097 × 10–4 (and therefore <5 × 10–4), indicates that the mass transfer resistance mainly exists in the gas film, which controls the entire absorption process. This result is consistent with the experimental results, confirming that the absorption process was dominated by gas film resistance.
As the temperature was an important factor affecting the absorption process, its effect on mass transfer was also investigated (Table 2). The gas- and liquid-phase mass transfer coefficients decreased with increasing absorption temperature. The dependence of the mass transfer coefficient on the temperature was determined by a linear regression using the data shown in Table 2. The correlation coefficient test showed that the logarithm of mass transfer coefficient (ln KL and ln KG) and 1/T have a linear relationship (R2 = 0.99, p < 0.05). Thus, the influence of the absorption temperature on the gas- and liquid-phase mass transfer coefficients can be described by the following equations at constant pressure.
| 8 |
| 9 |
Table 2. Gas- and Liquid-Phase Mass Transfer Coefficients at Various Temperatures.
| temperature (°C) | KL × 10–6 (m/s) | KG × 10–6 (mol/(m2·s·kPa)) |
|---|---|---|
| 25 | 3.64 | 1.92 |
| 30 | 3.51 | 1.85 |
| 35 | 3.31 | 1.74 |
| 40 | 3.16 | 1.66 |
| 45 | 2.97 | 1.56 |
3.4. Separation of VOCs and Recovery of SHS
It was reported previously that SHSs can be reversibly switched from being hydrophobic to hydrophilic by bubbling CO2 through the solvent. To test whether the mixture of SHS and VOC can be efficiently separated, a switching experiment with CO2 as a trigger was performed. As shown schematically in Figure 1B, the mixture of CyNMe2 with toluene was separated upon mixing with carbonated water into two layers, where the upper layer was toluene and the lower layer was a mixture of CyNMe2 (as its bicarbonate salt) and carbonated water. After removal of the toluene-rich phase, the aqueous phase (carbonated water and CyNMe2) separated into two layers upon N2 being bubbled through the mixture, where the upper layer was primarily CyNMe2 while the lower layer was mostly water. The following desorption experiments were conducted to test this postulation and to optimize the operation conditions on the separation efficiency of toluene/CyNMe2.
3.4.1. Desorption of Toluene from SHS by Polarity Switching of SHSs via CO2
As listed in Table 3, there is a notable separation of CyNMe2 and toluene when treated with water and CO2, and the desorption efficiency of toluene was up to 93.7%, which verifies the feasibility of the desorption process by CO2 control. Subsequently, we performed experiments to probe the effects of optimal conditions on the desorption efficiency of toluene in CyNMe2 by varying the CO2 flow rate, CO2 purity, desorption temperature, volume ratio of mixtures to water, and toluene concentration. The desorption efficiencies of toluene in the mixtures versus the CO2 flow rate for 50 min experiments are listed in Table 3. The desorption efficiencies of toluene were found to be approximately 97.7, 93.7, and 93.0% for CO2 flow rates of 45, 30, and 15 mL/min, respectively, implying that a high CO2 flow rate promotes the desorption of toluene in the mixed system. Although the desorption efficiency of toluene at 45 mL/min was the highest of the three flow rates, the amount of CO2 consumption was also the highest. In addition, the desorption efficiencies of toluene with CO2 flows at 30 and 15 mL/min were nearly equal, while the desorption time required at 15 mL/min was much longer. We concluded that the CO2 flow rate at 30 mL/min was the most economical for the desorption processes, and hence, a 30 mL/min CO2 flow rate was used in the subsequent experiments.
Table 3. Efficiency of Desorption of Toluene from CyNMe2 Using CO2 and Water under Different Conditionsa.
| parameters | desorption of toluene (%) | bubbling time (min) | CO2 consumption (mL) |
|---|---|---|---|
| CO2 Flow Rate (mL/min) | |||
| 45 | 97.7 | 50 | 2250 |
| 30 | 93.7 | 60 | 1800 |
| 15 | 93.0 | 115 | 1725 |
| Temperature (°C) | |||
| 20 | 92.5 | 80 | 2400 |
| 25 | 93.7 | 60 | 1800 |
| 35 | 92.9 | 70 | 2100 |
| 45 | 89.3 | 85 | 1951 |
| 55 | 73.7 | 105 | 3150 |
| Toluene Concentration (g/L) | |||
| 65 | 92.8 | 75 | 2250 |
| 130 | 93.7 | 60 | 1800 |
| 260 | 92.0 | 85 | 2550 |
| bVolume Ratio (Lmix/Lup) | |||
| 10:50 | 96.0 | 45 | 1350 |
| 10:25 | 93.7 | 50 | 1500 |
| 10:16.7 | 93.0 | 50 | 1500 |
| 10:12.5 | 93.9 | 55 | 1650 |
| 10:10 | 93.7 | 60 | 1800 |
| CO2 Purity (%) | |||
| 100 | 93.7 | 60 | 1800 |
| 90 | 91.5 | 65 | 1950 |
| 80 | 89.1 | 70 | 2100 |
| 50 | 80.3 | 80 | 2400 |
| 10 | 65.7 | 150 | 4500 |
| 5 | 53.2 | 195 | 5850 |
| 1 | 48.2 | 210 | 6300 |
Unless otherwise stated, CO2 (100% purity, 30 mL/min) was bubbled into the mixtures of water (10 mL) and 10 mL of standard solution containing CyNMe2 and toluene (130 g/L) at 25 °C.
Lmix/Lup represents the volume ratio of mixed standard solution to pure water.
Table 3 also lists the effect of temperature on the desorption of toluene from CyNMe2 in the range of 20–55 °C. As the temperature was increased from 20 to 55 °C, the desorption efficiency of toluene generally decreased. It is known that an increase of temperature accelerates the volatilization of toluene and leads to a decreased amount of toluene obtained, which might explain the decrease in the desorption efficiency of toluene. Moreover, amine group bicarbonates22,27,42 are not stable and decompose easily at high temperatures, leading to reduced formation of bicarbonates,43 poorer toluene desorption, and likely greater losses of CyNMe2 to the toluene phase. Therefore, high temperatures are not conducive to the separation of toluene and CyNMe2. Based on the desorption time and CO2 consumption at different temperatures, we concluded that the temperature of 25 °C was the best for desorption, and this desorption temperature was chosen for subsequent experiments.
As can be seen in Table 3, at different initial concentrations of toluene, the desorption efficiencies of toluene were comparable. Nevertheless, at 130 g/L of toluene, the desorption process had a relatively short desorption time and low CO2 consumption, indicating that this concentration was beneficial for the desorption of toluene from CyNMe2. The effect of the proportions of the mixtures and water on the desorption process of toluene was investigated (Table 3). Upon increasing the mixture to water ratio (Lmix/Lup), the desorption efficiencies of toluene were similar, but the bubbling time and CO2 consumption increased slightly. However, the consumption of water decreased at high mixture to water ratios (Lmix/Lup), leading to decrease of wastewater production. Thus, we concluded that a high ratio of the mixture to water is preferred for the desorption process.
We also performed experiments to examine the effects of CO2 purity on the desorption of toluene by altering the flow proportion of N2 and CO2. As indicated by Table 3, the desorption efficiency of toluene from CyNMe2 increased with the increase of CO2 purity. For example, when 100% CO2 was used, the desorption efficiency of toluene was determined to be 93.7%. However, even if the purity of CO2 was only 1%, this was sufficient to switch the polarity of CyNMe2, resulting in the desorption of toluene as high as 48.2%. This indicates that a broad spectrum of purities of CO2 can be used as triggers to switch the polarity of CyNMe2 in production and practical applications.
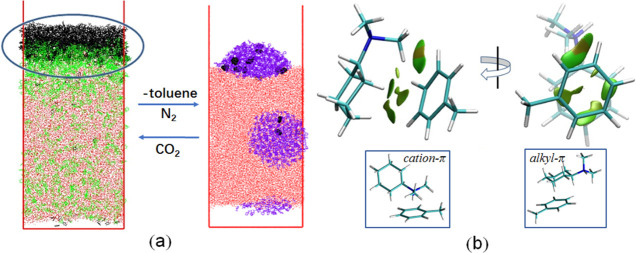
The desorption process mechanism for toluene from CyNMe2 was probed by MD simulation. It can be seen from Figure 5a that CyNMe2H+ spontaneously entered the aqueous water phase from the organic phase, resulting in the separation of toluene and CyNMe2. During this separation process, we observed that CyNMe2H+ was initially adsorbed at the oil/water interface forming an adsorption layer prior to entering into the aqueous phase, indicating that CyNMe2H+ would bind with toluene molecules through different interaction modes in this adsorption layer. Consequently, we selected the configurations from the MD results for further QM calculation. As shown in Figure 5b, noncovalent interaction occurred between CyNMe2H+ and toluene, which can be attributed to the alkyl−π interactions. Moreover, cation−π interactions formed between the CyNMe2H+ headgroup and the toluene aromatic ring also exist. However, these weak interactions cannot prevent the phase transfer of CyNMe2H+ from the organic phase to the aqueous phase because of the great hydrophilicity of the [CyNMe2H+][HCO3–] salt. Therefore, CyNMe2 partitions into the aqueous phase and therefore separates from toluene upon addition of CO2 as a trigger.
Figure 5.
(a) Configuration from MD simulation. CyNMe2, CyNMe2H+, toluene, and water are shown in violet, green, black, and red, respectively. (b) Gradient isosurfaces for CyNMe2H+/toluene system.
3.4.2. Recovery of SHS from the Aqueous Phase upon Removal of CO2
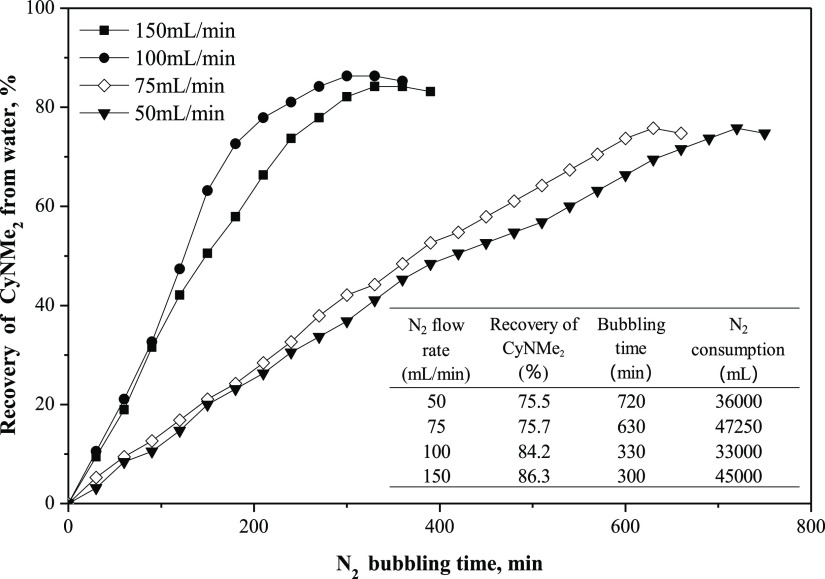
To recover CyNMe2 from the carbonated CyNMe2/water mixed solution, N2 was bubbled through the mixture to remove CO2 and change the hydrophilic [CyNMe2H+][HCO3–] salt back into hydrophobic CyNMe2, which caused the mixture to split into two liquid phases as shown in Figure 5a. As shown in Figure 6, the recovery efficiency of CyNMe2 increased with the increase of N2 flow rate from 50 to 150 mL/min. For N2 flow rates of 100 and 150 mL/min, the recovery efficiencies of CyNMe2 were comparable. Despite the somewhat short desorption time at 150 mL/min, the consumption of N2 was considerable (Figure 6). Thus, we conclude that a N2 flow rate of 100 mL/min was the best for the desorption process of CyNMe2.
Figure 6.
Comparison of the recovery of CyNMe2 from carbonated water under various N2 flow rates at 40 °C. N2 was bubbled into a single-phase mixture of CyNMe2 (10 mL) and ultrapure water (10 mL) that had been previously carbonated by bubbling of CO2 (30 mL/min, 60 min) at 25 °C.
A previous study found that the separation of CyNMe2 and water can also be achieved by heating.22 We conducted experiments to investigate the effects of temperature on the desorption process. The effect of temperature on the recovery of CyNMe2 in water (Figure S5) showed that the final recovery of CyNMe2 at high temperatures was greater than that at low temperatures. Moreover, at high temperatures, the consumption of N2 and the desorption time decreased dramatically. Consequently, it can be concluded that elevated temperatures significantly accelerate the desorption process, presumably because mass transfer of CO2 out of the liquid mixture is accelerated and the rate of decomposition of amine bicarbonates increases.22,27
3.5. Absorption Performance of Recovered SHS toward Toluene
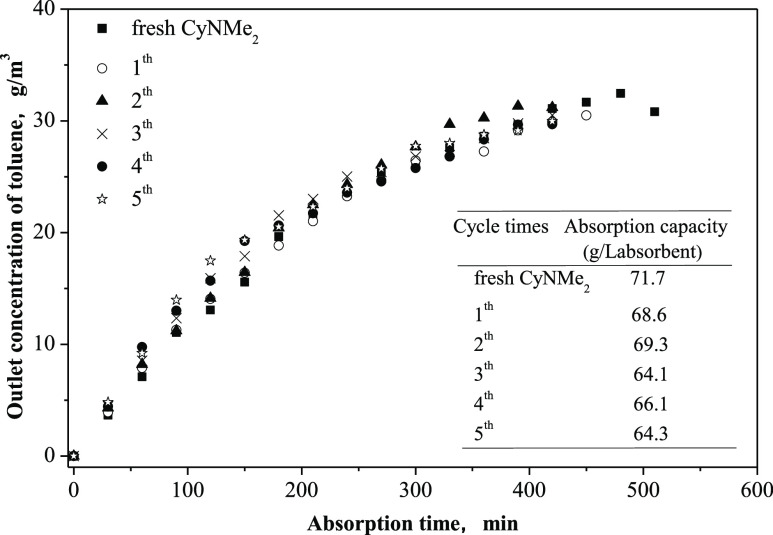
Recycling absorption experiments were performed to evaluate the absorption capacity of the recovered CyNMe2. The absorption capacity of recovered CyNMe2 slightly decreased with increasing number of cycles (Figure 7). After five absorption–desorption cycles, the absorption capacity had decreased by 10.3% compared to that of fresh CyNMe2. This indicates that CyNMe2 is a good reusable absorbent for the removal of VOCs. Since the CyNMe2 recovered contained a certain amount of water,44 the effect of water on the absorption capacity of CyNMe2 recovered was investigated (Text S2.4). The results showed that the absorption capacity decreased with the increase of water content in CyNMe2 (Figure S6). With 13.8% water content, for instance, the absorption capacity was found to be 66.2 g/LAbsorbent, which is 8% lower than that of fresh CyNMe2 (71.7 g/LAbsorbent). The water content may therefore be responsible for the lower absorption capacity of recovered CyNMe2 compared to fresh CyNMe2.
Figure 7.
Effect of multiple cycles of CyNMe2 on its absorption capacity for toluene at 25 °C. 430 mL/min N2 carried 27 g/m3 toluene absorbed by 20 mL of CyNMe2 that was recovered using water, CO2 (30 mL/min, 25 °C), and N2 (100 mL/min, 60 °C).
The amount of water in CyNMe2 carried into each cycle is controlled by the solubility limit of water at the temperature of the decarbonation step of the preceding cycle. For this reason, the water content will level out at a constant value after a few cycles rather than continue to climb. The water content can be reduced, if needed, by raising the temperature of the decarbonation step or by pretreating the wet (i.e., water-containing) recycled CyNMe2 with CO2 before use in the next cycle in order to trigger a separation of the water from the bulk of the amine.
If wet CyNMe2 is exposed to a waste gas stream that contains CO2, then much of the water in the amine is expelled from the organic liquid phase. This was demonstrated by bubbling 10 wt % CO2 in N2 gas mixture through a solution of 8.1 wt % water in CyNMe2. A small aqueous phase, about 10% of the total liquid volume, separated from the organic phase and could be easily decanted.
The longevity of the amine is important to consider before commercial application of this technology. Decomposition should be avoided not only because of the loss of absorbent but also because the secondary amines that result from decomposition may convert to carcinogenic nitrosamines. In CO2-capture processes, for example, the chemical decomposition of the amine is a significant problem. The amines used in such processes are ethanolamines, which typically decompose by loss of acetaldehyde (eq 10).
| 10 |
However, CyNMe2 is not an ethanolamine and is therefore incapable of decomposing in this way. Additionally, the temperatures used in the toluene absorption process are significantly lower than those used in the regeneration stage of CO2 capture processes. For both reasons, the rate of decomposition of CyNMe2 should be significantly lower than that observed in CO2-capture processes.
3.6. Engineering Implications
This study found that the SHS CyNMe2 exhibited an excellent absorption performance for toluene compared to many common organic absorbents, and CyNMe2 can be regenerated by bubbling CO2 to alter the polarity of the SHS. It was reported that an organic absorbent should have the following properties to be attractive for an industrial process:10,24 (i) a high absorption capacity, (ii) a low viscosity and a high diffusion coefficient, (iii) a low vapor pressure to reduce the loss of absorbent by stripping and to prevent possible air pollution, (iv) no toxicity or fire or explosion risks, and (v) a low cost. As indicated by Table S3, CyNMe2 is less viscous than traditional absorbents, which is indicative of good diffusion ability. For the vapor pressure, Vanderveen et al.24 reported that most SHS are less volatile than the two common VOCs, toluene and hexane, which may reduce the risk of worker exposure even if the acute toxicity (oral, rat) is greater than that of toluene and hexane. In addition, CyNMe2 has a significantly higher flash point (43 °C) than toluene (4 °C) and hexane (−22 °C), indicating that the SHS is much safer than the two solvents. As for the cost of CyNMe2, its price per kilogram (¥882) is comparable to or higher than many common absorbents according to the quoted price from J&K Scientific (https://www.jkchemical.com/CH/Index.html). For example, the price per kilogram of CyNMe2 is slightly lower than those of diisodecyl phthalate (¥990) and silicone oil (¥984) and is higher than those of WO (¥30), di(2-ethylhexyl) adipate (¥448), diisobutyl phthalate (¥448), diisobutyl phthalate (¥184), and polyethylene glycol 400/300 (¥483/580). Based on these discussions, CyNMe2 may be an alternative to common absorbents for organic waste gas. Other inexpensive SHSs exist, having even higher flash points and lower toxicity than CyNMe2. They have not yet been evaluated for this application, but such screening of a range of SHS would be an important step before application of this technology.
In engineering practice, the cost for absorption of organic waste gas includes the costs of the absorbents themselves, the absorption process, and the process for the separation of the absorbents from the absorbed VOCs. In a comparison of traditional absorbents versus SHSs, the key difference is the process for separating the absorbents from the captured VOCs. In the case of traditional absorbents, the separation of absorbents is typically realized by distillation of the monophasic mixture of absorbents and VOCs. This distillation process is energy intensive and may lead to the release of volatile compounds into environment, resulting in secondary pollution. However, for the SHSs, the mixture of absorbents with VOCs can be separated by simply bubbling CO2 at ambient temperature in the presence of water (Figure 1B). All things taken together, despite the relatively high cost of the SHSs, the SHS-based absorption process may be preferable considering the simple absorbent separation processes and the relatively low pollutant release. Of course, to guarantee compliance with emission standards related to the release of volatile pollutants during the separation processes, it may be even better to combine the SHS-based absorption process with advanced oxidation processes (e.g., photocatalysis, catalytic oxidation, and adsorption).45
In industrial practice, the use of air or nitrogen may not be the preferred method of stripping the CO2 from the carbonated water because of the cost of recovering the CO2 from the resulting gas mixture. Steam stripping or other nondiluting methods of removing CO2 from water would likely be preferred because the recovered gas would not then be diluted by another gas, facilitating the recycling of CO2 and thereby greatly reducing the consumption of gases.
The key advantage to this unusual absorbent is that, in contrast to most liquid absorbents for VOCs, SHS such as CyNMe2 can be used as absorbents and regenerated without a distillation step, which may avoid the key disadvantages related to distillation including high energy consumption and low recovery rates. In terms of the absorption capacities of fresh and recycled CyNMe2, the recovery efficiency of CyNMe2, and the thermodynamic equilibrium parameters, CyNMe2 proved to be a suitable absorbent for the removal of VOCs from gas. The absorption capacities of SHSs are comparable to those of many common absorbents. Many absorption conditions (e.g., initial concentration of toluene, flow rate of N2, and temperature) can impact the absorption capacities of SHSs for VOCs. A low Henry’s law constant was beneficial for the absorption of SHSs for VOCs. The desorption experiments demonstrated that in a mixture containing SHSs, VOCs, and water, VOCs were easily separated from the mixture upon bubbling of CO2. The resulting aqueous stream containing SHSs and water could be separated upon bubbling of N2. The SHS absorbents can be reused even after five absorption–desorption cycles. Thus, SHSs are attractive for the removal of VOCs from industrial exhaust gases.
Although some operating parameters in the industrial absorption–desorption scale, such as volumetric energy input and space velocity, have not been determined in current laboratory conditions, this study provides evidence that the developed reversible absorption process based on SHSs is feasible for removal of VOCs, and the mixture of SHSs and VOCs can be separated simply by altering the polarity of SHSs. In addition, for the high vapor pressure portion of VOCs (e.g., alkane), the reversible absorption system may be modified by screening for more suitable SHSs and/or combining with catalytic/condensed technology. Further optimization of the reversible absorption–desorption process parameters for industrial exhaust gases will be carried out in a future study.
4. Conclusions
In terms of the absorption capacities of fresh and recycled CyNMe2, the recovery efficiency of CyNMe2, and the thermodynamic equilibrium parameters, CyNMe2 proved to be a suitable absorbent for the removal of VOCs from gas. The absorption capacities of SHSs are comparable to those of many common absorbents. Many absorption conditions (e.g., initial concentration of toluene, flow rate of N2, and temperature) can impact the absorption capacities of SHSs for VOCs. A low Henry’s law constant was beneficial for the absorption of VOCs by SHSs. The desorption experiments demonstrated that in a mixture containing SHSs, VOCs, and water, VOCs were easily separated from the mixture upon bubbling of CO2. The resulting aqueous stream containing SHSs and water could be separated upon bubbling of N2. The SHS absorbents can be reused even after five absorption–desorption cycles. Thus, SHSs are attractive for the removal of VOCs from industrial exhaust gases.
Acknowledgments
This study was accomplished with the financial support of the National Natural Science Foundation of China (41761072 and 21777064) and the High-Level Talent Foundation of Kunming University of Science and Technology (1411909411). P.G.J. thanks the Natural Sciences and Engineering Research Council (RGPIN-2017-04528) and the Canada Research Chairs Program.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04443.
Sources of chemicals, theory, evaluation criteria, mass transfer, KL calculations, KG calculations, experiments testing the effect of water, figures showing the apparatus for different experiments, a list of nomenclature and symbols, and graphs and tables presenting additional data (PDF)
The authors declare the following competing financial interest(s): PGJ is an inventor but not owner on patents covering SHS.
Supplementary Material
References
- Goldstein A. H.; Galbally I. E. Known and unexplored organic constituents in the earth’s atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
- Ng N. L.; Kroll J. H.; Chan A. W. H.; Chhabra P. S.; Flagan R. C.; Seinfeld J. H. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 2007, 7, 3909–3922. 10.5194/acp-7-3909-2007. [DOI] [Google Scholar]
- Rene E. R.; Murthy D. V. S.; Swaminathan T. Performance evaluation of a compost biofilter treating toluene vapours. Process Biochem. 2005, 40, 2771–2779. 10.1016/j.procbio.2004.12.010. [DOI] [Google Scholar]
- Wei W.; Wang S.; Hao J.; Cheng S. Projection of anthropogenic volatile organic compounds (VOCs) emissions in China for the period 2010–2020. Atmos. Environ. 2011, 45, 6863–6871. 10.1016/j.atmosenv.2011.01.013. [DOI] [Google Scholar]
- Phillips M.; Herrera J.; Krishnan S.; Zain M.; Greenberg J.; Cataneo R. N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B: Biomed. Sci. Appl. 1999, 729, 75–88. 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- Fourmentin S.; Landy D.; Blach P.; Piat E.; Surpateanu G. Cyclodextrins: a potential absorbent for VOC abatement. Global NEST J. 2006, 8, 324–329. 10.30955/gnj.000415. [DOI] [Google Scholar]
- Khan F. I.; Ghoshal A. K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. 10.1016/s0950-4230(00)00007-3. [DOI] [Google Scholar]
- Liu D. H. F.; Lipták B. G.. Environmental Engineers’ Handbook, 2nd ed.; CRC Press: Boca Raton; 1999; pp 90–134. [Google Scholar]
- Moretti E. C.Practical Solutions for Reducing Volatile Organic Compounds and Hazardous Air Pollutants; Center for Waste Reduction Technologies, American Institute of Chemical Engineers: New York, 2001. [Google Scholar]
- Heymes F.; Manno-Demoustier P.; Charbit F.; Fanlo J. L.; Moulin P. A new efficient absorption liquid to treat exhaust air loaded with toluene. Chem. Eng. J. 2006, 115, 225–231. 10.1016/j.cej.2005.10.011. [DOI] [Google Scholar]
- Pierucci S.; Rosso R. D.; Bombardi D.; Concu A.; Lugli G. An innovative sustainable process for VOCs recovery from spray paint booths. Energy 2005, 30, 1377–1386. 10.1016/j.energy.2004.02.017. [DOI] [Google Scholar]
- Poddar T. K.; Sirkar K. K. Henry’s Law constant for selected volatile organic compounds in high-boiling oils. J. Chem. Eng. Data 1996, 41, 1329–1332. 10.1021/je960064i. [DOI] [Google Scholar]
- Darracq G.; Couvert A.; Couriol C.; Amrane A.; Thomas D.; Dumont E.; Andres Y.; Le Cloirec P. Silicone oil: an effective absorbent for the removal of hydrophobic volatile organic compounds. J. Chem. Technol. Biotechnol. 2010, 85, 309–313. 10.1002/jctb.2331. [DOI] [Google Scholar]
- Xia B.; Majumdar S.; Sirkar K. K. Regenerative oil scrubbing of volatile organic compounds from a gas stream in hollow fiber membrane devices. Ind. Eng. Chem. Res. 1999, 38, 3462–3472. 10.1021/ie980657h. [DOI] [Google Scholar]
- Schmidt A.; Ulrich M. Absorptionsmittel zur Reinigung Iösemittelhaltiger Abluft. Chem. Ing. Tech. 1990, 62, 43–46. 10.1002/cite.330620112. [DOI] [Google Scholar]
- De Assuncao J. V.; Vasconcelos S. M. F.. Control of Toluene and Xylene by Absorption in Mineral Oil; Air and Waste Management Association: Pittsburgh, PA (United States), 1997. [Google Scholar]
- Darracq G.; Couvert A.; Couriol C.; Amrane A.; Le Cloirec P. Kinetics of toluene and sulfur compounds removal by means of an integrated process involving the coupling of absorption and biodegradation. J. Chem. Technol. Biotechnol. 2010, 85, 1156–1161. 10.1002/jctb.2414. [DOI] [Google Scholar]
- Kalina A.; Wanko H.; Weiss S. Untersuchungen zum einfluss des wasserdampfs auf die absorption organischer komponenten mit hochsiedenden waschflüssigkeiten. Chem. Tech. 1994, 46, 1–6. [Google Scholar]
- Augusto F.; Carasek E.; Silva R. G. C.; Rivellino S. R.; Batista A. D.; Martendal E. New sorbents for extraction and microextraction techniques. J. Chromatogr. A 2010, 1217, 2533–2542. 10.1016/j.chroma.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Heymes F.; Manno Demoustier P.; Charbit F.; Louis Fanlo J.; Moulin P. Hydrodynamics and mass transfer in a packed column: case of toluene absorption with a viscous absorbent. Chem. Eng. Sci. 2006, 61, 5094–5106. 10.1016/j.ces.2006.03.037. [DOI] [Google Scholar]
- Jessop P. G.; Phan L.; Carrier A.; Robinson S.; Dürr C. J.; Harjani J. R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809–814. 10.1039/b926885e. [DOI] [Google Scholar]
- Jessop P. G.; Kozycz L.; Rahami Z. G.; Schoenmakers D.; Boyd A. R.; Wechsler D.; Holland A. M. Tertiary amine solvents having switchable hydrophilicity. Green Chem. 2011, 13, 619–623. 10.1039/c0gc00806k. [DOI] [Google Scholar]
- Jessop P. G.; Phan L. N.; Carrier A. J.; Rui R.; Dominik W.. Switchable hydrophilicity solvents and methods of use thereof. U.S. Patent 8,900,444 B2, 2014.
- Vanderveen J. R.; Durelle J.; Jessop P. G. Design and evaluation of switchable-hydrophilicity solvents. Green Chem. 2014, 16, 1187–1197. 10.1039/c3gc42164c. [DOI] [PubMed] [Google Scholar]
- Durelle J.; Vanderveen J. R.; Quan Y.; Chalifoux C. B.; Kostin J. E.; Jessop P. G. Extending the range of switchable-hydrophilicity solvents. Phys. Chem. Chem. Phys. 2015, 17, 5308–5313. 10.1039/c4cp05313c. [DOI] [PubMed] [Google Scholar]
- Gil R. R.; Ruiz B.; Lozano M. S.; Martín M. J.; Fuente E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. 10.1016/j.cej.2014.02.012. [DOI] [Google Scholar]
- Yang Y.; Miller D. J.; Hawthorne S. B. Toluene solubility in water and organic partitioning from gasoline and diesel fuel into water at elevated temperatures and pressures. J. Chem. Eng. Data 1997, 42, 908–913. 10.1021/je960395v. [DOI] [Google Scholar]
- Gaudillat P.; Jurin F.; Lakard B.; Buron C.; Suisse J.-M.; Bouvet M. From the solution processing of hydrophilic molecules to polymer-phthalocyanine hybrid materials for ammonia sensing in high humidity atmospheres. Sensors 2014, 14, 13476–13495. 10.3390/s140813476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar A. D. F.; Richardson T. H.; McNaughton A. J.; Hutchinson J.; Hunter C. A. Investigation of free base, Mg, Sn, and Zn substituted porphyrin LB films as gas sensors for organic analytes. J. Phys. Chem. B 2006, 110, 16646–16651. 10.1021/jp0626059. [DOI] [PubMed] [Google Scholar]
- Lin H.; Jang M.; Suslick K. S. Preoxidation for colorimetric sensor array detection of VOCs. J. Am. Chem. Soc. 2011, 133, 16786–16789. 10.1021/ja207718t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-H.; Kim K.-H. A simple method for the accurate determination of the Henry’s law constant for highly sorptive, semivolatile organic compounds. Anal. Bioanal. Chem. 2016, 408, 775–784. 10.1007/s00216-015-9159-3. [DOI] [PubMed] [Google Scholar]
- Robbins G. A.; Wang S.; Stuart J. D. Using the static headspace method to determine Henry’s law constants. Anal. Chem. 2002, 65, 3113–3118. 10.1021/ac00069a026. [DOI] [Google Scholar]
- Jiang L.; Tian S.; Ning P.; Mo H. Solubilization absorption of toluene vapor by formation of oil-in water microemulsions with cation surfactants. Sep. Sci. Technol. 2010, 45, 508–514. 10.1080/01496390903526378. [DOI] [Google Scholar]
- Wang J.; Lu P.; Wang Z.; Yang C.; Mao Z.-S. Numerical simulation of unsteady mass transfer by the level set method. Chem. Eng. Sci. 2008, 63, 3141–3151. 10.1016/j.ces.2008.03.018. [DOI] [Google Scholar]
- Hess B.; Kutzner C.; van der Spoel D.; Lindahl E. GROMACS 4: Algorithms for highly efficient load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- Oostenbrink C.; Soares T. A.; van der Vegt N. F. A.; van Gunsteren W. F. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. 10.1007/s00249-004-0448-6. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford CT, 2016.
- Lu T.; Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Gossett J. M. Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 1987, 21, 202–208. 10.1021/es00156a012. [DOI] [Google Scholar]
- Tu J. L.; Wu Z. Q.. Absorption Operation in Chemical Industry: Engineering and Technology of Gas Absorption; East China University of Science and Technology Press: Shanghai, 1994; pp 42–75. [Google Scholar]
- Jessop P. G.; Heldebrant D. J.; Li X.; Eckert C. A.; Liotta C. L. Green chemistry: reversible nonpolar-to-polar solvent. Nature 2005, 436, 1102. 10.1038/4361102a. [DOI] [PubMed] [Google Scholar]
- Pellegrini G.; Strube R.; Manfrida G. Comparative study of chemical absorbents in postcombustion CO2 capture. Energy 2010, 35, 851–857. 10.1016/j.energy.2009.08.011. [DOI] [Google Scholar]
- Stephenson R. M. Mutual solubilities: water + cyclic amines, water + alkanolamines, and water + polyamines. J. Chem. Eng. Data 1993, 38, 634–637. 10.1021/je00012a041. [DOI] [Google Scholar]
- Yang C.; Miao G.; Pi Y.; Xia Q.; Wu J.; Li Z.; Xiao J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. 10.1016/j.cej.2019.03.232. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.