Abstract
Given the preventable morbidity and mortality associated with atrial fibrillation (AF), increased awareness of undiagnosed AF, and advances in mobile electrocardiogram (ECG) technology, there is a critical need to assess the effectiveness of using such technology to routinely screen for AF in clinical practice. VITAL-AF is a pragmatic trial that will test whether screening for AF using a single-lead handheld ECG in individuals 65 years or older during primary care visits will lead to an increased rate of AF detection. The study is a cluster-randomized trial, with 8 primary care practices randomized to AF screening and 8 primary care practices randomized to usual care. We anticipate studying approximately 16,000 patients in each arm. During the 1-year enrollment period, practice medical assistants will screen eligible patients who agree to participate during office visits using a single-lead ECG device. Automated screening results are documented in the electronic health record, and patients can discuss screening results with their provider during the scheduled visit. All single-lead ECGs are overread by a cardiologist. Screen-detected AF is managed at the discretion of the patient’s physician. The primary study end point is incident AF during the screening period. Key secondary outcomes include new oral anticoagulation prescriptions, incident ischemic stroke, and major hemorrhage during a 24-month period following the study start. Outcomes are ascertained based on electronic health record documentation and are manually adjudicated. The results of this pragmatic trial may help identify a model for widespread adoption of AF screening as part of routine clinical practice.
Background
Atrial fibrillation (AF) is a common arrhythmia associated with a 2- to 5-fold increased risk of ischemic stroke.1 Oral anticoagulation reduces stroke risk by approximately two-thirds in individuals with AF.2,3 However, AF is frequently undiagnosed,4,5 and many patients with AF do not receive treatment with oral anticoagulation.6–8 Indeed, about 20% of patients with strokes and AF have been reported to be first diagnosed with AF at the time of the stroke,9,10 and between 1% and 5% of patients with AF are estimated to present with stroke as the first manifestation of the arrhythmia.11
Current clinical practice guidelines have offered variable guidance on the appropriateness of screening for AF. European Society of Cardiology guidelines acknowledge the potential utility of screening for AF, recommend opportunistic screening for AF “by pulse taking or ECG rhythm strip in patients >65 years of age” (class I),8 and further state that “systematic ECG [electrocardiogram] screening may be considered to detect AF in patients aged >75 years, or those at high stroke risk” (class IIb).8 The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand recommend opportunistic point-of-care screening in the clinic or community in individuals ≥65 years with pulse palpation followed by an ECG, if irregular, or by an ECG rhythm strip using a handheld ECG.12 The American Heart Association/American College of Cardiology guidelines have not directly addressed screening to date.6,7 By contrast, the United States Preventive Services Task Force concluded recently that “current evidence is insufficient to assess the balance of benefits and harms of screening for atrial fibrillation with ECG.”13
In recent years, technology has evolved to enable efficient screening using handheld single-lead ECG systems with automated algorithms. The AliveCor KardiaMobile ECG (AliveCor, Inc), which communicates with a smartphone or tablet, is one such device and is cleared for use by the Food and Drug Administration. Such handheld technology may have greater sensitivity and specificity for AF than pulse palpation and requires minimal medical training to perform.14,15 The efficiency, portability, and convenience of such technological developments enable the deployment and reuse of single-lead ECG systems for population-based screening of patients in a variety of settings. Office-based encounters are a potential site to augment routine clinical assessments by closely linking screening with the ability to intervene if AF is detected. Given recognition of the public health burden of AF, increased awareness of undiagnosed AF, and advances in mobile ECG technology, there is a critical need to assess the utility of integrating such technology into clinical practice.
In the United States, preventive health measures are typically delivered via primary care practices. Several studies, 4 of which were randomized trials,16–19 have reported the feasibility of screening for AF and were associated with increased detection of AF compared to usual care.16–22 To date, 216,18 randomized trials have tested a strategy of opportunistic pulse palpation embedded into routine clinical care, both of which observed increased rates of AF detection. None have compared the effectiveness of mass screening for AF using handheld ECG technology in a routine clinical setting. Two trials,17,19 including the only randomized controlled trial conducted in the United States to date, included long-term assessments of AF outside of clinical practice with a focus on paroxysmal rather than more persistent forms of AF that are likely to be diagnosed at the time of a routine clinical assessment. VITAL-AF will build upon these studies to evaluate use of mobile ECG technology for population-level screening for undiagnosed AF as part of routine clinical care.
The VITAL-AF trial was designed as a pragmatic trial23,24 to assess the feasibility and effectiveness of embedding AF screening using a single-lead ECG into routine care for individuals 65 years or older attending a primary care practice visit at a single health care system in the United States. The age cutoff enriches for patients with an increased risk of thromboembolism and for whom prophylaxis with oral anticoagulation would be indicated according to clinical practice guidelines if a diagnosis of AF was made.7 Performing screening prior to a clinic visit should enable a patient’s personal physician to rapidly respond to the information and increase adherence with management decisions. VITAL-AF will provide a contrast between contemporary practice of cardiac physical examination with or without pulse palpation versus contemporary practice augmented by mobile cardiac rhythm monitoring technology. This study tests the primary hypothesis that routine screening for AF using a single-lead handheld ECG in individuals 65 years or older at a primary care practice visit will lead to an increased rate of AF detection compared to usual care over a 12-month period.
Trial design
Participants
The trial population consists of patients from 16 of the 22 primary care practices within the Massachusetts General Hospital Practice Based Research Network who are invited and agree to participate. Patients are included in the study population if they are 65 years and older and attend an outpatient clinic appointment at a participating primary care practice with a primary care physician, nurse practitioner, or physician’s assistant (ie, visits where vital signs are routinely assessed and the patient is seen by a provider who can manage a positive screen for AF). The screening threshold of age ≥65 years was chosen to preferentially capture patients at both higher risk of AF and higher risk of stroke if AF is detected. We did not incorporate any additional selection criteria to make screening for AF as simple as possible for the clinic staff. Patients with prevalent AF are not excluded from screening. Patients are excluded if they do not visit their primary care practice during the study period. Based on historical data, approximately 35,000 patients from the 16 participating practices will be eligible for AF screening. The trial enrollment period will last for 12 months, and approximately 32,000 patients are expected to have at least 1 primary care clinic visit during the study period based on historical visit data.
Enrollment procedures
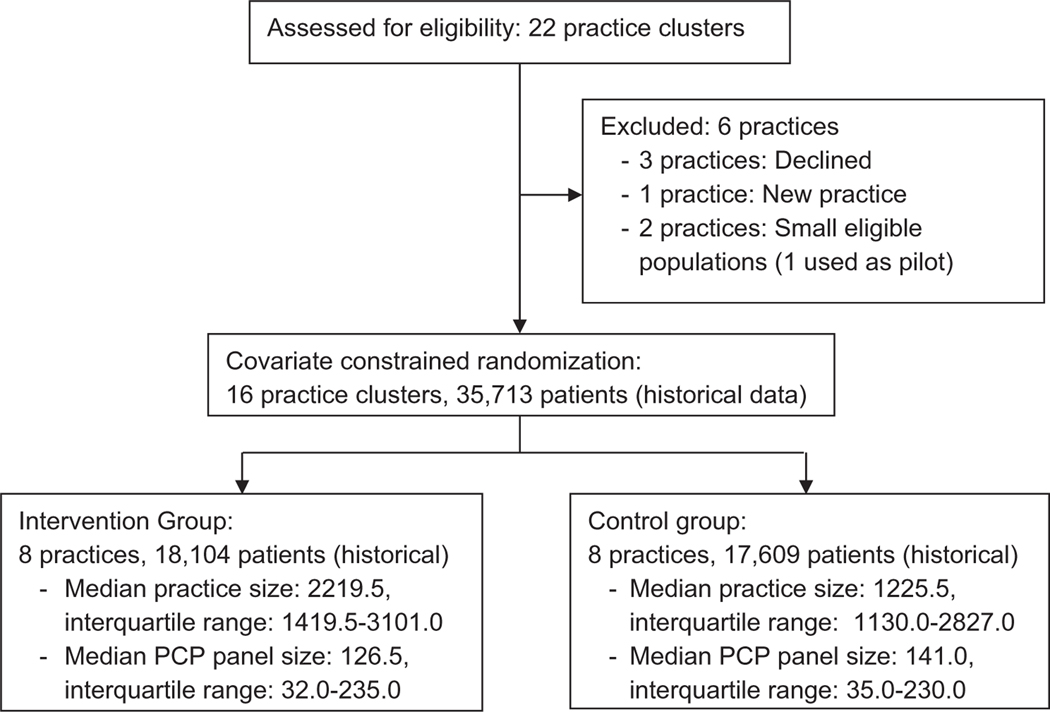
The procedure for enrolling practices included an initial presentation of the study concept to practice leaders and managers, followed by a detailed presentation at scheduled practice meetings to identify practices interested in participating. Among the 22 network practices, 1 was not invited because it was a new practice that was still implementing routine clinical practice operations, and 3 declined. Of the remaining 18 practices, 2 had small eligible populations, and 1 of these was selected as a pilot site; the other practice was not included (Figure 1).
Figure 1.
Practice clusters assessed for eligibility (n = 22) and included in covariate constrained randomization scheme (n = 16).
Practice randomization
The study is a pragmatic cluster-randomized controlled trial (Supplement 1) in which the individual practices are the units of randomization. Randomizing at the level of the practice (1) assesses whether AF screening can be embedded as part of routine care and (2) is less likely to result in contamination of the control group than patientor physician-level randomization. Among 16 participating practice sites, 8 were randomly selected for the AF screening intervention. A total of 12,870 possible allocations of 8 intervention practices from 16 total practices were first reduced to 3,696 to ensure that independent practices with 2 locations within the same geographic location (n = 4) would not be in the same grouping (ie, practices that share an organizational and physical structure but operate independently). Because the patient characteristics of the practices from the previous year were known, a constrained randomization approach25 was used to achieve balance for important characteristics that may influence the primary and secondary study end points (see below). Using historical data, we selected among the 3,696 combinations that provided balance between intervention and control groups in terms of patient age, gender, race, AF prevalence, AF incidence, anticoagulation rate, comorbidities, and sample size. The absolute difference was limited to within 2% for factors with prevalence ≥20% (aged ≥75 years, female, white race, obesity, hypertension, coronary artery disease, and diabetes), within 0.5% for factors with prevalence between 5% and 20% (congestive heart failure, AF prevalence, and anticoagulation within the prior year), within 0.1% for AF incidence within the prior year, and within 1,000 for sample size. Using these criteria, 1 combination that met all criteria was identified. The combination also preserved balance among all covariates both when all practice patients were included as well as when patients with prevalent AF were excluded from the sample (Table I). We flipped a coin to randomly assign 1 of the 2 groups of practices to the screening intervention.
Table I.
Characteristics of patients ≥65 years with completed visits to intervention and control practices in the year prior to study implementation
| Intervention (n = 18,104) | Control (n = 17,609) | |
|---|---|---|
| Age, mean (SD) | 74.6 (7.2) | 74.8 (7.3) |
| Age, % ≥75 y | 40.6 | 41.6 |
| Gender, female | 57.5 | 55.9 |
| Race, white | 83.9 | 83.3 |
| Obese | 31.5 | 33.0 |
| Hypertension | 69.2 | 70.3 |
| Coronary artery disease | 18.5 | 18.8 |
| Diabetes | 17.1 | 18.5 |
| Congestive heart failure | 8.4 | 8.1 |
| AF prevalence | 13.1 | 13.2 |
| AF incidence | 1.34 | 1.45 |
Values presented as percentages unless otherwise specified.
Intervention implementation
In intervention practices, AF screening is performed using an FDA-cleared single-lead ECG device (KardiaMobile ECG, AliveCor Inc, San Francisco, CA) by practice medical assistants as part of the usual primary care checkin procedure when other vital signs are recorded (Figure 2). The sensitivity (71%−98%) and specificity (91%−99%) of the AliveCor automated algorithm for detection of atrial fibrillation and atrial flutter has varied in prior studies.21,26–28 All patients in the practices randomized to the control arm receive their care during outpatient visits at the direction of their primary care provider.
Figure 2.
AliveCor KardiaMobile device paired with an iPad and a customized case used in intervention practices to complete screening for undiagnosed AF.
This study was granted a waiver of documentation of written informed consent by the Partners Human Research Committee given that the rhythm assessment represented no more than minimal risk to subjects, all patients continued to receive standard care under the direction of their primary care provider, and the research could not practicably be conducted without a waiver given the goal of population-based screening. Patients in intervention practices are provided an information sheet describing the study, informing the patient that they can decline to undergo screening without adversely affecting their care, and containing study investigator contact information. Information sheets are sent via US mail 2 weeks in advance of an appointment and are displayed in office waiting rooms. Additional signage summarizing the study protocol is also displayed in practices. Prior to performing the screening, practice medical assistants ask each patient if she/he would like to participate and be screened for AF. Patients requesting more information at the time are provided with information sheets containing study investigator contact information.
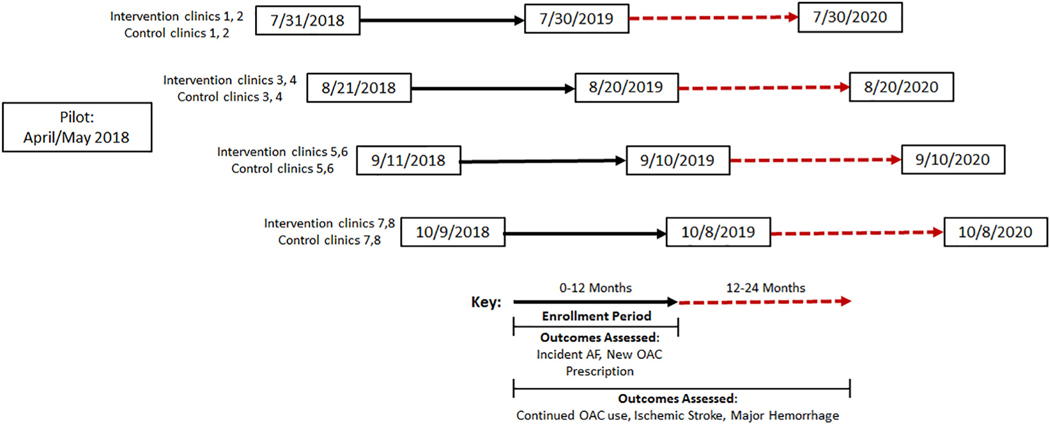
At each of the 8 intervention sites, study staff met with practice leadership and personnel prior to the start of the intervention to discuss workflow and the optimal way to embed screening for AF into the practice. Standard medical assistants within the practice perform the screening intervention as part of routine previsit vital signs assessment. Intervention practices were provided with funds to hire an additional medical assistant to support the additional work required for screening. Study staff conducted training sessions with medical assistants that included an introduction to the study background and goals and instruction in how to describe the test to patients, how to use the AliveCor device, how to document results in the electronic health record (EHR), and how and when to inform primary care providers about screening results. Study personnel were available either on-site or via page for intervention practices as a resource for medical assistants and to confirm appropriate screening procedures. All other practice personnel, including clinicians and support staff, were informed about the study goals, procedures, and support available by study personnel at regular practice meetings. To facilitate training and implementation, a 3-week pilot phase was conducted in a Massachusetts General Hospital primary care practice not included in our randomization scheme (Figure 3). The purpose of this pilot phase was to evaluate screening workflows, identify technical and informatics obstacles, evaluate medical assistant performance, determine the impact of screening on the workflow of the practice, develop an AliveCor tracing review workflow (see “Single-lead ECG Adjudication” section), and identify other unanticipated obstacles.
Figure 3.
Overview of study timing, including pilot, practice enrollment, and outcome assessment.
Screening intervention
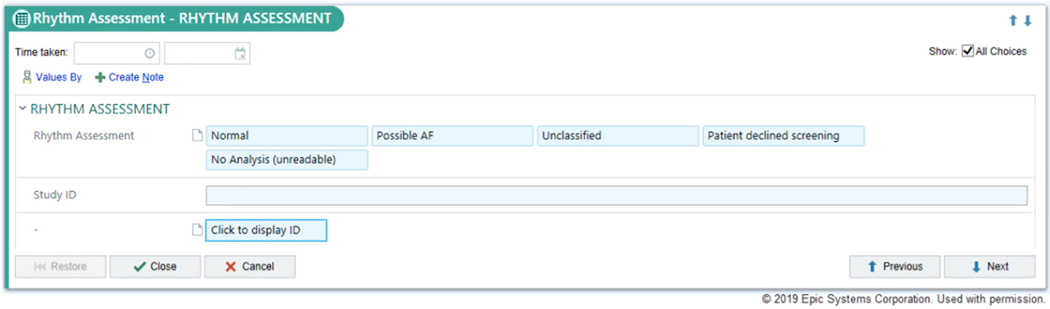
During the enrollment period at each intervention practice, medical assistants will screen eligible, consenting patients for undiagnosed AF during all regularly scheduled office visits using an AliveCor Kardia device, a single-lead ECG device with an FDA-cleared algorithm designed to identify AF. Following screening, medical assistants document the screening result in the EHR (Epic, Verona, WI) using a custom-designed module (Figure 4) along with other vital signs. Results are visible to providers by viewing vital signs in the rooming tab or by viewing the encounter summary where the results are also displayed. If a screening results in a “possible atrial fibrillation” reading, medical assistants are trained to notify the primary care provider directly. Primary care providers are instructed that the AliveCor automated result is considered a screening test and is not considered diagnostic, and that confirmatory testing (eg, a 12-lead ECG) is recommended to establish a new diagnosis of AF. Decisions to order a 12-lead ECG are at the discretion of the primary care provider. All AliveCor tracings are overread by a study cardiologist (see “Single-Lead ECG Adjudication” section). Eligible patients who have more than 1 visit during the 12-month period may be screened at each visit. Practice start dates were staggered over 70 days (4 different start dates each including 2 intervention and 2 control sites matched by number of potentially eligible patients) (Figure 3). Study enrollment began July 31, 2018, in 2 intervention and 2 control sites, and all 16 practices were enrolling patients as of October 9, 2018. As of September 30, 2018, a total of 2,628 patients have completed visits in the intervention arm, with 2,171 (83%) completing screening. Baseline characteristics of the first 2,628 individuals enrolled in intervention sites are provided in Table II. Enrolled patients have a substantial burden of risk factors for AF and stroke based on their age, demographics, and comorbidities.
Figure 4.
Modified rooming tab in Epic EHR used in 8 intervention primary care practices at MGH for medical assistants to record AF screening results.
Table II.
Characteristics of 2,628 patients ≥65 years old with completed primary care visits in intervention sites as of September 30, 2018
| Patients with a visit to an intervention site (n = 2628) | |
|---|---|
| Age, mean (SD) | 75.0 (7.0) |
| Age, % ≥75 y | 43.6 |
| Gender, female | 64.0 |
| Race, white | 85.6 |
| Obese | 32.9 |
| Hypertension | 69.8 |
| Coronary artery disease | 18.0 |
| Diabetes | 19.4 |
| Congestive heart failure | 8.8 |
| AF prevalence | 12.5 |
| CHA2DS2-VASc, median (interquartile range) | 3.0 (2.0–4.0) |
Values presented as percentages unless otherwise specified. The CHA2DS2-VASc score was based on derived EHR features by summing 1 point each for an age between 65 and 74 years, congestive heart failure, hypertension, diabetes, vascular disease, and female sex, and two points each for age of at least 75 years, or a prior stroke, transient ischemic attack, or systemic embolism.29
Single-lead ECG adjudication
All single-lead ECG tracings are transmitted with a study identifier to a Web-based portal (AliveCor KardiaPro). A team of trained cardiologists uses this portal to access and overread the single-lead ECG tracings. If any tracing is read as AF or if any other concerning rhythm disturbance is identified, the primary care provider is notified by a study nurse if medical record review indicates that the provider is not aware of the rhythm finding. The overread validates the automated AliveCor reading and ensures that primary care providers are aware of actionable findings. The initial automated AliveCor result entered into the Rhythm Assessment module of the EHR is not modified. Per institutional review board protocol, cardiologists are required to complete overreads within 7 days of the initial tracing.
Outcomes.
The primary outcome is incident AF during the screening period. The eligible study population comprising the denominator will be assessed in 2 ways: (1) the whole population, defined as all patients aged ≥65 years presenting for a primary care visit with a physician, nurse practitioner, or physician assistant during the enrollment period, and (2) the whole population excluding patients with an AF diagnosis prior to their first visit during the study period (prevalent AF).
Key secondary outcomes include change in AF incidence proportion from the 12-month period prior to the screening period in intervention compared to control practices, incident AF associated with a primary care encounter (because AF can also be diagnosed in multiple other settings), new oral anticoagulation prescriptions, incident ischemic stroke, and incident major hemorrhage according to the International Society on Thrombosis and Haemostasis criteria.30 We will assess whether AF screening leads to increased use of oral anticoagulation by looking at new prescriptions for an oral anticoagulant during the study period among (1) the whole population, (2) the whole population excluding patients with a prescription for an oral anticoagulant in the year prior to their first visit during the study period, (3) patients with incident AF, and (4) patients with prevalent AF. In addition, we will evaluate continued prescription of oral anticoagulation at 12 months among those started on oral anticoagulation during the study period. We will assess whether screening for AF is associated with a reduced rate of ischemic stroke or an increased rate of major hemorrhage within 24 months of the study start. Additionally, we will report the proportion of eligible patients screened and a breakdown of AliveCor automated ECG classifications in the intervention arm.
Outcome ascertainment.
Primary and secondary outcomes will be ascertained in intervention and control arms based on electronic case identification. Electronic health record data will be assessed using the Partners HealthCare Research Patient Data Registry, which is a centralized data warehouse of inpatient and outpatient health data from various different Partners hospital systems.31 AF will be ascertained on the basis of a new AF diagnosis entered in the EHR identified by a sensitive electronic algorithm searching for a billing diagnosis for AF or atrial flutter (International Classification of Diseases, 10 Revision [ICD-10]) (Supplement 2), problem list entry, or reported on a 12-lead ECG. Oral anticoagulation will be assessed using prescription order data. Ischemic stroke and major hemorrhage events will be ascertained on the basis of new inpatient and outpatient ICD-10 codes (Supplement 2).
Outcome adjudication.
Potential new AF, stroke, and hemorrhage events will be manually adjudicated by a clinical end point committee. The clinical end point committee consists of 2 trained research nurse reviewers. Unaffiliated specialty clinicians serve as expert reviewers for specific study end points for cases in which reviewers are uncertain or discrepancies exist after conference between reviewers. Adjudication will occur via a direct search of prespecified elements within the EHR without blinding. Prevalent AF is identified using a validated algorithm which requires 2 billing codes or problem list entries for AF in the prior 3 years.32 Patients identified by the prevalent AF algorithm who also have a prescription for an oral anticoagulant in the prior year will be considered high-probability prevalent AF cases, which will not be reviewed. The positive predictive value of this method of ascertaining high-probability prevalent AF was found to be 98.4% in a manual review of 125 patients. All other cases identified as prevalent AF by the algorithm will be adjudicated to confirm the diagnosis.
Statistical considerations
Primary analyses comparing intervention and control groups will use an intention-to-treat approach including all eligible patients in the analysis regardless of whether patients receive the intended intervention. A secondary analysis will use the complier average causal effect approach33 to address noncompliance issues (eg, patients in the control group receiving screening or patients in the intervention group with no screening). Unadjusted and adjusted logistic regression models that include established AF risk factors will be used to compare the 2 groups and to explore the heterogeneity of treatment effect by age, heart rate, predicted risk of AF (low, intermediate, or high as assessed by the CHARGE-AF score34) and number of visits. Because outcomes obtained from patients cared for by the same clinician are not expected to be entirely independent, generalized estimating equations techniques will be used to take into account the clustering of patient data within providers in all analyses. For the secondary outcome of change in AF incidence proportion from the 12-month period prior to the screening period, we will include a time by group interaction in the models. In addition, we will compare time to incident AF between the 2 groups using a survival analysis approach. Statistical significance will be defined as a 2-tailed P value <.05.
The study was designed to provide sufficient statistical power to address the primary outcome (ie, to detect differences in the proportion with incident AF detected in practices assigned to AF screening vs those assigned to usual care). Based on preliminary data over a 1-year period, the expected sample size is approximately 17,500 per group among all patients 65 years or older and approximately 15,225 per group excluding those with a previous diagnosis of AF, and it was assumed that 92.6% of eligible patients will have an outpatient visit during a 1-year study period, which provides a sample size of 16,212 per group. The intraclass correlation within provider clusters was estimated to be 0.0007 from historical data; therefore, the effective sample size is ~14,569 per group.35 For the intervention group, a simulation study was used to estimate the proportion of patients who will receive screening based on patient visit data from 2016 to 2017. It was assumed that 80% of eligible patients will be invited to participate by medical assistants during each clinic visit, 85% of these patients will consent to screening during the first encounter, and 50% will consent to screening after previous refusal. There will be repeated opportunities for screening for patients who have more than 1 clinic visit during the study period. The simulation results show that 87% of the patients would be screened on at least 1 occasion by the end of a 1-year study period. The AF incidence rate among those who are not screened is estimated to be 1.6% among those without a prior AF diagnosis (and 1.39% for the whole population including those with a prior AF diagnosis). The incidence rate was assumed to be increased to 2.16% (a 35% increase) among those without a prior AF diagnosis if patients were screened. Table III summarizes the power of the study based on several different assumptions of the proportion of patients screened in the intervention group using a 2-sided significance level of .05. The study will have sufficient power to detect a 0.42% difference in AF incidence in the whole population or a 0.48% difference in AF incidence among the whole population excluding prevalent AF.
Table III.
Study power according to different assumptions of the proportion of patients screened in the intervention group using a 2-sided significance level of .05
| Population | Screened rate | 85% | 87% | 89% |
|---|---|---|---|---|
| All aged ≥65 y | Intervention group AF incidence rate | 1.81% | 1.82% | 1.83% |
| Power | 0.80 | 0.81 | 0.83 | |
| Aged ≥65 y without prior AF diagnosis | Intervention group AF incidence rate | 2.08% | 2.09% | 2.10% |
| Power | 0.80 | 0.82 | 0.83 |
Study organization
In accordance with Harvard Clinical and Translational Science Center guidance36 and given the short period of the screening intervention, nontherapeutic nature of the screening intervention, and minimal risk nature of the screen, we do not plan to use a Data Safety Monitoring Board and will not use formal interim analyses or guidelines for early termination of this trial. A data and safety monitoring protocol has been developed for the study by the investigative team to track study-related activities on a regular basis. Data and safety monitoring is performed by the study investigators who review study conduct (eg, accrual, dropouts, protocol deviations, adverse events) on a monthly basis. On a weekly basis, the principal investigator, and study staff involved in notifications to providers for potentially actionable tracings, will review the process and any concerning issues. The Bristol-Myers Squibb/Pfizer Alliance provided funding for this investigator-initiated study. The authors are solely responsible for the design and conduct of this trial, all data analyses, and the final contents of this and future manuscripts. ClinicalTrials.gov Identifier: NCT03515057.
Discussion
Atrial fibrillation is often asymptomatic and may be first diagnosed at the time of a stroke. Efficient and scalable screening methods for AF detection may facilitate the early identification of AF and enable appropriate initiation of oral anticoagulation to prevent strokes.
AF screening studies have been implemented in a variety of settings, including pharmacies,21,37 influenza vaccination encounters,38 primary care16,18 or other screening clinics,20,22 and remotely in individuals’ homes.17,19,39 VITAL-AF will involve screening a large population of patients, with approximately 16,000 eligible patients expected in the intervention arm, at routine primary care office visits. In the United States, primary care clinics are ideally suited for AF screening because they are the delivery setting for most preventive care. Furthermore, primary care clinics that are part of a practice network enable population-level screening where providers can efficiently effect treatment changes (Table IV) based on the results of screening.
Table IV.
Potential settings to screen for undiagnosed atrial fibrillation
| Setting | Identify individuals benefitting from treatment change | Enable provider to effect change | Enact change efficiently and with minimal handoffs | Reach mass population |
|---|---|---|---|---|
| Primary care office | ✓ | ✓ | ✓ | ✓ |
| Emergency department | ✓ | ✓ | ||
| Pharmacy based | ✓ | ✓ | ||
| Influenza vaccination setting | ✓ | ✓ | ||
| Community-based invitation | ✓ | ✓ | ||
| Home (wearable embedded) | ✓ | ✓ |
Advances in mobile ECG technology provide an opportunity to assess whether integrating efficient ECG screening for undiagnosed AF into routine clinical care is feasible and effective. Such technologies may be implemented in several ways, such as at dedicated screening visits or screening outside the context of a clinic visit.17–19,39 In contrast to prior studies, VITAL-AF will assess whether a rhythm assessment using mobile ECG technology added to routine collection of vital signs at outpatient visits is an efficient way to identify undiagnosed AF in patients. Use of a handheld single-lead ECG in population-level primary care practice screening may be preferable to screening with 12-lead ECGs for reasons of cost and efficiency. Handheld single-lead ECGs may be more sensitive and specific than pulse palpation. Standardizing rhythm assessments at the time of vital signs may also ensure that all patients are screened in routine practice, where competing clinical demands may otherwise preclude a thorough clinical pulse palpation by primary care clinicians. Restricting screening to patients aged ≥65 years will select a population at elevated risk of both AF and ischemic stroke.
This trial aims to achieve population-level screening by assessing all eligible patients in multiple primary care practices at every clinician encounter. The approach is implementable at scale by using clinical medical assistants, rather than research personnel, to screen for AF at the time of other routine vital sign assessments. The inclusion of all patients aged ≥65 years, including those with prevalent AF, will allow estimation of the frequency of newly detected AF in a primary care population and simplify medical assistant workflow. We hypothesize that linking screening for undiagnosed AF prior to evaluation by a provider who can initiate timely management will not only increase detection of AF but lead to higher rates of anticoagulation. The ultimate benefit may be reduced rates of AF-related ischemic stroke.
VITAL-AF contrasts with other randomized trials of AF screening in several important ways. As compared to prior trials in ambulatory settings conducted approximately a decade ago,16,18 VITAL-AF is larger and will be the first to embed AF screening into routine ambulatory care in the United States. Moreover, VITAL-AF will use a contemporary handheld single-lead ECG device and will involve integration of results into the EHR. VITAL-AF also enables repeated assessments, which may facilitate AF detection because many patients may have multiple encounters during the screening period. As compared to prior trials of prolonged heart rhythm monitoring,17,19 VITAL-AF will likely identify patients with more persistent AF, as compared to paroxysmal forms of AF, and therefore at presumably higher risk of ischemic stroke.40 Throughout the implementation of VITAL-AF, we have endeavored to minimize the impact of AF screening on the efficiency of clinic workflow. Success with this aspect of the study will greatly add to the acceptance and scalability of AF screening.
Limitations
VITAL-AF has potential limitations. First, the trial is being conducted within a single, urban academically linked health care system in the United States. The incremental value of AF screening will depend on how attentive physicians are to AF in their usual care. We anticipate that our findings will be generalizable to contemporary primary care practices that are hospital and community based as well as health centers in communities with economic and cultural barriers to care. Second, randomization was at the practice level for practical implementation purposes. Despite efforts to ensure balance in potential confounders between study arms, there may be imbalances at the end of the study period. Third, the intervention is implemented in a reallife clinic setting. Some patients may not be screened. However, routine use of AF screening at all patient visits will increase the probability that patients with multiple visits are likely to be screened on at least 1 occasion. Fourth, in the VITAL-AF trial, recording of outcome events is dependent on electronic ascertainment in the EHR. Physician documentation may be incomplete. Furthermore, outcome events occurring outside the trial’s health care system may be missed. In addition, using a 1-time 30-second screen, VITAL-AF will disproportionately pick up cases of more persistent AF and will miss patients with lower AF burden. However, the case for using anticoagulants is stronger for patients with more persistent AF.40 VITAL-AF will not lead to increased AF detection in patients that do not present for a primary care visit. Future trials should examine the effectiveness of remote extended monitoring outside of clinical visits on AF detection and stroke prevention.
The VITAL-AF trial will help determine if routine screening for AF with a single-lead ECG in the primary care setting will increase AF detection compared to usual care and will assess whether increased use of appropriate anticoagulation results. In addition, as an intervention administered by clinical staff within a clinic setting, this study may serve as a model for widespread adoption of AF screening in routine clinical care.
Supplementary Material
Acknowledgements
Partners eCare Research Core contributed to this study by leading EHR integration and creating queries and reports within the electronic health record to identify and track the study population.
Dr McManus receives research support from grants U54HL143541, R01HL126911, R01HL137734, R01HL137794, and R01HL135219 from the National Heart, Lung, and Blood Institute and National Center for Complementary and Integrative Health (National Institutes of Health), and grant NSF-12-512 from the National Science Foundation. D. Ellinor is supported by the National Institutes of Health grants 1RO1HL092577, R01HL128914, and K24HL105780; by the American Heart Association (18SFRN34110082); and by the Fondation Leducq (14CVD01). Dr Lubitz is supported by the National Institutes of Health grant 1R01HL139731 and the American Heart Association 18SFRN34250007.
Disclosures
Dr Ellinor is supported by a grant from Bayer AG to the Broad Institute focused on the genetics and therapeutics of cardiovascular disease. Dr Lubitz receives sponsored research support from Bristol Myers Squibb/Pfizer, Bayer AG, and Boehringer Ingelheim and has consulted for Abbott, Quest Diagnostics, and Bristol Myers Squibb/ Pfizer. Dr McManus has received research support from Bristol Myers Squibb, Care Evolution, Samsung, Apple Computer, Pfizer, Biotronik, Boehringer Ingelheim, and Philips Research Institute; has consulted for Bristol Myers Squibb, Pfizer, Philips, Samsung Electronics, and FlexCon; and has inventor equity in Mobile Sense Technologies, LLC. Drs Ashburner and Atlas receive sponsored research support from Bristol Myers Squib/Pfizer and Boehringer Ingelheim. Dr Singer receives research support from Boehringer Ingelheim and Bristol-Myers Squibb and has served as a consultant to Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Johnson and Johnson, Medtronic, and Pfizer.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2019.06.011.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22(8):983–8. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146(12):857–67. [DOI] [PubMed] [Google Scholar]
- 3.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383(9921):955–62. [DOI] [PubMed] [Google Scholar]
- 4.Healey JS, Alings M, Ha A, et al. Subclinical atrial fibrillation in older patients. Circulation 2017;136(14):1276–83. [DOI] [PubMed] [Google Scholar]
- 5.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366(2): 120–9. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130 (23):2071–104. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol 2019;74(1):104–32. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37(38):2893–962. [DOI] [PubMed] [Google Scholar]
- 9.Borowsky LH, Regan S, Chang Y, et al. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc Dis 2017;43(3–4): 192–9. [DOI] [PubMed] [Google Scholar]
- 10.Lin HJ, Wolf PA, Benjamin EJ, et al. Newly diagnosed atrial fibrillation and acute stroke. The Framingham Study Stroke 1995;26(9):1527–30. [DOI] [PubMed] [Google Scholar]
- 11.Lubitz SA, Yin X, McManus DD, et al. Stroke as the initial manifestation of atrial fibrillation: the Framingham Heart Study. Stroke 2017;48(2):490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group NCAFGW, Brieger D, Amerena J, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018;27 (10):1209–66. [DOI] [PubMed] [Google Scholar]
- 13.Force USPST, Curry SJ, Krist AH, et al. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA 2018;320(5):478–84. [DOI] [PubMed] [Google Scholar]
- 14.Soni A, Earon A, Handorf A, et al. High burden of unrecognized atrial fibrillation in rural India: an innovative community-based cross-sectional screening program. JMIR Public Health Surveill 2016;2(2), e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21(29):1–236. [DOI] [PubMed] [Google Scholar]
- 16.Fitzmaurice DA, Hobbs FD, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335(7616):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136(19):1784–94. [DOI] [PubMed] [Google Scholar]
- 18.Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. The British journal of general practice : the journal of the Royal College of General Practitioners 2002;52(478):373–4. 377–80. [PMC free article] [PubMed] [Google Scholar]
- 19.Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a homebased wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320(2):146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engdahl J, Andersson L, Mirskaya M, et al. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation 2013;127(8):930–7. [DOI] [PubMed] [Google Scholar]
- 21.Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111(6):1167–76. [DOI] [PubMed] [Google Scholar]
- 22.Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131(25):2176–84. [DOI] [PubMed] [Google Scholar]
- 23.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 24.Roland M, Torgerson DJ. What are pragmatic trials? BMJ 1998;316(7127):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivers NM, Halperin IJ, Barnsley J, et al. Allocation techniques for balance at baseline in cluster randomized trials: a methodological review. Trials 2012;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desteghe L, Raymaekers Z, Lutin M, et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2017;19(1):29–39. [DOI] [PubMed] [Google Scholar]
- 28.Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165(1):193–4. [DOI] [PubMed] [Google Scholar]
- 29.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137(2):263–72. [DOI] [PubMed] [Google Scholar]
- 30.Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3 (4):692–4. [DOI] [PubMed] [Google Scholar]
- 31.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc 2006;1044. [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner JM, Singer DE, Lubitz SA, et al. Changes in use of anticoagulation in patients with atrial fibrillation within a primary care network associated with the introduction of direct oral anticoagulants. Am J Cardiol 2017;120(5):786–91. [DOI] [PubMed] [Google Scholar]
- 33.Imbens GW, Rubin DB. Bayesian inference for causal effects in randomized experiments with noncompliance. Ann Stat 1997;25(1):305–27. [Google Scholar]
- 34.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2(2), e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutterford C, Copas A, Eldridge S. Methods for sample size determination in cluster randomized trials. Int J Epidemiol 2015;44(3):1051–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakshminarayan K, Solid CA, Collins AJ, et al. Atrial fibrillation and stroke in the general medicare population: a 10-year perspective (1992 to 2002). Stroke 2006;37(8):1969–74. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu RK, Dolovich L, Deif B, et al. High prevalence of modifiable stroke risk factors identified in a pharmacy-based screening programme. Open Heart 2016;3(2), e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaasenbrood F, Hollander M, Rutten FH, et al. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace 2016;18(10):1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turakhia MP, Desai M, Hedlin H, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the Apple Heart Study. Am Heart J 2019;207:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2(5):474–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.