Highlights
-
•
Whole-brain intensity modulated proton therapy capably spares hippocampal volumes.
-
•
Hippocampal avoidance whole-brain radiotherapy may benefit pediatric populations.
-
•
Intensity modulated proton therapy provides superior target-dose homogeneity relative to modulated x-rays.
Keywords: Intensity modulated proton therapy, Hippocampal sparing, Whole brain radiation therapy, RTOG 0933
Abstract
Background and purpose
Intensity modulated proton therapy (IMPT) allows for modulation parameterized for individual beamlets by position, intensity, and depth. This modulation capability is ideally suited for sparing organs at risk intermediate of the radiation target, such as hippocampal volumes within the whole brain. This work compared IMPT relative to volumetric modulated arc therapy (VMAT) during hippocampal avoidance whole brain radiation therapy (HA WBRT).
Materials and methods
Ten adult and ten pediatric patients previously treated for central nervous system malignancies were identified. IMPT and VMAT treatment plans employing HA WBRT were generated for each patient, delivering 30 GyE (Gray Equivalent) in 10 fractions for adults and 36 GyE in 20 fractions for pediatrics. Dose indices, including dose volume histogram metrics and homogeneity index HI = [D5% − D95%]/[Dmean] × 100, were used to assess plan quality and describe target coverage and normal-tissue sparing.
Results
IMPT offered significant benefits relative to VMAT for hippocampal sparing. Hippocampal mean dose was reduced from 13.7 ± 0.8 Gy with VMAT to 5.4 ± 0.3 GyE using IMPT for pediatrics, and was reduced from 11.7 ± 0.9 Gy with VMAT to 4.4 ± 0.2 GyE using IMPT for adults. IMPT similarly lowered left hippocampal mean dose. Dose to 95% of the clinical target volume was statistically equivalent for both groups; however IMPT reduced the homogeneity index by roughly half.
Conclusion
This manuscript demonstrates that HA IMPT can match or exceed dosimetric benefits offered with modulated X-rays. Inclusion of IMPT in future prospective studies is warranted.
1. Introduction
The role of whole brain radiation therapy (WBRT) in the treatment of brain metastasis has evolved over the past decade. While there has been growing interest in the use of radiosurgery to treat metastasis, there are still indications for whole brain radiation therapy due to multiple metastatic sites, failed prior radiosurgery, leptomeningeal disease, and in patients with poor performance status. Much of the concern over WBRT relates to associated toxicities which include cognitive deficits, fatigue and diminished quality of life [1], [2]. This paper presents the dosimetric advantages of using Intensity Modulated Proton Therapy (IMPT) during WBRT to reduce radiation-induced cognitive deficits, building on the Radiation Therapy Oncology Group (RTOG) study number 0933.
Radiation-induced brain injury can present as both anatomic and functional deficits, clinically expressed in terms of acute, early delayed, and late delayed injury [3]. Neurocognitive impairment remains a prominent side effect of cranial irradiation, impacting attention, executive function, information processing and memory [4], [5], [6]. Several hypotheses exist attempting to explain the pathophysiology of these injuries, including vascular damage, demyelination, deficits in neurogenesis etc.; however many questions remain [7]. Specific to cognitive decline, the hippocampus has emerged in recent years as a region potentially more sensitive to radiation therapy compared to other regions of the brain [8]. Human and animal studies have previously indicated a dependence of cognitive function on neurogenesis; Olsson et al., administered unilateral radiation to juvenile rat brains which demonstrated a radiation-induced reduction to hippocampal volume and neurogenesis [9]. Interference of adult neurogenesis within the hippocampus and stem cell recesses in the periventricular region is believed to be associated with radiation-related cognitive deficits and regulation of various neurocognitive functions: the subventricular zone laterally along the lateral ventricle and the subgranular zone within the dentate gyrus (DG) [1], [10], [11], [12], [13], [14].
Radiotherapeutic dose to the hippocampi can be reduced using intensity modulated radiation therapy. RTOG study number 0933 demonstrated hippocampal avoidance within the radiation treatment plans with the goal of maintaining 100% of each hippocampus to less than 9 Gy and maximal dose below 16 Gy, without significantly compromising treatment efficacy [15], [16]. Utilizing these constraints, they found that patients treated had an improvement in neurologic function with mean relative decline in the Hopkins Verbal Learning Test-Delayed Recall of 7% at 4 months compared to historical controls of 30% over the same time period. Most recently, Tsai and colleagues demonstrated stability neurocognitive outcomes in patients receiving hippocampal sparing during WBRT following volumetric modulated arc therapy (VMAT). At 4 months, functional preservation was significantly observed in Wechsler Memory Scale-III Word List immediate recall with the EQD2 ranging from a maximal dose of 12.6 Gy, to a minimal dose of 5.8 Gy delivered to both hippocampi [17]. Extension of hippocampal avoidance to pediatric populations may yield matching or improved results compared to previous adult studies.
Since this impetus was established, several other studies have investigated the optimal approaches for hippocampal sparing for various diagnoses and treatment modalities [6], [18], [19], [20], [21], [22]. To date, Tomotherapy arguably has presented as the most compelling modality for hippocampal sparing [23] however, the capability of IMPT to spare the hippocampal region during either whole or partial brain irradiation has received only limited reporting [24]. With its capability to modulate proximal and intermediate doses, in addition to distal target conformity, we hypothesized that IMPT is ideally suited to spare organs at risk (OARs), such as hippocampi without compromising target volume coverage.
2. Materials and methods
2.1. Study design
VMAT and IMPT plans were generated for ten adult and ten pediatric patients consecutively identified from patients previously treated at Mayo Clinic Arizona. All patients underwent computed tomography (CT)-based simulation in the supine position. The head and upper neck were immobilized with a thermoplastic mask and contoured headrest. The selected headrest ensured that posterior beams incident upon the cranium were not subjected to sharp changes in radiological path length. For pediatric patients requiring anesthesia, thermoplastic masks were modified to allow for placement of nasal cannula. All patient data sets were anonymized prior to institutional review board study approval.
Target structures as well as OARs were delineated by a staff radiation oncologist, with the exception of hippocampi, which was delineated by staff radiologist in accordance with RTOG/NRG guidelines [25]. Contouring of the hippocampus was performed around the gray matter (T1 hypo-intense) signal in the medial temporal lobe on thin coronal and axial images. Although there are other sequences that allow better differentiation between gray and white matter, the ubiquity of T1 weighted sequences on treatment planning scans makes them desirable.
2.2. Hippocampal anatomy
The hippocampus is a comma shaped structure along the medial border of the temporal lobe consisting of the Cornu Ammonis (Hippocampus Proper) and DG in an interlocking “u” configuration. The lateral border was defined by the medial ependymal surface of the temporal horn. The inferior border was delineated by the adjacent gyrus as it borders the subiculum and CA1. The superior border was defined by the choroidal fissure and fimbria. Posteriorly, it was bounded by the splenium of the corpus callosum adjacent to the quadrigeminal plate cistern. More anteroinferiorly, the medial border was defined by the ambient cistern. The uncal recess of the temporal horn helped differentiate the hippocampus from the amygdala.
The clinical target volume (CTV) consisted of the brain, including meninges. The hippocampi, including a modality-specific planning organ at risk volume (PRV) expansion, were excluded from the CTV. For IMPT planning, pre-optimization spot placement was permitted within a 0.8-cm expansion of the CTV, which allowed for 1 proton beam spot outside the CTV. A dose-limiting annulus surrounding the CTV facilitated shaping the dose gradient outside the target. All adult plans were normalized so that 95% of CTV received a 30 radiobiological Gray Equivalent (GyE) dose, based on a 10-fraction course. Pediatric plans were similarly normalized, except using 36 GyE delivered using a 20-fraction course.
2.3. Field arrangement and beam parameters
VMAT plans consisted of two full arcs oriented coplanar to axial patient plane, and two half arcs with their axes of rotation tilted an equal but opposite 30 degrees along the coronal plane, such that the half arcs are mirrored in the sagittal plane. VMAT plan constraints were matched to RTOG 0933 planning requirements, and benchmarked against Ref. [20]. RTOG 0933 planning constraints were scaled by 120% for pediatric cases to match the change in prescribed dose from 30 to 36 GyE. IMPT plans were generated using multi-field optimization to meld dose distributions of a posterior-anterior field and a craniocaudal field angled 25 degrees anteriorly from patient vertex. IMPT coverage of the CTV was subject to a 3% range and 2 mm setup uncertainty robustness evaluation, requiring a worst-case 95% dose coverage to 95%. The CTV was kept identical across both modalities to validate dosimetric comparison. Each field used an acrylonitrile butadiene styrene range shifter with a water-equivalent thickness (WET) of 4.5 cm. The range shifter (ERS45) was mounted on a frame extended from the proton nozzle. For a 117.1-MeV beam (10.0-cm WET range to distal 80%) this extended position of the ERS45 results in an approximately 20% smaller beam sigma (8.0 mm vs. 10.2 mm) at isocenter relative to a non-extended range shifter. Fluence and dose were determined using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA), with spot spacing based on an energy-dependent lookup table.
2.4. Dosimetric evaluation
Patients were divided into adult and pediatric groupings. Plan quality was evaluated by an homogeneity index derived from the equation HI = [D5% − D95%]/[Dmean] × 100, where D95% is the dose-volume histogram (DVH) curve dose representing 95% of target volume, and D5% is the DVH curve dose representing 5% of target volume. The ideal value for HI is 0, with increasing values for the metric indicative of declining homogeneity throughout the volume. Volumes of total CTV, as well as volumes of brain and cribriform plate portions of the CTV receiving at least 95% of prescribed dose were also recorded. Mean, along with maximum and/or minimum dose for hippocampi, cochlea and lenses were also evaluated.
2.5. Statistical evaluation
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc.). Significance was determined by Wilcoxon matched-pairs signed ranks test. The Wilcoxon matched-pairs signed ranks test is a nonparametric test which tests the equality of matched pairs of observations with the null hypothesis that both distributions are the same.
3. Results
The CTV D95% was statistically equivalent for both modalities for pediatrics and adults. However, VMAT delivery significantly increased the mean and maximum brain dose (see Table 1), resulting in a homogeneity indices that were roughly double when compared to IMPT for pediatrics and adults. Cross sectional anatomic views highlighting the relative homogeneities and proximity of the 50% isodose line to hippocampal structures are shown in Fig. 1 (pediatric) and Fig. 2 (adult).
Table 1.
Target volume coverage and dose heterogeneity.
| Pediatric patients |
Adult patients |
|||
|---|---|---|---|---|
| Mean IMPT ± SD | Mean VMAT ± SD | Mean IMPT ± SD | Mean VMAT ± SD | |
| D5 (Gy or GyE) whole brain | 38.6 ± 0.4 | 42.2 ± 3.1** | 32.0 ± 0.2 | 36.6 ± 2.5** |
| D5 (Gy or GyE) CTV | 38.6 ± 0.4 | 42.2 ± 3.1** | 32.1 ± 0.2 | 36.3 ± 2.4** |
| D5 (Gy or GyE) brainstem | 38.6 ± 0.7 | 41.3 ± 2.6** | 32.0 ± 0.3 | 35.8 ± 2.2** |
| D95 (Gy or GyE) whole brain | 28.2 ± 3.0 | 32.8 ± 1.8* | 23.9 ± 2.5 | 26.4 ± 1.6* |
| D95 (Gy or GyE) CTV | 36.1 ± 0.3 | 36.1 ± 0.8 | 30.0 ± 0.2 | 30.1 ± 1.3 |
| D95 (Gy or GyE) brainstem | 27.7 ± 6.0 | 32.6 ± 2.6 | 24.0 ± 6.5 | 25.6 ± 6.1 |
| HI – whole brain | 28.9 ± 7.6 | 24.0 ± 7.2 | 27.1 ± 8.0 | 30.5 ± 9.7 |
| HI – CTV | 6.8 ± 0.8 | 15.1 ± 6.3** | 6.8 ± 0.4 | 18.3 ± 8.5** |
| Mean (Gy or GyE) CTV | 37.3 ± 0.3 | 39.6 ± 2.0** | 31.0 ± 0.2 | 33.6 ± 1.3** |
| Mean (Gy or GyE) brainstem | 35.8 ± 1.0 | 38.0 ± 1.2** | 29.9 ± 1.0 | 31.9 ± 1.2** |
| V95% cribriform plate | 97.8 ± 2.3 | 94.0 ± 13.2 | 98.1 ± 2.5 | 98.3 ± 3.0 |
Abbreviations: DX% = Dose (GyE or Gy) received by at least X% of volume; CTV = clinical target volume; HI homogeneity index (D5%–D95%)/(mean dose) × 100; V95% = percentage of volume receiving at least 95% of prescribed dose. Data compared using Wilcoxon matched-pairs signed ranks test.
Differences were significant (P < 0.05).
Differences were highly significant (P ≪ 0.01).
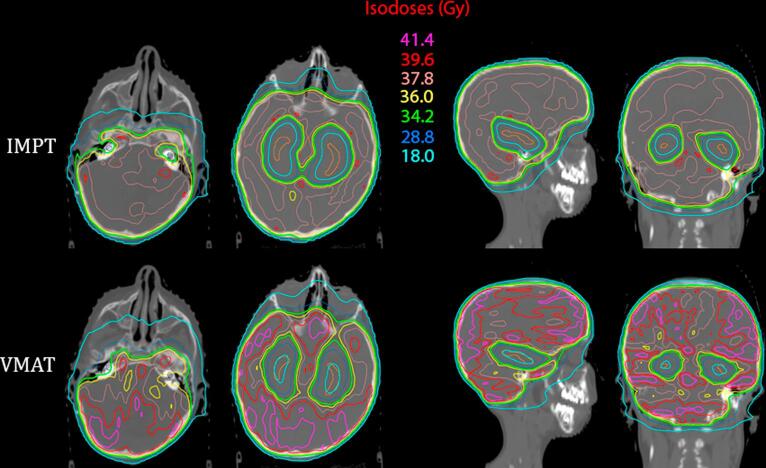
Fig. 1.
Comparison of the dose distributions between an intensity modulated proton therapy (IMPT) plan on the left and a volumetric modulated arc therapy (VMAT) plan on the right for a pediatric patient receiving hippocampal-avoidance whole brain irradiation. The dose delivered is 36 Gy delivered in twenty 1.8 Gy fractions. Axial slice A depicts the cochlea contoured in dark blue, while axial, sagittal, and coronal slices B, C, and D depict the hippocampi contoured in orange. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
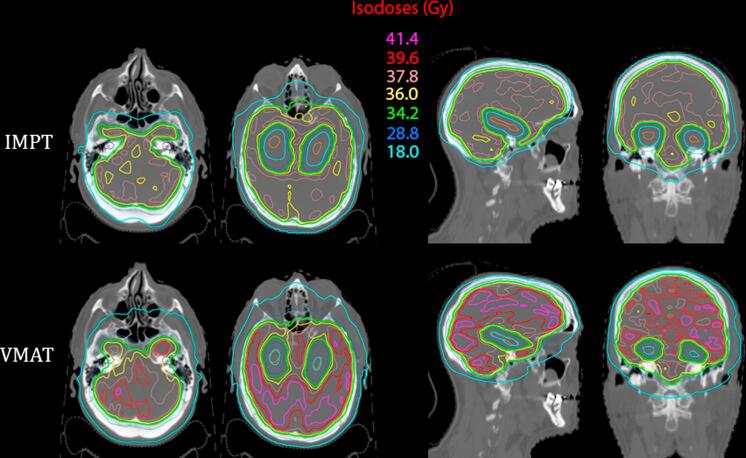
Fig. 2.
Comparison of the dose distributions between an intensity modulated proton therapy (IMPT) plan on the left and a volumetric modulated arc therapy (VMAT) plan on the right for an adult patient receiving hippocampal-avoidance whole brain irradiation. The dose delivered is 30 Gy delivered in ten 3.0 Gy fractions. Axial slice A depicts the cochlea contoured in dark blue, while axial, sagittal, and coronal slices B, C, and D depict the hippocampi contoured in orange. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
IMPT offered significant benefit relative to VMAT for hippocampal, lens, and cochlear sparing. Table 2 highlights these significant improvements. These data are shown for a representative patient in Fig. 3. For Pediatric right hippocampal mean dose was reduced from 13.7 ± 0.8 Gy with VMAT to 5.4 ± 0.3 GyE for IMPT, with the left mean dose similarly reduced. IMPT also lowered adult right hippocampal mean dose from 11.7 ± 0.9 Gy with VMAT to 4.4 ± 0.2 GyE. Minimum and maximum hippocampal dose advantages observed for IMPT were of similar magnitude across both age groups. Lens dose was reduced by roughly 50% with IMPT, while maintaining cribriform plate coverage (see Table 1, Table 2). Dose sparing for the cochlea was statistically significant though less pronounced, dropping about 15–18% with IMPT.
Table 2.
Selected dose metrics (GyE or Gy) for principal organs at risk.
| Pediatric patients |
Adult patients |
|||
|---|---|---|---|---|
| Mean IMPT ± SD | Mean VMAT ± SD | Mean IMPT ± SD | Mean VMAT ± SD | |
| Brain | ||||
| Minimum | 3.8 ± 0.4 | 11.1 ± 0.5** | 3.2 ± 0.3 | 9.0 ± 0.9** |
| Maximum | 40.4 ± 0.7 | 44.1 ± 3.6** | 33.4 ± 0.3 | 38.0 ± 2.8** |
| Mean | 36.3 ± 0.5 | 38.9 ± 19.9** | 30.2 ± 0.2 | 33.0 ± 1.3** |
| Left Cochlea | ||||
| Minimum | 28.5 ± 5.0 | 35.1 ± 2.2** | 22.8 ± 2.1 | 28.6 ± 3.2** |
| Maximum | 32.9 ± 4.5 | 39.3 ± 2.3** | 26.7 ± 1.6 | 32.6 ± 1.5** |
| Mean | 30.6 ± 4.7 | 37.2 ± 1.9** | 24.7 ± 1.8 | 30.7 ± 2.1** |
| Right Cochlea | ||||
| Minimum | 28.3 ± 4.5 | 35.1 ± 2.0** | 22.7 ± 2.4 | 29.7 ± 2.7** |
| Maximum | 33.1 ± 4.5 | 39.7 ± 2.4** | 26.8 ± 1.9 | 33.1 ± 1.8** |
| Mean | 31.0 ± 4.8 | 37.5 ± 2.1** | 24.8 ± 2.0 | 31.4 ± 2.2** |
| Left Lens | ||||
| Maximum | 8.3 ± 3.5 | 14.6 ± 3.9** | 3.3 ± 2.8 | 13.4 ± 3.4** |
| Mean | 6.0 ± 2.9 | 13.0 ± 3.6** | 2.0 ± 1.6 | 11.9 ± 3.0** |
| Right Lens | ||||
| Maximum | 8.2 ± 4.0 | 15.5 ± 5.8** | 3.2 ± 2.6 | 13.6 ± 3.6** |
| Mean | 5.7 ± 3.3 | 13.8 ± 5.2** | 1.9 ± 1.5 | 12.3 ± 3.4** |
| Left Hippocampus | ||||
| Minimum | 4.0 ± 0.4 | 11.2 ± 0.5** | 3.3 ± 0.3 | 9.4 ± 0.4** |
| Maximum | 8.5 ± 0.8 | 18.4 ± 1.3** | 6.8 ± 0.4 | 15.8 ± 0.6** |
| Mean | 5.4 ± 0.2 | 13.7 ± 0.8** | 4.4 ± 0.2 | 11.8 ± 0.8** |
| Volume (cc) | 1.6 ± 0.7 | 1.5 ± 0.7 | 1.8 ± 0.7 | 1.8 ± 0.7 |
| Right Hippocampus | ||||
| Minimum | 3.9 ± 0.4 | 11.2 ± 0.4** | 3.3 ± 0.3 | 9.3 ± 0.5** |
| Maximum | 8.4 ± 0.7 | 18.9 ± 1.5** | 7.2 ± 1.1 | 16.1 ± 0.5** |
| Mean | 5.4 ± 0.3 | 13.7 ± 0.8** | 4.4 ± 0.2 | 11.7 ± 0.9** |
| Volume (cc) | 1.4 ± 0.7 | 1.4 ± 0.7 | 2.1 ± 0.9 | 2.1 ± 0.9 |
Abbreviations: IMPT = intensity modulated proton therapy; VMAT = volumetric modulated arc therapy. GyE = Gray Equivalent; Data compared using Wilcoxon matched-pairs signed ranks test.
Differences were highly significant (P ≪ 0.01).
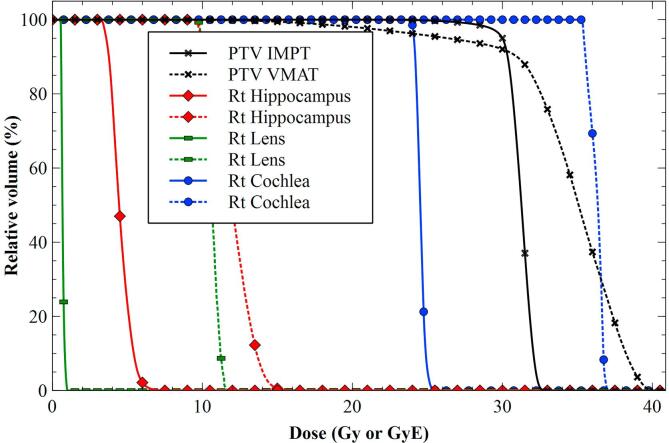
Fig. 3.
Dose Volume Histogram demonstrating difference in target coverage and normal tissue sparing for VMAT and IMPT representative patient. IMPT results are shown as solid lines; VMAT results are shown as dashed lines.
4. Discussion
This manuscript represents the first published work to date highlighting the significant dosimetric impact IMPT can have on HA WBRT. Also of merit is the demonstrated capability of IMPT to maintain target coverage while substantially improving dose homogeneity within the whole brain target, as well as reducing dose to critical structures including cochlea and lenses. Somewhat deceptively, HI values for whole brain appear improved for VMAT; this is a product of the substantially lower hippocampal doses achieved with IMPT. The HI values reported for the CTV, which consists of the whole brain volume absent of the hippocampi, are a better indicator of the improved dose homogeneity achieved using IMPT.
Hippocampal sparing has demonstrated clinical benefit in preliminary studies. Gondi et al. prospectively investigated a potential predictive relationship between neurocognitive impairment and hippocampal dosimetric parameters. Hippocampi receiving less than 7.3 Gy to 40% volume demonstrated statistically significant cognitive benefit; this study demonstrates that IMPT achieves this important threshold with HA WBRT [26]. Currently an ongoing randomized trial is formally assessing the benefits of hippocampal sparing. This trial also builds upon a RTOG prospective randomized placebo controlled trial for patients with brain metastases receiving WBRT that found improved cognitive outcomes with memantine [27]. In a phase III trial, run by NRG, patients will be randomized to receive hippocampal avoidance WBRT with memantine or standard WBRT with memantine and neurocognitive function assessed at set time points. Patients eligible for this trial include patients with metastatic disease to the brain. If HA is shown to improve cognitive function and hence quality of life in survivors it may be expected that this would be adopted as standard of care; IMPT would be a preferred modality in this case, based on capability to deliver homogenous target dose amidst superior HA.
In addition to ongoing work at NRG, the current study assessed HA IMPT in patients treated for primary CNS malignancies. In general, these patients are expected to have superior survival outcomes when compared to patients treated for metastatic disease to the brain. Because cognitive decline typically progresses with time, such patients might be expected to benefit from HA IMPT to an even greater extent. Also, unique to our study is the inclusion of pediatric patients. Pediatric patients are especially sensitive to the radiation induced cognitive deficits, perhaps much more so than adult patients [28]. Here again the cognitive and quality of life benefits of HA IMPT in pediatric patients could be substantial. A recent study recently reported a significant association between the hippocampus volume receiving 20 GyE However, it is important to note that HA either with photon or IMPT would carry the risk of increased rates of disease recurrence within the excluded volumes [28]. Hence such studies would have to be done as part of prospective clinical trials with built in early stopping rules and close attention payed to patterns of failure in order to ensure that disease control was not significantly compromised.
On a technical note, accurate delineation of the hippocampi is necessary in order to achieve intended clinical outcomes. A recent review reported on various deviations identified from the Quality Assurance Results of RTOG 0933 [29]. Of 113 physicians, eight (6.8%) failed hippocampal contouring on 1st attempt; with only three of the eight achieving approval on the 2nd attempt. However, with the availability of a hippocampal atlas and continuing instruction, one would hope for quality to improve with time.
This is the first work to assess the benefits of HA using IMPT in pediatric primary brain tumor patients requiring WBRT. This manuscript demonstrates that HA IMPT can match or exceed dosimetric benefits offered with modulated X-rays. This increased dosimetric benefit to OAR may warrant inclusion of the IMPT modality as part of any upcoming clinical investigations into hippocampal avoidance for pediatric populations.
Conflict of interest
The authors certify that they have no affiliations or involvement in any organization or entity with any financial interest relating to the subject matter herein.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2018.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brown P.D., Ballman K.V., Cerhan J.H., Anderson S.K., Carrero X.W., Whitton A.C. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC. 3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow E., Davis L., Holden L., Tsao M., Danjoux C. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18–23. doi: 10.1016/j.jpainsymman.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Constine L.S., Konski A., Ekholm S., McDonald S., Rubin P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988;15:319–330. doi: 10.1016/s0360-3016(98)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Packer R.J., Sutton L.N., Atkins T.E., Radcliffe J., Bunin G.R., D'Angio G. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–713. doi: 10.3171/jns.1989.70.5.0707. [DOI] [PubMed] [Google Scholar]
- 5.Copeland D.R., Fletcher J.M., Pfefferbaum-Levine B., Jaffe N., Ried H., Maor M. Neuropsychological sequelae of childhood cancer in long-term survivors. Pediatrics. 1985;75:745–753. [PubMed] [Google Scholar]
- 6.Prokic V., Wiedenmann N., Fels F., Schmucker M., Nieder C., Grosu A.L. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. Int J Radiat Oncol Biol Phys. 2013;85:264–270. doi: 10.1016/j.ijrobp.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Chan K.C., Khong P.L., Cheung M.M., Wang S., Cai K.X., Wu E.X. MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging. 2009;29:1013–1020. doi: 10.1002/jmri.21736. [DOI] [PubMed] [Google Scholar]
- 8.Monje M.L., Mizumatsu S., Fike J.R., Palmer T.D. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 9.Olsson E., Eckerstrom C., Berg G., Borga M., Ekholm S., Johannsson G. Hippocampal volumes in patients exposed to low-dose radiation to the basal brain. A case-control study in long-term survivors from cancer in the head and neck region. Radiat Oncol. 2012;7:202. doi: 10.1186/1748-717X-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Bonaguidi M.A., Song J., Ming G.L., Song H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr Opin Neurobiol. 2012;22:754–761. doi: 10.1016/j.conb.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene-Schloesser D., Moore E., Robbins M.E. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z., Li Y.Q., Wong C.S. Effects of aging on hippocampal neurogenesis after irradiation. Int J Radiat Oncol Biol Phys. 2016;94:1181–1189. doi: 10.1016/j.ijrobp.2015.12.364. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Gondi V., Tome W.A., Marsh J., Struck A., Ghia A., Turian J.V. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gondi V., Pugh S.L., Tome W.A., Caine C., Corn B., Kanner A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai P.F., Yang C.C., Chuang C.C., Huang T.Y., Wu Y.M., Pai P.C. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol. 2015;10:253. doi: 10.1186/s13014-015-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padovani L., Chapon F., Andre N., Boucekine M., Geoffray A., Bourdeau F. Hippocampal sparing during craniospinal irradiation: what did we learn about the incidence of perihippocampus metastases? Int J Radiat Oncol Biol Phys. 2018;100:980–986. doi: 10.1016/j.ijrobp.2017.12.265. [DOI] [PubMed] [Google Scholar]
- 19.Zhang I., Antone J., Li J., Saha S., Riegel A.C., Vijeh L. Hippocampal-sparing and target volume coverage in treating 3 to 10 brain metastases: a comparison of Gamma Knife, single-isocenter VMAT, CyberKnife, and TomoTherapy stereotactic radiosurgery. Pract Radiat Oncol. 2017;7:183–189. doi: 10.1016/j.prro.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Gondi V., Tolakanahalli R., Mehta M.P., Tewatia D., Rowley H., Kuo J.S. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu F., Carolan H., Nichol A., Cao F., Nuraney N., Lee R. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1–3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;76:1480–1485. doi: 10.1016/j.ijrobp.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Pinkham M.B., Bertrand K.C., Olson S., Zarate D., Oram J., Pullar A. Hippocampal-sparing radiotherapy: the new standard of care for World Health Organization grade II and III gliomas? J Clin Neurosci. 2014;21:86–90. doi: 10.1016/j.jocn.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez A.N., Westerly D.C., Tome W.A., Jaradat H.A., Mackie T.R., Bentzen S.M. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodin N.P., Munck af Rosenschold P., Blomstrand M., Kiil-Berthlesen A., Hollensen C., Vogelius I.R. Hippocampal sparing radiotherapy for pediatric medulloblastoma: impact of treatment margins and treatment technique. Neuro Oncol. 2014;16:594–602. doi: 10.1093/neuonc/not225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran W.J., Jr., DiSaia P.J., Wolmark N. NRG Oncology research opportunities within the new National Clinical Trials Network. Semin Oncol. 2014;41:553–555. doi: 10.1053/j.seminoncol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gondi V., Hermann B.P., Mehta M.P., Tome W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83:e487–e493. doi: 10.1016/j.ijrobp.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown P.D., Pugh S., Laack N.N., Wefel J.S., Khuntia D., Meyers C. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zureick A.H., Evans C.L., Niemierko A., Grieco J.A., Nichols A.J., Fullerton B.C. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018 doi: 10.1002/cncr.31143. [DOI] [PubMed] [Google Scholar]
- 29.Gondi V., Cui Y., Mehta M.P., Manfredi D., Xiao Y., Galvin J.M. Real-time pretreatment review limits unacceptable deviations on a cooperative group radiation therapy technique trial: quality assurance results of RTOG 0933. Int J Radiat Oncol Biol Phys. 2015;91:564–570. doi: 10.1016/j.ijrobp.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.