Abstract
Background and purpose
Prognosis of locally advanced non-small cell lung cancer remains poor despite chemoradiation. This planning study evaluated a stereotactic boost after concurrent chemoradiotherapy (30 × 2 Gy) to improve local control. The maximum achievable boost directed to radioresistant primary tumor subvolumes based on pre-treatment fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) (pre-treatment-PET) and on early response monitoring 18F-FDG-PET/CT (ERM-PET) was compared.
Materials and methods
For ten patients, a stereotactic boost (VMAT) was planned on ERM-PET (PTVboost;ERM) and on pre-treatment-PET (PTVboost;pre-treatment), using a 70% SUVmax threshold with 7 mm margin to segmentate radioresistant subvolumes. Dose was escalated till organ at risk (OAR) constraints were met, aiming to plan at least 18 Gy in 3 fractions (EQD2 84 Gy/BED 100.8 Gy).
Results
In five patients, PTVboost;ERM was 9–40% smaller relative to PTVboost;pre-treatment. Overlap of PTVboost;ERM with OARs decreased also compared to overlap of PTVboost;pre-treatment with OARs. However, any overlap with OAR remained in 4/5 patients resulting in minimal differences between planned dose before and during treatment. Median dose (EQD2) covering 99% and 95% of PTVboost;ERM were 15 Gy and 18 Gy respectively. Median boost volume receiving a physical dose of ≥ 18 Gy (V18) was 88%. V18 was ≥ 80% for PTVboost in six patients.
Conclusions
A significant stereotactic boost to volumes with high initial or persistent 18F-FDG-uptake could be planned above 60 Gy chemoradiation. Differences between planned dose before and during treatment were minimal. However, as an ERM-PET also monitors changes in tumor position, we recommend to plan the boost on the ERM-PET.
Keywords: Non-small cell lung cancer, Early response monitoring fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET), Stereotactic radiation boost
1. Introduction
Dose escalation up to a total dose of 60 Gy yields a greater proportion of disease control and better survival compared to 40–50 Gy for the treatment of irresectable locally advanced non-small cell lung cancer (NSCLC) [1]. However, about 30% of patients treated with 60 Gy radiotherapy have loco-regional recurrence in absence of distant metastasis [2]. A meta-analysis showed that with combined sequential or concurrent chemoradiotherapy, dose escalation beyond 60 Gy does not lead to further improvements in overall survival [3]. The RTOG 0617 trial demonstrated that 74 Gy in 2 Gy fractions concurrent chemoradiation might even result in a survival decrement compared to 60 Gy in 2 Gy fractions [4]. In case of radiation therapy alone, higher radiation dose results in longer survival without an upper dose level above which there is no further benefit [3]. Therefore, radiation dose intensification combined with chemotherapy should not be discouraged based on the RTOG 0617 results. Especially since in RTOG 0617 compliance with normal tissue dose constraints was not mandatory, older (less conformal) radiotherapy techniques were allowed, and the prolonged overall treatment time could be associated with poorer survival because of accelerated repopulation [4], [5].
Intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) enable more conformal irradiation, thereby lowering dose to organs at risk (OAR) [6]. Currently, it is possible to identify subvolumes within the planning target volume (PTV) that are more radioresistant [7], [8], [9]. Usmanij et al. demonstrated that NSCLC metabolic non-responders, as determined by a poor decrease in total lesion glycolysis (TLG) on fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) at the beginning of third week of radiotherapy, have a worse progression-free survival compared to early metabolic responders [8]. Thus, early response measurement using 18F-FDG-PET/CT enables the identification of patients that may benefit most from dose escalation.
Stereotactic body radiotherapy (SBRT) delivers very conformal high radiation doses resulting in excellent local control rates (>90%) with low toxicity in inoperable stage I-II NSCLC patients [10]. It was shown that a biologically effective dose (BED) prescription of at least 100 Gy is required for acceptable tumor control probability [11]. Therefore, a SBRT radiation boost directed towards 18F-FDG-PET/CT defined radioresistant subvolumes may increase local control in locally advanced NSCLC. Furthermore, by using SBRT, high maximum dose (Dmax) within the PTV is achieved with limited dose to OAR due to steep dose decline just outside the PTV, enabling higher dose escalation compared to other approaches. Limiting prolongation of overall treatment time (OTT) is another advantage of SBRT boosting as the booster dose is delivered in only a few fractions.
The aim of this study was to compare the maximum achievable dose escalation for locally advanced NSCLC treated with concurrent chemoradiation by using a stereotactic boost directed to radioresistant subvolumes of the primary tumor as determined by an 18F-FDG-PET/CT before start of chemoradiation (pre-treatment PET) and an early response monitoring 18F-FDG-PET/CT (ERM-PET).
2. Material and methods
2.1. Patients
18F-FDG-PET/CTs acquired for the ERM study by Usmanij et al. were used for this planning study [8]. This ERM study was approved by the Institutional Review Board of the Radboud university medical center. All patients gave written informed consent. Twenty-eight patients with stage IIIA-B NSCLC eligible for concomitant chemoradiotherapy were enrolled in this study. A prescription dose of 60 Gy in 2 Gy fractions was applied in this planning study for the entire tumor and involved lymph nodes with margin (PTV60Gy). Detailed information upon radiation treatment planning for PTV60Gy is described in Supplementary material 1.
For every patient, a pre-treatment 18F-FDG-PET/CT was acquired with a median interval of 11 days (range 1–27 days) before start of chemoradiation and an ERM 18F-FDG-PET/CT was performed at the beginning of the third week of treatment (after a median dose of 20 Gy; range 20–24 Gy). Timing of the ERM-PET was specifically chosen at the beginning of third week of treatment. A decrease in uptake early during chemoradiation reflects tumor response, whereas this decrease in uptake may disappear later in the course of chemoradiation due to the onset of treatment induced inflammation [8].
We selected those fourteen patients with a poor response to treatment as assessed by a TLG decrease < 45% on the ERM-PET relative to the pre-treatment PET. These poor responders showed worse disease-free survival, possibly due to the fact that they harbour more radioresistant tumors [8]. Three patients were ineligible for this planning study, because they had only a small primary tumor with the bulk of gross tumor volume (GTV) located in the mediastinum. Furthermore, one radiotherapy CT and radiation treatment plan could not be restored. So, ten patients were included in this planning study. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics.
| Patient | Sex | Age | cTNM | Pathology | Location primary tumor |
|---|---|---|---|---|---|
| 1 | Female | 55 | T2N2M0 | AC | RUL; PTV0 and PTV3 not near PRV |
| 2 | Male | 61 | T2N2M0 | SCC | LLL; PTV0 and PTV3 overlap aorta PRV, near spinal cord and bronchial tree |
| 3 | Male | 49 | T2N2M0 | AC | ML; PTV0 and PTV3 overlap heart PRV |
| 4 | Male | 60 | T3N2M0 | SCC | RUL; PTV0 overlaps bronchial tree PRV |
| 5 | Female | 49 | T3N2M0 | AC | LUL; PTV0 and PTV3 overlap heart, aorta and bronchial tree PRV |
| 6 | Female | 52 | T4N3M0 | NSCLC NOS | LH; PTV0 and PTV3 overlap heart, aorta and bronchial tree PRV |
| 7 | Male | 70 | T3N2M0 | AC | RUL; PTV0 and PTV3 overlap heart PRV |
| 8 | Male | 66 | T4N0M0 | SCC | LUL; PTV0 and PTV3 overlap brachial plexus and great vessels PRVs. Near spinal cord and esophagus |
| 9 | Male | 61 | T1N2M0 | AC | ML; PTV0 and PTV3 not near PRV |
| 10 | Female | 49 | T2N2M0 | NSCLC NOS | LUL; PTV0 and PTV3 overlap heart PRV |
Abbreviations: AC: adenocarcinoma; cTNM: clinical tumor node metastasis staging system 7th edition; LLL: left lower lobe; LUL: left upper lobe; LH: left hilum; ML: middle lobe; NOS: not otherwise specified; PTV0: boost planning target volume determined on pre-treatment 18F-FDG-PET/CT; PTV3: boost planning target volume determined on the early response monitoring 18F-FDG-PET/CT; PRV: planning organ at risk volume; RUL: right upper lobe; SCC: squamous cell carcinoma
2.2. 18F-FDG-PET/CT image acquisition
All PET scans were performed with a hybrid PET/CT scanner (Biography Duo Siemens Medical Solutions, USA, Inc.). Patients fasted for at least six hours. A venous blood sample was drawn to measure blood glucose level (<8.2 mmol/L in all patients (mean, 6.0 mmol/L)). Prior to the PET scan, a low dose CT during free-breathing was acquired for PET attenuation correction and anatomical matching. Sixty minutes after intravenous injection of 18F-FDG (3.45 MBq/kg; Covidien) and furosemide (10 mg), static emission scans in three-dimensional mode were obtained with an acquisition time of four minutes per bed position. Images were iteratively reconstructed in 128x128 matrices by ordered subsets expectation maximization (OSEM) algorithm using four iterations/sixteen subsets (4i/16 s) with a 5 mm Gaussian filter. Correction for photon attenuation (by using the low dose CT) and decay of 18F-FDG was performed for images. Rigid co-registration (starting with a bone match and visually checking the plausibility of the match regarding tumor and surrounding normal tissue) of the PET scans to the radiotherapy planning CT was performed.
2.3. Boost volume definition
Radioresistant subvolumes of the primary tumor, to which the boost must be directed, were delineated on the pre-treatment PET and ERM-PET. For automated segmentation of biological target boost volumes (BTVboost), a threshold of 70% of maximum intensity level was used to identify tumor subvolumes at greatest risk of relapse [9]. Adding a 7 mm circumferential margin to BTVboost created PTVboost. Volumes (cm3) of PTVboost based on the pre-treatment PET (PTVboost;pre-treatment) and ERM-PET (PTVboost;ERM) were recorded. To assess the effect of timing of PET scans on the planned dose to PTVboost, a stereotactic boost was planned for all ten patients on both the pre-treatment PET and the ERM-PET.
2.4. Organs at risk definition and constraints
The bronchial tree (up to and including lobar bronchi), heart, great vessels, esophagus, lungs minus GTV60Gy (i.e., lung volume minus the volume of the GTV planned to receive 60 Gy), spinal cord and brachial plexus were considered OAR. Adding a 5 mm margin to the first four OAR contours created the planning OAR volumes (PRV) [12]. For the latter two OAR, PRVs were created adding a 2 mm margin as breathing induced movement is assumed to be smaller/absent for this nerve tissue. No PRV margin was used for the lungs. The following constraints were applied: Lungs minus GTV60Gy: mean lung dose < 20 Gy, V20 < 35% (Vx is the relative volume receiving x Gy); V5 < 65–70% for lungs minus GTV60Gy and V5 < 55% for contralateral lung (‘soft’ constraint) [13], [14], [15]. PRV esophagus: Dmax 70 Gy equivalent dose in 2 Gy fractions (EQD2) (α/β-value 3 Gy) [16] PRV brachial plexus: Dmax 66 Gy EQD2 (α/β-value 2 Gy) [12]. PRV heart, great vessels, bronchial tree: Dmax 94 Gy EQD2 (α/β-value 3 Gy) [12]. PRV spinal cord: Dmax 53 Gy EQD2 (α/β-value 2 Gy) [12].
2.5. Boost planning
Doses to OARs were determined for the 60 Gy treatment plan. Except for the Vx doses and mean dose to the lungs, these doses can be converted into EQD2 doses using the formula EQD2 = total dose*((fraction dose+ α/β)/(2+α/β)). Thereafter, the extra allowed EQD2 dose to OAR was calculated (i.e., maximum allowed EQD2 minus maximum EQD2 delivered after 60 Gy). Subsequently, this extra allowed EQD2 was converted into a physical dose (planned to be delivered in three fractions). This physical dose was calculated for every separate OAR and used as maximum allowed dose for boost treatment planning. This strategy was performed for both the whole OAR and the part of the OAR in close proximity to the boost volume, to take into account the spatial component of the maximum dose of the 60 Gy treatment plan. So, also the maximum dose of the OAR subvolume near PTVboost was determined in the conventionally fractionated 60 Gy radiotherapy plan. This dose was used to calculate the maximum tolerable dose for that subvolume of the OAR bordering the PTVboost. Higher dose escalation of the boost volume could be achieved with this strategy. Boosts were planned using the Pinnacle3 (Version 8.0–9.2; Philips Radiation Oncology Systems, Fitchburg, WI) treatment planning system.
A BED prescription dose of at least 100 Gy is required for acceptable tumor control probability [11]. A dose of 60 Gy in 2 Gy fractions is 60 Gy EQD2 and is equal with a BED of 72 Gy (α/β-value = 10 Gy for tumor). Delivering 18 Gy in three fractions results in a boost of 24 Gy EQD2 (total EQD2 84 Gy) and a BED of 28.8 Gy (total BED 100.8 Gy). Therefore, it was attempted to plan a boost with a minimum dose of 18 Gy in three fractions. The final planned dose to PTVboost depended on the maximum tolerable dose for the OAR. The majority of tumors in this study were located near critical organs at risk, as is often the case in irresectable stage III NSCLC, resulting in a small therapeutic window for planning a stereotactic radiation boost. Radiation dose was escalated till OAR constraints were met. In case a higher dose than 18 Gy could be planned this was done. However, in case 18 Gy could not be planned due to critical OAR, a lower dose had to be accepted.
All boost plans were generated using a single VMAT arc avoiding the contralateral lung. To ensure a rapid dose decline outside the PTV, a ring contour (1 cm) around the PTV was created. In case of overlap of PTVboost with PRV, two separate PTVs were created: PTV inside PRV and PTV outside PRV. This enabled better dose coverage for the PTV outside the PRV, thereby limiting underdosage of the PTV. The optimization objectives for the PTVboost and the ring contours were individually set according to calculated constraints for OAR. No hard constraints were set for the maximum allowed dose within PTVboost, because the maximum doses reached in this setting will never approach the maximum allowed (EQD2) dose that is clinically accepted in SBRT for limited stage lung cancer. We allowed a maximum dose as high as needed to enable a steep dose decline outside PTVboost, without exceeding the maximum total dose (EQD2) accepted in SBRT for limited stage lung cancer.
3. Results
3.1. PTVboost volumes
In only two of ten patients, PTVboost volumes did not overlap with any of the PRVs (Table 1). In five of ten patients, PTVboost;ERM was 9–40% smaller relative to PTVboost;pre-treatment. However, for the other five patients, PTVboost;ERM remained stable or increased compared to PTVboost;pre-treatment (range 0–50%) (Table 2).
Table 2.
Boost planning target volume determined on pre-treatment and early response monitoring 18F-FDG-PET/CT.
| Patient | PTV0 (cm3) | PTV3 (cm3) | Absolute difference (cm3) | Relative difference (%) |
|---|---|---|---|---|
| 1 | 19 | 19 | 0 | 0 |
| 2 | 214 | 183 | −31 | −15 |
| 3 | 14 | 21 | 7 | 50 |
| 4 | 164 | 98 | −66 | −40 |
| 5 | 94 | 56 | −38 | −40 |
| 6 | 127 | 133 | 6 | 5 |
| 7 | 43 | 34 | −9 | −21 |
| 8 | 208 | 189 | −19 | −9 |
| 9 | 14 | 15 | 1 | 7 |
| 10 | 15 | 16 | 1 | 7 |
Abbreviations: PTV0: boost planning target volume determined on pre-treatment 18F-FDG-PET/CT; PTV3: boost planning target volume determined on the early response monitoring 18F-FDG-PET/CT; Absolute difference: PTV3 volume minus PTV0 volume; Relative difference: (absolute difference/PTV0)*100.
Furthermore, it was examined whether the changes in PTVboost resulted in less overlap with PRVs (Supplementary material 2). Overlap did decrease in five patients with 6–100%. Unfortunately, overlap with PRVs disappeared in only one of these five patients (Fig. 1, Supplementary material 2). Overlap with PRVs increased for two patients (Supplementary material 2). Dose limiting OAR for boost planning, allowed dose to OAR and planned dose to OAR are shown in Table 3 and Supplementary material 3.
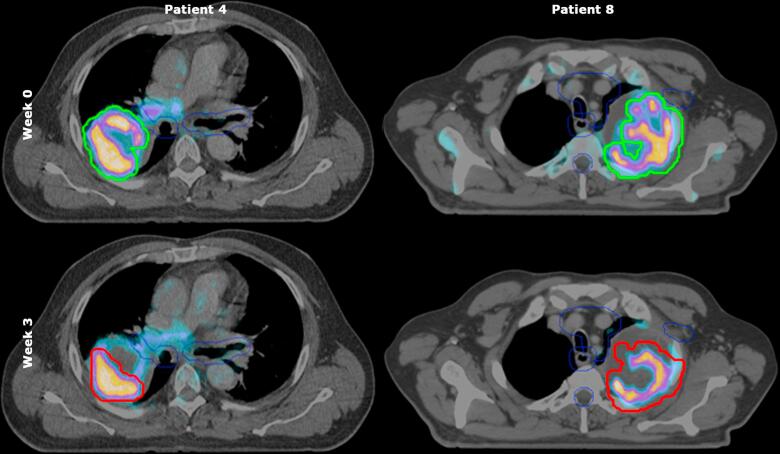
Fig. 1.
Boost PTVs of two patients delineated on 18F-FDG-PET/CT scans before start of treatment and at the beginning of week 3 during treatment. 18F-FDG-PET/CT scans of patient number 4 and 8. The green line represents PTVboost before start of treatment (upper panel), the red line represents PTVboost at the beginning of third week of treatment (lower panel). For patient number 4 (left), there was a remarkable decrease in PTVboost volume in contrast to PTVboost volume of patient number 8 (right) whose PTVboost volume was similar for both time points. The blue line indicates the planning organs at risk volumes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Dose limiting organs at risk for boost planning.
| Patient | Dose limiting OAR |
|---|---|
| 1 | Esophagus |
| 2 | Esophagus |
| 3 | Heart |
| 4 | Lungs |
| 5 | Heart, bronchial tree and esophagus |
| 6 | Esophagus |
| 7 | Heart |
| 8 | Heart, esophagus, brachial plexus and spinal cord |
| 9 | Lungs |
| 10 | Heart |
3.2. PTVboost and BTVboost dose
Dose delivered to 99% of PTVboost (D99; EQD2), dose delivered to 95% of PTVboost (D95; EQD2), Dmax (EQD2), and percentage of PTVboost receiving ≥ 18 Gy (V18; physical dose) were assessed for PTVboost;pre-treatment and PTVboost;ERM. Median D99 and D95 of PTVboost;pre-treatment were 17 Gy (range 4–31) and 19 Gy (range 7–42), respectively. Median V18 of PTVboost;pre-treatment was 93% (range 56–100). Median D99 and D95 of PTVboost;ERM were 15 Gy (range 3–30) and 18 Gy (range 6–32), respectively. Median V18 of PTVboost;ERM was 88% (range 51–100).
Differences between the planned dose in week 0 and 3 were minimal due to the fact that overlap of PTVboost;ERM with PRVs remained in most patients. V18 in week 3 was higher in five patients compared to V18 in week 0. However, planning results were somewhat worse for four patients in week 3 relative to week 0. For patient number 3, differences in coverage of PTVboost;pre-treatment and PTVboost;ERM were very large due to changes in atelectasis and thereby a shift in tumor position towards the heart in week 3, resulting in overlap of PTVboost;ERM with heart PRV limiting dose escalation (Table 4; Fig. 2; Supplementary material 2).
Table 4.
Dose planned to PTVboost.
| Patient | PTVboost |
|||||||
|---|---|---|---|---|---|---|---|---|
| D99 (EQD2) |
D95 (EQD2) |
Dmax (EQD2) |
V18 |
|||||
| week 0 | week 3 | week 0 | week 3 | week 0 | week 3 | week 0 | week 3 | |
| 1 | 24 | 25 | 26 | 28 | 92 | 85 | 99 | 100 |
| 2 | 18 | 19 | 22 | 21 | 41 | 41 | 93 | 91 |
| 3 | 31 | 13 | 42 | 18 | 87 | 80 | 100 | 91 |
| 4 | 23 | 30 | 28 | 32 | 51 | 48 | 99 | 100 |
| 5 | 5 | 11 | 8 | 13 | 68 | 55 | 63 | 60 |
| 6 | 5 | 7 | 7 | 9 | 55 | 41 | 56 | 51 |
| 7 | 17 | 18 | 17 | 19 | 48 | 59 | 49 | 61 |
| 8 | 4 | 3 | 7 | 6 | 47 | 51 | 72 | 73 |
| 9 | 30 | 28 | 32 | 31 | 75 | 77 | 100 | 100 |
| 10 | 15 | 12 | 17 | 16 | 66 | 66 | 82 | 84 |
Dose in EQD2, except for V18. Abbreviation: PTVboost: boost planning target volume; D99: dose planned to 99% of PTVboost (EQD2); D95: dose planned to 95% of PTVboost (EQD2); Dmax: maximum dose (EQD2); V18: percentage of planning target volume receiving ≥18 Gy (physical dose); week 0: boost plan based on pre-treatment 18F-FDG-PET/CT; week 3: boost plan based on early response monitoring 18F-FDG-PET/CT.
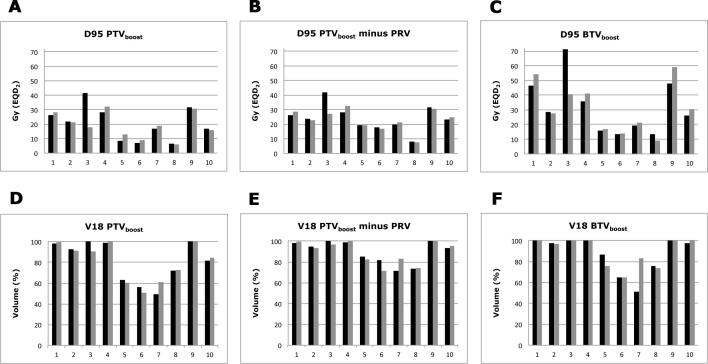
Fig. 2.
SBRT dose planned to PTVboost;pre-treatment and PTVboost;ERM. (A) Dose (EQD2) planned to 95% of PTVboost (D95) for individual patients. (B) Dose (EQD2) planned to 95% of PTVboost (D95) minus overlap with planning organ at risk volume for individual patients. (C) Dose (EQD2) planned to 95% of BTVboost (D95) for individual patients. (D) Percentage of PTVboost volume planned to receive ≥18 Gy (V18; physical dose) for individual patients. (E). Percentage of PTVboost volume minus overlap with planning organ at risk volume planned to receive ≥18 Gy (V18; physical dose) for individual patients. (F) Percentage of BTVboost volume planned to receive ≥18 Gy (V18; physical dose) for individual patients. Grey bars indicate dose planned to PTVboost based on early response monitoring 18F-FDG-PET/CT (PTVboost;ERM). Black bars indicate dose planned to PTVboost based on pre-treatment 18F-FDG-PET/CT (PTVboost;pre-treatment). X-axis represents individual patients. SBRT: stereotactic body radiotherapy.
In seven patients, D95 PTVboost was substantially higher than the prescribed dose (74 Gy) of the RTOG 0617 study. The total D95 PTVboost;pre-treatment was ≥ 80 Gy in five patients and for four patients a total D95 PTVboost;ERM ≥ 80 Gy could be planned (Fig. 2A; Table 4). The summed D95 of PTVboost minus overlap with PRV was ≥ 80 Gy in seven patients and for another patient this summed D95 was 79 Gy (Fig. 2B). V18, which equals an ablative dose, was ≥ 80% for the whole PTVboost in six patients (Fig. 2D) and ≥ 80% in eight patients for PTVboost minus overlap with PRV (Fig. 2E).
In clinical practice, clinical target volume (CTV) coverage is also evaluated in case of overlap with OAR. D95 BTVboost (considering BTVboost as CTV) was considerably larger than D95 PTVboost (Fig. 2C): for seven patients a summed BTV dose of ≥ 80 Gy could be planned. BTVboost;ERM V18 was (almost) 100% in six patients (Fig. 2F). In the other patients, BTV-PRV overlap hampered planning of an ablative dose for the complete BTVboost.
4. Discussion
This study compared the maximum achievable dose escalation for locally advanced NSCLC treated with concurrent chemoradiation by using a stereotactic boost directed to radioresistant subvolumes of the primary tumor as determined by a pre-treatment PET and an ERM-PET. In five of ten patients, PTVboost;ERM was 9–40% smaller relative to PTVboost;pre-treatment. However, differences between the planned dose before and during treatment were minimal due to the fact that overlap of PTVboost;ERM with PRVs remained in most patients. V18, which equals an ablative dose, was ≥ 80% for PTVboost in six patients.
Some studies have investigated the feasibility of dose escalation in locally advanced NSCLC, all with different treatment strategies [12], [17], [18]. For example, a prospective single institution trial examined stereotactic boosting (two fractions of 10 Gy, or three fractions of 6.5 Gy) of residual primary tumor < 5 cm 1–2 months after chemoradiation (60 Gy). Mean coverage of SBRT boost was 96.4%, but was not described for patients individually [17]. Disadvantage of this strategy is prolonging of OTT, which is biologically less effective. Furthermore, only small tumors were eligible. Delivering three additional SBRT boost fractions immediately after 60 Gy results in a minimal prolongation of the OTT and the boost dose is not delivered simultaneously with chemotherapy.
Another study, the RTOG 1106 study, is an ongoing phase 2 randomised trial comparing 60 Gy in thirty fractions (IMRT) versus adaptive radiotherapy to residual tumor based on during-treatment 18F-FDG-PET (fraction 18–19) to deliver a boost in the final nine fractions (2.2–3.8 Gy/fraction) to a maximum total physical dose of 80.4 Gy in thirty fractions. Primary goal of this study is to determine whether dose can be escalated to improve locoregional control. Contrary to our study, a simultaneously integrated boost (SIB) is planned. The SBRT boost may be advantageous over this SIB procedure due to its dose inhomogeneity with high Dmax resulting in a higher biologically effective tumor dose.
Van Elmpt et al reported on the PET-boost randomized phase II trial that randomized patients between dose-escalation (IMRT-SIB) of the entire primary tumor or dose-escalation of the high FDG-uptake region (>50% SUVmax) inside the primary tumor [12]. Mean boost dose was 79.2 Gy for the entire tumor and 86.9 Gy for the high FDG-uptake area (p = 0.001). However, in case of overlap of PTV with an OAR, PTV was allowed to have a reduced coverage for 15% of the volume. D95-99 for boost volumes were not described.
In general, the feasibility of dose escalation fully depends on the accepted dose to OAR and related toxicity. For example, it is suggested that the negative result of the 0617 trial is due to cardiac toxicity as compliance with normal tissue dose constraints was encouraged but not necessary. The effect of heart dose on overall survival is complex. It is advised to keep heart V50 < 25% [19]. This constraint was met in our study (Supplementary material 4). Hepel and colleagues tried to deliver a SBRT boost in two fractions with a total boost dose of 16–28 Gy on primary and nodal disease after 50.4 Gy concurrent chemoradiation (phase I dose escalation trial) [18]. There was no dose constraint for the proximal bronchial-vascular tree. One of twelve patients (8.3%) died due to fatal bronchopulmonary hemorrhage. Dose delivered to 4 cm3 of bronchial-vascular tree was substantially higher in this patient: 20.3 Gy (EQD2 53.4 Gy, α/β-value 3 Gy) for SBRT boost and total dose of 73.5 Gy (EQD2 105.5 Gy, α/β-value 3 Gy). So, a mediastinal SBRT boost may increase the risk of fatal toxicity substantially and therefore a dose constraint to the bronchial-vascular tree is mandatory. Our maximum dose of 94 Gy (EQD2) delivered to the bronchial-vascular tree PRV is considered safe [12]. Severe late esophageal toxicity (stenosis and fistula) is observed in 6% of patients receiving a maximum dose of ≥ 70 Gy [16]. Based on these results we set a maximum of 70 Gy to the esophagus with 0.5 cm margin. However, the RTOG 1106 protocol allows a maximum dose of 74–76 Gy.
Besides the above mentioned issue regarding treatment-related toxicity, some technical aspects such as boost volume definition and tumor motion management need further discussion. It is not known which segmentation method is optimal for boost volume segmentation. Aerts et al. conclude that residual metabolic-active areas after (chemo)radiation have a high overlap with pre-treatment volume defined by 50% SUVmax [7]. However, defining the boost volume using a threshold of 50% SUVmax may result in too large boost volumes because this threshold is in general regarded as a segmentation method to quantify 18F-FDG-avid areas of the entire tumor [20]. Therefore, it is likely that residual metabolic active disease remains within this 50% SUVmax volume. Calais et al. propose a 70% SUVmax threshold on pre-treatment 18F-FDG-PET/CT scans to define treatment resistant tumor subvolumes [9]. This smaller volume will facilitate radiotherapy dose escalation. Therefore we decided to use this segmentation method notwithstanding that a threshold of 80–90% could be sufficient as well resulting in even smaller boost volumes.
For adequate radiotherapy delivery, determination of tumor movement is important. The ERM study by Usmanij et al., however, was performed when four-dimensional planning CT was not standard of care yet. Therefore, for this planning study, three-dimensional planning CTs were used. It was therefore not possible to assess individual PTV margins for BTVboost such as with the midventilation approach in stereotactic radiotherapy [21]. We decided to use a 7 mm PTV margin for BTVboost, as this is a common PTV margin for the midventilation concept in our experience. However, in case of implementation of this stereotactic boost planning study into clinical practice, a four-dimensional CT should be performed for all patients to assess individual PTV margins for the BTVboost [21].
In conclusion, a stereotactic boost to primary tumor subvolumes with initial high or persistent 18F-FDG uptake (poor-responding areas) could be planned in combination with 60 Gy concurrent chemoradiation. V18, which equals an ablative dose, was ≥ 80% for PTVboost in six/ten patients. Therefore, a stereotactic boost to regions with high 18F-FDG-uptake is an attractive treatment strategy to optimize NSCLC therapy. Differences between the planned dose before and during treatment were minimal due to the fact that overlap of PTVboost;ERM with PRVs remained in most patients. However, as an ERM-PET also monitors changes in tumor position, planning the boost on the ERM-PET should be considered.
5. Compliance with ethical standards
5.1. Disclosure of potential conflicts of interest
All authors declare no conflict of interest. This study was not funded by external sources.
5.2. Research involving human participants
This ERM study was approved by the Institutional Review Board of the Radboud university medical center. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
5.3. Informed consent
Written informed consent was obtained from all individual participants included in the study.
6. Author’s contributions
TM designed the study, did the radiation treatment planning, collected the data, interpreted the results, and wrote the manuscript.
RW: designed the study, did the radiation treatment planning, collected the data, interpreted the results, and wrote the manuscript.
EU: designed the study, and interpreted the results.
OS: designed the study, and interpreted the results.
PK: supervised the radiation treatment planning.
LB: supervised the radiation treatment planning.
JB: designed the study, interpreted the results, and helped to write the manuscript.
LG: designed the study, interpreted the results, and helped to write the manuscript.
All authors read and approved the final manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.08.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Perez C.A., Bauer M., Edelstein S., Gillespie B.W., Birch R. Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys. 1986;12(4):539–547. doi: 10.1016/0360-3016(86)90061-1. [DOI] [PubMed] [Google Scholar]
- 2.Kalman N.S., Weiss E., Walker P.R., Rosenman J.G. Local radiotherapy intensification for locally advanced non-small-cell lung cancer – a call to arms. Clin Lung Cancer. 2018;19:17–26. doi: 10.1016/j.cllc.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ramroth J., Cutter D.J., Darby S.C., Higgins G.S., McGale P., Partridge M. dose and fractionation in radiation therapy of curative intent for non-small cell lung cancer: meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2016;96(4):736–747. doi: 10.1016/j.ijrobp.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belderbos J., Walraven I., van Diessen J., Verheij M., de Ruysscher D. Radiotherapy dose and fractionation for stage III NSCLC. Lancet Oncol. 2015;16(4):e156–e157. doi: 10.1016/S1470-2045(15)70121-X. [DOI] [PubMed] [Google Scholar]
- 6.Grills I.S., Yan D., Martinez A.A., Vicini F.A., Wong J.W., Kestin L.L. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57(3):875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 7.Aerts H.J., Bussink J., Oyen W.J., van Elmpt W., Folgering A.M., Emans D. Identification of residual metabolic-active areas within NSCLC tumours using a pre-radiotherapy FDG-PET-CT scan: a prospective validation. Lung Cancer. 2012;75(1):73–76. doi: 10.1016/j.lungcan.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Usmanij E.A., de Geus-Oei L.F., Troost E.G., Peters-Bax L., van der Heijden E.H., Kaanders J.H. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J Nucl Med. 2013;54(9):1528–1534. doi: 10.2967/jnumed.112.116921. [DOI] [PubMed] [Google Scholar]
- 9.Calais J., Thureau S., Dubray B., Modzelewski R., Thiberville L., Gardin I. Areas of high 18F-FDG uptake on preradiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for non-small cell lung cancer. J Nucl Med. 2015;56(2):196–203. doi: 10.2967/jnumed.114.144253. [DOI] [PubMed] [Google Scholar]
- 10.Grills I.S., Mangona V.S., Welsh R., Chmielewski G., McInerney E., Martin S. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 11.Brown J.M., Brenner D.J., Carlson D.J. Dose escalation, not “New Biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85(5):1159–1160. doi: 10.1016/j.ijrobp.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Elmpt W., De Ruysscher D., van der Salm A., Lakeman A., van der Stoep J., Emans D. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol. 2012;104(1):67–71. doi: 10.1016/j.radonc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Khalil A.A., Hoffmann L., Moeller D.S., Farr K.P., Knap M.M. New dose constraint reduces radiation-induced fatal pneumonitis in locally advanced non-small cell lung cancer patients treated with intensity-modulated radiotherapy. Acta Oncologica. 2015;54(9):1343–1349. doi: 10.3109/0284186X.2015.1061216. [DOI] [PubMed] [Google Scholar]
- 14.Yom S.S., Liao Z., Liu H.H., Tucker S.L., Hu C.S., Wei X. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(1):94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z.Q., Yang K., Komaki R., Wei X., Tucker S.L., Zhuang Y. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83(1):332–339. doi: 10.1016/j.ijrobp.2011.06.1963. [DOI] [PubMed] [Google Scholar]
- 16.Chen C., Uyterlinde W., Sonke J.J., de Bois J., van den Heuvel M., Belderbos J. Severe late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapy. Radiother Oncol. 2013;108(2):337–341. doi: 10.1016/j.radonc.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Feddock J., Arnold S.M., Shelton B.J., Sinha P., Conrad G., Chen L. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2013;85(5):1325–1331. doi: 10.1016/j.ijrobp.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Hepel J.T., Leonard K.L., Safran H., Ng T., Taber A., Khurshid H. Stereotactic body radiation therapy boost after concurrent chemoradiation for locally advanced non-small cell lung cancer: a phase 1 dose escalation study. Int J Radiat Oncol Biol Phys. 2016;96(5):1021–1027. doi: 10.1016/j.ijrobp.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Speirs C.K., DeWees T.A., Rehman S., Molotievschi A., Velez M.A., Mullen D. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 20.Boellaard R., Delgado-Bolton R., Oyen W.J., Giammarile F., Tatsch K., Eschner W. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolthaus J.W., Sonke J.J., van Herk M., Belderbos J.S., Rossi M.M., Lebesque J.V. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008;70(4):1229–1238. doi: 10.1016/j.ijrobp.2007.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.