1. Introduction
Deep-inspiration breath-hold (DIBH) is a gated treatment technique which delivers radiation at near full inspiration in order to reduce the dose to organs at risk (OARs). It is of particular clinical benefit for reducing both cardio- and pulmonary toxicity in left-sided breast cancer patients by increasing the distance between the chest wall and heart and reducing the relative volume of the ipsilateral lung exposed [1], [2].
Assessing the breath-hold for DIBH can be achieved with a variety of approaches, ranging from the observation of a light field or laser line on the patient’s skin to video based skin surface monitoring and breathing motion detection systems [3], [4], [5]. The ultimate quantity of interest is the internal alignment of the field border with the patient’s anatomy. This is generally only verified a limited number of times during the course of the treatment using individual port film images. For the majority of the treatment time, the above mentioned surrogates are relied on to accurately describe the position of the anatomy.
In previous studies the position of the anatomy was evaluated using single MV images that were acquired on a daily basis [6], [7]. Other studies have used real-time MV fluoroscopy imaging to monitor the open field segments of combined open field/IMRT DIBH treatments [8].
This work used comprehensive MV cine imaging to investigate the performance of a widely used clinical DIBH monitoring technique in terms of its ability to ensure that consistently the intended patient anatomy is irradiated. In a retrospective analysis of clinical DIBH cases treated at our facility the quality of the achieved breath-holds throughout the treatment was assessed. MV cine images collected during each treatment fraction were used to analyse the position of the anatomy relative to the treatment beam. To investigate the dosimetric implications of deviations from the ideal (planned) breath-hold depth, a simplified model was used to calculate dose volume histogram (DVH) parameters for each patient reflecting the actual delivered dose to the breast and the OARs. Using the latter, the change in risk of major coronary events due to deviations from the planned patient setup was estimated.
This is the first study that used continuous daily portal imaging during gated DIBH treatments to estimate the delivered DVH parameters and to predict the change in risk of major coronary events.
2. Materials and methods
2.1. Simulation, treatment, and imaging
MV cine images have been collected for all fractions (16 or 25) of ten left sided breast cancer patients treated routinely with DIBH in our clinic. For simulation patients have been scanned using a Toshiba Aquilion CT scanner (Canon Medical Systems Corporation, Otawara-shi, Tochigi, Japan), in the supine position, inclined on a breast board and with both arms elevated. Reference marks were positioned at the effective centre of the tangential fields on the anterior chest on midline and laterally at the approximate posterior tangent edge location. Once the patient was positioned, the Real-Time Position Management (RPM, Varian Medical Systems, Palo Alto, CA, USA) system was used to assess the patient’s ability to perform a DIBH, to establish breath-hold parameters, and to scan the patient under DIBH. Audio coaching was used to guide patients’ breath-hold for simulation and treatment.
Patients were planned using a standard 6 MV, field in field, tangential technique (Varian Eclipse AAA algorithm V11). Two dose regimes were used, with two patients being treated to 50 Gy in 25 fractions and all others receiving 42.5 Gy in 16 fractions. To enable comparison of internal anatomy positioning during treatment with planned positioning, the distance between the posterior field edge and the breast-lung interface at the centre of the field (superior-inferior) was measured for each patient’s treatment fields using the complete irradiated area outline. This is referred to as the Expected Lung Depth, ELD (Fig. 1 in Supplementary Material).
Gated treatments were delivered on a Varian Clinac 21IX Linear Accelerator, with the treatment beam automatically turning on 1 s after the patient’s respiratory trace entered a defined 5 mm gating threshold.
Per clinical routine single medial – lateral (ML) MV images were acquired for isocentre verification during the first three fractions for all patients and again for fractions 14 – 16 for those patients on a 25 fraction treatment regime. A departmental offline protocol utilising an in-house program was then used to correct the treatment isocentre if based on these three images the average deviation from the planned position equalled 3 mm or more (similar to the no-action-level protocol proposed by de Boer [9] but with a 3 mm action level). This procedure is standard departmental clinical practice and was performed as it would be for other patients. For any fields and at every treatment fraction where this standard departmental imaging was not required, MV cine images were acquired throughout the entire beam-on time at a frame rate of 11 frames per second. Using the standard system workflow, the MV cine images were transferred to the patient database.
2.2. Image analysis
For the retrospective analysis the acquired MV cine images were retrieved from the patient database in DICOM format. The lung depth during treatment (LD) was determined in each of the images for all fractions of a patient’s treatment using a custom software written in MATLAB (MathWorks, Natick, USA) [10]. As part of the interactive analysis superior and inferior field edges were chosen manually in the first image of a treatment fraction. From the intensity profile along the midline of the field the LD was calculated for all acquired images as the distance between the posterior inflection point of the profile, representing the posterior field edge, and the next local maximum, representing the chest wall (Fig. 1).
Fig. 1.
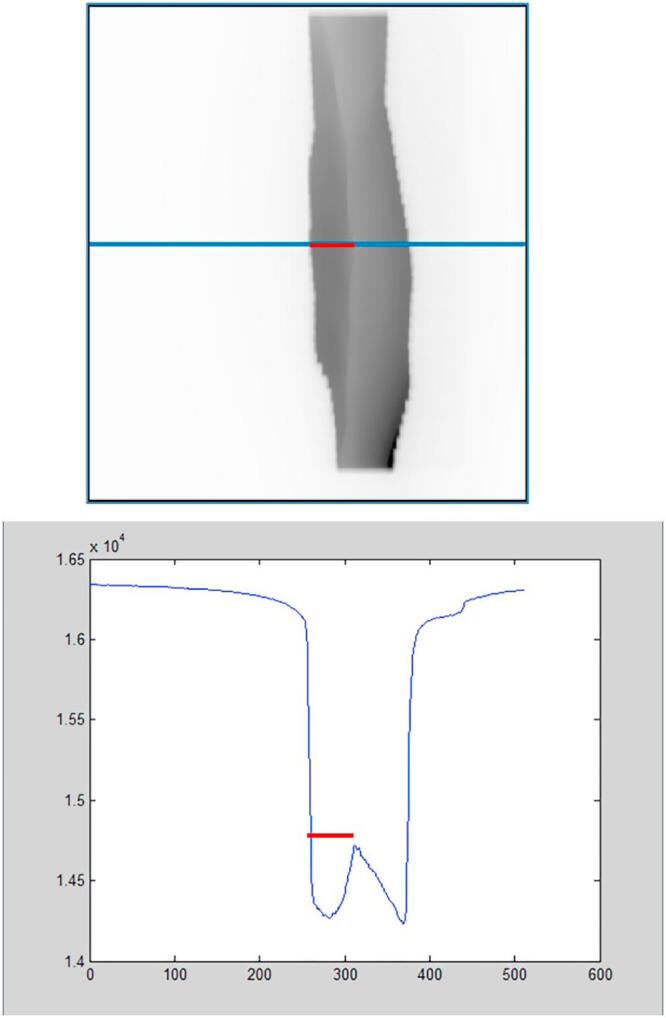
Illustration of the measured quantity lung depth (LD) in a MV cine image (top) and the intensity profile (bottom, arbitrary units).
In order to estimate actually delivered doses to target and OARs based on the observed LDs and in comparison with the ELD and the original treatment plan a simplified model was used, assuming a rigid motion of the patient. This approach, which, for practical reasons, shifted the isocentre of the beam rather than the patient, closely resembled the often in patients observed arching of the back. In the process up to ten additional treatment plans were created for each patient retrospectively. The change in LD was approximated by shifting the isocentre, i.e. the two treatment fields in increments of 2 mm perpendicular to the original posterior field edge to cover the range of observed LDs.
For the original plan as well as for each of the modified plans the following DVH parameters were selected for their relevance in the clinical plan evaluation process: mean dose to the heart (MHD), percentage of the volume of the heart that received at least 25% of the dose (V25), mean dose to both lungs (MLD), percentage of the volume of the left lung that received at least 20% of the dose (V20 left lung), percentage of the volume of the PTV that received at least 95% of the dose (V95 PTV), as well as maximum dose of the plan (Plan Max). Each plan was then weighted with the percentage of images of the whole course showing a LD in the respective range to determine the retrospective DVH parameters for the actual treatment. The original plan was assumed to reflect the range ELD − 1 mm < LD < ELD + 1 mm. In addition, all DVH parameters were also calculated assuming that the entire treatment delivery was performed with the observed LD that deviated most from the ELD (worst case scenario).
Using the MHDs from the delivered and the original plan the change in risk of major coronary events was determined using the linear dose – risk relationship (7.4%/Gy with no threshold) found by Darby et al. [11]. Due to this proportionality between dose and risk any relative change between planned and delivered MHD therefore will directly translate to give the same relative change in risk. The same was done for the worst case scenario.
3. Results
3.1. Determination of the lung depth
Analysis of more than 15,000 patient cine images using the described approach proved feasible. The LDs could be determined reliably for all patients. A fraction-by-fraction view showed that in some patients the LD varied substantially between different fractions (Fig. 2, patient 1). In comparison, the LD distribution for others was fairly uniform and the values remained within a smaller range (Fig. 2, patient 2).
Fig. 2.
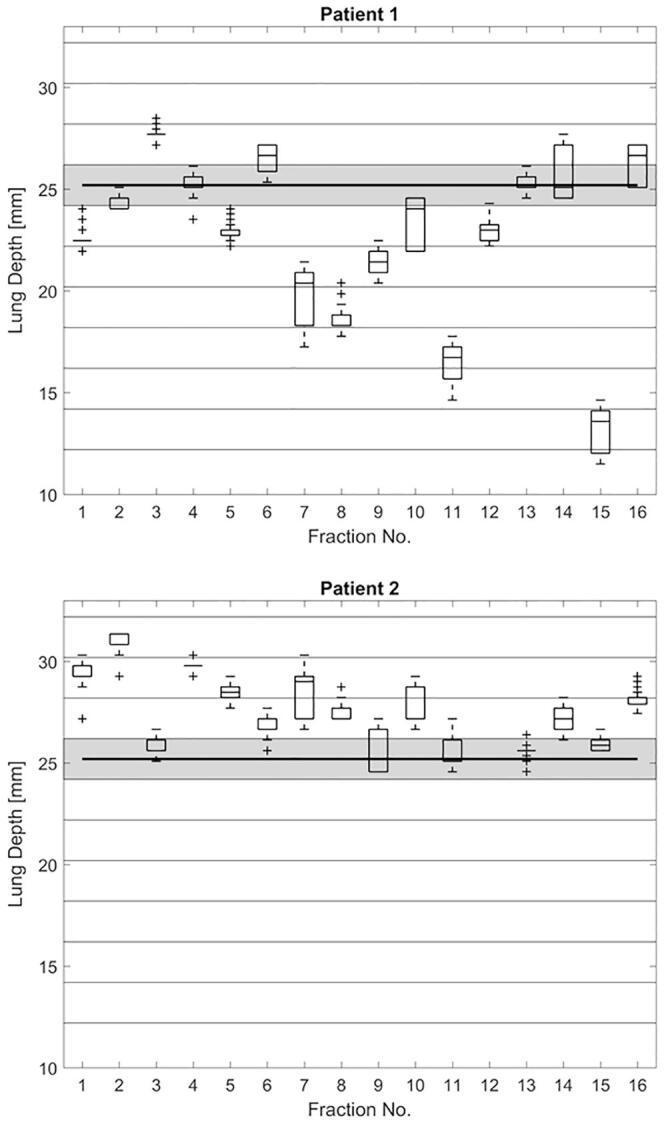
LD distribution for all fractions of two patients for both tangential fields combined. The horizontal bands reflect additional plans created using 2 mm shift increments for LDs in the specified range. The shaded area marks the LDs corresponding to the original treatment plan. Both patients exhibited the same ELD during planning (shown as black horizontal line).
Fig. 3 shows the range of observed deviations from each respective ELD for all patients. Deviations of more than 10 mm were encountered for some patients (1 and 5) while others remained closer to the ELD for all fractions (i.e. patients 2 and 4). In every patient the range of LDs observed during the overall course of the treatment exceeded the range set in the gating system (5 mm).
Fig. 3.
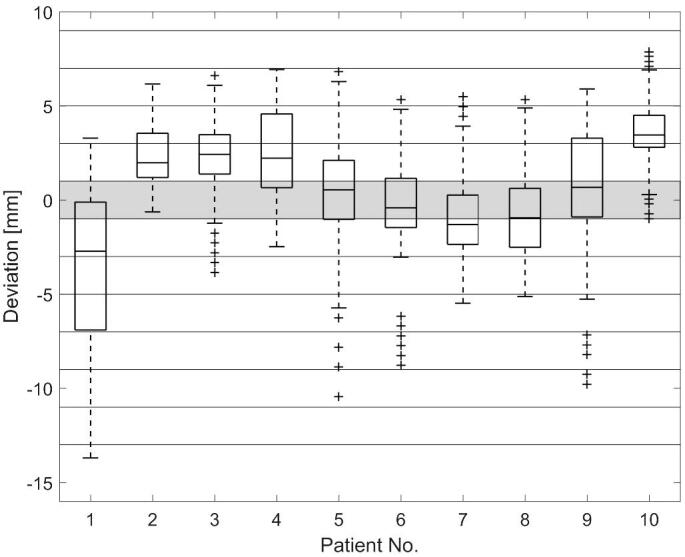
Observed deviation from the expected lung depth (ELD) for all patients (95% percentile, 75% percentile, median, 25% percentile, 5% percentile, and outliers). The horizontal bands reflect additional plans created using 2 mm shift increments for LDs in the specified range. The shaded area marks the LDs corresponding to the original treatment plan.
3.2. Analysis of dose distribution during gated DIBH
The difference between the delivered and the planned dose to both lungs varied between -15% and 24% (patients 1 and 10, respectively, see Table 1). At the same time the delivered dose remained well below the planning constraint of 18 Gy in all cases. Even the maximum MLD found in the worst case scenario did not exceed 3.8 Gy (patient 2).
Table 1.
DVH parameters for planned and delivered treatments as well as for the worst case scenario. The planning constraint for each parameter used at our institute is given below the respective parameter name.
| Patient No. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| MLD <18 Gy | Planned [Gy] | 2.4 | 2.9 | 2.4 | 1.8 | 2.3 | 2.2 | 2.0 | 2.3 | 2.0 | 2.2 |
| Delivered [Gy] | 2.1 | 3.2 | 2.6 | 2.1 | 2.4 | 2.2 | 1.9 | 2.2 | 2.0 | 2.7 | |
| Relative change [%] | −15 | 11 | 11 | 17 | 3 | −2 | −6 | −4 | 4 | 24 | |
| Worst case [Gy] | 2.9 | 3.8 | 3.1 | 2.5 | 3.2 | 3.0 | 2.8 | 3.2 | 2.6 | 3.7 | |
| Relative change [%] | 20 | 29 | 30 | 41 | 39 | 37 | 36 | 38 | 35 | 66 | |
| V20 Left lung <30% | Planned [%] | 9 | 13 | 10 | 7 | 8 | 9 | 7 | 8 | 8 | 8 |
| Delivered [%] | 7 | 14 | 11 | 9 | 8 | 9 | 6 | 8 | 8 | 10 | |
| Relative change [%] | −20 | 15 | 14 | 24 | 5 | −3 | −9 | −5 | 7 | 33 | |
| Worst case [%] | 12 | 17 | 14 | 11 | 12 | 14 | 10 | 12 | 11 | 15 | |
| Relative change [%] | 27 | 38 | 41 | 58 | 57 | 52 | 55 | 51 | 51 | 96 | |
| Plan Max <107% | Planned [%] | 105 | 106 | 106 | 105 | 106 | 108 | 107 | 104 | 105 | 108 |
| Delivered [%] | 108 | 106 | 106 | 105 | 107 | 108 | 108 | 105 | 105 | 106 | |
| Relative change [%] | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | −2 | |
| Worst case [%] | 112 | 106 | 108 | 106 | 109 | 111 | 111 | 107 | 107 | 106 | |
| Relative change [%] | 7 | 1 | 2 | 0 | 3 | 3 | 5 | 3 | 2 | −1 | |
| V95 PTV None | Planned [%] | 89 | 86 | 91 | 84 | 92 | 87 | 83 | 87 | 84 | 84 |
| Delivered [%] | 83 | 88 | 91 | 87 | 91 | 86 | 81 | 85 | 83 | 85 | |
| Relative change [%] | −6 | 2 | 0 | 3 | −1 | −1 | −2 | −3 | −1 | 1 | |
| Worst case [%] | 70 | 88 | 88 | 81 | 74 | 73 | 74 | 74 | 68 | 84 | |
| Relative change [%] | −21 | 2 | −4 | −4 | −19 | −16 | −11 | −14 | −20 | −1 | |
| MHD <4Gy | Planned [Gy] | 0.7 | 0.7 | 0.9 | 0.6 | 1.1 | 0.9 | 0.8 | 0.7 | 0.8 | 0.7 |
| Risk (planned) [%] | 5.3 | 5.5 | 6.6 | 4.3 | 8.1 | 6.8 | 5.9 | 5.5 | 5.7 | 5.4 | |
| Delivered [Gy] | 0.6 | 0.9 | 1.0 | 0.7 | 1.1 | 0.9 | 0.8 | 0.7 | 0.8 | 0.9 | |
| Risk (delivered) [%] | 4.8 | 6.4 | 7.5 | 4.9 | 8.5 | 6.7 | 5.8 | 5.4 | 6.0 | 6.7 | |
| Absolute change in risk [%] | −0.5 | 0.9 | 0.9 | 0.6 | 0.4 | 0.0 | −0.2 | −0.1 | 0.3 | 1.4 | |
| Relative change in dose and risk [%] | −9 | 16 | 13 | 14 | 5 | −1 | −3 | −2 | 5 | 25 | |
| Worst case [Gy] | 0.8 | 1.1 | 1.3 | 0.8 | 1.6 | 1.2 | 1.0 | 1.0 | 1.0 | 1.5 | |
| Risk (worst case) [%] | 6.0 | 8.5 | 9.6 | 5.9 | 11.7 | 9.1 | 7.1 | 7.3 | 7.6 | 10.9 | |
| Absolute change in risk [%] | 0.8 | 3.0 | 3.0 | 1.7 | 3.6 | 2.3 | 1.2 | 1.8 | 1.9 | 5.5 | |
| Relative change in dose and risk [%] | 14 | 54 | 46 | 39 | 45 | 34 | 20 | 33 | 33 | 102 | |
| V25 Heart None | Planned [%] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Delivered [%] | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The deviations varied from −20% to 33% between the delivered and the planned V20 of the left lung (patients 1 and 10, respectively). The worst case scenario showed a possible maximum increase of 96% (patient 10). In all the cases the V20 remained well below the planning constraint of 30%. The maximum V20 was calculated as 17% (patient 2).
In all cases except for patient 10 the calculated plan maximum was larger than the maximum of the original plan. All patients with the exception of patients 6 and 10 were originally planned to remain below the ICRU constraint of 107%. Patients 1, 6, and 7 were calculated to have exceeded this planning constraint on average slightly. In the worst case scenario, however, the calculated plan maximum reached up to 112% in the case of patient 1. Patients 1, 3, 5, 6, and 7 would have exceeded the planning constraint.
While planning constraints are not applied to the V95 of the PTV at our clinic the calculated values showed that the coverage of the PTV on average differed only by small amounts from the value of the original plan. Deviations of up to −6% were found (patient 1). When comparing the original planned value to the worst case scenario the coverage decreased by about 20% in some cases (patients 1, 5, and 9).
The MHD remained well below the planning constraint of 4 Gy in all cases. The deviations varied between −9% and 25% (patients 1 and 10, respectively). It’s is notable, however, that in patient 10 the dose that would have been delivered in the worst case scenario exceeded the planned MHD by more than 100%.
The results for the estimated change in risk of major coronary events suggested that while for all patients the absolute increase in risk for the delivered MHD remained below 2% the maximum absolute risk increase in the worst case scenario was found to be 5.5% (patient 10, relative increase of 102%).
All patients were originally planned with a V25 of 0%. The respective delivered values were in very close agreement.
4. Discussion
The purpose of this study was to evaluate the reproducibility and accuracy of a marker block based gating system triggering the treatment beam for breast DIBH treatments using continuous MV portal imaging for analysis of the anatomy position. From the measured LD DVH parameters of the actual treatment have been derived and compared to the planned parameters. Based on the change of the dose to the heart the change in risk of major coronary events was calculated using published correlations.
As the treatment beam was triggered by monitoring a marker block on the chest and the analysed MV cine images were taken with the treatment beam, every image represented an acceptable DIBH as assessed by the gating system. Considering the 5 mm window set for the DIBH, the range of LDs found during each single fraction appeared reasonable for most patients with some outliers, i.e. in most cases the range observed per fraction was less than 5 mm. However, the observed LDs varied greatly for some patients. The ranges of LDs within a treatment fraction indicated a reasonable performance of the DIBH monitoring system. The differences between fractions and the overall deviations of the LD from the ELD point to problems with the approach.
In general, the DVH parameters calculated from the EPID images acquired during treatment showed that DIBH is well suited to control the dose to organs at risk. Especially the planned as well as the calculated V25 were well within the QUANTEC guideline of 10% [12]. Based on our results the normal tissue complication probability (NTCP) for the heart was far below 0.01% for all DIBH patients in this study which compares well with other studies [13], [14].
The reported findings apply only to the technique employed for DIBH monitoring in this study. While its use is widespread, there are other approaches which use different surrogates to assess the depth of breath-hold and which might yield different findings. Conceptually, the use of surface image guidance systems could bring an improvement as it avoids the uncertainties associated with the marker block and its setup by monitoring the body surface of the patient directly. The connection between outside patient surface and internal anatomy of interest, as well as any inaccuracies or fluctuations in the monitoring procedure, could still result in deviations and would be very interesting to investigate in a future study [2], [15].
The presented methodology for LD calculation and evaluation is subject to several sources of uncertainty: (1) The overall uncertainty of the deviation of the LD from the ELD due to variations in planning, definition of field boundaries, curved surfaces, voxel size as well as MLC leaf position and direction was estimated to be approximately 2 mm. (2) Displacement of the patient relative to the field in the superior-inferior dimension or through rotation could lead to disproportionate changes in the LD. (3) The LD methodology cannot distinguish between geometric displacement and variations in DIBH level/lung volume. Therefore a deviation in LD from ELD could be caused by the patient being in the perfect posture and breath-hold but in the wrong spot relative to isocentre or by the patient having taken a deeper breath than on the planning scan. The LD would give the same deviation from ELD in both cases. This is unlikely going to affect the desired situation of a correct LD (in agreement with ELD), unless the patient happens to be in the wrong position and performs an insufficient breath-hold. (4) There could be gross changes in the breast shape, due to anatomical changes or arm positioning, or the patient positioning in the superior-inferior dimension that are not accounted for by the LD. (5) Different methods exist to correct for setup errors during tangential breast treatments. Verification images can be acquired to adjust for deviations from the planned setup online on a daily basis. This has the advantage to provide for a more accurate setup than offline protocols [16], [17], [18].
In order to determine if any LD deviations resulted in significant changes in the dose delivered to the patient compared to the treatment plan additional plans were created by shifting the treatment isocentre, i.e. both treatment fields perpendicular to the posterior field edge. This approach to assess the impact of the change in LD on the delivered dose distribution was an approximation. It assumed a shift of the patient’s anatomy in that direction, for example a lifting up of the patient’s body in case of LD > ELD (rather than additional inhale). We consider this a valid approximation as such behaviour has been frequently observed for patients in our and other clinics.
Several authors have used similar isocentre shift methods to evaluate changes in dose. McIntosh et al. [7] determined a rigid heart shifting method to estimate heart doses when using the same system that was investigated in our study. This technique was then applied by Mittauer et al. [19] when evaluating DIBH using an active breathing coordinator (ABC)-assisted breath-hold technique (Elekta Oncology Systems, Crawley, UK). The same LD can be achieved on imaging for very different levels of inspiration, which may represent a different heart dose when MV imaging is used to evaluate the position of the target with respect to the treatment unit because the marker block technique measures the distance moved from a baseline that is independent from the treatment unit position.
This study showed that observing the alignment of the field border with the patient’s anatomy using MV cine imaging allowed for direct assessment of the quantity of interest, the LD, without any additional imaging dose or equipment. The marker block motion did not seem to be a sufficient surrogate to consistently determine the level of inspiration during treatment.
Conflicts of interest
No conflicts of interest exist.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.02.006.
Contributor Information
Marcus Doebrich, Email: Marcus.Doebrich@calvarymater.org.au.
Janine Downie, Email: Janine.Downie@calvarymater.org.au.
Joerg Lehmann, Email: Joerg.Lehmann@calvarymater.org.au.
Appendix A. Supplementary data
Supplementary Fig. S1.
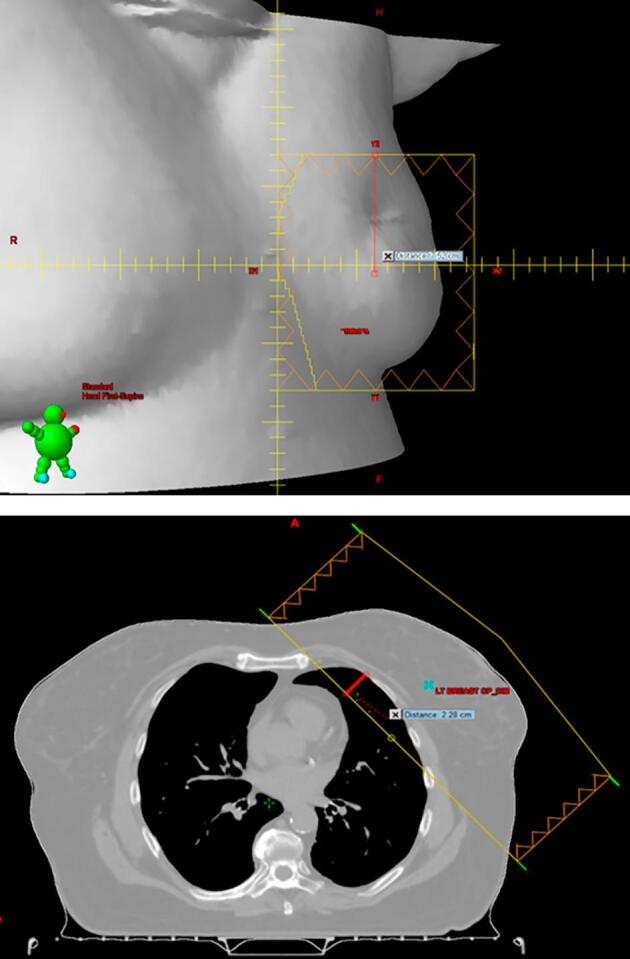
Beams-eye-view of a treatment field with the centre of the field marked (top) and a transverse view showing both tangential fields with the expected lung depth (ELD, red, bottom).
References
- 1.Sixel K.E., Aznar M.C., Ung Y.C. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 2.Schönecker S., Walter F., Freislederer P. Treatment planning and evaluation of gated radiotherapy in left-sided breast cancer patients using the Catalyst/Sentinel system for deep inspiration breath-hold (DIBH) Radiat Oncol. 2016;11(1):143. doi: 10.1186/s13014-016-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathpal M., Tinnel B., Sun K. Deep inspiration breath hold with electromagnetic confirmation of chest wall position for adjuvant therapy of left-sided breast cancer: technique and accuracy. Pract Radiat Oncol. 2016;6(5):e195–e202. doi: 10.1016/j.prro.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett F.R., Colgan R.M., Donovan E.M. Voluntary breath-hold technique for reducing heart dose in left breast radiotherapy. J Vis Exp. 2014;89:51578. doi: 10.3791/51578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy L., Yeung R., Watt E. Evaluation of target and cardiac position during visually monitored deep inspiration breath-hold for breast radiotherapy. J Appl Clin Med Phys. 2016;17(4):25–36. doi: 10.1120/jacmp.v17i4.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett F.R., Donovan E.M., McNair H.A. The UK HeartSpare Study (Stage II): multicentre evaluation of a voluntary breath-hold technique in patients receiving breast radiotherapy. Clin Oncol. 2017 Mar;29(3):e51–e56. doi: 10.1016/j.clon.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh A., Shoushtari A.N., Benedict S.H. Quantifying the reproducibility of heart position during treatment and corresponding delivered heart dose in voluntary deep inhalation breath hold for left breast cancer patients treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(4):e569–e576. doi: 10.1016/j.ijrobp.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Borst G.R., Sonke J.J., den Hollander S. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int J Radiat Oncol Biol Phys. 2010 Dec 1;78(5):1345–1351. doi: 10.1016/j.ijrobp.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.de Boer H.C.J., Heijmen B.J.M. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001 Aug 1;50(5):1350–1365. doi: 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed] [Google Scholar]
- 10.Doebrich M., Downie J., Stanton C., Dempsey C., Lehmann J. Feasibility of MV cine imaging for monitoring of DIBH treatments. Aust Phys Eng Sci Med. 2016;39:336. [Google Scholar]
- 11.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi G., Constine L.S., Moiseenko V. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–S85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 13.Moiseenko V., Einck J., Murphy J. Clinical evaluation of QUANTEC guidelines to predict the risk of cardiac mortality in breast cancer patients. Acta Oncol. 2016;55(12):1506–1510. doi: 10.1080/0284186X.2016.1234067. [DOI] [PubMed] [Google Scholar]
- 14.Zagar T.M., Kaidar-Person O., Tang X. Utility of deep inspiration breath hold for left-sided breast radiation therapy in preventing early cardiac perfusion defects: a prospective study. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):903–909. doi: 10.1016/j.ijrobp.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Tang X., Zagar T.M., Bair E. Clinical experience with 3-dimensional surface matching-based deep inspiration breath hold for left-sided breast cancer radiation therapy. Pract Radiat Oncol. 2014;4(3):e151–e158. doi: 10.1016/j.prro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Lutz C.M., Poulsen P.R., Fledelius W. Setup error and motion during deep inspiration breath-hold breast radiotherapy measured with continuous portal imaging. Acta Oncol. 2016;55(2):193–200. doi: 10.3109/0284186X.2015.1045625. [DOI] [PubMed] [Google Scholar]
- 17.Korreman S.S., Juhler-Noettrup T., Persson G.F. The role of image guidance in respiratory gated radiotherapy. Acta Oncol. 2008;47(7):1390–1396. doi: 10.1080/02841860802282786. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S.B., Wolfgang J., Mageras G.S. Quality assurance challenges for motion-adaptive radiation therapy: Gating, breath holding, and four-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2008;71(1 Suppl):S103–S107. doi: 10.1016/j.ijrobp.2007.07.2386. [DOI] [PubMed] [Google Scholar]
- 19.Mittauer K.E., Deraniyagala R., Li J.G. Monitoring ABC-assisted deep inspiration breath hold for left-sided breast radiotherapy with an optical tracking system. Med Phys. 2015 Jan;42(1):134–143. doi: 10.1118/1.4903511. [DOI] [PubMed] [Google Scholar]


