Highlights
-
•
PSMA PET/CT allows visualisation of salivary glands with good spatial and contrast resolution.
-
•
Evaluable glands include minor and less well-known gland locations.
-
•
PSMA PET/CT can visualise damage to salivary glands from external beam radiotherapy.
-
•
PSMA PET/CT has the potential to guide optimisation of radiotherapy to the head and neck.
-
•
The potential benefit of PSMA PET/CT is to avoid salivary gland toxicity and preserve quality of life.
Keywords: Salivary glands, Toxicity, PSMA, Radiotherapy, Case series, Head and neck neoplasms
Abstract
Evaluation of salivary gland damage after head and neck radiotherapy (RT) is difficult with current tools, such as subjective patient-reported outcome measures. We demonstrate the use of prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) as an objective non-invasive tool to visualize damage to salivary glands resulting from RT. In three clinical cases, the PSMA-ligand distribution correlates to the RT dose distribution including intra-gland dose gradients and matches patient-reported toxicity, suggesting a dose-response relation. These findings support further exploration of PSMA PET/CT to guide and evaluate RT, with the ultimate aim to reduce salivary gland toxicity.
1. Introduction
External beam radiotherapy (RT) can cause damage to salivary glands, resulting in xerostomia and reduction of quality of life [1], [2]. Evaluation of salivary gland function is currently limited to patient-reported outcomes measures, or to laborious and poorly quantifiable procedures such as gland cannulation or planar scintigraphy during stimulated salivation [3]. This limits objective evaluation of toxicity, for example voxel-based evaluations or anticipated comparison of regular intensity modulated radiotherapy (IMRT) with new modalities like proton treatment.
Based on clinical observations, our hypothesis was that a molecular imaging of the prostate-specific membrane antigen (PSMA) using radiolabelled ligands and positron emission tomography combined with computed tomography (PET/CT) can be used to visualise and quantify viable gland cells in salivary glands. Functional imaging with PSMA PET/CT is generally applied for sensitive and specific (re)staging of prostate carcinoma [4], [5]. Normal salivary and seromucous glands have recently been shown to consistently demonstrate high uptake of PSMA-ligand (Fig. 1) [6], [7]. The function of the PSMA epitope in salivary gland tissue has not yet been elucidated, but the expression is reported to be located in secretory glandular (acinar) cells [8]. This theoretically enables sensitive and selective imaging of the presence of acinar gland cells in both major and previously undetectable minor salivary glands, and to quantify loss of these cells in a gland-specific and voxel-based approach [9].
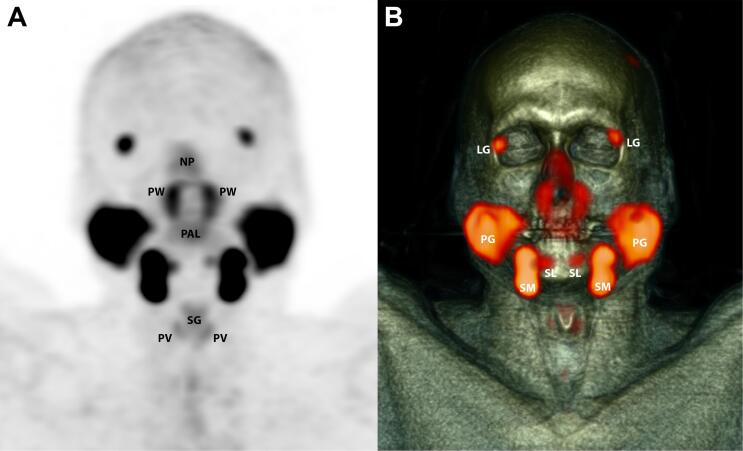
Fig. 1.
Example PSMA PET/CT of the head and neck with healthy salivary glands. Coronal maximum intensity projection of normal PSMA distribution in a 60-year-old male with no history of disease in the head and neck, who was scanned for staging of prostate cancer using PET (A) in combination with CT (B). NP = nasal and nasopharyngeal mucosa; PW = posterior pharyngeal wall; PAL = soft palate; SG = supraglottic larynx; PV = plica vocalis; LG = lacrimal gland; PG = parotid gland; SM = submandibular gland; SL = sublingual gland.
We aimed to identify and introduce PSMA PET/CT as a possible new application to guide radiotherapy, in a first observational report.
2. Materials and methods
Three patients who previously received external beam radiotherapy to the head and neck region are presented. These patients were selected and underwent PSMA PET/CT, for a pilot study approved by the local ethical committee. Case A, a 60-year old female with a T3N0 tonsillar carcinoma, was treated with radiotherapy (70 Gy) and cisplatin (CCRT). Case B was a 50-year old male treated with CCRT (tumour 70 Gy; bilateral neck 54 Gy) for a T4N2 nasopharyngeal carcinoma. Case C was a 68-year old male treated for a T3N1 oral cavity carcinoma with CCRT (70 Gy, large field involving all macroscopic salivary gland locations except the lips) and a left sided salvage neck dissection.
All patients underwent a PET/CT after intravenous administration of the radiolabelled PSMA-ligand [68Ga]-HBED-CC-Glu-NH-CO-NH-Lys(Ahx), either 100 MBq for whole-body imaging (2 min/bed position) or 50 MBq for the head-neck alone (4 min/bed position). Images were acquired at 45–60 min p.i. using a Gemini TOF PET/CT (Philips, Cleveland). The tracer distribution was visually evaluated and compared to the previously irradiated anatomical area and radiotherapy dose distribution. Xerostomia was scored according to the Common Terminology Criteria for Adverse Events version 4 (CTCAE v4).
3. Results
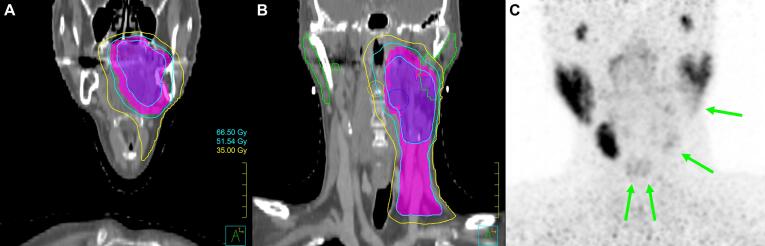
In case A, PSMA PET/CT showed significantly decreased ligand uptake in the irradiated area, suggesting extensive damage to the left submandibular gland and caudal part of the left parotid gland which received 70 Gy (Fig. 2). The sublingual glands which received 46 Gy showed partial loss of uptake. All other glands showed normal uptake, with standard uptake values (SUV) comparable to the earlier described values for healthy salivary glands [9]. Clinically there had been grade II xerostomia after treatment, but this had resolved completely at the time of imaging (12 months after RT).
Fig. 2.
Case A. Coronal slices of radiotherapy dose distribution at the level of the oral cavity (A) and parotid glands (B). Coronal maximum intensity projection of PSMA PET (C), showing the correlation between dose distribution and reduced PSMA uptake in salivary glands: left parotid gland, left submandibular gland, and both sublingual glands (green arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
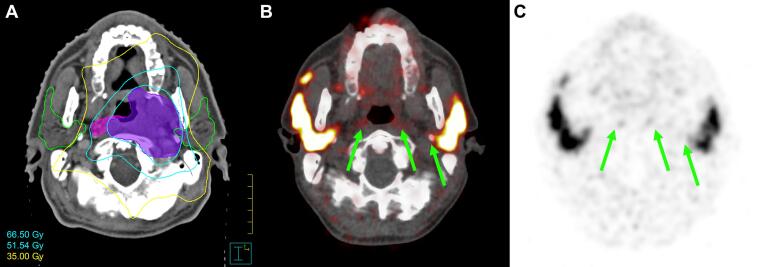
In case B, PET/CT showed loss of PSMA-ligand uptake along the posterior pharyngeal wall glands and in the deep lobe of the left parotid gland, all of which received 70 Gy (Fig. 3). All other gland locations showed normal uptake. Patient reported persistent grade II xerostomia at the time of imaging (11 months after RT).
Fig. 3.
Case B. Transverse slices of radiotherapy dose distribution (A), fused PET/CT (B) and PSMA PET (C) at the level of the parotid and retropharyngeal glands, illustrating complete loss of PSMA uptake in pharyngeal wall glands and in the deep lobe of the left parotid gland (green arrows), which received a high dose. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
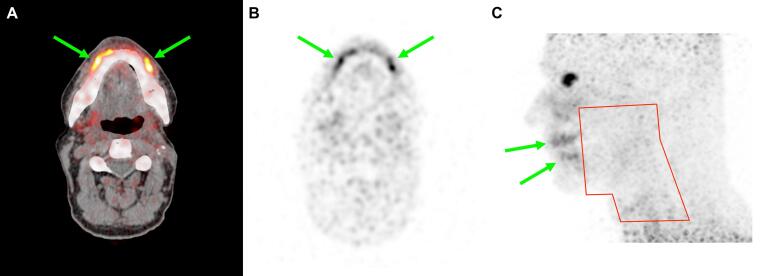
In case C, PSMA PET/CT showed complete loss of signal in all major and minor salivary gland locations (Fig. 4), except for notably high uptake in the area of the mucosal lip glands that had not been irradiated. At the time of imaging (240 months after RT) patient reported grade II xerostomia, but with sufficient salivation in the ventral part of the mouth for normal speech and intake and without the need for artificial saliva or other measures.
Fig. 4.
Case C. Transverse slice of fused PET/CT (A) and PSMA PET (B), and lateral maximum intensity projection of PSMA PET with indication of prior radiotherapy field in red (C). Complete loss of PSMA uptake is seen in most salivary glands after radiotherapy. Remarkably, PSMA PET indicates hypertrophy of the spared minor salivary glands in the lips (green arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In addition to the familiar role of PSMA PET/CT in the assessment of prostate cancer, we propose a new and very different use for this molecular imaging modality. Our clinical findings show that PSMA PET/CT can visualise areas with healthy salivary and seromucous glands after RT, and identify damage by showing specific glands and gland areas with loss of PSMA expression coherent with the dose distribution from prior RT.
PSMA PET/CT is able to detect subclinical damage in case A. The signal gradient over the left parotid gland is in concordance with the dose distribution, and visually has an inverse relationship between the treatment dose and tracer accumulation. This also indicates a spatial resolution that is sufficient for interpretation of intra-gland variations. This finding could be elucidated further with prospective research that includes voxel-based evaluations of the dose-effect relation.
In case B, a relationship between clinical symptoms and the distribution of damage in specific gland locations on PSMA PET/CT is suggested. This helps to clarify previously unexplained toxicity, in a patient where the major salivary glands were spared an indeed appear to be otherwise unaffected.
The remarkable findings in case C suggest functional compensation by hypertrophy of minor glands in the mucosa of the lips on both sides, to explain the residual salivation capacity after complete loss of PSMA-ligand binding in the major salivary glands after RT. This finding suggests the relevance of gland locations that are currently not considered in RT planning, especially in case of damage to the major salivary glands, and provides more insight in the functional compensation capacity of minor mucosal gland locations.
The spatial resolution of PET images (generally in the order of 2–5 mm FWHM in the head-neck area) allows evaluation of inhomogeneous loss of ligand binding within larger salivary glands. In combination with the high contrast resolution of PET, more and smaller gland locations can be evaluated than with common methods such as planar scintigraphy or functional magnetic resonance imaging (fMRI) [9]. As a result, PET can for the first time allow quantitative assessment of glandular damage using gland-based or voxel-based approaches.
As stated before, the expression of PSMA is reported to be located in the secretory glandular (acinar) cells [8]. Although the function of the epitope in the salivary glands is unknown, the level of PSMA-expression per acinar gland cell is thought to be stable. Therefore, the observed reduction in local PSMA-ligand binding is thought to indicate loss of secretory cells in the affected glands, but this remains to be confirmed in upcoming studies [10]. This method for quantification of cell loss would potentially be more objective and specific than patient-reported outcomes, e.g. independent from factors such as patient experience, medication use, examiner experience etc., and more convenient and potentially better reproducible than salivary gland cannulation or scintigraphy during stimulated salivation. If the suggested relationship can be confirmed, PSMA PET/CT may contribute to the development of new and more accurate dose-effect relations and RT planning objectives, for the first time with opportunities to derive these per gland subtype and including previously unevaluable gland locations. However, this will require evaluation of potentially interfering factors such as the role of renewed PSMA expression related to (long term) regeneration of irradiated salivary glands from stem cells.
There are other PET-tracers that accumulate in salivary gland tissues to allow visualization, with examples including F-18-FDG (metabolic activity), F-18-choline (cell membrane synthesis) or Gallium-68-citrate (non-specific cell activity). However, PSMA-ligands have some unique advantages for this specific application. The uptake in healthy glands is very high, and together with a virtually negative background in all surrounding normal organs this simplifies signal contouring and quantification [9]. But most importantly, histopathological evaluation has proven that PSMA-ligands bind the most relevant cell population, the acinar secretory cells. The distribution of less specific tracers in normal salivary glands is currently not as well understood and is likely to involve other cell types, which brings a risk on signal contamination due to e.g. infiltration with inflammatory cells, and this makes them less suited for measuring salivary gland damage. Tracers for scintigraphy and single photon emission computed tomography (SPECT) imaging like Tc-99 m-pertechnetate have a well-understood uptake mechanism, but suffer from low spatial resolution and poor signal quantification, and they rely on poorly reproducible stimulated salivation to yield a measurable signal. These factors have led to the identification of PSMA PET/CT as a new and promising instrument to quantify specific cell loss in salivary glands, with a better understood biological relation, better spatial resolution, and lower patient burden than currently available alternatives. In conclusion, PSMA PET/CT has the potential to further elucidate the effects of radiation to salivary and seromucous glands. These findings support further exploration of PSMA PET/CT to guide and evaluate RT, with the ultimate aim to reduce salivary gland toxicity.
Conflicts of interest
None.
Grants
Dutch Cancer Society-grant; number: 10606/2016-2. The Dutch Cancer Society had no role in decisions regarding the study design, the collection, analysis and interpretation of data, in the writing of the report, nor in the decision to submit the article for publication.
References
- 1.Pinna R., Campus G., Cumbo E., Mura I., Milia E. Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage. Ther Clin Risk Manag. 2015;11:171–188. doi: 10.2147/TCRM.S70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellema A., Slotman B., Doornaert P., Leemans C., Langendijk J. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:751–760. doi: 10.1016/j.ijrobp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Cheng S., Wu V., Kwong D., Ying M. Assessment of post-radiotherapy salivary glands. Br J Radiol. 2011;84:393–402. doi: 10.1259/bjr/66754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira J., Gomes C., Faria D., Vieira T., Silva F., Vale J. 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography for prostate cancer imaging: a narrative literature review. World J Nucl Med. 2017;16:3–7. doi: 10.4103/1450-1147.198237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera M., Papa N., Christidis D., Wetherell D., Hofman M., Murphy D. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Demirci E., Sahin O., Ocak M., Akovali B., Nematyazar J., Kabasakal L. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl Med Commun. 2016;37:1169–1179. doi: 10.1097/MNM.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 7.Prasad V., Steffen I., Diederichs G., Makowski M., Wust P., Brenner W. Biodistribution of [(68)Ga]PSMA-HBED-CC in patients with prostate cancer: characterization of uptake in normal organs and tumour lesions. Mol Imaging Biol. 2016;18:428–436. doi: 10.1007/s11307-016-0945-x. [DOI] [PubMed] [Google Scholar]
- 8.Wolf P., Freudenberg N., Bühler P., Alt K., Schultze-Seemann W., Wetterauer U. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate. 2010;70(5):562–569. doi: 10.1002/pros.21090. [DOI] [PubMed] [Google Scholar]
- 9.Klein Nulent T.J.W., Valstar M.H., de Keizer B., Willems S.M., Smit L.A., Al-Mamgani A. Physiological distribution of PSMA-ligand in salivary and seromucous glands of the head and neck on PET/CT. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:478–486. doi: 10.1016/j.oooo.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Backhaus P., Noto B., Avramovic N., Grubert L., Huss S., Bögemann M. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45:860–877. doi: 10.1007/s00259-017-3922-y. [DOI] [PubMed] [Google Scholar]