Abstract
Background and purpose
Automated configurations are increasingly utilised for radiotherapy treatment planning. This study investigates whether automated treatment planning configurations are adaptable across clinics with different treatment planning protocols for prostate radiotherapy.
Material and methods
The study comprised three participating centres, each with pre-existing locally developed prostate AutoPlanning configurations using the Pinnacle3® treatment planning system. Using a three-patient training dataset circulated from each centre, centres modified local prostate configurations to generate protocol compliant treatment plans for the other two centres. Each centre applied modified configurations on validation datasets distributed from each centre (10 patients from 3 centres). Plan quality was assessed through DVH analysis and protocol compliance.
Results
All treatment plans were clinically acceptable, based off relevant treatment protocol. Automated planning configurations from Centre’s A and B recorded 2 and 18 constraint and high priority deviations respectively. Centre C configurations recorded no high priority deviations. Centre A configurations produced treatment plans with superior dose conformity across all patient PTVs (mean = 1.14) compared with Centre’s B and C (mean = 1.24 and 1.22). Dose homogeneity was consistent between all centre’s configurations (mean = 0.083, 0.077, and 0.083 respectively).
Conclusions
This study demonstrates that automated treatment planning configurations can be shared and implemented across multiple centres with simple adaptations to local protocols.
Keywords: Prostate, Automatic, VMAT, Treatment planning, Pinnacle, Multi-centre
1. Introduction
Conventional treatment planning for Intensity Modulated Radiotherapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT) require many manual processes, with treatment planners iteratively adjusting optimisation goals within a treatment planning system to develop clinically acceptable treatment plans. This process is not only time-consuming, but treatment plan quality is inherently dependent on the individual skill of the planner [1], [2], [3], [4], [5]. The importance of high-quality treatment planning on clinical outcomes has been demonstrated during clinical trials [6], [7], [8].
Both automated treatment planning methods and knowledge-based optimisation engines are now available within most commercial treatment planning systems [9], [10], [11], and have demonstrated improvements in planning efficiency and plan quality compared to current practice [12], [13]. Multiple institutions have investigated the efficacy of automated treatment planning for head and neck [14], [15], [16], [17], [18], [19], oesophageal [20], and prostate cancers [21], [22], [23], [24], and found the automatically generated plan to be noninferior and often superior to manual planning quality, while significantly reducing treatment planning times. Additionally, previously complex and time-consuming stereotactic treatments for liver cancer have had automatic treatment plans developed [25], while quantitative tools have been constructed to automatically identify poorer quality treatment plans [26], [27].
Previous automated treatment planning studies have been conducted either within a single institution, or during clinical trials using a single protocol across multiple centres [28], [29]. As most radiotherapy patients are not treated within a clinical trial, they are planned according to a wide variety of local protocols and practices developed at different centres. Consequently, comparisons in treatment plan quality between developed automated techniques difficult, with no common baseline for plan comparison between centres.
This study investigated whether locally developed automatic treatment planning configurations for prostate radiotherapy could be adapted to meet distributed protocols shared amongst multiple centres. It provides an explicit example of how protocol sharing between centres could proceed, allowing smaller clinics to benefit from the work of larger centres during future clinical trials.
2. Materials and methods
2.1. AP Configuration development
The three participating centres (two European, one Australian, denominated A, B, and C) had previously developed automated treatment planning techniques utilising the AutoPlanning (AP) module within Pinnacle3® treatment planning system (Philips Radiation Oncology, Madison, WI). All centres distributed local protocol details on prescription, contouring and dose constraints for prostate radiotherapy (Tables 1, A.1). Evaluation criteria were specified by the host-centre as either low, medium, high, or constraint priority. Additionally, three patient datasets (one easy, one medium, and one difficult to plan) were provided by each centre as training datasets. All distributed datasets contained target volumes and organs-at-risk previously contoured by each centre.
Table 1.
Target and high/constraint priority OAR prescriptions and evaluations. Complete lists of evaluation criteria can be found within Table A.1(supplementary material).
| Protocol A | Protocol B | Protocol C | ||||||
|---|---|---|---|---|---|---|---|---|
| Target | Prescription | Target | Prescription | Target | Prescription | |||
| PTV1 | 35 × 2 Gy | PTV1 | 39 × 2 Gy | PTV1 | 39 × 2 Gy | |||
| PTV2 | 35 × 2.2 Gy | |||||||
| Target | Evaluation | Priority | Target | Evaluation | Priority | Target | Evaluation | Priority |
| PTV1 | V95% > 99% | Constraint | PTV1 | D95% > 100% | Medium | PTV1imrt | D99% > 95% | Medium |
| PTV2 | V95% > 99% | Constraint | PTV1 | D2% < 105% | Medium | PTV1imrt | D0% < 107% | Medium |
| PTV1/PTV2 | D1% < 107% | Constraint | CTV1 | D99% > 100% | High | PTV1imrt | D95% > 95% | High |
| PTV1/PTV2 | D1% < 105% | Medium | PTV1 | Dmax < 107% | Medium–Soft | PTV1 | D99.9% > 66.5 Gy | High |
| PTV_prostate_imrt | D99% > 95% | Medium | ||||||
| PTV_prostate_imrt | D97% > 95% | High | ||||||
| OAR | Evaluation | Priority | OAR | Evaluation | Priority | OAR | Evaluation | Priority |
| Rectal Wall | V75Gy < 10% | High | Rectum | V65Gy < 20% | Constraint | Rectum | V74Gy < 1 cm3 | Constraint |
| Rectal Wall | V64Gy < 35% | High | Rectum | V70Gy < 10% | Constraint | Circumference of rectum | Dmax < 50 Gy | Constraint |
| Anal Sphincter | Dmean < 45 Gy | High | Rectum | V75Gy < 5% | High | |||
| 90% − 95% isodose tight around target | High | RECT_IN_PTV | V79Gy < 1 cm3 | High | ||||
| RECT_78_03_NS | V80Gy < 1 cm3 | Constraint | ||||||
Each centre modified their locally developed clinical AP configurations using the training datasets to meet other centre’s distributed protocols. Only dose objectives and AP optimisation parameters were to be adjusted during configuration development (i.e. beam configuration, energy, etc. were to remain as used clinically, see Tables A.2(a–c)). It should be noted that all centres’ AP configurations incorporated similar VMAT treatment techniques, although the number of arcs utilised (single or dual) varied.
Consequently, each centre generated three distinct VMAT AP configurations to meet the dose objectives for each of the three protocols. Naming convention is shown in Fig. 1, with the protocol name corresponding to the origin of the patients and planning objectives. Conversely, the naming of the AP configuration corresponds to the centre the AP technique was developed.
Fig. 1.
Schematic of the study design. Participating centres had a local prostate radiotherapy protocol and associated AP configuration. Each centre created new AP configurations for the other two protocols through modification of their local AP configuration, based on a training dataset of three patients provided by each centre (pre-contoured CT datasets).
2.2. Treatment planning
An additional ten prostate patient datasets with target volume and organ-at-risk contours were distributed by each centre as validation datasets, with each patient previously planned using the host-centre’s local AP configuration. Treatment planning system setups and patient selection criteria are found in Tables A.3 and A.4 respectively. Centres applied modified AP configurations to the corresponding centre’s validation datasets, resulting in three AP plans each for the thirty patients. Post-optimisation following AP was allowed, based on each centre’s standard clinical practice. All treatment plans were exported as DICOM RT files and uploaded to a single host for analysis.
2.3. AP configuration evaluation and statistics
Quantitative analyses between AP configurations were performed by dose-volume histogram (DVH) analysis, and total protocol deviations. Population median DVH for planning target volumes (PTVs) and OARs across all ten patients per centre were generated, utilising previously developed software [16], [20]. Conformity and homogeneity indices (CI = V95%/VPTV, HI = (D2% – D98%)/DPrescription) were calculated for all patient treatment plans. Two-sided Wilcoxon matched-pair sign rank probability curves, previously described by Bertelsen et al. [30], were used to illustrate differences between AP configuration DVH distributions. It should be noted that while individual p-values are not a solid statistical test of differences at a given dose level, given these parameters are highly correlated, they can be used to visualize where differences between the population median DVHs exist.
3. Results
All centres successfully developed AP configurations that met each centre’s protocols utilising the validation datasets (Table A.2(a–c), supplementary material). Mean and standard deviations of target volume (Table 2), as well as constraint, high priority (Table 2), and medium and low priority OAR evaluations (Table A.5, supplementary material) were recorded across all AP configurations.
Table 2.
Target and high/constraint priority OAR mean and standard deviations (S.D.) for all protocols. Conformity and homogeneity indices are shown for all PTVs. Differences in metrics considered significant (p < 0.05) are bolded. Complete list of objectives can be found within Table A.4(supplementary material).
| Centre A |
Centre B |
Centre C |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | p-value | Mean | S.D. | p-value | Mean | S.D. | p-value | |
| Protocol A/Configuration A (Host) | Protocol A/Configuration B | Protocol A/Configuration C | |||||||
| PTV1: V95% > 99% | 99.9% | 0.1% | 100.0% | 0.1% | 0.08 | 100.0% | < 0.1% | 0.002 | |
| PTV2: V95% > 99% | 99.7% | 0.2% | 99.9% | 0.2% | 0.04 | 100.0% | < 0.1% | 0.002 | |
| PTV2: D1% < 107% | 103.1% | 0.5% | 102.6% | 0.6% | 0.03 | 102.4% | 0.2% | 0.010 | |
| Rectal Wall: V75Gy < 10% | 4.2% | 2.2% | 5.1% | 3.0% | 0.11 | 6.6% | 2.6% | 0.002 | |
| Rectal Wall: V64Gy < 35% | 14.8% | 5.8% | 16.2% | 5.3% | 0.002 | 17.2% | 6.0% | 0.002 | |
| Anal Sphincter: Dmean < 45 Gy | 9.1 Gy | 7.7 Gy | 10.4 Gy | 8.7 Gy | 0.004 | 10.0 Gy | 9.4 Gy | 0.23 | |
| PTV1 Conformity Index | 1.19 | 0.04 | 1.33 | 0.06 | 0.002 | 1.31 | 0.06 | 0.002 | |
| PTV2 Conformity Index | 1.12 | 0.05 | 1.29 | 0.10 | 0.002 | 1.29 | 0.08 | 0.002 | |
| PTV1 Homogeneity Index | 0.13 | 0.01 | 0.10 | 0.01 | 0.002 | 0.09 | 0.01 | 0.002 | |
| PTV2 Homogeneity Index | 0.07 | < 0.01 | 0.05 | 0.01 | 0.002 | 0.05 | < 0.01 | 0.002 | |
| Protocol B/Configuration A | Protocol B/Configuration B (Host) | Protocol B/Configuration C | |||||||
| PTV1: D95% > 100% | 99.2% | 1.4% | 0.23 | 99.6% | 0.9% | 99.2% | 1.6% | 0.50 | |
| PTV1: D2% < 105% | 104.0% | 0.2% | 0.69 | 104.0% | 0.3% | 104.4% | 0.3% | 0.02 | |
| CTV1: D99% > 100% | 101.0% | 0.7% | 0.83 | 101.0% | 0.5% | 101.3% | 0.7% | 0.32 | |
| PTV1: Dmax < 107% | 105.2% | 0.3% | 0.13 | 105.5% | 0.7% | 105.7% | 0.3% | 0.70 | |
| Rectum: V65Gy < 20% | 12.6% | 2.8% | 0.11 | 13.6% | 4.0% | 11.7% | 3.3% | 0.002 | |
| Rectum: V70Gy < 10% | 8.5% | 2.1% | 0.004 | 11.0% | 3.6% | 8.1% | 2.4% | 0.002 | |
| Rectum: V75Gy < 5% | 3.6% | 1.2% | 0.002 | 7.6% | 2.7% | 3.5% | 1.2% | 0.002 | |
| RECT_IN_PTV: V79Gy < 1 cm3 | 0.1 cm3 | 0.1 cm3 | 0.004 | 0.5 cm3 | 0.4 cm3 | 0.1 cm3 | 0.1 cm3 | 0.006 | |
| RECT_78_03_NS: V80Gy < 1 cm3 | 0.3 cm3 | 0.2 cm3 | 0.49 | 0.3 cm3 | 0.3 cm3 | 0.5 cm3 | 0.3 cm3 | 0.13 | |
| PTV1 Conformity Index | 1.32 | 0.07 | 0.63 | 1.33 | 0.08 | 1.27 | 0.05 | 0.04 | |
| PTV1 Homogeneity Index | 0.07 | 0.02 | 0.006 | 0.06 | 0.01 | 0.08 | 0.03 | 0.006 | |
| Protocol C/Configuration A | Protocol C/Configuration B | Protocol C/Configuration C (Host) | |||||||
| PTV1imrt: D99% > 95% | 93.9% | 0.7% | 0.006 | 94.9% | 0.9% | 0.85 | 95.0% | 0.6% | |
| PTV1imrt: D0% < 107% | 103.7% | 0.5% | 0.002 | 106.1% | 0.4% | 0.05 | 105.5% | 0.8% | |
| PTV1imrt: D95% > 95% | 96.2% | 0.3% | 0.002 | 97.5% | 0.6% | 0.04 | 97.1% | 0.4% | |
| PTV1: D99.9% > 66.5 Gy | 69.1 Gy | 0.5 Gy | 0.002 | 69.9 Gy | 1.0 Gy | 0.004 | 67.9 Gy | 0.4 Gy | |
| PTV_prostate_imrt: D99% > 95% | 94.0% | 0.9% | 0.02 | 95.0% | 0.9% | 1.000 | 94.9% | 0.6% | |
| PTV_prostate_imrt: D97% > 95% | 95.6% | 0.4% | 0.004 | 96.7% | 0.7% | 0.16 | 96.3% | 0.5% | |
| Rectum: V74Gy < 1 cm3 | 0.5 cm3 | 0.2 cm3 | 0.16 | 0.8 cm3 | 0.3 cm3 | 0.38 | 0.7 cm3 | 0.3 cm3 | |
| PTV1 Conformity Index | 0.99 | 0.02 | 0.002 | 1.11 | 0.02 | 0.70 | 1.11 | 0.03 | |
| PTV1 Homogeneity Index | 0.11 | 0.01 | 0.002 | 0.12 | 0.01 | 0.70 | 0.12 | 0.01 | |
3.1. Protocol A
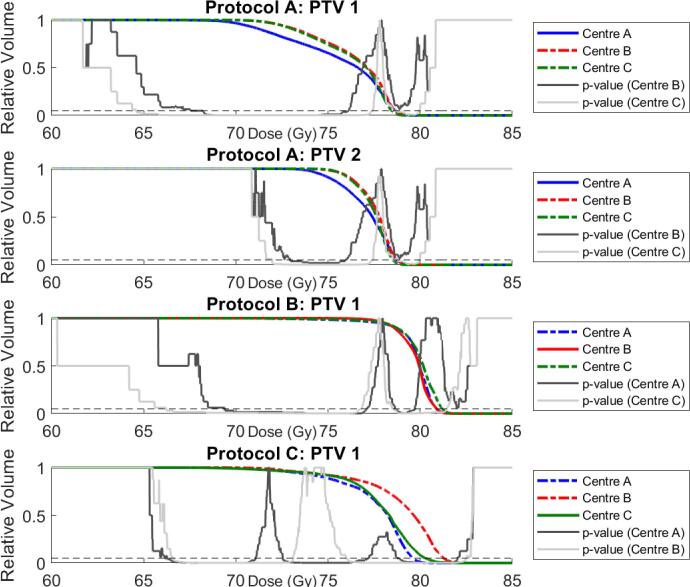
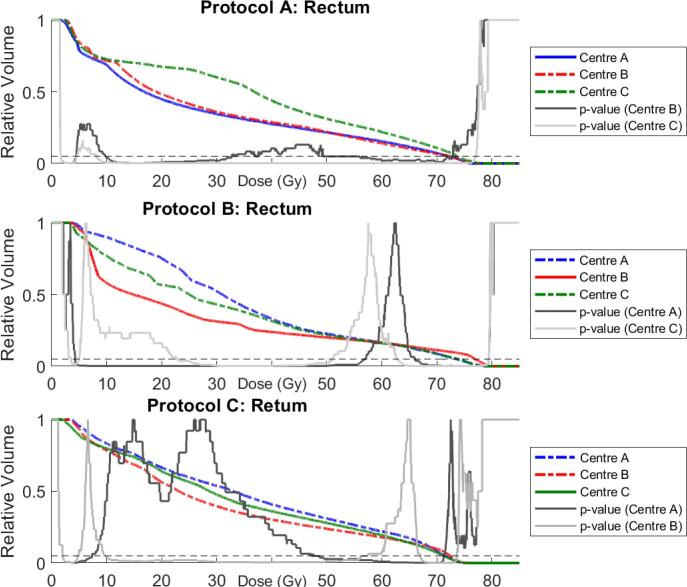
AP Configurations B and C for protocol A patients recorded superior dose coverage across PTV1 and PTV2, shown by an elevated shoulder region within the DVH curve (Fig. 2). The PTV coverage was compensated by superior rectal wall and anal sphincter sparing by the host AP configuration (Table 2). A large difference between AP Configurations A and C is evident in the rectum DVH curves between 10 and 60 Gy (Fig. 3), with V30Gy varying by over 20% (30.4% vs. 52.4%). Both modified AP configurations produced superior femoral head coverage (Table A.5, Figs. A.1, A.2).
Fig. 2.
Median PTV DVHs for all patients for AP Configurations A (blue), B (red), and C (green). Note that the scale begins at 60 Gy for clarity. Solid lines correspond to host-centre AP configuration, modified configurations are dashed. Solid and dotted grey p-value curves, indicating significant differences between population median DVHs, are also illustrated. The dashed black line shows p = 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Median Rectum DVHs for all patients for AP Configurations A (blue), B (red), and C (green). Solid and dotted grey p-value curves again indicate significant differences between population median DVHs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Protocol B
AP Configurations A and C for protocol B recorded statistically significant improvements in comparison to the original treatment plans for rectum V70Gy (8.5%, 8.1% vs. 11.0%) and V75Gy (3.6%, 3.5% vs. 7.6%). Conversely, rectum V40Gy and V50Gy were superior for the host AP configuration (Fig. 3). Dose coverage across PTV1 and CTV1 were superior for the host configuration treatment plans, however differences were generally not statistically significant. AP Configurations A and C produced superior femoral head coverage, although AP Configuration A produced inferior bladder V50Gy and V60Gy (Table A.5, Figs. A.1–A.3).
3.3. Protocol C
AP Configuration A modified for protocol C recorded significantly poorer D95% and D99% for PTV1imrt (96.2%, 93.9%), and D97% and D99% for PTV_prostate_imrt (95.6%, 94.0%) compared to the host configuration treatment plans (97.1%, 95.0%, 96.3%, 94.9% respectively). Rectum V70Gy, V65Gy, V60Gy, and V50Gy were consistently hotter for AP Configuration A (Table A.5). AP Configuration B plans produced superior dose coverage for PTV1imrt (D95% = 97.5%) and PTV1 (D99.9% = 69.9 Gy) compared to the host treatment plans (D95% = 97.1%, D99.9% = 67.9 Gy), although produced hotter PTVimrt (D0% = 106.09%, 105.51% respectively). High rectal dose sparing was superior for the host configuration compared to modified AP Configuration B (V70Gy = 7.19%, 9.5% respectively), but poorer V50Gy (Table A.5, Fig. 3). Femoral Head maximum doses were significantly higher for the modified AP Configurations A and B (Table A.5, Figs. A.1, A.2), although no significant differences between AP configurations was observed for Bladder V50Gy, V60Gy, or V70Gy.
Protocol A and C patients planned with AP Configuration A displayed significantly increased conformity compared to AP Configurations B and C, although for Protocol A this was at the cost of reduced homogeneity (Table 2). Homogeneity and conformity for AP Configurations B and C were consistent across protocols A and C. Protocol B patients displayed the least conformal plans across all AP configurations, with the significant improvements in the modified AP Configuration C plans resulting in reduced homogeneity.
Total constraint/high priority deviations recorded by AP Configurations A, B, and C were 2, 18, and 0 respectively (Table 3). All constraint and high priority deviations occurred for Protocol B patients. Additionally, AP Configurations B and C recorded no deviations for Protocol A patients, compared to 5 medium priority deviations recorded by the host centre. AP Configuration A recorded a significantly larger number of medium priority deviations (35, 15, and 17 respectively), while low priority deviation numbers were consistent between all AP configurations. Specific deviations are given in Table A.6 (supplementary material).
Table 3.
Total deviations for each AP configuration. Specific deviations can be found in Table A.5(supplementary material).
| AP Configuration A |
AP Configuration B |
AP Configuration C |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocol A | Protocol B | Protocol C | Protocol A | Protocol B | Protocol C | Protocol A | Protocol B | Protocol C | |
| Constraint | 1 | 8 | |||||||
| High | 1 | 10 | |||||||
| Medium | 5 | 7 | 23 | 4 | 11 | 4 | 13 | ||
| Low | 1 | 11 | 13 | 11 | |||||
4. Discussion
With only a three-patient training dataset utilised in this study, each centre was successfully able to modify clinical AP configurations to meet distributed prostate radiotherapy protocols. By ensuring the training datasets contained a spread with regards to planning difficulty, the modified AP configurations were able to account for the expected anatomical variety that was encountered in the validation datasets. The ability for developed AP configurations to be modified using a small training dataset has major potential advantages in improving quality assurance during clinical trials, where poor quality manual treatment planning quality can significantly impact clinical trial efficacy [3], [6]. While this improvement is inherently dependent on centres possessing the same treatment planning system, it still represents an improvement in reducing treatment plan variation in these trials.
Across all patients, only a single constraint and high priority deviation were recorded by the modified AP configurations (Table 3). Both deviations occurred for a single patient from centre B, with this patient exhibiting a large overlap (12.5%) between PTV and rectum. As the original clinically accepted treatment plan for this patient also recorded identical protocol deviations, all treatment plans developed from modified AP configurations were considered clinically acceptable.
Treatment plans developed by AP Configuration B delivered higher doses to PTV than those developed by AP Configurations A and C for Protocol C (Table 2, Fig. 2). This contributed in hotter doses being delivered to the rectum (Fig. 3), although differences were often marginal and not significant. Significant differences were seen between AP configurations for intermediate rectum doses, with host-centre treatment plans delivering reduced dose at these volumes for all protocols, except for Protocol C, where AP Configuration B produced lower doses. This is reflective of the different emphasis centres placed on meeting constraint and high priority deviations, with less importance placed on moderate and low priority deviations. Similarly, Figs. A.1 and A.2 show significantly reduced dose to the femoral heads across all protocols by AP Configuration C. Table A.2 shows that AP Configuration C was the only AP configuration that included the femoral heads as objectives.
Multiple constraint and high priority deviations were recorded for protocol B by the host AP configuration. Further inspection revealed a discrepancy between the protocol requirements submitted by centre B for the study, and what was utilised clinically. ln particular, rectum V65Gy, V70Gy, and V75Gy were assigned higher priorities for this study than applied clinically. To account for this, the host AP configuration for protocol B was modified for further analysis to bring it in line with the distributed protocol. The modified AP configuration is given in Table A.7, with new DVH parameters shown in Table A.8 (supplementary material). The modified AP configuration significantly reduced V65Gy, V70Gy, and V75Gy, meeting distributed protocol for all metrics except V75Gy, at the expense of poorer dose coverage. It should be noted that these rectum doses were still significantly warmer than those achieved by AP Configurations A and C for this protocol.
A concern with this type of analysis, particularly with multiple metrics with an inherent dependence to one another, is the detection of false positives from multiple testing. Bonferroni corrections are often employed to account for this, however these corrections risk being too conservative. Just as with the median DVHs, the results presented here are illustrative of differences that arise between AP configurations for the same protocol. As the main aim of this investigation was to assess whether AP configurations could be adapted to meet these protocols, rather than document the extent that these plans differ, it was decided by the authors not to proceed with multiple testing procedures.
A limitation of this study is the lack of qualitative assessment from an experienced radiation oncologist to assess the clinical acceptability of treatment plans developed from the modified AP configurations. As this was an investigation assessing whether AP configurations could be adapted between centres to meet local protocol, quantitative analysis only was proposed. Future work investigating improvements in treatment plan quality through sharing of multiple centre’s AP configurations would require such a qualitative assessment.
Automated treatment planning for prostate radiotherapy is a high interest field, with multiple studies investigating implementation of knowledge-based [22], [23], [29] and template derived [21] treatment planning protocols. Investigations validating the use of RapidPlan (Varian Medical Systems, Palo Alto, USA) have demonstrated the feasibility of sharing models across multiple centres [10], [11]. This study provides further evidence for the viability of sharing clinical protocols and training datasets to aid template based automatic treatment planning of prostate cancer.
Through the distribution of clinical protocols and three patient training datasets centres were able to adapt local AP configurations to satisfy other centre’s protocols. This study shows that AP configurations are readily adaptable to different prostate radiotherapy protocols, and provides a methodology for the sharing of AP configurations across centres to assist efficient implementation and increased uptake of AP in the clinic for the benefit of patients. Future work can then investigate adapting and improving local AP configurations based on the collaboration of multiple centre’s configurations.
Funding
This project was partly funded by NHMRC project grant number 1077788, Danish Cancer Society grant, University of Southern Denmark scholarship and Odense University Hospital scholarship.
Conflict of interest statement
None.
Acknowledgements
The authors would like to thank Lois Holloway and Michael Jameson for their feedback.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.04.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Batumalai V., Jameson M.G., Forstner D.F., Vial P., Holloway L.C. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Pract Radiat Oncol. 2013;3:e99–e106. doi: 10.1016/j.prro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Nelms B.E., Robinson G., Markham J., Velasco K., Boyd S., Narayan S. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2:296–305. doi: 10.1016/j.prro.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Berry S.L., Boczkowski A., Ma R., Mechalakos J., Hunt M. Interobserver variability in radiation therapy plan output: Results of a single-institution study. Pract Radiat Oncol. 2016;6:442–449. doi: 10.1016/j.prro.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore K.L., Brame R.S., Low D.A., Mutic S. Quantitative metrics for assessing plan quality. Semin Radiat Oncol. 2012 doi: 10.1016/j.semradonc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Hansen C., Sykes J., Barber J., West K., Bromley R., Szymura K. Multicentre knowledge sharing and planning/dose audit on flattening filter free beams for SBRT lung. J Phys Conf Ser. 2015 doi: 10.1088/1742-6596/573/1/012018. [DOI] [Google Scholar]
- 6.Peters L.J., O'Sullivan B., Giralt J., Fitzgerald T.J., Trotti A., Bernier J. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 7.Abrams R.A., Winter K.A., Regine W.F., Safran H., Hoffman J.P., Lustig R. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704—a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2012;82:809–816. doi: 10.1016/j.ijrobp.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dühmke E., Franklin J., Pfreundschuh M., Sehlen S., Willich N., Rühl U. Low-dose radiation is sufficient for the noninvolved extended-field treatment in favorable early-stage Hodgkin’s disease: long-term results of a randomized trial of radiotherapy alone. J Clin Oncol. 2001;19:2905–2914. doi: 10.1200/JCO.2001.19.11.2905. [DOI] [PubMed] [Google Scholar]
- 9.Xhaferllari I., Wong E., Bzdusek K., Lock M., Chen J.Z. Automated IMRT planning with regional optimization using planning scripts. J Appl Clin Med Phys. 2013;14:176–191. doi: 10.1120/jacmp.v14i1.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogliata A., Belosi F., Clivio A., Navarria P., Nicolini G., Scorsetti M. On the pre-clinical validation of a commercial model-based optimisation engine: application to volumetric modulated arc therapy for patients with lung or prostate cancer. Radiother Oncol. 2014;113:385–391. doi: 10.1016/j.radonc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Schubert C., Waletzko O., Weiss C., Voelzke D., Toperim S., Roeser A. Intercenter validation of a knowledge based model for automated planning of volumetric modulated arc therapy for prostate cancer. The experience of the German Rapid Plan Consortium. PLoS One. 2017;12:e0178034. doi: 10.1371/journal.pone.0178034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore K.L., Kagadis G.C., McNutt T.R., Moiseenko V., Mutic S. Vision 20/20: automation and advanced computing in clinical radiation oncology. Med Phys. 2014;41 doi: 10.1118/1.4842515. [DOI] [PubMed] [Google Scholar]
- 13.Heijmen B., Voet P., Fransen D., Penninkhof J., Milder M., Akhiat H. Fully automated, multi-criterial planning for Volumetric Modulated Arc Therapy–an international multi-center validation for prostate cancer. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Hazell I., Bzdusek K., Kumar P., Hansen C.R., Bertelsen A., Eriksen J.G. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;17 doi: 10.1120/jacmp.v17i1.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gintz D., Latifi K., Caudell J., Nelms B., Zhang G., Moros E. Initial evaluation of automated treatment planning software. J Appl Clin Med Phys. 2016;17 doi: 10.1120/jacmp.v17i3.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen C.R., Bertelsen A., Hazell I., Zukauskaite R., Gyldenkerne N., Johansen J. Automatic treatment planning improves the clinical quality of head and neck cancer treatment plans. Clin Transl Radiat Oncol. 2016;1:2–8. doi: 10.1016/j.ctro.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krayenbuehl J., Norton I., Studer G., Guckenberger M. Evaluation of an automated knowledge based treatment planning system for head and neck. Radiat Oncol. 2015;10:226. doi: 10.1186/s13014-015-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusters J., Bzdusek K., Kumar P., van Kollenburg P.M., Kunze-Busch M., Wendling M. Automated IMRT planning in Pinnacle. Strahlenther Onkol. 2017;193:1031–1038. doi: 10.1007/s00066-017-1187-9. [DOI] [PubMed] [Google Scholar]
- 19.Tol J.P., Delaney A.R., Dahele M., Slotman B.J., Verbakel W.F. Evaluation of a knowledge-based planning solution for head and neck cancer. Int J Radiat Oncol Biol Phys. 2015;91:612–620. doi: 10.1016/j.ijrobp.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Hansen C.R., Nielsen M., Bertelsen A.S., Hazell I., Holtved E., Zukauskaite R. Automatic treatment planning facilitates fast generation of high-quality treatment plans for esophageal cancer. Acta Oncol. 2017;56:1495–1500. doi: 10.1080/0284186X.2017.1349928. [DOI] [PubMed] [Google Scholar]
- 21.Nawa K., Haga A., Nomoto A., Sarmiento R.A., Shiraishi K., Yamashita H. Evaluation of a commercial automatic treatment planning system for prostate cancers. Med Dosim. 2017 doi: 10.1016/j.meddos.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Nwankwo O., Mekdash H., Sihono D.S.K., Wenz F., Glatting G. Knowledge-based radiation therapy (KBRT) treatment planning versus planning by experts: validation of a KBRT algorithm for prostate cancer treatment planning. Radiat Oncol. 2015;10:111. doi: 10.1186/s13014-015-0416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powis R., Bird A., Brennan M., Hinks S., Newman H., Reed K. Clinical implementation of a knowledge based planning tool for prostate VMAT. Radiat Oncol. 2017;12:81. doi: 10.1186/s13014-017-0814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chanyavanich V., Das S.K., Lee W.R., Lo J.Y. Knowledge-based IMRT treatment planning for prostate cancer. Med Phys. 2011;38:2515–2522. doi: 10.1118/1.3574874. [DOI] [PubMed] [Google Scholar]
- 25.Gallio E., Giglioli F.R., Girardi A., Guarneri A., Ricardi U., Ropolo R. Evaluation of a commercial automatic treatment planning system for liver stereotactic body radiation therapy treatments. Phys Med. 2018;46:153–159. doi: 10.1016/j.ejmp.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Heijmen B.J., Petit S.F. Prospective clinical validation of independent DVH prediction for plan QA in automatic treatment planning for prostate cancer patients. Radiother Oncol. 2017;125:500–506. doi: 10.1016/j.radonc.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Janssen T.M., Kusters M., Wang Y., Wortel G., Monshouwer R., Damen E. Independent knowledge-based treatment planning QA to audit Pinnacle autoplanning. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Wu B., Kusters M., Kunze-busch M., Dijkema T., McNutt T., Sanguineti G. Cross-institutional knowledge-based planning (KBP) implementation and its performance comparison to Auto-Planning Engine (APE) Radiother Oncol. 2017;123:57–62. doi: 10.1016/j.radonc.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Good D., Lo J., Lee W.R., Wu Q.J., Yin F.-F., Das S.K. A knowledge-based approach to improving and homogenizing intensity modulated radiation therapy planning quality among treatment centers: an example application to prostate cancer planning. Int J Radiat Oncol Biol Phys. 2013;87:176–181. doi: 10.1016/j.ijrobp.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Bertelsen A., Hansen C.R., Johansen J., Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol. 2010;95:142–148. doi: 10.1016/j.radonc.2010.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.