Abstract
Background and purpose
Focal tumour boosting is currently explored in radiotherapy of prostate cancer to increase tumour control. In this study we applied dose response models for both tumour control and normal tissue complications to explore the benefit of proton therapy (PT) combined with focal tumour boosting, also when accounting for inter-fractional motion.
Materials and methods
CT scans of seven patients fused with MRI-based index volumes were used. Two volumetric modulated arc therapy (VMAT) plans were created for each patient; one with conventional dose (77 Gy) to the entire prostate, and one with an additional integrated boost (total dose of 95 Gy) to the index lesion. Two corresponding intensity modulated PT (IMPT) plans were created using two lateral opposing spot scanning beams. All plans were evaluated using an MRI-based tumour control probability (TCP) model and normal tissue complication probability (NTCP) models for the rectum and bladder. Plan robustness was evaluated using dose re-calculations on repeat cone-beam CTs.
Results
Across all plans, median TCP increased from 86% (range: 59–98%) without boost to 97% (range: 96–99%) with boost. IMPT plans had lower rectum NTCPs (e.g. 3% vs. 4% for boost plans) but higher bladder NTCPs (20% vs. 18% for boost plans), yet only the bladder NTCPs remained different in the cone beam CT-based re-calculations.
Conclusions
Focal tumour boosting can be delivered with either VMAT or protons, and increases the predicted TCP. The small benefit of IMPT when assessing the planned dose distributions was lost when accounting for inter-fractional motion.
1. Introduction
Radiotherapy (RT) is an important treatment modality for both localised and locally-advanced stages of prostate cancer [1], [2]. Around 35% of intermediate- and high-risk prostate cancer patients present with disease relapse following RT, but trials have shown that this rate can be reduced by RT dose escalation [3], [4], [5]. It has also been shown that local recurrences tend to occur at the location of the macroscopic tumour [6], [7]. The emerging concept of focal tumour boosting is currently explored in clinical prostate cancer trials [8], [9], [10] and involves a radiation boost limited to the macroscopic tumour within the prostate to improve local control without increasing the risk of toxicity of the organs at risk (OARs). Focal boosting of the prostate may become established clinical practise, in turn, making it essential to explore the optimal treatment approach and modality.
The on-going focal prostate tumour boosting trials are performed using photon-based RT. However, proton therapy (PT) may be advantageous due to the physical properties of charged particles, with a sharp increase in depth prior to reaching their well-defined range. Using photons, dose conformity surrounding the tumour is achieved by a re-distribution of low to intermediate doses to a greater volume of the normal tissues. A highly conformal dose around the target volume can be achieved with fewer fields when using PT, which in turn reduces the integral dose thus avoiding the low dose bath [11], [12], [13].
Recent treatment planning studies have compared the feasibility of focal boosting in different RT modalities such as volumetric modulated arc therapy (VMAT), intensity-modulated proton therapy (IMPT) and brachytherapy, and found that this was equally feasible for all modalities up to a certain dose. Andrzejewski et al. found that although OAR doses were higher using focal boosting than for standard treatment, the risk levels were reasonably low for all these three modalities [14]. Kuang et al. investigated focal boosting with VMAT and concluded that planning objectives and dose constraints could be met in the 30 included prostate patients [15]. Yeo et al. compared protons with intensity-modulated RT (IMRT) in a focal boost scenario and found that protons delivered comparable doses to targets and spared normal tissues from intermediate-to-low doses better than IMRT [11].
Internal anatomical variations can have a large impact on the delivered proton dose distributions [16], [17], in particular for focal boost treatments, shifting the high dose volumes away from the tumour and possibly into the normal tissues and ORs [11], [14], [15]. However, none of the previous focal boosting planning studies investigated the robustness towards inter-fractional motion for VMAT or IMPT [11], [14], [15]. Furthermore, biological models might be particularly useful when evaluating the potential clinical implications of dose re-distributions. In recent studies we have therefore developed an apparent diffusion coefficient (ADC) based tumour control probability (TCP) model [18], [19], [20], particularly suited for evaluation of tumour dose escalation/boosting/painting strategies.
The aim of this study was therefore to compare the use of photon- vs. proton-based RT for focal tumour boosting using biological models. More specifically we investigated the robustness of both photons and protons to inter-fractional motion, both in a tumour boost and non-boost scenario.
2. Materials and methods
2.1. Patient and image materials
The study included a cohort of seven prostate cancer patients that received RT at Aarhus University Hospital, Aarhus, Denmark (Group 1), matched to seven patients that had undergone MRI scanning including diffusion weighted imaging (DWI) prior to prostatectomy at Haukeland University Hospital, Bergen, Norway (Group 2). All patients in Group 1 had a planning CT (pCT) with delineations of all relevant volumes of interest (VOIs), as well as weekly cone beam CT (CBCT) scans during the course of RT. All patients in Group 2 had ADC maps calculated from the DWI data, combined with MRI-based masks of the index lesion (GTVindex) and the whole prostate outlined for each patient [18]. We rigidly matched the patient CT data from Group 1 with patient ADC maps from Group 2 with respect to prostate volume and shape (the pairs of seven cases were selected to obtain a minimal prostate volume difference) using the MIM Maestro software (MIM Software Inc., Cleveland, Ohio) in order to obtain seven complete patient data sets consisting of CT scans, repeat CBCTs as well as rectum, bladder and GTV delineations, combined with the MRI-based GTVindex delineations and ADC maps.
2.2. Delineation of volumes of interest
The rectum, bladder, and gross target volume (GTV) of the prostate were delineated in all planning CT scans by a radiation oncologist. For three of the seven patients the same VOIs were delineated for their respective CBCTs. The planning target volume (PTV) was created by adding margins of 7 mm (anterior/posterior/left/right) and 9 mm (cranial/caudal) to the prostate GTV (based on VMAT procedures at our institution). The GTVIndex contours were transferred from the delineations of the multiparametric MRI. Additionally, the normal prostate PTV, i.e. the volume of the prostate PTV outside the index lesion, was defined as the substraction of the GTVIndex from the PTV (PTVsubGTVIndex).
2.3. Treatment planning
Plans with and without boost using both VMAT and IMPT were created for all seven patients using the Eclipse treatment planning system (Varian Medical Systems v13.7, Inc. Palo Alto, CA, USA. For VMAT the anisotropic analytical algorithm v13.7.14 was used, and for IMPT the proton convolution superposition algorithm v13.7.15 was used. All proton plans were coplanar, and the proton plan beam angle configuration was 90°/270° (lateral opposing beams) (Fig. 1). The PTVs for both VMAT and IMPT were prescribed to receive a mean dose of 77 Gy/35 fx, while GTVindex were prescribed (in the boost plans) to receive a mean dose of 95 Gy/35 fx. For all plans dose constraints, as described in Table 1, were fulfilled whenever possible.
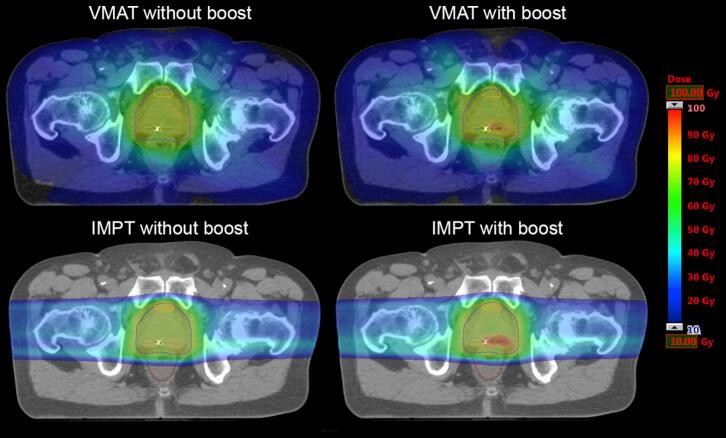
Fig. 1.
Dose distributions. Dose distributions for each modality and arm for the same patient.
Table 1.
Dose constraints and goals for rectum and PTV.
| Priority | Structure | Constraint/goal |
|---|---|---|
| 1 | Rectum | D1cm3 < 74 Gy |
| 1 | V70 Gy ≤ 10% | |
| 2 | PTV | D95% ≥ 73.15 Gy |
2.4. Robustness towards inter-fractional motion
Plans with and without boost using both VMAT and IMPT were created for all seven patients using the Eclipse treatment planning system (Varian Medical Systems v13.7, Inc. Palo Alto, CA, USA. Dose calculations for VMAT were performed using the anisotropic analytical algorithm v13.7.14, while for IMPT the proton convolution superposition algorithm v13.7.15 was used. All proton plans were coplanar, and with a 90°/270° (lateral opposing) two-beam configuration (Fig. 1). The PTVs for both VMAT and IMPT were prescribed to receive a mean dose of 77 Gy in 35 fractions, while GTVindex were prescribed (in the boost plans) to receive a mean dose of 95 Gy in 35 fractions. Dose constraints, as described in Table 1, were fulfilled whenever possible for all plans.
2.5. Data analysis
Dose volume histograms (DVHs) were extracted for all plans and analysed with the tool DVHmetrics [21]. To analyse differences between VMAT and IMPT plans, several parameters were used for the organs at risk: D1cm3, V70Gy as well as the normal tissue complication probability (NTCP) for both the rectum and the bladder. We also compared PTV coverage (D95%) and PTV mean dose between modalities using the Wilcoxon Signed-Rank test when possible (note that PTV coverage for boost plans was evaluated separately at the PTVsubGTVIndex and GTVIndex volumes). Photon-based Lyman-Kutcher-Burman NTCP models were assumed to be applicable for both protons and protons, and were used for both modalities. The rectum parameter applied (TD50 = 76.9 Gy, n = 0.09, and m = 0.13) were for endpoint grade ≥ 2 late toxicity or rectal bleeding [22], while for the bladder (TD50 = 91.0 Gy, n = 0.01, and m = 0.19) the endpoint was obstruction [23]. Differences in tumor control between the different treatment modalities and arms were evaluated using an ADC-based TCP model, including patient-specific tumour densities [18]. TCPs were calculated on the dose distribution of the pCT for seven patients and also for the CBCT-based re-calculations in the three patients.
3. Results
3.1. Treatment plan comparisons on the planning CT
The planned dose distributions (on the pCT) fulfilled the planning constraints for all patients, for both modalities (VMAT and IMPT) and for both arms. However, statistically significant improved PTV coverage for IMPT plans compared to VMAT was found in the boost arm (median D95%: 73.9 Gy vs. 73.4 Gy; p < 0.05). The median TCP increased from 86% (range: 59–98%) without boost to 97% (range: 96–99%) with boost (p < 0.01, Suppl. Fig. 1). Rectum V70Gy was significantly lower in the IMPT plans compared to VMAT for both arms (median: 6.4% vs. 9.3% for non-boost, and 6.4% vs. 8.1% for boost; p < 0.05). The rectum NTCPs were marginally lower for IMPT plans (median: 3% vs. 4% for VMAT, for both arms; p < 0.05). However, VMAT resulted in lower bladder NTCPs (median: 19% vs. 22% for non-boost, and 18% vs. 20% for boost; p < 0.05) (Table 2).
Table 2.
Statistical analysis (Wilcoxon Signed-Rank test) results of the comparison between the pCT metrics of VMAT and IMPT (boost and non-boost cases) for all seven patients. The value in each cell is the median of the metric values for IMPT and VMAT for both arms. The bold-faced values indicate a significant difference (p < 0.05) in favour of IMPT, while the underlined values are in favour of VMAT.
| Rectum |
Bladder | PTV |
Index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arm | Modality | NTCP (%) |
D1cm3 (Gy) |
V70Gy (%) |
NTCP (%) |
D95% (Gy) |
DMEAN (Gy) |
D95% (Gy) |
DMEAN (Gy) |
| Boost | VMAT | 4 | 73.8 | 8.1 | 18 | 73.4 | 77.5 | 92.0 | 96.3 |
| IMPT | 3 | 73.5 | 6.4 | 20 | 73.9 | 77.0 | 91.1 | 94.6 | |
| Non-boost | VMAT | 4 | 73.6 | 9.3 | 19 | 73.7 | 77.4 | – | – |
| IMPT | 3 | 73.3 | 6.4 | 22 | 73.8 | 76.7 | – | – | |
3.2. Dose re-calculations on CBCTs
In the dose re-calculations on the CBCTs, there was a small difference between IMPT and VMAT with respect to the mean dose of the PTV in the boost arm (median: 77.5 Gy (IMPT) and 76.9 Gy (VMAT)). As expected, there was a large difference between arms also when comparing TCPs in the CBCT re-calculations, with a median of 78% (range: 59–95%) for non-boost plans, which increased to a median of 95% (range: 93–97%) for boost plans (Suppl. Fig. 2). Across the re-calculations, VMAT resulted in better high-dose sparing of the rectum for both arms, e.g. with a median D1cm3 of 73.6 Gy compared to 75.6 Gy for IMPT for the boost arm. Also, the bladder NTCPs were lower for VMAT across the repeat scan re-calculations for both arms; e.g. for the boost arm the median NTCP was 17% for VMAT and 20% for IMPT). No other metrics showed any significant differences between IMPT and VMAT (Table 3).
Table 3.
Results of the comparison between the CBCT metrics of VMAT and IMPT (boost and non-boost cases) for three patients. The value in each cell is the median of the metric values for IMPT and VMAT for both arms.
| Rectum |
Bladder | PTV |
Index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arm | Modality | NTCP (%) |
D1cm3 (Gy) |
V70Gy (%) |
NTCP (%) |
D95% (Gy) |
DMEAN (Gy) |
D95% (Gy) |
DMEAN (Gy) |
| Boost | VMAT | 5 | 74.6 | 8.7 | 17 | 73.0 | 76.9 | 92.3 | 96.3 |
| IMPT | 5 | 75.6 | 7.8 | 20 | 73.2 | 77.5 | 92.6 | 96.6 | |
| Non-boost | VMAT | 5 | 73.2 | 9.5 | 18 | 73.3 | 76.5 | – | – |
| IMPT | 4 | 74.7 | 7.9 | 22 | 72.7 | 76.6 | – | – | |
The comparison between planned distributions (on pCTs) and re-calculated distributions (on CBCTs) did not show any particular difference, for none of the modalities nor arms, except for a small difference in mean PTV dose for non-boost IMPT plans (Suppl. Table 3).
4. Discussion
In this study, we have explored the potential benefit of spot scanning PT (IMPT) compared to contemporary photon-based RT (VMAT) in a focal tumour boosting scenario for patients with prostate cancer, and in particular how robust the modalities are towards inter-fractional motion. Despite some variations, the overall finding was that the modalities performed equally well with respect to achieving the prescribed dose and coverage of the PTV and GTVIndex. We found that the small benefit of IMPT when assessing the planned dose distributions (in the pCTs) was lost when considering the re-calculated doses.
A considerably higher TCP was found for the boost arm, for both IMPT and VMAT, compared to plans without boost. Similar results were also seen in the paper by Kuang et al. [15] and Zamboglou et al. [24] in the case of VMAT, using a different TCP model than ours. In our case, the large difference between the boost arms and non-boost arms might be due to the inclusion of tumour-index information together with ADC maps-based cell density, which increases the inter-patient tumour response differentiation, resulting in TCP curves with a larger range in D50% across the cohort compared with those based on uniform cell densities [18].
Previous planning studies (not accounting for inter-fractional motion) have also compared IMPT to VMAT for focal tumour boosting [14] and sparing of normal tissues/OARs [12], [13], [14] and found a similar performance for both modalities. Furthermore, other studies have shown that PT to the prostate alone can be robust towards inter-fractional changes, which was also what was seen in our study [17], [25], [26], [27]. It should be mentioned that the patients used in this study were treated with photons, i.e. the fixation and set-up methods were optimised for this modality. The robustness of IMPT might have been improved with a different and stricter patient fixation/set-up. Also, studies have shown that the robustness of proton beams change depending on the beam angles used [16], [26], [28]. Hence, the common practice of lateral beam angles also applied in the present study may not have been optimal in terms of robustness towards inter-fractional motion. In addition, accumulating evidence suggests that the relative biological effectiveness (RBE) of proton beams deviates from a constant factor of 1.1 as recommended by the ICRU (ICRU, 2007) but instead varies [29], [30] – in particular towards the distal end of the Bragg peak of the proton beam. In turn, this could potentially influence the outcome of PT both in terms of increased RBE-weighted doses to the normal tissue and OARs, as well as higher RBE to the prostate and index lesion. Also, different beam angle configurations might make proton beams more robust towards inter-fractional changes, however, studies have shown that use of lateral beam angle configurations might influence the RBE of the proton beams to a larger degree, in particular at the locations of the OARs [31].
Early results from the FLAME trial show no difference in toxicity and quality of life between the experimental (boost) arm and the standard arm [32]. Therefore, focal boosting of prostate tumours of up to 95 Gy with photon therapy seems safe. This was also what our planning study indicates for both photon therapy and PT, as there were no significant differences between boost and non-boost arms in terms of normal tissue sparing. Focal boosting of the prostate may become established clinical practise, making it essential to explore the optimal treatment approach and modality for focal boosting.
One weakness of the study is the fusing of the two cohorts based on the size and volume of the prostate in each group. This was done to provide “complete” data sets in the most realistic way, instead of e.g. using randomly generated/shaped index lesions. In addition, the rigid transfer of index lesions was also done to achieve a more realistic set of placements for the lesions compared to randomly placing them in the prostate.
Another limitation of the study is the HU override in the repeat CBCTs. This was done to avoid dose calculation issues caused by the limited image quality of the CBCTs (e.g. scattering). This way we could better isolate the effects of inter-fractional motion. It should also be pointed out that the plan comparison DVH metrics used in this study were based on our departmental photon therapy protocol. The proton plans were also made without the use of robust optimisation, and also without evaluating the plans for robustness towards range uncertainties (e.g. with the tool for this in our treatment planning system); this was outside the scope of our study. From a statistical point of view, the small sample size makes it hard to draw strong conclusions, in particular on the investigations of inter-fractional motion.
In conclusion, focal tumour boosting can be delivered with either VMAT or protons, and increases the predicted TCP. There were minor differences between the two modalities, also with respect to robustness to inter-fraction motion. The small benefit of IMPT when assessing the planned dose distributions was lost when considering re-calculated doses on repeat CBCTs.
Acknowledgments
Acknowledgements
Per Rugaard Poulsen, Department of Medical Physics, Aarhus University Hospital/Aarhus University, Aarhus, Denmark, is thanked for his advice and feedback in the study. This study was supported by The Danish Cancer Society (Grant number: R150-A10071).
Disclosure statement
No potential conflict of interest was reported by the authors.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.06.002.
Appendix A. Supplementary data
References
- 1.Hamdy F.C., Donovan J.L., Lane J.A., Mason M., Metcalfe C., Holding P. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 2.Donovan J.L., Hamdy F.C., Lane J.A., Mason M., Metcalfe C., Walsh E. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters S.T.H., Heemsbergen W.D., Koper P.C.M., van Putten W.L.J., Slot A., Dielwart M.F.H. Dose-response in radiotherapy for localized prostate cancer: results of the dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 4.Widmark A., Klepp O., Solberg A., Damber J.-E., Angelsen A., Fransson P. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 5.Pollack A., Zagars G.K., Starkschall G., Antolak J.A., Lee J.J., Huang E. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 6.Cellini N., Morganti A.G., Mattiucci G.C., Valentini V., Leone M., Luzi S. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 7.Pucar D., Hricak H., Shukla-Dave A., Kuroiwa K., Drobnjak M., Eastham J. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69:62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 8.Lips I.M., van der Heide U.A., Haustermans K., van Lin E.N.J.T., Pos F., Franken S.P.G. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials. 2011;12:255. doi: 10.1186/1745-6215-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack A, Abramowitz M. A Phase III Trial of Hypofractionated External Beam Image-Guided Highly Targeted Radiotherapy: The HEIGHT Trial, https://clinicaltrials.gov/ct2/show/study/NCT01411332 [accessed 31 May 2018].
- 10.Chung P. Tumor TARGET Prostate Cancer, https://clinicaltrials.gov/ct2/show/NCT01802242?term=NCT01802242&rank=1 [accessed 31 May 2018].
- 11.Yeo I., Nookala P., Gordon I., Schulte R., Barnes S., Ghebremedhin A. Passive proton therapy vs. IMRT planning study with focal boost for prostate cancer. Radiat Oncol. 2015;10:213. doi: 10.1186/s13014-015-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle L.A., Studenski M., Harvey A., Dicker A.P., Xiao Y. Dosimetric comparison of VMAT, IMRT and proton therapy for post-prostatectomy radiation therapy for prostate cancer (abstr) Int J Radiat Oncol Biol Phys. 2010;78:806. [Google Scholar]
- 13.Rana S., Cheng C., Zheng Y., Risalvato D., Cersonsky N., Ramirez E. Proton therapy vs. VMAT for prostate cancer: a treatment planning study. Int J Part Ther. 2014;1:22–33. [Google Scholar]
- 14.Andrzejewski P., Kuess P., Knäusl B., Pinker K., Georg P., Knoth J. Feasibility of dominant intraprostatic lesion boosting using advanced photon-, proton- or brachytherapy. Radiother Oncol. 2015;117:509–514. doi: 10.1016/j.radonc.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Kuang Y., Wu L., Hirata E., Miyazaki K., Sato M., Kwee S.A. Volumetric modulated arc therapy planning for primary prostate cancer with selective intraprostatic boost determined by 18F-choline PET/CT. Int J Radiat Oncol Biol Phys. 2015;91:1017–1025. doi: 10.1016/j.ijrobp.2014.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen A.G., Casares-Magaz O., Muren L.P., Toftegaard J., Bentzen L., Thörnqvist S. A method for evaluation of proton plan robustness towards inter-fractional motion applied to pelvic lymph node irradiation. Acta Oncol. 2015;54:1643–1650. doi: 10.3109/0284186X.2015.1067720. [DOI] [PubMed] [Google Scholar]
- 17.Thörnqvist S., Muren L.P., Bentzen L., Hysing L.B., Hoyer M., Grau C. Degradation of target coverage due to inter-fraction motion during intensity-modulated proton therapy of prostate and elective targets. Acta Oncol. 2013;52:521–527. doi: 10.3109/0284186X.2012.752860. [DOI] [PubMed] [Google Scholar]
- 18.Casares-Magaz O., van der Heide U.A., Rørvik J., Steenbergen P., Muren L.P. A tumour control probability model for radiotherapy of prostate cancer using magnetic resonance imaging-based apparent diffusion coefficient maps. Radiother Oncol. 2016;119:111–116. doi: 10.1016/j.radonc.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Raidou R.G., van der Heide U.A., Dinh C.V., Ghobadi G., Kallehauge J.F., Breeuwer M. Visual analytics for the exploration of tumor tissue characterization. Comput Graph Forum. 2015;34:11–20. [Google Scholar]
- 20.Casares-Magaz O., Raidou R.G., Rørvik J., Vilanova A., Muren L.P. Uncertainty evaluation of image-based tumour control probability models in radiotherapy of prostate cancer using a visual analytic tool. Phys Imag Radiat Oncol. 2018;5:5–8. doi: 10.1016/j.phro.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollschlager D, Karle H, Schmidberger H. DVHmetrics: Analyze Dose-Volume Histograms and Check Constraints, https://cran.r-project.org/package=DVHmetrics [accessed 31 May 2018].
- 22.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:123–129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thor M., Olsson C., Oh J.H., Petersen S.E., Alsadius D., Bentzen L. Urinary bladder dose-response relationships for patient-reported genitourinary morbidity domains following prostate cancer radiotherapy. Radiother Oncol. 2016;119:117–122. doi: 10.1016/j.radonc.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboglou C., Sachpazidis I., Koubar K., Drendel V., Wiehle R., Kirste S. Evaluation of intensity modulated radiation therapy dose painting for localized prostate cancer using 68 Ga-HBED-CC PSMA-PET/CT: a planning study based on histopathology reference. Radiother Oncol. 2017;123:472–477. doi: 10.1016/j.radonc.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Vees H., Dipasquale G., Nouet P., Zilli T., Cozzi L., Miralbell R. Pelvic lymph node irradiation including pararectal sentinel nodes for prostate cancer patients: treatment optimization comparing intensity modulated X-rays, volumetric modulated arc therapy, and intensity modulated proton therapy. Technol Cancer Res Treat. 2015;14:181–189. doi: 10.7785/tcrt.2012.500405. [DOI] [PubMed] [Google Scholar]
- 26.Cuaron J.J., Harris A.A., Chon B., Tsai H., Larson G., Hartsell W.F. Anterior-oriented proton beams for prostate cancer: a multi-institutional experience. Acta Oncol. 2015;54:868–874. doi: 10.3109/0284186X.2014.986288. [DOI] [PubMed] [Google Scholar]
- 27.Trofimov A., Nguyen P.L., Coen J.J., Doppke K.P., Schneider R.J., Adams J.A. Radiotherapy treatment of early-stage prostate cancer with imrt and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch K., Andersen A.G., Casares-Magaz O., Petersen J.B.B., Bentzen L., Thörnqvist S. Biological modelling based assessment of organ motion during photon vs. proton therapy for locally advanced prostate cancer. Acta Oncol. 2017;56:839–845. doi: 10.1080/0284186X.2017.1317107. [DOI] [PubMed] [Google Scholar]
- 29.Grassberger C., Paganetti H. Varying relative biological effectiveness in proton therapy : knowledge gaps versus clinical significance versus clinical significance. Acta Oncol. 2017;56:761–762. doi: 10.1080/0284186X.2017.1316516. [DOI] [PubMed] [Google Scholar]
- 30.Paganetti H., Niemierko A., Ancukiewicz M., Gerweck L.E., Goitein M., Loeffler J.S. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen J., Petersen J.B.B., Stokkevåg C.H., Ytre-Hauge K.S., Flampouri S., Li Z. Biological dose and complication probabilities for the rectum and bladder based on linear energy transfer distributions in spot scanning proton therapy of prostate cancer. Acta Oncol. 2017;56:1413–1419. doi: 10.1080/0284186X.2017.1373198. [DOI] [PubMed] [Google Scholar]
- 32.Van Vulpen M., Van Loon J., Pos J., Haustermans K., Smeenk R., Van den Bergh L. FLAME randomised trial: 95Gy MRI-boost vs 77Gy prostate radiotherapy: toxicity and quality of life (abstr) Radiother Oncol. 2016;119:132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.