Abstract
Background and purpose
Spinal stereotactic body radiotherapy (SBRT) involves large dose gradients and high geometrical accuracy is therefore required. The aim of this work was to assess residual intra-fraction error with a tracking robotic system for non-immobilized patients. Shifts from the first alignment (i.e. mimicking the unavailability of tracking) were also quantified.
Materials and methods
Forty-two patients treated for spinal metastasis (128 fractions, 4220 images) were analyzed. Residual error was quantified as the difference between translations/rotations referring to consecutive x-ray images during delivery (tracking) and to the initial set-up (no-tracking). The error distribution for each fraction/patient and the entire population was assessed for each axis/rotation angle. The impact of lesion sites, fractionation and patient’s pain (VAS score) were investigated. Finally, the dosimetric impact of residual motion was quantified in the four most affected fractions.
Results
Mean overall errors (OE) were near 0 (SD < 0.1 mm). Residual translations/rotations >1 mm/1° were found in less than 1.5%/1% of measurements. Lesion site and fractionation showed no impact. The dosimetric impact in the most affected fractions was negligible. For “no-tracking”, mean OE was <1 mm/0.5°; less than 2% of displacements were >2 mm/1° within 10 min from the start of treatment with an increasing probability of shifts >2 mm over time. A significantly higher fraction of OE ≥ 2 mm was found for patients with pain in case of no-tracking.
Conclusions
Spine tracking with a latest-generation robotic system is highly efficient for non-immobilized patients: residual error is time independent and close to 0. For delivery times >7–8 min, tracking should be considered as mandatory for non-immobilized patients.
Keywords: Spine SBRT, Residual error, Cyberknife
1. Introduction
3D-conformal radiotherapy (3DCRT) delivering palliative doses (i.e.: in the range of 8–10 Gy in one fraction up to 30 Gy in 10–15 fractions), is a well-recognized standard of care for symptomatic patients with spinal metastases. However, a relatively low rate of complete response and a limited pain relief after 3–6 months were correlated to this treatment approach [1], [2], [3], [4].
Higher doses and individualized treatments have been recommended in this patient setting. The safety and efficacy of stereotactic body radiotherapy (SBRT) for spinal metastases have been demonstrated in retrospective and prospective phase I/II trials, showing higher rates of local control and faster and longer pain relief [5], [6], [7], [8], [9]. The issue is of particular relevance in oligometastatic patients even due to the potentially relevant impact on the oncological outcome [5], [8], [9]
Given the delivery of high doses in a small number of fractions (typically 1–5) and the proximity of the spinal cord dose distributions with steep dose gradients outside the planning target volume (PTV) need to be delivered. Consequently, in order to avoid potential adverse effects [10], [11], [12], [13], sufficiently high geometric accuracy is required.
Advanced in-room image-guidance techniques are intended to reduce the impact of inter-and intra-fraction movements during treatment delivery. Tracking, widely used in the treatment of spinal lesions [14], [15], [16], [17], [18], [19], enables to continuously monitor any vertebral target motion. Clearly, these potential misalignments and their impact on the delivered dose distributions are time and patient-dependent. Although invasive immobilization devices could help reduce intra-fraction shifts, non-invasive devices may be advisable for better patient comfort and ease of use, especially for pain-prone patients. A simple and easy positioning was generally chosen for patients treated with robotic spinal SBRT, due to both the relatively long time spent in treatment position (typically 30–60 min overall) and to frequently reported pain.
The aim of this study was to assess residual intra-fraction patient motion in the delivery of spinal radiosurgery with a robotic system for non-immobilized patients using a fiducial–free tracking approach. Possible correlations between residual errors and clinical/geometrical parameters were also explored. The impact of the residual motion on the planned dose distribution was investigated for representative fractions, by considering both the effect on clinical target volume (CTV) coverage and on organ-at-risk (OARs) sparing. Moreover, the intra-fraction motion relative to the first alignment (i.e. mimicking the absence of tracking) was quantified and its time dependency assessed.
2. Materials and methods
2.1. Patient characteristics, contouring and planning
Data from 42 patients treated from October 2017 through November 2018 for thoracic (27 patients), lumbar (21 patients), cervical (2 patients) and sacral (3 patients) spinal metastases using SBRT with a robotic system were retrospectively analyzed. Seven patients received multiple treatments to different sites. Fifty-four treatments were analyzed overall: 27 delivered in a single fraction, 17 in three fractions and 10 in five fractions. All treated patients signed an informed consent form for therapy and permission for publication of disease-related information in accordance with the Declaration of Helsinki.
No immobilization devices were used for the patients considered, apart from two patients treated for cervical lesions that were immobilized with a thermoplastic mask; patients were supine-positioned with arms down at the sides with a knee fix cushion. Primary tumor site and localization of the metastases treated were reported in Table S1. In the same table, the Visual Analog Scale (VAS) score for patient pain [20] registered by the physician before SBRT was reported.
CTV and OAR contouring followed the RTOG 0631 guideline [21]. Both CT and T1-T2 weighted MR scans were generally available. If MRI scans were not available, the spinal cord was defined as the spinal canal (6/42 patients). A 2 mm expansion from CTV was used for PTV definition, and a 2 mm expansion was used to generate the planning organ at risk volume (PRV) from the spinal cord. A median prescribed daily dose of 18, 8 and 5 Gy was delivered for single, three and five fraction schedules, respectively.
The prescribed dose generally referred to the 70%-80% isodose level aiming to obtain V100%>95% for PTV coverage. Due to the proximity of the spinal cord, part of the PTV could have been underdosed. OAR constraints for spinal cord and other OARs (mostly esophagus, cauda, trachea) were based on the AAPM Task Group 101 [22]. Treatment times were generally within 45–50 min (single fraction) or 30–35 min (multifraction).
2.2. Image guidance for spine tracking
The S7 Cyberknife® (CK) system [Accuray, Inc] was used in this study. The image-guided targeting system was combined with a 6MV linear accelerator coupled to a robotic manipulator that allowed six degrees of freedom. The imaging system used two x-ray generators mounted to the ceiling of the treatment room and two amorphous silicon x-ray detectors [23], [24], [25], [26]. A fiducial-free tracking approach was used: stereoscopic x-ray live images of the patients’ spine were compared with planned DRRs using a grid-based, automatic non rigid technique [27], [28]. In short, displacements to be applied were computed at an increased resolution level by means of an intensity-based image registration evaluated on an ROI grid (mesh) with 81 nodes positioned on the skeletal structure near the target. The algorithm calculated the overall displacements by averaging the detected offsets on each node for all translational and rotational coordinates: x (cranio-caudal), y (left-right), z (anterior-posterior), roll, pitch and yaw. The time between consecutive corrections could be chosen by the operator and was generally adapted to consider the specific compliance and pain of every patient.
For the investigated cohort, the time between consecutive controls ranged between 30 s and 2 min (median value 1 min). Translations >10 mm and rotations >1° (roll, pitch) and 3° (yaw) were set to stop the delivery, and generally, required a user operation to manually move the couch and/or reposition the patient.
2.3. Intra-fraction patient motion quantification: the “tracking” and “no-tracking” approach
Residual error analysis during tracking dealt with the shifts between consecutive images: for each control, the residual error was quantified as the difference between measured translational and rotational displacements and the previous values, as previously described [19]. In this way, any potential effect of shifts between two consecutive corrections was safely taken into account.
Similarly, intra-fraction motion was also investigated, simulating the patient’s position as if tracking were not being employed. Here shifts were quantified as the difference between translational and rotational displacements at a given treatment time and at time zero (alignment). All displacements due to treatment interruptions or to couch movements were individually verified and corrected, in order to take into account only patient motion during delivery. An overall number of 4220 images were analyzed, with a number of controls/corrections per session between 15 and 68.
2.4. Data analysis
The error distribution for each single session, each patient and the entire population was assessed for all translational and rotational coordinates, for both the tracking and no-tracking scenarios. The mean residual error for the population and its standard deviation were calculated by averaging the mean residual errors of every treatment session, weighting each treatment session of multi-fraction patients so as to be the same as a single fraction session. Possible correlations between residual errors and clinical/geometrical parameters were explored: bone lesion site, fractionation (first fraction vs others) and patient pain (VAS = 0 vs VAS > 0) were considered. Overall 3D-error defined as sqrt(x^2 + y^2 + z^2) was considered for these evaluations. The non-parametric Mann Whitney test was used to test the significance of the investigated correlations.
2.5. Dosimetric impact of intra-fractional patient motion
The dosimetric impact of intra-fraction patient motion was quantified considering the worst case scenarios within our population. Four sessions referring to four patients were chosen: the two sessions with the highest mean residual error and the two sessions with the highest single offsets occurring at least one time during delivery. The translational coordinates of the original planned beams were edited based on the estimated displacements generating a new beamset. The displacements were then applied assuming that every beam delivered between two consecutive images was affected by the same (maximum) error. Based on the new beamset, the original optimized plan was then recalculated by obtaining a dose distribution representative of those actually delivered. Planned and recalculated dose distributions were then compared, both in terms of target coverage and critical organ sparing.
3. Results
3.1. Description of intra-fraction motion
Single session data clearly showed that a fraction of patients experienced large (>5mm) shifts during delivery (counterbalanced by the tracking) at increasing treatment times. These effects were visible when tracking was not considered and showed some continuous shift over time, leading to systematic deviations in a few patients or to random large isolated shifts. Results are shown in the Supplementary material (Figs. S1 and S2): as an example, in Fig. S2, three typical patterns were shown for stable patients (76/128 sessions) (Fig. S2a), large isolated shifts (16/128, 14/16 after 10 min from the start of the treatment) (Fig. S2b) and systematic trends in one direction (36/128) (Fig. S2c). The large amount of information was summarized below, mostly focusing on the population errors considering and not considering tracking correction.
3.2. Intra-fraction patient motion: “no tracking” approach
A mean shift <1 mm and <0.5° was estimated for translations and rotations respectively (Table 1). The maximum average displacement was 0.6 mm for the left-right direction (Δy). Of note, the population SDs for translations ranged between 0.9 and 1.3 mm, reflecting a non negligible intra-fraction motion if tracking was not applied.
Table 1.
Mean residual error and SD with and without considering tracking corrections. x, y and z refer to cranio-caudal, lateral and anterior-posterior translational coordinates, respectively.
| Mean | SD | |
|---|---|---|
| Without tracking | ||
| Δx [mm] | −0.1 | 0.9 |
| Δy [mm] | 0.6 | 1.3 |
| Δz [mm] | 0.4 | 1.1 |
| Δroll [°] | 0.0 | 0.3 |
| Δpitch [°] | 0.0 | 0.3 |
| Δyaw [°] | −0.1 | 0.4 |
| With tracking | ||
| Δx [mm] | 0.0 | 0.1 |
| Δy [mm] | 0.0 | 0.1 |
| Δz [mm] | 0.0 | 0.1 |
| Δroll [°] | 0.0 | <0.1 |
| Δpitch [°] | 0.0 | <0.1 |
| Δyaw [°] | 0.0 | <0.1 |
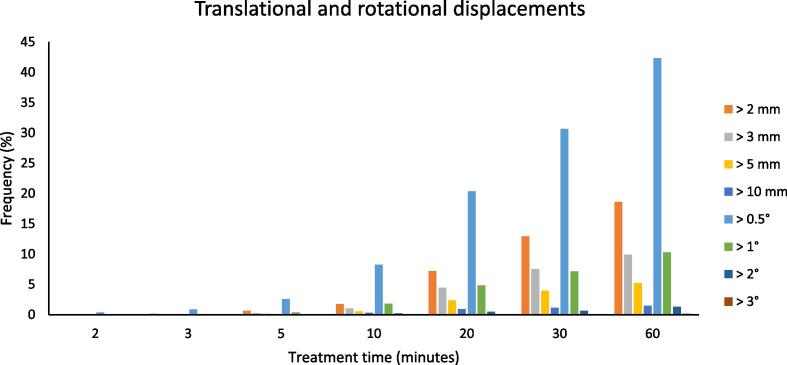
Investigating the dependence between recorded displacements and delivery time from the first alignment (Fig. 1), can be noted that less than 2% of shifts were >2 mm (and rotations >1°) within 10 min from the start of the treatment. Instead, for longer treatment times the increase in frequency of the largest displacements is evident: after 30 min from the first alignment, the frequency of translations >2 mm was around 13% and the frequency of rotations >1° around 10%.
Fig. 1.
Data in the case of tracking was not applied: frequency of translational shifts larger than 2, 3, 5 and 10 mm vs treatment time (all translations have been grouped together) and Frequency of rotational shifts larger than 0.5, 1, 2, 3° vs treatment time (all rotations have been grouped together).
3.3. Intra-fraction patient motion: tracking approach
A mean error close to 0 was found with a SD < 0.1 mm, for the tracking scenario and for the entire population (Table 1).
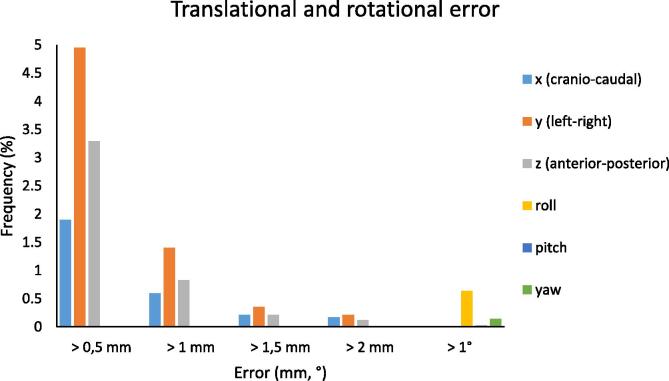
As reported in Fig. 2, translational displacements >0.5 mm occurred with 2%, 5% and 3% frequency in the cranio-caudal, lateral and anterior-posterior directions, respectively; frequency of shifts >1 mm drops to 0.6%, 1.4% and 0.9% correspondently, while shifts >1.5 mm and 2 mm were nearly negligible. For rotations, displacements >1° occur within a frequency lower than 0.7%. Lateral shifts (y coordinate) and roll displacements were the most affected.
Fig. 2.
Data in the case of tracking. Frequency of translational shifts larger than 0.5, 1, 1.5 and 2 mm (all translations have been grouped together) and frequency of rotational shifts larger than 1° (all rotations have been grouped together).
No significant differences (Mann Whitney test p > 0.05) were found for the mean 3D-error estimated at the first fraction compared with that found in each of the following sessions. A mean 3D-error of around 0.1 mm was estimated for multi-fractions scheduling (3–5 fractions), with a mean 3D-error of 0.1 mm, for both the first session and the third and final session.
Similar considerations can be made regarding the lesion site; no statistically significant differences were found between thoracic (T3-T12) and lumbar treatment sites (L1-L5). Similar shifts <0.1 mm and <0.01° were found in both patient groups for translational and rotational displacements, with a 3D-error of around 0.1 mm.
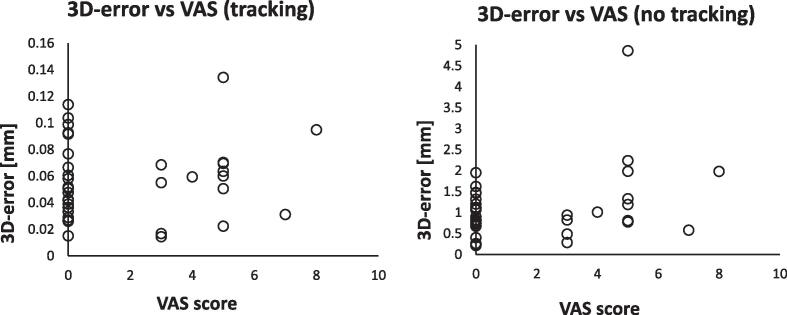
Concerning patient pain, no significant differences (p > 0.05) were found comparing patients with VAS = 0 and with VAS > 0 in the case of tracking approach; a mean 3D-error of 0.1 mm was estimated in both groups. Differently, a significantly (p = 0.03) higher fraction of 3D-errors ≥ 2 mm were found for patients with VAS score > 0 (4/14) compared to those with VAS = 0 (0/23) in the case of no-tracking approach (Fig. 3).
Fig. 3.
3D-error vs VAS score for the tracking and no-tracking scenario.
3.4. Dosimetric impact of residual error with tracking
Two sessions showing the highest mean residual errors (0.13 mm and 0.12 mm for the left-right coordinate) and two sessions including the highest single offsets (7.6 mm, 10.7 mm and 10.9 mm for the first patient and 6.5 mm, 12.3 mm and 12.9 mm for the second patient referring to the cranio-caudal, left-right and anterior-posterior coordinates respectively) were considered.
No clinically significant differences between the planned and delivered dose distributions were found in the selected fractions: for all patients, D0.03cc for spinal cord differed less than 0.5 Gy, V100 for targets differed less than 1% (Table S3). In Fig. 4 an example of the effect on the difference between planned and effectively delivered dose distributions is shown.
Fig. 4.
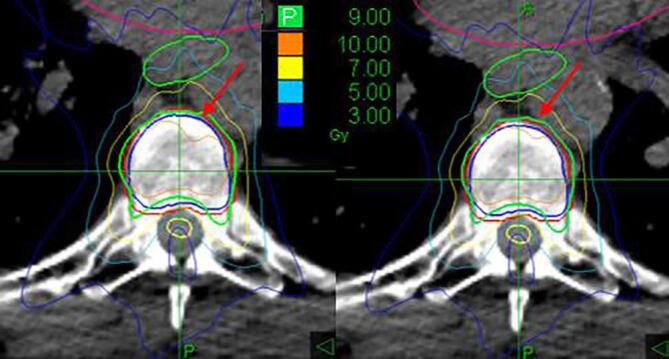
Recalculated dose distribution (left) and planned dose distribution (right). The green line corresponds to the 9 Gy isodose (prescription). The blue contour represents the CTV and the red contour the PTV (CTV + 2 mm).
4. Discussion
The current investigation focused on quantifying residual error for spine SBRT patients treated with a latest generation robotic system without using immobilization devices. Tracking images were used to calculate residual error both after tracking and also simulating the case that tracking was not available. The dosimetric impact of residual error was quantified in four selected “worst” fractions.
A mean shift <1 mm and <0.5° was found for translations and rotations, respectively, when tracking was not considered. A mean error close to 0 with an SD within 0.1 mm was estimated when considering tracking. A negligible dosimetric impact was found.
The quantification of residual error for SBRT treatments to spinal metastasis has been the focus of several published works [19], [29], [30], [31], [32], [33], [34], [35], [36], [37], most referring to immobilized patients. The availability of tracking data for the current investigation provided information on the patients’ movements throughout the entire treatment. This is useful not only to test our delivery procedure and the effectiveness of the tracking technique but also to estimate the amount of time for which treatment delivery without tracking may be considered reasonably safe. Consequently, our results should be useful also for institutions delivering SBRT without tracking.
Considering the “no tracking” approach, we found a very low rate (~2%) of large shifts (translation >2 mm, rotations > 1°) within 10 min from the start of the treatment. This result is quite consistent with those of Hoogeeman et al. [31], where 95% of the observed displacements were within 3 mm for an interval of 15 min. Similar conclusions were also recently reported in a paper by Wu et al. [36], where treatment was stopped due to intra-fractional observed motion >2 mm in 6/1019 treatment sessions (median treatment time ~ 29 min).
Not surprisingly, our results clearly show a significantly increased number of large shifts if patients are not immobilized, showing a 13% rate of large translations (vs < 1% of the Wu paper) after 30 min, if tracking is not applied. This result suggests the need for extreme caution in avoiding immobilization if tracking is not available: the time between the first set-up and the occurrence of large shifts is estimated at around 10 min. Similar conclusions were reported by Svestad et al. [35] and Hyde et al. [32]: Svestad et al. [35] did not report intra-faction shifts >2 mm within 10 min using CBCTs. A slightly longer “safe” time was reported by Hyde et al. [32], suggesting intra-fraction imaging and correction every 15–20 min for immobilized patients.
Based on our results, patients with pain (VAS > 0) have a higher chance of experiencing larger residual error whitout tracking. A stricter imaging protocol should be considered in such cases; this is indeed the case for non-immobilized patients for whom, to our knowledge, no studies have attempted to separately analyze residual error according to “pain scores” such as the VAS.
A major result of our study was that the residual intra-fraction error with a latest generation robotic accelerator and spine tracking is almost negligible, with mean 3D-errors close to zero for both translations and rotations and SD < 0.1 mm and <0.02° respectively. These results were consistent with most results reported in similar studies [19], [32], [34], [35], considering both different patient immobilization systems and different imaging monitoring approaches and confirmed that tracking is highly effective even without immobilization. This finding is not completely new [19], [37] but represents a valuable confirmation using updated technology and, in our opinion, confirms the benefit of tracking in rendering the set-up and delivery of spine SBRT easier and more comfortable for patients (especially those with pain). Similarly to Fürweger et al. [19] and Hyde et al. [32], lateral shifts and roll displacements proved to be the most affected components.
Results referring to thoracic and lumbar spine were did not show significant differences, as similarly reported by others [19], [32]. Significantly greater intra-fraction motion was, however, reported for cervical spine lesions by Yamoah et al. [33] suggesting greater C-spine mobility and/or a suboptimal mask immobilization system. As only two patients with cervical lesions were included in our cohort, this point could not be investigated.
Based on the large set of patient treatments considered, including both single and multiple sessions, a comparison between mean residual errors in different treatment sessions for patients treated with multi-fractions scheduling (3–5 sessions) was performed and no significant differences were found between the residual error estimated at the first fraction compared to the subsequent sessions. This confirms the accuracy of the tracking technique, which is able to guarantee the same, safe patient position even in the first treatment session, when patients may feel less relaxed.
Although the median overall residual error is quite low, large sudden shifts may occur, with a potential impact on the delivered dose distribution compared to the planned one. To investigate this, two kinds of worst case scenarios (in four different patients) were considered, including sudden shifts above 10 mm. The translational shifts were included in the treatment plan assuming, in a very conservative approach, that every beam included between two consecutive images was affected by the same final error. Similar to that reported by Fürweger [19], no significant dosimetric impact was found for the two worst case patient, with maximum differences of around 1% between planned and delivered dose distributions. A larger impact was, however, reported in an early work by Chuang et al. [30], where differences of up to 4% were estimated, above all for critical structures. As a robust approximation, we recalculated the effective delivered dose distribution taking only translations into account, assuming a very slight impact from rotational displacements: following the approach by Fürweger et al. [19], the maximum possible contribution of rotations in terms of translations of the target was calculated. To do so, we considered the highest roll, pitch and yaw registered shifts (5.5°, 2°, 3.1°, respectively) within our entire population, and calculated the equivalent translational displacements for a target point 25 mm from the tracking center, which is located centrally to the lesion. In this extreme condition, a maximum translational error of 1.5 mm was estimated (compared to the 7.9–12.9 mm translations of the worst cases considered), supporting the robustness of the applied (only translation) approximation for recalculation.
Although the negligible dosimetry impact of intra-fraction residual error based on worst case scenarios, cannot cover all the situations, it clearly corroborated the safety of the margin (2 mm) applied at our Institute. On the other hand, this margin should be sufficient to include any targeting error due to the intrinsic geometric accuracy of the CK spine tracking system, estimated to be within 0.5 mm (±0.2 mm), as confirmed by our periodic QA.
In conclusion, residual error in the case of spine SBRT after tracking with a last generation robotic system is almost null and does not affect dose distribution. Maintaining a margin of 2 mm for non-immobilized patients with a robotic spine tracking system is redundantly safe, even considering the intrinsic uncertainty of the system. Based on our findings, when delivery time exceeds 7–8 min tracking should be considered as mandatory for non-immobilized patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper. The research leading to these results has received funding from AIRC under IG 2019 – ID. 23150 project – P.I. Fiorino Claudio.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2020.09.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Howell D.D., James J.L., Hartsell W.F., Suntharalingam M., Machtay M., Suh J.H. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97–14. Cancer. 2013;119:888–896. doi: 10.1002/cncr.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Party B.P.T. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- 3.Rades D., Lange M., Veninga T., Stalpers L.J.A., Bajrovic A., Adamietz I.A. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 4.Roos D.E., Turner S.L., O'Brien P.C., Smith J.G., Spry N.A., Burmeister B.H. Trans-tasman radiation oncology group, TROG 96.05. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75:54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 6.Tseng C.L., Soliman H., Myrehaug S., Lee Y.K., Rushin M., Atenafu E.G. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiation therapy (SBRT) Int J Radiat Oncol Biol Phys. 2018;102:499–507. doi: 10.1016/j.ijrobp.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Sprave T., Verma V., Förster R., Schlampp I., Brukner T., Bostel T. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128:274–282. doi: 10.1016/j.radonc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Zeng K.L., Tseng C.L., Soliman H., Weiss Y., Shagal A., Myrehaug S. Stereotactic body radiotherapy (SBRT) for oligometastatic spine metastases: an overview. Front Oncol. 2019;9:337. doi: 10.3389/fonc.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glicksman R.M., Tjong M., Neve W.P.F., Jr., Spratt D.E., Chua K.L.M., Mansouri A. Stereotactic ablative radiotherapy for the management of spinal metastatses. A review. JAMA Oncol. 2020;6:567–577. doi: 10.1001/jamaoncol.2019.5351. [DOI] [PubMed] [Google Scholar]
- 10.Bishop A.J., Tao R., Rebueno N.C., Christensen E.N., Allen P.K., Wang X.A. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–1026. doi: 10.1016/j.ijrobp.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Lovelock D.M., Zhang Z., Jackson A., Keam J., Bekelman J., Bilsky M. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77:1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsoulakis E., Jackson A., Cox B., Lovelock M., Yamada Y. A detailed dosimetric analysis of spinal cord tolerance in high-dose spine radiosurgery. Int J Radiat Oncol Biol Phys. 2017;99:598–607. doi: 10.1016/j.ijrobp.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 13.Lijun M., Wang L., Lee Y., Tseng C.L., Soltys S., Braunstein S. Correlation between small-volume spinal cord doses for spine stereotactic body radiotherapy (SBRT) J Radiosurg SBRT. 2018;5:229–236. [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon G.J., Nasr N.M., Liao J.J., Molzahn I., Marsh D., McRae D. Treatment of Spinal Tumors using Cyberknife fractionated stereotactic radiosurgery: pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery. 2009;64:297–306. doi: 10.1227/01.NEU.0000338072.30246.BD. [DOI] [PubMed] [Google Scholar]
- 15.Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases: clinical experience in 500 Cases from a single institution. Spine. 2007;32:193–999. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 16.Sahgal A., Larson D.A., Chang E.L. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 17.Gerszten P.C., Burton S.A., Welch W.C., Brufsky A.M., Lembersky B.C., Ozhasoglu C. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104:2244–2254. doi: 10.1002/cncr.21467. [DOI] [PubMed] [Google Scholar]
- 18.Kim N., Lee H., Kim J.S., Baek J.G., Lee C.G., Chang S.K. Clinical outcomes of multileaf collimator- based Cyberknife for spine stereotactic body radiation therapy. Br J Radiol. 2017;90:20170523. doi: 10.1259/bjr.20170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furweger C., Drexler C., Kufeld M., Muacevic A., Wowra B., Schlaefer M.K. Patient motion and targeting accuracy in robotic spinal radiosurgery: 260 single-fraction fiducial-free cases. Int J Radiat Oncol Biol Phys. 2010;78:937–945. doi: 10.1016/j.ijrobp.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 20.McCormack H.M., Horne D.J., Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 21.Ryu S., Pugh S.L., Gerszten P.C., Yin F.F., Timmerman R.D., Hitchcock V.J. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1–3) spine metastases: phase 2 results. Pract Radiat Oncol. 2014;4:76–81. doi: 10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict S.H., Yenice K.M., Followill D., Galvin J.M., Hinson W., Kavanagh B. Stereotactic body radiation therapy: the report of AAPM task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 23.Yu C., Main W., Taylor D., Kuduvalli G., Apuzzo M.L., Adler J.R. An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Jr Neurosurgery. 2004;55:1138–1149. doi: 10.1227/01.neu.0000141080.54647.11. [DOI] [PubMed] [Google Scholar]
- 24.Ho A.K., Fu D., Cotrutz C., Hancock S.L., Chang S.D., Gibbs I.C. A study of the accuracy of cyberknife spinal radiosurgery using skeletal structure tracking. Neurosurgery. 2007 doi: 10.1227/01.NEU.0000249248.55923.EC. [DOI] [PubMed] [Google Scholar]
- 25.Muacevic A., Staehler M., Drexler C., Wowra B., Reiser M., Tonn J.C. Technical description, phantom accuracy, and clinical feasibility for fiducial-free frameless real-time image-guided spinal radiosurgery. J Neurosurg Spine. 2006;5:303–312. doi: 10.3171/spi.2006.5.4.303. [DOI] [PubMed] [Google Scholar]
- 26.Fürweger C., Drexler C., Kufeld M., Muacevic A., Wowra B. Advances in fiducial –free image –guidance for spinal radiosurgery – a phantom study. J Appl Clin Med Phys. 2010;12:3446. doi: 10.1120/jacmp.v12i2.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M.J., Cox R.S. The accuracy of dose localization for an image-guided frameless radiosurgery system. Med Phys. 1996;23:2043–2049. doi: 10.1118/1.597771. [DOI] [PubMed] [Google Scholar]
- 28.Murphy M.J. An automatic six-degree-of-freedom image registration algorithm for image-guided frameless stereotaxic radiosurgery. Med Phys. 1997;24:857–866. doi: 10.1118/1.598005. [DOI] [PubMed] [Google Scholar]
- 29.Murphy M.J., Chang S.D., Gibbs I.C., Le Q.T., Hai J., Kim D. Pattern of patient movement during frameless image-guided radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1400–1408. doi: 10.1016/s0360-3016(02)04597-2. [DOI] [PubMed] [Google Scholar]
- 30.Chuang C., Sahgal A., Lee L., Larson D., Huang K., Petti P. Effects of residual taget motion for image-tracked spine radiosurgery. Med Phys. 2007;348:4484–4490. doi: 10.1118/1.2790587. [DOI] [PubMed] [Google Scholar]
- 31.Hoogeman M.S., Nuyttens J.J., Levendag P.C., Heijmen B.J.M. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–618. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 32.Hyde D., Lochray F., Korol R., Davidson M., Wong C.S., Ma L. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degree of freedom. Int J Radiat Oncol Biol Phys. 2012;82:e555–e562. doi: 10.1016/j.ijrobp.2011.06.1980. [DOI] [PubMed] [Google Scholar]
- 33.Yamoah K., Zaorsky N.G., Siglin J., Shi W., Werner-Wasik M., Andrews D.W. Spine stereotactic body radiation therapy residual setup errors and intra-fraction motion using stereotactic x-ray image guidance verification system. Int J Med Phys Clin Eng Radiat Oncol. 2014;3:1–8. doi: 10.4236/ijmpcero.2014.31001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Z., Bondenson J.C., Lewis J.H., Mannarino E.G., Friesen S.A., Wagar M.M. Evaluation of initial setup accuracy and intrafraction motion for spine stereotactic body radiation therapy using stereotactic body frames. Pract Radiat Oncol. 2016;6:e17–e24. doi: 10.1016/j.prro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Svestad J.G., Ramberg C., Skar B., Hellebust T.P. Intrafractional motion in stereotactic body radiotherapy of spinal metastases utilizing cone beam computed tomography image guidance. Phys Imag Radiat Oncol. 2019;12:1–6. doi: 10.1016/j.phro.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Wu J., Ballangrud A., Mechalakos J., Yamada J., Lovelock D.M. Frequancy of large intrafractional target motions during spine stereotactic body radiation therapy. Pract Radiat Oncol. 2020;10:e45–e49. doi: 10.1016/j.prro.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Hazelaar C., Dahele M., Mostafavi H., van der Weide L., Slotman B.J., Verbakel W.F.A.R. Subsecond and submillimetre resolution positional verification for stereotactic irradiation of spinal lesions. Int J Radiat Oncol Biol Phys. 2016;94:1154–1162. doi: 10.1016/j.ijrobp.2016.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.