Abstract
Background and purpose
Adjuvant radiation therapy (RT) of the whole breast (WB) is still the standard treatment for early breast cancer. A variety of radiation techniques is currently available according to different delivery strategies. This study aims to provide a comparison of six treatment planning strategies commonly adopted for breast-conserving adjuvant RT and to use the Pareto concept in an attempt to assess the degree of plan optimization.
Materials and methods
Two groups of six left- and five right-sided cases with different dose prescriptions were involved (22 patients in total). Field-in-Field (FiF), two and four Fields static-IMRT (sIMRT-2f and sIMRT-4f), Volumetric-Modulated-Arc-Therapy (VMAT), Helical Tomotherapy (HT) and Static-Angles Tomotherapy (TomoDirect™ – TD) were planned. Dose volume constraints were taken from the RTOG protocol 1005. Pareto fronts were built for a selected case to evaluate the reliability of the plan optimization process.
Results
The best target dose coverage was observed for TD able to improve significantly (p < 0.01) the V95% in a range varying from 1.2% to 7.5% compared to other techniques. The V105% was significantly reduced up to 2% for HT (p < 0.05) although FiF and VMAT produced similar values. For the ipsilateral lung, V5Gy, V10Gy and Dmean were significantly lower than all other techniques (p < 0.02) for TD while the lowest value of V20Gy was observed for HT. The maximum dose to contralateral breast was significantly lowest for TD (p < 0.02) and for FiF (p < 0.05). Minor differences were observed for the heart in left-sided patients. Plans for all tested techniques were found to lie on their respective Pareto fronts.
Conclusions
Overall, TD provided significantly better results in terms of target coverage and dose sparing of ipsilateral lung with respect to all other evaluated techniques. It also significantly minimized dose to contralateral breast together with FiF. Pareto front analysis confirmed the reliability of the optimization for a selected case.
Keywords: Treatment planning, Whole-breast irradiation, Planning comparison, Pareto front
1. Introduction
Post-operative whole breast (WB) irradiation is the standard treatment for early-stage breast cancer and it reduces the 10-year risk of recurrence by one third and the 15-year risk of breast cancer death of almost one fifth [1], yet the use of radiation is still potentially associated to an increased risk of pneumonitis [2], fatal cardiac effects [3], acute and late skin reactions [4], as well as of a second breast cancer [5].
The treatment of WB using a photon tangential wedged field technique is still widely used within radiotherapy departments [6]. Although this approach gives excellent local control [7], in general it does not provide good results in terms of planning target volume (PTV) homogeneity and this issue becomes significant when hypofractionated schemes are adopted [8]. Intensity Modulated Radiation Therapy (IMRT) demonstrated its potential to improve PTV dose homogeneity [9], [10], [11] together with the dose reduction to organs at risk (OAR) [12] and, more recently, this was further improved by means of modern IMRT solutions such as Volumetric Modulated Arc-Therapy (VMAT) [13], [14], [15], [16], [17], Helical Tomotherapy (HT) [17], [18], [19], [20] and Static-Angles Tomotherapy, TomoDirect™ (TD) [17], [21], [22], [23].
When comparing irradiation techniques, the clinical implications of the resulting dosimetry are rarely conclusive since different factors play an important role, from the ability of the planner to the accuracy of the calculation algorithm. In principle, dose comparison studies should be designed to compare plans calculated with the same dose algorithm and optimized according to the Pareto concept [24]. Even if huge improvements have been made in treatment planning systems (TPS) to provide optimization with the Pareto concept, suboptimal results have been documented [25]. Therefore, manual Pareto optimization remains the best method to obtain accurate optimized plans albeit this is too time-consuming to use on all cases in clinical practice. However, it can be used for a limited number of cases as a quality control of the plan optimization process.
The aim of this study was to compare the treatment plans designed for WB irradiation with six radiotherapy techniques commonly implemented in clinical practice: Field-in-Field (FiF), static-IMRT using two and four fields (s-IMRT-2f and s-IMRT-4f, respectively), VMAT, HT and TD. Comparison was focused on PTV coverage and homogeneity as well as on OARs sparing according to pre-defined dose-volume objectives. In order to improve the robustness of the comparison, the degree of optimization of s-IMRT-4f, VMAT, HT and TD plans for one selected case was assessed according to their respective bi-dimensional Pareto fronts built for the tradeoff between PTV coverage and ipsilateral lung mean dose. Although several planning comparisons concerning breast treatment have already been published, they included only some of the techniques presented in this paper. To our knowledge, this is the first time they are compared all together.
2. Materials and methods
2.1. Patient selection
Twenty-two patients were randomly selected from our database to populate two groups (G1 and G2) of eleven patients each, according to different fractionation: 50 Gy in 25 fractions (G1) and 42.4 Gy in 16 fractions (G2). These schemes are currently adopted in our clinical practice, in particular the hypofractionated treatment is preferred for patients of age >60 years old. Each group was populated by six left- and five right-sided cases. No ethics committee approval was needed for the study where patients’ planning CT scans were retrospectively involved. An expert radiation oncologist delineated all contours in the axial CT slices using Velocity software (Varian, PaloAlto, USA). The Clinical Target Volume (CTV) of the WB was considered to be all glandular breast tissue and the tissue encompassed in a wire placed clinically at the time of CT acquisition taken in free breathing conditions. PTV was defined by adding a 5-mm isotropic margin to the CTV. The mean PTV volumes and SD were 1240 ± 644 cm3 and 1060 ± 249 cm3 for G1 and G2, respectively, thus covering a full range of breast volumes [26]. A further “PTVeval” structure was introduced by retracting the PTV 5 mm from the body surface to be used for PTV dose comparison purposes in order to avoid any surface dose calculation differences between algorithms. The OARs were the ipsilateral lung (IL), contralateral lung (CL), contralateral breast (CB) and heart (H). Mean lung volumes (MLV) and SD were 2368 ± 509 cm3 and 2877 ± 621 cm3 for G1 and G2, respectively. DVH constraints were taken from the RTOG 1005 protocol [27] and were adapted according to the fractionation schemes as summarized in Table 1. The original values were modified to be stricter, because no additional dose was planned for the lumpectomy cavity.
Table 1.
Dose volume objectives defined for PTVeval and OARs in terms of ideal (first column) and acceptable (second column) values to be achieved. Dmax is defined in one calculation voxel. VxGy <y% stands for “the volume receiving x Gy should be less than y% of its total”.
| Structure | Ideal value | Acceptable value |
|---|---|---|
| PTV eval | V95% > 95% | V90% > 90% |
| D2% < 105% | D2% < 110% | |
| Contralateral Breast | Dmax < 310 cGy | Dmax < 496 cGy |
| V186cGy < 5% | V310cGy < 5% | |
| Ipsilateral Lung | V20Gy < 20% | V20Gy < 25% |
| V10Gy < 25% | V10Gy < 30% | |
| V5Gy < 40% | V5Gy < 50% | |
| Contralateral Lung | V5Gy < 5% | V5Gy < 10% |
| Heart (left sided) | V20Gy < 5% | V25Gy < 5% |
| V10Gy < 15% | V10Gy < 20% | |
| Dmean < 4 Gy | Dmean < 5 Gy |
2.2. Treatment planning techniques
Six treatment plans were generated on each CT study resulting in 132 plans overall. A medical physicist with a strong experience in treatment planning and knowledge of the TPS designed all plans involved in the study. To avoid any possible bias, no plan template nor class solutions were applied, that is each plan was optimized by choosing the most appropriate dose volume constraints and gantry angle. The radiation oncologist finally approved each treatment plan.
FiF, s-IMRT-2f, s-IMRT-4f and VMAT plans were created for Elekta Synergy Linac (Elekta AB, Stockholm, Sweden) with a 10-mm leaf width MLC.
FiF plans were generated with six to eight 6MV photon tangential beams using XiO TPS (version 5.00.01, Elekta AB, Stockholm, Sweden). The planning technique used two classic tangential fields at a first instance, with the sequential addition of further opposing tangential beam couples sharing the same isocenter and gantry position in order to reduce PTV overdosage. Additional tangential beams were reduced in size keeping a minimum equivalent field size >3 × 3 cm2. Dose was calculated using the superposition algorithm with a dose grid size of 3 × 3 × 3 mm3.
sIMRT-2f and sIMRT-4f plans were generated with Monaco TPS (version 5.00.04, Elekta AB, Stockholm, Sweden) employing Monte Carlo algorithm with a dose-grid size of 3 × 3 × 3 mm3 and a statistical uncertainty of 0.5%. Two and four 6MV photon tangential fields were used, respectively. For sIMRT-4f, beams were spaced 10° apart from one another at each breast side. The step-and-shoot segmentation option with a minimum segment area of 2 cm2 and a minimum number of MU per segment of 4 was used.
VMAT plans were also generated with Monaco TPS using two to four 6MV photon arcs with a span of 210° with 120 control points per arc and a minimum segment width of 2 cm.
HT and TD plans were created for the TomoHDA system (Accuray, Sunnyvale, USA) with TomoEdge™ option [28]. For both techniques, plans were generated using the convolution/superposition algorithm (Version 5.1.0.4, Accuray, Sunnyvale, USA) with a dose-grid size of 2.2 × 2.2 × 3 mm3.
HT plans were designed with a field width of 2.5 cm, a pitch of 0.287, and a modulation factor between 2 and 3. No blocking structure was employed to avoid angular beam irradiation.
TD plans were designed using four tangential beams, two at each breast side with 10° angular spacing with a field width of 2.5 cm, a pitch of 0.25, and a modulation factor between 2 and 3.
For all techniques except HT, gantry angles were chosen to minimize the direct irradiation of the CB.
All plans were designed by the same planner who is typically involved in clinical planning using the aforementioned techniques. For inverse planning techniques, the optimization workflow was divided in three sequential steps: optimization of the PTV until an ideal DVH in terms of coverage and homogeneity is obtained, optimization of all OARs in order to fulfill the dose-volume objectives reported in Table 1, and further optimization of the PTV if compromised without violating the OARs dose-volume objectives.
2.3. Plan analysis
To avoid possible differences caused by various DVH computing algorithms, dose distributions were exported to Velocity where the DVHs of the treatment plans were recomputed.
Mean values of parameters reported in Table 1 were used to compare the different planning techniques and for statistical analysis. In addition, for the PTVeval, the conformity index (CI) and the homogeneity index (HI) were also computed (see Supplementary material for definitions).
2.4. Statistical analysis
The Wilcoxon matched-paired signed-rank test was used to compare the dose-volume results calculated from the planning techniques with a significance level p ≤ 0.05.
2.5. Plan quality analysis
To summarize the differences between techniques a Plan Quality Score (PQS) was arbitrarily chosen and assigned to each plan. A score of ±1 (+ best, − worst result) was assigned to calculated parameters that had a difference that was statistically significant with respect to all others and a score of ±0.5 (+ best, − worst result) to calculated parameters statistically significant compared to at least four others. Otherwise, 0 was assigned. Techniques that performed better returned higher scores.
2.6. Pareto front evaluation
Pareto fronts were built for four techniques (s-IMRT 4f, VMAT, HT and TD) for one selected case of G1. They were not built for FiF (forward planning technique) and s-IMRT 2f (smaller number of available beamlets). Bidimensional Pareto fronts represented the trade-off between V95% of PTVeval and the mean dose of the IL. The dose to all others OARs was held constant according to the original plan so that a dose deviation of a maximum of 0.5 Gy on average and maximum doses was accepted. The plans were obtained by gradually increasing the penalty values for the IL objective [29], [30].
If the original plan lied on the front, its optimization was considered mathematically optimal at least concerning the dose tradeoff evaluated.
3. Results
Overall, all the techniques were able to meet the acceptable dose volume objectives with the exception of CB maximum dose which was found slightly higher than the acceptable value for some of the techniques, as it will be summarized in the following.
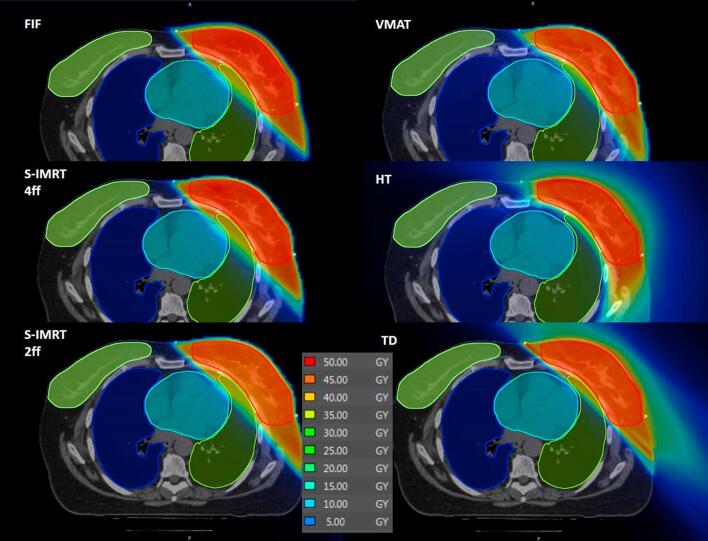
Fig. 1 shows the dose distributions of a single left-sided patient. The lowest number of Monitor Units (MUs) was observed for FiF technique in G1 and G2 groups (360/422 ± 43/55 MU, respectively, p < 0.05), VMAT required 875/1010 ± 95/99 MU, respectively, to deliver the treatments while the two IMRT techniques lied in between. In terms of treatment time, HT techniques required 660/710 s ± 58/63 s as the longest beam-on-time (BOT) (p < 0.01), followed by TD with 390/411 s ± 90/110 s. FiF resulted as the fastest technique in terms of BOT with 96/111 s ± 15/18 s (p < 0.01).
Fig. 1.
Dose distributions of the six different techniques for a patient of G1.
3.1. PTVeval
No significant difference (p = 0.42) was observed between the mean PTVeval volumes.
All techniques fulfilled the dose objectives of D2% and V90% in both groups (Table 2, Table 3). In contrast, the objective V95% >95% was not achieved by FIF in G1 and by s-IMRT 2f in both groups.
Table 2.
Detailed comparison of dose distributions in G1 and statistically significant differences (p < 0.05) for each technique versus alternatives: a Test vs FiF; b Test vs s-IMRT 4f; c Test vs s-IMRT 2f; d Test vs VMAT; e Test vs HT; f Test vs TD. Best results are reported in bold while worst results are reported in italic. Values are underlined if they were statistically significant versus at least four out of five alternative techniques.
| Objective |
FIF (a) | s-IMRT 4f (b) | s-IMRT 2f (c) | VMAT (d) | HT (e) | TD (f) | |||
|---|---|---|---|---|---|---|---|---|---|
| Ideal | Acceptable | ||||||||
| PTVeval | D2% (Gy) | 52.5 | 55 | 52.0 ± 0.2b,e | 52.3 ± 0.5a,e | 52.4 ± 0.5e | 52.1 ± 0.5e | 51.1 ± 0.4a,b,c,d,f | 52.0 ± 0.5e |
| V90% (%) | – | 90 | 99.1 ± 1.6 | 99.5 ± 0.4 | 98.7 ± 1.6 | 99.4 ± 0.7 | 99 ± 0.6 | 99.6 ± 0.5 | |
| V95% (%) | 95 | – | 94.5 ± 4.2c,f | 95.0 ± 2.2c,f | 91.3 ± 5.9a,b,d,e,f | 96.7 ± 3.2c | 96.5 ± 2.1c,f | 98.8 ± 1.5a,b,c,e | |
| Contralateral Breast | D5% (cGy) | <186 | <310 | 70.5 ± 31.7b,c,d,e | 174.9 ± 73.1a,d,e,f | 179.0 ± 65.2a,d,e,f | 337.8 ± 81.0a,b,c,f | 324.8 ± 62.6a,b,c,f | 77.0 ± 21.6b,c,d,e |
| Dmax (cGy) | <310 | <496 | 691.3 ± 200.5 | 510.4 ± 264.5f | 464.4 ± 187.6e,f | 617.0 ± 209.8f | 636.7 ± 195.2c,f | 290.9 ± 103.2b,c,d,e | |
| Ipsilateral Lung | V5Gy (%) | <40 | <50 | 24.1 ± 5.7d,e,f | 26.6 ± 6.0 d,e,f | 24.3 ± 6.9d,e,f | 32.5 ± 7.4f | 37.1 ± 8.0f | 20.1 ± 3.9a,b,c,d,e |
| V10Gy (%) | <25 | <30 | 19.3 ± 4.9f | 18.3 ± 6.3f | 16.9 ± 5.6d,f | 19.8 ± 5.8c,f | 18.1 ± 6.0f | 14.2 ± 5.0a,b,c,d,e | |
| V20Gy (%) | <20 | <25 | 15.1 ± 4.2b,c,d,e,f | 12.1 ± 5.0a | 11.7 ± 5.1a,f | 10.9 ± 4.3a | 9.1 ± 3.4a | 9.6 ± 3.6a,c | |
| Contralateral Lung | V5Gy (%) | <5 | <10 | 0.0 ± 0.0d,e | 0.0 ± 0.0d,e | 0.0 ± 0.0d,e | 8.5 ± 10.4a,b,c,f | 6.8 ± 2.5a,b,c,f | 0.2 ± 0.5d,e |
| Heart (left-sided) | V20Gy (%) | <5% | – | 7.0 ± 4.5d,e,f | 4.3 ± 2.3e | 3.4 ± 1.9e | 2.4 ± 2.1a | 1.3 ± 1.8a,b,c,f | 3.2 ± 3.6a,e |
Table 3.
Detailed comparison of dose distributions in G2 and statistically significant differences (p < 0.05) for each technique versus alternatives: a Test vs FiF; b Test vs s-IMRT 4f; c Test vs s-IMRT 2f; d Test vs VMAT; e Test vs HT; f Test vs TD. Best results are reported in bold while worst results are reported in italic. Values are underlined if they were statistically significant versus at least four out of five alternative techniques.
| Objective |
FIF (a) | s-IMRT 4f (b) | s-IMRT 2f (c) | VMAT (d) | HT (e) | TD (f) | |||
|---|---|---|---|---|---|---|---|---|---|
| Ideal | Acceptable | ||||||||
| PTVeval | D2% (Gy) | 44.2 | 46.3 | 44.2 ± 0.2b,e | 44.3 ± 0.2a,e | 44.4 ± 0.4e | 44.1 ± 0.4e | 43.1 ± 0.3a,b,c,d,f | 43.6 ± 0.3e |
| V90% (%) | – | 90 | 99.8 ± 0.2 | 99.7 ± 0.3 | 99.6 ± 0.5 | 99.9 ± 0.1 | 99.3 ± 0.5 | 99.8 ± 0.2 | |
| V95% (%) | 95 | – | 95.5 ± 1.4b,f | 97.7 ± 0.8c,f | 94.2 ± 3.1a,b,d,e,f | 98.0 ± 0.8c | 96.9 ± 0.1c,f | 99.2 ± 0.4a,b,c,e | |
| Contralateral Breast | D5% (cGy) | <186 | <310 | 37.4 ± 18.8b,c,d,e,f | 165.2 ± 50.1a,c,d,e | 124.8 ± 27.8a,b,d,e | 355.5 ± 97.9a,b,c,f | 297.8 ± 37.3a,b,c,f | 87.4 ± 72.3a,d,e |
| Dmax (cGy) | <310 | <496 | 252.6 ± 215.1b,d,e | 539.7 ± 155.6a,f | 427.0 ± 255.0d | 620.9 ± 206.7a,c,f | 551.5 ± 179.2a,f | 290.8 ± 103.8b,d,e | |
| Ipsilateral Lung | V5Gy (%) | <40 | <50 | 25.4 ± 5.5d,e | 29.2 ± 7.3 d,f | 24.7 ± 6.8 d | 36.7 ± 4.6a,b,c,f | 30.3 ± 8.9a,f | 19.1 ± 3.6b,d,e |
| V10Gy (%) | <25 | <30 | 20.2 ± 5.1 e,f | 20.1 ± 4.7f | 17.1 ± 5.9 | 21.5 ± 5.7e,f | 14.9 ± 5.5 a,d,f | 13.0 ± 4.4a,b,d | |
| V20Gy (%) | <20 | <25 | 15.0 ± 3.7b,c,d,e,f | 12.4 ± 3.6a,e,f | 11.8 ± 4.5a,f | 11.3 ± 5.1a,e,f | 8.0 ± 3.2a,b,d | 7.9 ± 3.8a,b,c,d | |
| Contralateral Lung | V5Gy (%) | <5 | <10 | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 3.4 ± 6.8e | 5.6 ± 1.3a,b,c,d,f | 0.2 ± 0.5e |
| Heart (left-sided) | V20Gy (%) | <5% | – | 2.7 ± 2.3b,c,d,e,f | 1.4 ± 2.1a | 1.0 ± 1.2a,f | 0.1 ± 0.2a,f | 0.5 ± 0.2a | 1.3 ± 1.0a,c,d |
The lowest significant mean PTVeval dose value was observed for HT in both groups (p < 0.05). The best significant PTVeval dose coverage was achieved for TD in both groups (p < 0.01). s-IMRT 2f produced the lowest significant values of D98% and V95% in both groups (p < 0.01). HT significantly produced the lowest values of D2% in both groups (p < 0.05). The highest value of CI was obtained for VMAT and it was significant in G1 (p < 0.05). TD achieved the best HI value while s-IMRT 2f achieved the worst one in both groups.
3.2. Contra-lateral breast (CB)
In both groups, the D5% objective was achieved in FiF, s-IMRT 4f, s-IMRT 2f and TD while it was slightly higher than the acceptable value for VMAT and HT. The lowest D5% values were found in FiF (significant in G2, p < 0.05) and TD. For Dmax, TD was found to be well within the ideal value in both groups as well as FiF in G2. The other techniques did not meet the acceptable value and, in particular, the two rotational techniques resulted in the lowest dose-sparing of the CB.
3.3. Ipsilateral lung (IL)
All techniques satisfied the objective for V5Gy, V10Gy and V20Gy in both groups. The lowest V5Gy and V10Gy were found in TD in both groups (significant in G1, p < 0.02). The higher V5Gy values were found in VMAT and HT. The highest significant value of V20Gy was found for FiF (p < 0.02) and the lowest value for HT in both groups. Lowest Dmean was achieved in TD in both groups while the highest value was observed in FIF in G1 and VMAT in G2.
3.4. Contra-lateral lung (CL)
FiF, s-IMRT 4f, s-IMRT2f and TD largely satisfied the dose objective of V5Gy <5% being equal or very close to zero in both groups. VMAT and HT gave significantly higher values for V5Gy as well as Dmean and Dmax (p < 0.001).
3.5. Heart (H)
For left-sided patients, the objective V20Gy <5% was achieved for all techniques in both groups apart from FiF in G1. Its lowest value, was found in VMAT and HT in both groups (statistically significant for HT in G1, p < 0.001). No significant differences were observed for V10Gy nor for Dmean in both groups.
For right-sided patients, major differences were found for Dmean: FiF and TD were able to reduce up to 2.5 Gy the mean dose in both groups with respect to VMAT and HT (statistically significant for FiF in both groups, p < 0.05).
3.6. Plan quality analysis
The results of the plan quality analysis (Tables 1 and 2 of Supplementary material) showed that in G1, TD resulted in the highest PQS (4) while s-IMRT 2f resulted in the lowest one (−1.5). The other techniques were equivalent. Similarly, in G2, TD resulted in the highest PQS (2.5) while HT showed the lowest results (−3). VMAT and s-IMRT 2f scored -1. FIF and s-IMRT 4f were found to be nearly equivalent (−0.5 and 0, respectively).
3.7. Pareto front evaluation
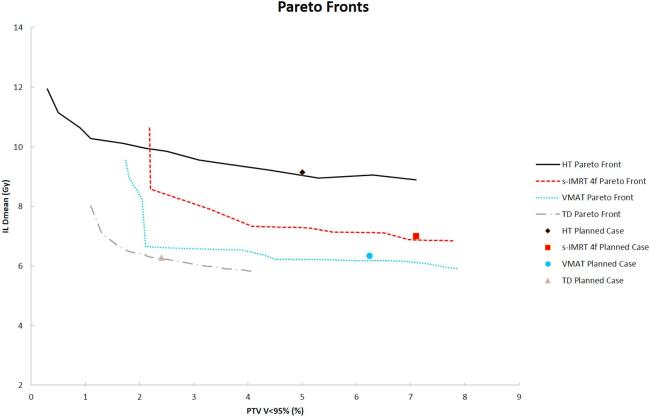
Fig. 2 presents the Pareto fronts obtained for the four techniques together with the corresponding DVH point value of the planned case. The planned cases lied on their Pareto fronts for all techniques except for VMAT where the Dmean of the IL was found to be 0.3 Gy greater than the Pareto front value. The best Pareto front was found for TD while the worst for HT.
Fig. 2.
Pareto fronts comparison for a selected case of G1. The plot also reports the values for the planned case.
4. Discussion
Treatment planning studies should require improved standards for designing the studies and reporting the results [31], Pareto fronts were built for four techniques in order to evaluate the degree of plan optimization. Our analysis suggests that our plans are all Pareto optimal for the specific dose trade-off evaluated increasing the reliability of our conclusions. To our knowledge, this is the first time an attempt to evaluate the quality of the optimization is conducted in a planning comparison study.
We considered it useful to report dose-volume data for a well-established hypofractionated radiation schedule [32] due to the lack of such data in the literature. Further in this discussion, differences between techniques will be discussed regardless of the group unless specified.
TD produced the best PTVeval coverage with a significant difference compared to all techniques except for VMAT. Both TD and VMAT coverage values were similar to those previously reported for a standard fractionation schedule [14], [15], [16], [21], [23]. On the other hand, s-IMRT 2f significantly resulted in the worst value. In G1, where the mean breast volume was larger, the PTVeval coverage did not fulfill the ideal objective. This is due to the small number of degrees of freedom available during the optimization that caused a reduction of coverage to prevent hot spots. Although such a low value corresponds well with other findings [33], two other papers [14], [16] have reported better results in terms of PTV coverage. In both studies, the mean breast volume was smaller (537 cc and 360 cc, respectively) than those herein reported. However, FiF showed better coverage, suggesting that the segmentation process implemented in Monaco TPS could be improved at least when only two fields are employed. The difference between optimal and segmented dose when only two fields were employed was observed to be quite large if compared to the same difference observed when four fields were used. The lowest significant value of D2% observed for HT in both groups confirmed results of other studies [10], [34] demonstrating the increase of target homogeneity according to the increased number of beamlets.
The highest CI value returned by VMAT (significant in G1) and, in turn, by s-IMRT 4f demonstrated how the method implemented in Monaco TPS to improve the dose conformality was more effective than the ring-based optimization usually employed in the Accuray TPS. Although the lowest CI values were observed for TD and FiF they were slightly superior to those published earlier [10], [14]. The highest HI returned for TD was a consequence of the best PTVeval coverage obtained for such technique.
IMRT techniques may increase CB doses, exposing patients to an increased risk of developing a secondary malignancy [35]. Our results show that both D5% and Dmax were significantly higher for the two rotational techniques. TD was the only IMRT technique that was able to almost reach the best results obtained with FiF. Although the CB dose reported in the literature is quite different, no significant differences were observed compared to previously published data [10], [15]. CB dose-sparing increases when irradiating small breast volumes or when cutting the PTV at its edges [14], [36]. The overexposure of the CB for HT may have been reduced by the use of blocking geometric structures. However, this may improve the OARs dose-sparing at the expense of the CI and the beam-on time [18] because of the small number of beamlets and the reduced irradiation angle. In this study, plans were designed to fulfill the clinical goals of our department that privilege the conformity of the high dose to the target volume.
The occurrence of radiation pneumonitis after breast irradiation has been associated with the age of the patient, the use of concomitant chemotherapy, and the dose delivered to the IL (V20Gy and mean dose) [37], [38], [39]. Another study also demonstrated that the V5Gy might lead significantly to the development of pneumonitis if greater than 42% [40]. Therefore, reducing IL V20Gy, V5Gy, and mean dose remains important endpoints. All plans in our study were able to maintain these three parameters well below the RTOG dose limits. HT and VMAT plans resulted in higher V5Gy, although their mean dose values were similar or even smaller than the other techniques. Overall, TD was significantly superior to all other techniques in terms of IL sparing. Compared to previous studies, IL mean values obtained from our study in G1 were similar for FiF [10], [15], [16], [32], s-IMRT 2f [14], [15], [16], [31], s-IMRT 4f [14], [31], [32], VMAT [10], [14], [15], [16] and HT [10], [18], [32] while for TD it was lower than those previously published [23], [32].
Rotational techniques significantly increased the low dose spillage to the contra-lateral lung as highlighted in particular by the V5Gy values that still remained well below the dose constraints as well as the endpoint for radio-induced pneumonitis.
For left-sided breast irradiation, cardiovascular late toxicity progresses over time and may manifest after decades [41]. Darby et al. reported that the rate of ischemic heart disease increases linearly with the mean dose to the heart by 7.4%/Gy, with no apparent threshold [42]. Furthermore, several studies observed substantial radiation-induced heart disease when the heart receives more than 40 Gy [43], [44]. Although the use of IMRT has demonstrated to reduce doses delivered to the heart by 25–75% when compared with standard tangential fields [45], [46], an increase in cardiovascular morbidity was still observed [47], [48]. Hence, the use of technological advances, such as VMAT or TD to reduce cardiac dose, may be of increasing importance particularly when associated to deep inspiration breath-hold (DIBH) techniques [49]. In the present study, volumetric modulated irradiation techniques provided worse results in terms of mean dose, as expected, even though they were still within acceptable values and aligned with values found in literature [10], [15], [16], [18], [31]. On the other hand, it is worth noting that the higher degree of fluence modulation lowered the V20Gy. The significant increase of the mean dose observed in this study when using HT and VMAT for right-sided patients suggests to avoid such techniques for these patients since this may lead to a potential increase of the risk of ischemic disease >20%. However, patients included in Darby’s study were irradiated with old techniques supposed to deliver either very high or very low doses to heart. Therefore, the linearity between heart disease and mean dose may be mitigated or even no longer exists with the use of modern RT techniques as VMAT and HT.
TD returned the highest PQS in both groups. This was explained by its superior results in terms of target coverage and IL dose-sparing and good results also for CB, CL and heart. The poorest score was obtained by s-IMRT 2f and was somewhat expected due to the lower degree of modulation available. However, this latter mainly affected the PTVeval coverage while the technique showed results similar to the others for doses to the OARs.
Homogeneous results were found between groups for all techniques except for HT. The poorest results obtained by HT for the CL returned in G2 were probably due to the smaller mean target volume compared to G1. It reduced the amount of attenuating tissue, leading to an increase of the transmitted dose to the CL.
Six different treatment planning techniques used for whole-breast irradiation were compared in terms of calculated dose to PTV and OARs. Although all techniques were able to fulfill the dose limit criteria adapted from RTOG 1005, TD was significantly superior to others in terms of target coverage and sparing of the ipsi-lateral lung. It also resulted, together with FiF, the best option to spare contra-lateral breast. HT and VMAT were observed to significantly increase the dose spillage to contra-lateral OARs. Pareto front analysis confirmed the reliability of the optimization for a selected case.
Conflict of interest statement
R. Moeckli is holding a grant from Accuray Inc. for a research project in Tomotherapy. However, the present work is not directly related to that grant.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke M., Collins R., Darby S., Davies C., Elphinstone P., Evans V. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-years survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J., Stewart H., Rutqvist L., Houghton J., Edwards R., Redmond C. Cause-specific mortaliy in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 4.Turesson I., Nyman J., Holmberg E., Odén A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Rad Oncol Biol Phys. 1996;36:1065–1075. doi: 10.1016/S0360-3016(96)00426-9. [DOI] [PubMed] [Google Scholar]
- 5.Boice J.D., Harvey E.B., Blettner M., Stovall M., Flannery J.T. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–785. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- 6.Mo J.C., Huang J., Gu W.D., Gao M., Ning Z.H., Mu J.M. A dosimetric comparison of double-arc volumetric arc therapy, step-shoot intensity modulated radiotherapy and 3D-CRT for left-sided breast cancer radiotherapy after breast-conserving surgery. Technol Health Care. 2017;25:851–858. doi: 10.3233/THC-160746. [DOI] [PubMed] [Google Scholar]
- 7.Bartelink H., Horiot J.C., Poortmans P.M., Struilmans H., Van den Bogaert W., Fourquet A. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer:10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 8.START Trialists' Group, Bentzen S.M., Agrawal R.K., Aird E.G., Barrett J.M., Barrett-Lee P.J., Bliss J.M. The UK standardization of breast radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicini F.A., Sharpe M., Kestin L., Martinez A., Mitchell C.K., Wallace M.F. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:1336–1344. doi: 10.1016/S0360-3016(02)03746-X. [DOI] [PubMed] [Google Scholar]
- 10.Freedman G.M., Anderson P.R., Li J., Eisenberg D.F., Hanlon A.L., Wang L. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29:66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 11.McDonald M.W., Godette K.D., Butker E.K., Davis L.W., Johnstone P.A. Long-term outcomes of IMRT for breast cancer: a single institution cohort analysis. Int J Radiat Oncol Biol Phys. 2008;72:1031–1040. doi: 10.1016/j.ijrobp.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 12.Haciislamoglu E., Colak F., Canyilmaz E., Dirican B., Gurdalli S., Yilmaz A.H. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy. Phys Med. 2015;31:360–367. doi: 10.1016/j.ejmp.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Johansen S., Cozzi L., Olsen D.R. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and volumetric modulated arc treatment techniques. Acta Oncol. 2009;48:495–503. doi: 10.1080/02841860802657227. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H., Mingyuan H., Cheng G., Han D., Wu N., Shi D. A comparative dosimetric study of left sided breast cancer after breast-conserving surgery treated with VMAT and IMRT. Radiat Oncol. 2015;10:231. doi: 10.1186/s13014-015-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viren T., Heikkila J., Myllyoja K., Koskela K., Lahtinen T., Seppälä J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:79. doi: 10.1186/s13014-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin G.H., Chen L.X., Deng X.W., Liu X.W., Huang Y., Huang X.B. A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: conventional tangential field, field-infield, tangential-IMRT, multi-beam IMRT and VMAT. Radiat Oncol. 2013;8:89. doi: 10.1186/1748-717X-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koivumäki T., Fogliata A., Zeverino M., Boman E., Sierpowska J., Moeckli R. Dosimetric evaluation of modern radiation therapy techniques for left breast in deep-inspiration breath-hold. Phys Med. 2018;45:82–87. doi: 10.1016/j.ejmp.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Shiau A.C., Hsieh C.H., Tien H.J., Yeh H.P., Lin C.T., Shueng P.W. Left-sided whole breast irradiation with hybrid-IMRT and helical tomotherapy dosimetric comparison. BioMed Res Int. 2014;2014:7. doi: 10.1155/2014/741326. Article ID 741326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coon A.B., Dickler A., Kirk M.C., Liao Y., Shah A.P., Strauss J.B. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2010;78:104–110. doi: 10.1016/j.ijrobp.2009.07.1705. [DOI] [PubMed] [Google Scholar]
- 20.Goddu S.M., Chaudhari S., Mamalui-Hunter M., Pechenaya O.L., Pratt D., Mutic S. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys. 2009;73:1243–1251. doi: 10.1016/j.ijrobp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales V.J., Buchholz D.J., Langen K.M., Olivera G.H., Chauhan B., Meeks S.L. Evaluation of two tomotherapy-based techniques for the delivery of whole-breast intensity-modulated radiation therapy. Int J Radiat, Oncol, Biol Phys. 2006;65:284–290. doi: 10.1016/j.ijrobp.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 22.Reynders T., Tournel K., De Coninck P., Heymann S., Vinh-Hung V., Van Parijs H. Dosimetric assessment of static and helical TomoTherapy in the clinical implementation of breast cancer treatments. Radiother Oncol. 2009;93:71–79. doi: 10.1016/j.radonc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto H., Omura M., Matsui K., Mukai Y., Hongo H., Yamakabe W. Tangent field technique of TomoDirect improves dose distribution for whole-breast irradiation. J App Clin Med Phys. 2015;16:225–232. doi: 10.1120/jacmp.v16i3.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottosson R.O., Engstrom P.E., Sjöström D., Behrens C.F., Karlsson A., Knöös T. The feasibility of using pareto fronts for comparison of treatment planning systems and delivery techniques. Acta Oncol. 2009;48:233–237. doi: 10.1080/02841860802251559. [DOI] [PubMed] [Google Scholar]
- 25.Kyroudi A., Petersson K., Ghandour S., Pachoud M., Matzinger O., Ozsahin M. Discrepancies between selected Pareto optimal plans and final deliverable plans in radiotherapy multi-criteria optimization. Radiother Oncol. 2016;120:346–348. doi: 10.1016/j.radonc.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Harsolia A., Kestin L., Grills I., Wallace M., Jolly S., Jones C. Intensity-modulated radiotherapy results in significant decrease in clinical toxicitites compared with conventional wedge-based breast radiotherapy. Int J Radiat, Oncol, Biol Phys. 2007;68:1375–1380. doi: 10.1016/j.ijrobp.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 27.RTOG 1005: A Phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer. www.rtog.org, accessed March 3rd, 2014.
- 28.Sugie C., Manabe Y., Hayashi A., Murai T., Takaoka T., Hattori Y. Efficacy of the dynamic jaws mode in helical tomotherapy with static ports for breast cancer. Technol Cancer Res Treat. 2015;14:459–465. doi: 10.1177/1533034614558746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersson K., Ceberg C., Engstrӧm P., Benedek H., Nilsson P., Knöös T. Conversion of helical tomotherapy plans to step-and-shoot IMRT plans – Pareto from evaluation of plans from a new treatment planning system. Med Phys. 2011;38:3130–3138. doi: 10.1118/1.3592934. [DOI] [PubMed] [Google Scholar]
- 30.Petersson K., Nilsson P., Engstrӧm P., Knöös T., Ceberg C. Evaluation of dual-arc VMAT radiotherapy treatment plans automatically generated via dose mimicking. Acta Oncol. 2016;55:523–525. doi: 10.3109/0284186X.2015.1080855. [DOI] [PubMed] [Google Scholar]
- 31.Phillips M.H., Holdsworth C. When is better best? A multiobjective perspective. Med Phys. 2011;38:1635–1640. doi: 10.1118/1.3553404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whelan T.J., Pignol J.P., Levine M.N., Julian J.A., MacKenzie R., Parpia S. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 33.Borges C., Cunha G., Monteiro-Grillo I., Vaz P., Teixeira N. Comparison of different breast planning techniques and algorithms for radiation therapy treatment. Phys Med. 2014;30:160–170. doi: 10.1016/j.ejmp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Schubert L.K., Gondi V., Sengbusch E., Westerly D.C., Soisson E.T., Paliwal B.R. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol. 2011;100:241–246. doi: 10.1016/j.radonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Stovall M., Smith S.A., Langholz B.M., Boice J.D., Jr, Shore R.E., Andersson M. Dose to the contralateral breast from radiation therapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peulen H., Hanbeukers B., Boersma L., van Baardwijk A., van den Ende P., Houben R. Forward intensity-modulated radiotherapy planning in breast cancer to improve dose homogeneity: feasibility of class solutions. Int J Radiat Oncol Biol Phys. 2012;82:394–400. doi: 10.1016/j.ijrobp.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Taghian A.G., Assaad S.I., Niemerko A., Kuter I., Younger J., Schoenthaler R. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001;93:1806–1811. doi: 10.1093/jnci/93.23.1806. [DOI] [PubMed] [Google Scholar]
- 38.Lind P.A., Wennberg B., Gagliardi G., Rosfors S., Blom-Goldman U., Lideståhl A. ROC curves and evaluation of radiation-induced pulmonary toxicity in breast cancer. Int J Radiat Oncol Biol Phys. 2006;64:765–770. doi: 10.1016/j.ijrobp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Lind P.A., Marks L.B., Hardenbergh P.H., Clough R., Fan M., Hollis D. Technical factors associated with radiation pneumonitis after local +/- regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:137–143. doi: 10.1016/S0360-3016(01)01715-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang S., Liao Z., Wei X., Liu H.H., Tucker S.L., Hu C.S. Analysis of clinical and dosimetric factors associated with Treatment-Related Pneumonitis (TRP) in patients with Non-Small-Cell Lung Cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 41.Mousavi N., Nohria A. Radiation-induced cardiovascular disease. Curr Treat Options Cardio Med. 2013;15:507–517. doi: 10.1007/s11936-013-0259-0. [DOI] [PubMed] [Google Scholar]
- 42.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 43.Cosset J.M., Henry-Amar M., Pellae-Cosset B., Carde P., Girinski T., Tubiana M. Pericarditis and myocardial infarctions after Hodgkin’s disease therapy. Int J Radiat Oncol Biol Phys. 1991;21:447–449. doi: 10.1016/0360-3016(91)90794-5. [DOI] [PubMed] [Google Scholar]
- 44.Ishikura S., Nigei K., Ohtsu A., Boku N., Hironaka S., Mera K. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2013;21:2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 45.Mayo C.S., Urie M.M., Fitzgerald T.J. Hybrid IMRT plans-concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys. 2005;61:922–932. doi: 10.1016/j.ijrobp.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Landau D., Adams E.J., Webb S., Ross G. Cardiac avoidance in breast radiotherapy: A comparison of simple shielding techniques with intensity-modulated radiotherapy. Radiother Oncol. 2001;60:247–255. doi: 10.1016/S0167-8140(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 47.Harris E.E.R., Correa C., Hwang W.T., Liao J., Litt H.I., Ferrari V.A. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 48.Marks L.B., Yu X., Prosnitz R.G., Zhou S.M., Hardenbergh P.H., Blazing M. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 49.Peguret N., Ozsahin M., Zeverino M., Belmondo B., Durham A.D., Lovis A. Apnea-like suppression of respiratory motion: First evaluation in radiotherapy. Radiother Oncol. 2016;118:220–226. doi: 10.1016/j.radonc.2015.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.