Abstract
In magnetic resonance (MR) only radiotherapy, the target delineation needs to be performed without computed tomography (CT). We investigated in thirteen patients with prostate cancer, how the clinical target volume (CTV) was affected, when the target delineation procedure was changed from using both CT and MR images to using MR images only. The mean volume of the CTVCT/MR was 61.0 cm3 as compared to 49.9 cm3 from MR-only based target delineation, corresponding to an average decrease of 18%. Our results show that CTVMR-only was consistently smaller than CTVCT/MR, which has to be taken into consideration before clinical commissioning of MR-only radiotherapy.
1. Introduction
In modern radiotherapy, the weakest link for successful treatment has been the definition and delineation of the target volume [1]. One way to address this problem has been to introduce Magnetic Resonance (MR) imaging in the treatment planning process. For a long time computed tomography (CT) has been the standard imaging modality, but the excellent soft tissue contrast of the MR images enables a more accurate depiction of the anatomy. This is not only important for target delineation but also for outlining the organs at risk (OAR). For prostate cancer, MR imaging has been used in the radiotherapy treatment planning for more than a decade [2], [3]. The anterior, posterior and lateral borders of the prostate can often be clearly seen on CT, but MR is in particular valuable for depicting the prostate apex and its intersection with the bladder. The increasing use of MR imaging for prostate cancer in radiation oncology was recently the subject for a review [4].
Even if MR offers excellent imaging data, CT is still used in the treatment planning workflow. One obvious reason is to provide the density of the tissue needed by the treatment planning system (TPS) for dose calculation. In a combined MR and CT workflow, the delineation of target and risk organs is performed on the MR-images, and this information has to be transferred to the CT images. The combined workflow demands a registration between the MR data and the CT images. For prostate, this registration is most often based on intraprostatic fiducials. The registration techniques are marred by significant inaccuracies [5]. The treatment plan and the delivery of the treatment in a combined workflow is defined by the geometry given by the CT. Therefore, the target delineation after registration may have to be adjusted on the CT images due to registration limitations and changes in anatomy between the MR and CT scanning [6]. A workflow without CT, i.e. an MR-only workflow, will avoid these uncertainties, simplify the procedures, reduce the workload and therefore have the potential to become more cost effective [7]. To accomplish MR-only radiotherapy, i.e. with no attenuation data available from the CT, a density map referred to as synthetic CT (sCT), needs to be generated from MR-data. There are several methods presented to solve this issue [8] and a few are also available commercially [9], [10], and have recently been validated for prostate cancer radiotherapy planning [11], [12].
For clinics, which are about to implement an MR-only workflow, the target delineation will be carried out with no concerns to CT data. In our clinic, the radiation oncologists have many years of experience of target delineation from a combined MR/CT workflow. Many studies concerning the delineation of the prostate have revealed significant differences when using MR data instead of CT data [2], [3], [13], [14], [15]. The design of these studies has been organized so that the data from MR and CT have been used independently from each other, i.e. the knowledge from outlining on one modality was not used for the other. They were also mainly performed when MR was a relatively new modality for treatment planning, and the results may not reflect the present experience of radiation oncologists to MR images. A systematic smaller volume was reported when the prostate was defined on MR compared to CT. These studies do not compare a combined CT/MR workflow to MR-only workflow. In a combined CT/MR workflow, one also has to take into account that treatment is given using the CT frame of reference, and that the delineated target volume from the MR images may need adjustments. To the best of our knowledge, no study is available on how the delineated target volume is affected when a clinic with a well-established MR/CT workflow is converting to an MR-only workflow.
The aim of this study was to investigate how a transition from a MR/CT based workflow to an MR-only workflow affected the delineated prostate target volume.
2. Materials and methods
This study was approved by the regional ethics review board. For delineation T2 weighted (T2w) axial, sagittal and coronal MR images of the prostate region, were acquired on a 3.0 T Discovery 750 W scanner (GE Healthcare). Images were also acquired on CT-scanner (Siemens SOMATOM definition AS + ). The slice thickness was 3.0 mm for both MRI and CT images. All details of the imaging protocols are available in Supplementary information. Two radiation oncologists, with long-term experience (>10 years) of prostate delineation on both CT and combined CT/MR workflow, delineated the clinical target volume (CTVCT/MR) on two separate occasions, seven to eleven months apart. CTV was defined as the prostate gland and any extraprostatic tumor extension. In total 13 patients were included. One physician delineated six patients and the other physician seven patients. On the first occasion, the physicians delineated the CTVCT/MR according to normal clinical practice, i.e. using the T2w images, which was rigidly registered (translation and rotation) to the CT frame of reference using intraprostatic fiducial markers. The fiducials consisted of three in-house produced cylinder-shaped gold objects (length 5.0 mm and diameter 1.0 mm). The choice of fiducial marker type was according to the standard of the clinic and was manual identified using a MR gradient echo sequence [16]. The CTVCT/MR was adjusted, if needed, on the CT images to compensate for registration limitations and changes in anatomy between the MR and CT scanning. At this stage, the physicians were unaware of the upcoming second drawing exercise. At the second occasion, an MR-only workflow was simulated and the CTV (CTVMR-only) was delineated solely using the T2w images for the same patients and by the same physician. The potential difference in the delineated volumes between the two physicians were investigated using the non-parametric Mann-Whitney U test, since a normal distribution could not be assumed.
Using the CTVCT/MR as the reference the difference in absolute volume, equivalent sphere diameter and center of mass displacement were measured for CTVMR-only. The delineated CTVCT/MR structure were transferred to the T2w images prior evaluation to enable evaluation in a common coordinate system of both structures. In this process, the CT-data was reformatted to the spatial resolution of the MR-data.
3. Results
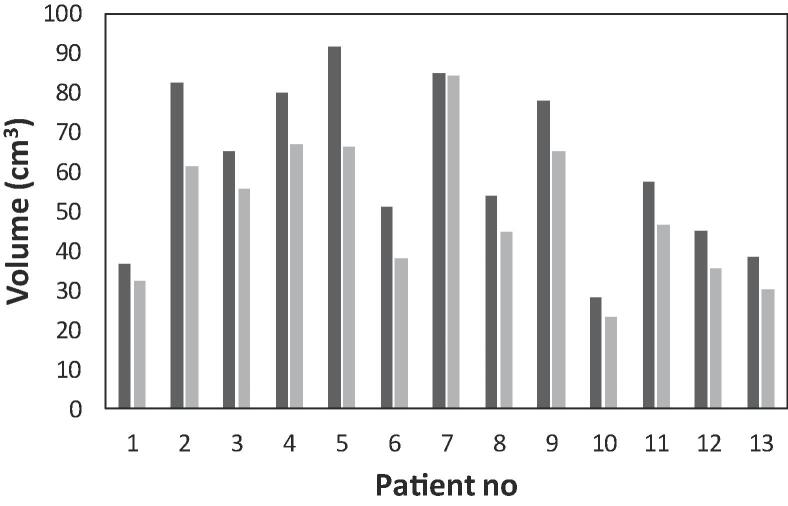
The CTVMR-only had a consistently smaller volume compared to CTVCT/MR (Fig. 1). Mean CTVCT/MR volume was 61.0 ± 20.1 ( ± sd) cm3 (range 27.9–91.8 cm3) while mean CTVMR-only was 49.9 ± 17.4 ( ± sd) cm3 (range 23.0–84.3 cm3). The ratio CTVCT/MR to CTVMR-only was 1.22 representing a 18% decrease in mean volume difference for CTVMR-only. The mean equivalent sphere diameter decreased by 3.0 ± 0.13 ( ± sd) mm, and the mean center of mass was displaced less than 0.5 ± 2 ( ± sd) mm in all directions. There were no significant (p = 0.35) differences between the results of the two physicians.
Fig. 1.
The CTV drawn on CT + MR images (black) and MR only images (gray) for 13 patients. The two physicians delineated patient 1–7 and patient 8–13, respectively. For all patients the volume was smaller when the volume was delineated on CT + MR compared to MR only images.
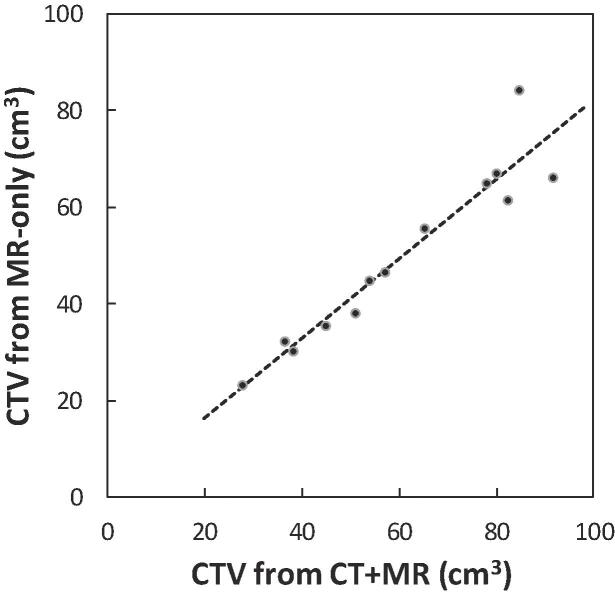
The CTVMR-only was linearly correlated (r2 = 0.91) to the CTVCT/MR (Fig. 2). The percentage volume difference was independent to the size of the prostate (all percentage differences within 95% confidence level in a Bland-Altman plot). Thereby, there was no systematic difference between the two delineations dependent on the absolute volume of the prostate
Fig. 2.
CTVs for volumes delineated on CT + MR images are linearly correlated (r2 = 0.91) to CTV delineated on MR only images.
4. Discussion
MR-only radiotherapy is an emerging technique, which has gained interest especially in the male pelvis, i.e. prostate cancer, for which synthetic CT generation methods are available. The geometric distortions in the MR images, which previously created concerns, are no longer considered an issue [17], [18]. In addition, techniques have been developed to identify the fiducial markers in MR-images [16], [19]. However, in the implementation of an MR-only workflow, there is also a need to study the local clinical conditions for target delineation using MR images only, which was the aim for this study.
We found an 18% smaller CTV when only MR data was used instead of combined CT/MR workflow. The initial drawing on the combined CT/MR data was performed without the knowledge that there would be another later session for an MR-only purpose. Thereby, the set-up had a relevant design for an implementation of an MR-only workflow. A limitation of the study is small number of patients. However, the subjects cover a good range of CTV volumes and the results showed only minor variation between subjects.
In previous studies comparing delineations of prostate CTV on CT and MR, respectively, there was a systematic smaller volume for MR. The CT to MR ratio varied between 1.16 and 1.5 [2], [13], [14], [15], [20]. It is obvious that there is range, which corresponds not only to different image contrasts in the CT compared to MR-images, but probably also a variation in local routines for target delineation. Our mean CT to MR ratio was 1.22, but it is important to underline that the set-up of this study was different compared to previous studies. The former studies compared scenarios using CT and MR data separately. This study compares the changes in target volume going from a combined CT/MR workflow to a MR-only workflow. Still there was a consistent decrease in target volume for all patients. One possible explanation is that the registration process MR/CT is never perfect. Recently, it was found that the uncertainties in MR/CT match was in the range 1.1–3.1 mm [6]. There may also be anatomical differences between the CT and MR-examination, which need to be taken into account. This is important since the treatment planning in a combined workflow is always performed on the CT-images. Therefore, the CTV may need, after the registration, to be slightly adjusted to the CT-geometry manually, which results in a larger CTV for the combined workflow. It was only very recently a consensus guideline for target volume delineation including MR was agreed [21]. Notably, these guidelines are for two different workflows: a CT-only, or a combined CT/MR workflow.
In conclusion, our results show that converting from a well-established MR/CT based workflow to an MR only workflow, the prostate target volume decreased. This demonstrates the need to evaluate these changes in target volumes prior to clinical commissioning of MR-only radiotherapy.
Acknowledgments
Acknowledgements
This study was partially funded by Vinnova, Sweden’s Innovation Agency supporting the Gentle Radiotherapy project (Grant no 2016-03847) and the local Cancer foundations at Lund University and Skåne University Hospital, Sweden.
Conflict of interest
None of the authors has any conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.03.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Segedin B., Petric P. Uncertainties in target volume delineation in radiotherapy – are they relevant and what can we do about them? Radiol Oncol. 2016;50:254–262. doi: 10.1515/raon-2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasch C., Barillot I., Remeijer P., Touw A., van Herk M., Lebesque J.V. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 3.Debois M., Oyen R., Maes F., Verswijvel G., Gatti G., Bosmans H. The contribution of magnetic resonance imaging to the three-dimensional treatment planning of localized prostate cancer. Int J Radiat Oncol Biol Phys. 1999;45:857–865. doi: 10.1016/s0360-3016(99)00288-6. [DOI] [PubMed] [Google Scholar]
- 4.Menard C., Paulson E., Nyholm T., McLaughlin P., Liney G., Dirix P. Role of prostate MR imaging in radiation oncology. Radiol Clin North Am. 2018;56:319–325. doi: 10.1016/j.rcl.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Ulin K., Urie M.M., Cherlow J.M. Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys. 2010;77:1584–1589. doi: 10.1016/j.ijrobp.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wegener D., Zips D., Thorwarth D., Weiss J., Othman A.E., Grosse U. Precision of T2 TSE MRI-CT-image fusions based on gold fiducials and repetitive T2 TSE MRI-MRI-fusions for adaptive IGRT of prostate cancer by using phantom and patient data. Acta Oncol. 2019;58:88–94. doi: 10.1080/0284186X.2018.1518594. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson M., Karlsson M.G., Nyholm T., Amies C., Zackrisson B. Dedicated magnetic resonance imaging in the radiotherapy clinic. Int J Radiat Oncol Biol Phys. 2009;74:644–651. doi: 10.1016/j.ijrobp.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 8.Edmund J.M., Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12:28–43. doi: 10.1186/s13014-016-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siversson C., Nordstrom F., Nilsson T., Nyholm T., Jonsson J., Gunnlaugsson A. Technical Note: MRI only prostate radiotherapy planning using the statistical decomposition algorithm. Med Phys. 2015;42:6090–6097. doi: 10.1118/1.4931417. [DOI] [PubMed] [Google Scholar]
- 10.Köhler M.V.T., Grootel M.V., Hoogeveen R., Kemppainen R., Renisch S. Philips White paper. 2015. MR-only simulation for radiotherapy planning. [Google Scholar]
- 11.Persson E., Gustafsson C., Nordstrom F., Sohlin M., Gunnlaugsson A., Petruson K. MR-OPERA: a multicenter/multivendor validation of magnetic resonance imaging-only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99:692–700. doi: 10.1016/j.ijrobp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi N., Fontenla S., Zhang J., Cloutier M., Kadbi M., Mechalakos J. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62:2961–2975. doi: 10.1088/1361-6560/aa5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzikas A., Karaiskos P., Papanikolaou N., Sandilos P., Koutsouveli E., Lavdas E. Investigating the clinical aspects of using CT vs. CT-MRI images during organ delineation and treatment planning in prostate cancer radiotherapy. Technol Cancer Res Treat. 2011;10:231–241. doi: 10.7785/tcrt.2012.500198. [DOI] [PubMed] [Google Scholar]
- 14.Smith W.L., Lewis C., Bauman G., Rodrigues G., D'Souza D., Ash R. Prostate volume contouring: a 3D analysis of segmentation using 3DTRUS, CT, and MR. Int J Radiat Oncol Biol Phys. 2007;67:1238–1247. doi: 10.1016/j.ijrobp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Seppala T., Visapaa H., Collan J., Kapanen M., Beule A., Kouri M. Converting from CT- to MRI-only-based target definition in radiotherapy of localized prostate cancer: a comparison between two modalities. Strahlenther Onkol. 2015;191:862–868. doi: 10.1007/s00066-015-0868-5. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson C., Korhonen J., Persson E., Gunnlaugsson A., Nyholm T., Olsson L.E. Registration free automatic identification of gold fiducial markers in MRI target delineation images for prostate radiotherapy. Med Phys. 2017;44:5563–5574. doi: 10.1002/mp.12516. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson C., Nordstrom F., Persson E., Brynolfsson J., Olsson L.E. Assessment of dosimetric impact of system specific geometric distortion in an MRI only based radiotherapy workflow for prostate. Phys Med Biol. 2017;62:2976–2989. doi: 10.1088/1361-6560/aa5fa2. [DOI] [PubMed] [Google Scholar]
- 18.Kapanen M., Collan J., Beule A., Seppala T., Saarilahti K., Tenhunen M. Commissioning of MRI-only based treatment planning procedure for external beam radiotherapy of prostate. Magn Reson Med. 2013;70:127–135. doi: 10.1002/mrm.24459. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson J.H., Garpebring A., Karlsson M.G., Nyholm T. Internal fiducial markers and susceptibility effects in MRI-simulation and measurement of spatial accuracy. Int J Radiat Oncol Biol Phys. 2012;82:1612–1618. doi: 10.1016/j.ijrobp.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Hentschel B., Oehler W., Strauss D., Ulrich A., Malich A. Definition of the CTV prostate in CT and MRI by using CT-MRI image fusion in IMRT planning for prostate cancer. Strahlenther Onkol. 2011;187:183–190. doi: 10.1007/s00066-010-2179-1. [DOI] [PubMed] [Google Scholar]
- 21.Salembier C., Villeirs G., De Bari B., Hoskin P., Pieters B.R., Van Vulpen M. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127:49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.