Abstract
Background and Purpose
Extensive radiation therapy quality assurance (RTQA) programs are needed when advanced radiotherapy treatments are used. As part of the RTQA four dimensional computed tomography (4DCT) imaging performance needs to be assessed. Here we present the RTQA data related to 4DCT procedures used within the context of stereotactic body radiotherapy (SBRT) of centrally located lung tumours. It provides an overview of the 4DCT acquisition methods and achievable accuracy of imaging lung tumour volumes.
Materials and Methods
3DCT and 4DCT images were acquired from a CIRS phantom with spheres of 7.5 and 12.5 mm radius using the institutional scan protocols. Regular asymmetric tumour motion was simulated with varying amplitudes and periods. Target volumes were reconstructed using auto-contouring with scanner specific thresholds. Volume and amplitudes deviations were assessed.
Results
Although acquisition parameters were rather homogeneous over the eleven institutions analysed, volume deviations were observed. Average volume deviations for the 12.5 mm sphere were 15% (−4% to 69%) at end of inspiration, 2% (−2% to 9.0%) at end of expiration and 12% (0% to 36%) at mid-ventilation. For the 7.5 mm sphere deviations were 13% (−99% to 65%), 16% (−34% to 66%) and 1% (−13% to 20%), respectively. The amplitude deviation was generally within 2 mm although underestimations up to 6 mm were observed.
Conclusions
The expiration phase was the most accurate phase to define the tumour volume and should be preferred for GTV delineation of tumours exhibiting large motion causing motion artefacts when using mid-ventilation or tracking techniques. The large variation found among the institutions indicated that further improvements in 4DCT imaging were possible. Recommendations for 4DCT QA have been formulated.
Keywords: Lung cancer, Radiotherapy quality assurance, 4DCT, Tumour motion, Clinical trial, EORTC
1. Introduction
Advanced radiotherapy techniques improve local tumour control and reduce treatment toxicity by delivering higher radiation doses to the targets while increasing the sparing of normal tissue. The benefit of advanced radiotherapy techniques using steep dose gradients and reduced margins is conditioned by an accurate target definition and volume delineation. Thus, the imaging quality needs to be high. To ensure treatment safety and standardization of treatment Radiation Therapy Quality Assurance (RTQA) procedures thus need to include an evaluation of the imaging techniques used. Respiration correlated Four Dimensional Computer Tomography (4DCT) is known to be the preferred CT imaging technique for treatment planning of stereotactic lung irradiation [1]. Although 4DCT is nowadays the tool of reference to determine the breathing motion of the tumour and enables a better quantification of the GTV than 3DCT imaging, the breathing motion may still cause imaging artefacts [2], [3]. In fact, the binning of the acquired images which can be done according to phase or amplitude might show artefacts or residual motion within a bin which may lead to errors in target volume and OAR delineation e.g., [4].
As mentioned above, several studies have already investigated the described issues of 4DCT image quality, mainly focussing on reduction of artefacts due to irregular motion. Some studies propose novel techniques such as prospective gating [5], [6], which comes at a cost of longer acquisition times, or multiple fast 3D scan acquisitions [7]. Wolthaus and co-workers have presented a method to reconstruct the time-averaged midposition CT using deformable registration which could also be used to reconstruct other phases [8]. Another approach is to use an advanced reconstruction method based on the breathing signal [9]. However, these advanced methods are not widely available.
Most studies report on single institution experience. Results of specific institutions are not necessarily generalizable to other institutions, as imaging equipment, acquisition protocols and imaging QA might vary widely between institutions.
The last European multicentre 4DCT respiratory phantom study was published by Hurkmans et al. in 2010 [10]. The authors performed a 4DCT phantom study with regular breathing patterns including eight different combinations of scanners and scanning parameters visiting nine institutions in The Netherlands. The results might not reflect the current state of 4DCT technology employed for radiotherapy as new CT models have been brought to market and moreover relies on results from a single country. Conversely, the current study provides data from eleven institutions throughout Europe. Thereby, the study will provide an overview of the achievable accuracy within European hospitals today and provides input on how to further improve clinical 4DCT imaging.
2. Materials and methods
To assess the effectiveness of stereotactic body radiotherapy (SBRT) of inoperable, centrally located NSCLC in a multicentre setting, the European Organisation for Research and Treatment of Cancer (EORTC) launched in 2014 the prospective multicentre Lungtech trial (EORTC 22113-08113, ClinicalTrials.gov Identifier:NCT01795521). The outline and main features of the trial have been described previously by Adebahr et al. [11]. The Lungtech trial aimed to show the effectiveness of SBRT in a multicentre setting within a wide variety of hospitals. As there is a delicate balance between the effectiveness of this treatment and the potential morbidity, an in-depth radiotherapy quality assurance (RTQA) procedure has been introduced [12]. Site-visits were conducted as part of the RTQA procedures within the Lungtech trial. These visits took three to five days and covered 4D PET-CT and 4DCT imaging, treatment planning and dose delivery. The institutions were visited by the same investigator using each time the same phantom and measurement equipment.
2.1. Phantom description
For this study the CIRS 008A phantom (Computerized Imaging Reference Systems, Norfolk, Virginia, USA) was used (See Fig. S1). This is a dynamic anthropomorphic phantom with two lung shaped regions with lung equivalent density material, a water-equivalent mediastinum and a vertebral structure. A lung equivalent density rod inserted in the phantom’s right lung contains one spherical target of water equivalent density simulating a lung lesion. A motion actuator moving the target according to a respiratory signal specified by the user is driving the rod in cranio-caudal direction. Two sphere sizes of 7.5 mm and 12.5 mm radius were used.
2.2. CT scanning of the phantom
It has been shown that a sinusoidal motion is not representative of respiration motion and may produce unrealistic results [2]. Thus, we simulated a breathing pattern in the cranio-caudal direction using a cos6 function as proposed by Lujan et al. [13]. The combinations of breathing periods (t) and peak-to-peak amplitudes (A) tested were I: t = 3 s with A = 15 mm, II: t = 6 s with A = 15 mm and III: t = 4 s with A = 25 mm. Due to mechanical limitations of the phantom, the combination of a three seconds breathing cycle with 25 mm amplitude was not possible and instead four seconds breathing cycle was used to represent a highly moving tumour. For the latter combination of amplitude and motion only the small sphere was scanned.
A 3DCT was acquired for each spherical insert with the phantom in static exhale condition. Thereafter, the three 4DCT scans were acquired with the CT acquisition parameters and respiratory measurement system used in clinical routine by the institution. The respiratory measurement system itself was not evaluated as the CIRS phantom was able to generate a proper signal from all respiratory measurement systems encountered.
2.3. Analysis
All CT scans were imported in a treatment planning system (Pinnacle v8, Philips Healthcare, The Netherlands) to enable auto-contouring of the reconstructed sphere. Knowing the exact volume of the sphere, an institution dependent Hounsfield Unit (HU) threshold was determined that resulted in the true volume when auto contouring the 3DCT images. This threshold was thereafter used for auto contouring the 4DCT datasets. Auto contouring was performed for all the phases of the 4DCT scans enabling the reconstructed sphere volumes to be calculated for each phase. The centre of mass of the auto-segmented contours in each phase was determined. The largest cranio-caudal distance between the centres of mass in a 4DCT scan was defined as the motion amplitude captured by that 4DCT scan. This result was evaluated and compared to the known motion amplitude.
For the three phases which have been reported to be used in treatment planning, namely end of inspiration, end of expiration and mid-ventilation together with the average scan (the image obtained by averaging over all the phases of the 4DCT images) the volume deviation as a function of the motion parameters and sphere sizes was assessed.
Amplitude deviations were calculated and analysed for each 4DCT acquisition separately. Thereafter, it was investigated whether there was a correlation between the cranio-caudal amplitude deviations and the pitch value used, as studies have shown that an inaccurate longitudinal position of the target volume as seen in some 4DCT images might be caused by an inadequate sampling related to the combination of pitch used, breathing frequency of the patient and scanner rotation speed [14]. In 4DCT imaging the helical pitch is defined as the table distance travelled in one rotation divided by the total collimated width of the X-ray beam at isocentre. To completely image a volume through a breathing cycle, the couch velocity must be low enough such that every (moving) voxel at least spends one breathing cycle within the imaging plane. For multi-slice CT’s the effective imaging plane is variable across the FOV. The maximum pitch at the isocentre plane can be expressed as follow [14]:
where pmax = maximum pitch, trot = rotation time in seconds, Tb = breathing period in s, FOV = reconstructed Field Of View, R = Focus to isocentre distance
The phantom was centrally positioned on the couch with the help of the in room and CT lasers. The tumour-like insert is located at a radial distance of 13 cm from the isocentre. The edge of the large sphere was at 14.25 cm from isocentre and thus a FOV value of 14.25 cm was used in our maximum pitch calculations.
For analysis purposes, the pitch employed by each institution for each breathing cycle was normalised to the pmax corresponding to the rotation speed and the breathing frequency used for the acquisition. The volume and positional deviations were thus analysed in comparison to the ratio pitch/pmax. To avoid overheating of the X-ray tube, manufacturers have limited the exposure time, hence limiting the minimal pitch to cover the length of the region of interest.
2.4. Statistics
Wilcoxon rank tests were performed between the volume deviations observed at each chosen phase, to established in a phase was giving a statistically more accurate volume.
3. Results
Eleven participating institutions were visited between December 2014 and November 2016. An overview of the CT scanners and scan protocols evaluated in the present study is given in Table 1. No GE scanner was used in the hospitals visited. No new 4DCT technology seems to have been introduced recently in the visited hospitals as the CT models used are already on the market for about ten years. All institutions used helical scanning modes. Two different types of data binning were used; phase binning in seven institutions and amplitude binning in four institutions. Eight centres used a rotation speed of 0.5 s whereas three other institutions used shorter rotation times. Slice thickness varied from 1.5 mm to 3 mm. All centres acquired their data using half scan reconstructions (180 degrees + fan angle). Some institutions used a lower tube current when scanning the phantom with motion t = 6 s than with t = 4 s while using the same pitch (institutions E, F and G). We did not prospectively register pitch values and unfortunately could not retrieve this for hospital 11. CTDIvol data was not available for all centres as this was retrieved from the dicom-CT dataset. If this dataset was not directly sent from the CT, but for first stored in a treatment planning system, this data element was sometimes empty.
Table 1.
Scanner models and acquisition parameters used in the 11 institutions participating in the Lungtech trial RTQA site visit.
| Institute | Scanner brand | FOV Acquisition diameter (mm)# | FOV Reconstruction diameter (mm) | bin technique & | kVp+ (V) | Expos-ure (mA/s) | Rotation speed (s) | Slice thickness (mm) | Scan length (mm) | CTDI vol(mGy) | Tube current (mA) | Pitch * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Toshiba Acquilion/LB | 550 | 550 | 10, P | 120 | 76 | 0.5 | 2 | 170 | NA | 250 | 0.12 |
| 170 | NA | 250 | 0.12 | |||||||||
| 170 | NA | 250 | 0.075 | |||||||||
| B | Philips Brillance big bore | 600 | 480 | 10, A | 120 | 600 | 0.5 | 2 | 162 | NA | 180 | 0.15 |
| 162 | NA | 132 | 0.11 | |||||||||
| 162 | NA | 90 | 0.075 | |||||||||
| C | Philips Gemini TF Big Bore | 600 | 600 | 10, P | 120 | 250 | 0.5 | 2 | 180 | 13.2 | 45 | 0.09 |
| 180 | 13.2 | 45 | 0.09 | |||||||||
| 180 | 13.2 | 45 | 0.09 | |||||||||
| D | Philips Brilliance big bore | 600 | 568 | 10, A | 120 | 801 | 0.44 | 3 | 240 | NA | 74 | 0.08 |
| 240 | NA | 74 | 0.041 | |||||||||
| 240 | NA | 74 | 0.041 | |||||||||
| E | Siemens Sensation open | 500 | 500 | 8, P | 120 | 400 | 0.5 | 1.5 | 79.5 | NA | 80 | 0.16 |
| 79.5 | NA | 80 | 0.1 | |||||||||
| 120 | NA | 40 | 0.1 | |||||||||
| F | Siemens sensation open | 500 | 500 | 8, P | 120 | 400 | 0.5 | 3 | 186 | 35.6 | 80 | 0.1 |
| 189 | 35.6 | 80 | 0.1 | |||||||||
| 276 | 36.0 | 40 | 0.15 | |||||||||
| G | Philips Brilliance big bore | 600 | 568 | 10, A | 120 | 801 | 0.44 | 3 | 171 | 47.4 | 74 | 0.08 |
| 171 | 47.4 | 132 | 0.041 | |||||||||
| 171 | 47.4 | 90 | 0.041 | |||||||||
| H | Philips Brilliance big bore | 600 | 480 | 10, A | 120 | 600 | 0.5 | 2 | 172 | 35.5 | 180 | 0.15 |
| 172 | 35.5 | 132 | 0.11 | |||||||||
| 172 | 25.5 | 90 | 0.075 | |||||||||
| I | Philips Brilliance big bore | 600 | 500 | 10, P | 120 | 350 | 0.5 | 2 | 226 | 20.7 | 105 | 0.15 |
| 226 | 20.7 | 53 | 0.11 | |||||||||
| 226 | 20.7 | 77 | 0.08 | |||||||||
| J | Toshiba Acquilion/LB | 550 | 550 | 10, P | 120 | 46 | 0.33 | 2 | 161 | 81.3 | 150 | 0.075 |
| 161 | 108.4 | 150 | 0.075 | |||||||||
| 161 | 108.4 | 150 | 0.075 | |||||||||
| K | Siemens Somatom Definition AS | 500 | 500 | 10, P | 120 | 80 | 0.5 | 3 | 176 | NA | 160 | NA |
| 176 | NA | 160 | NA | |||||||||
| 176 | NA | 80 | NA |
*Where 3 values are given, different values are used for breathing periods of 3, 4 and 6 s, respectively. The pitch values that are larger than pmax are given in bold. NA: Not available. $Number of bins are given and type of binning: P = phase binning, A = amplitude binning, #FOV = field of view, +KVP = kilovolt peak
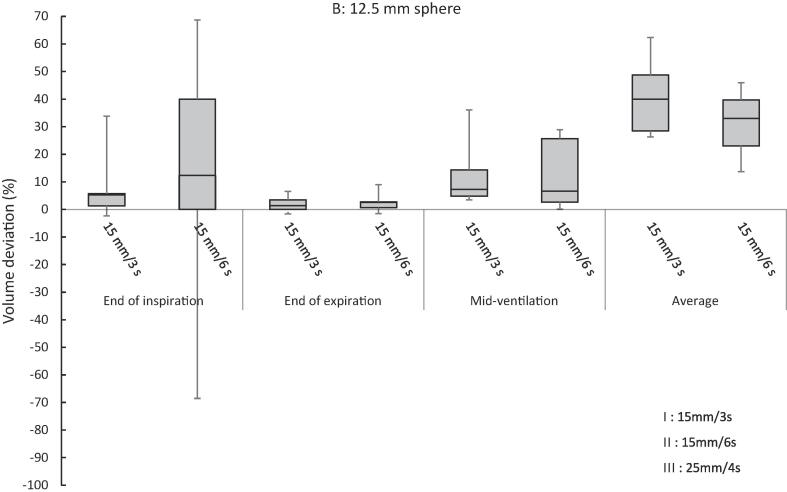
3.1. Volume deviation
Relative volume deviations determined in the end-inspiration, end-expiration, mid-ventilation defined as the phase the closest to the cos6 mathematical average longitudinal position and average scans, are depicted in Fig. 1. Further details about the volume deviations per institution are given in the supplement (Fig. S2). As the breathing motions tested were regular and known, the mid-ventilation position could also be determined for amplitude binned reconstructions. The volume deviations in the remaining phases of the breathing cycle were within the volume deviations presented here and are not given here for the sake of clarity.
Fig. 1.
Boxplot of volume deviation expressed in % of the true volume per sphere and motion type (minus signs mean an underestimation).
The volume deviations to the real volume ranged from as much as −99% to +65% for the 7.5 mm sphere and from −4% to +69% for the 12.5 mm sphere (Fig. 1 and Table 2). The underestimation of 99% means that the sphere almost disappeared. An example of a coronal slice of this scan is shown in Fig. 2.
Table 2.
Mean and range deviations in volume (in % of the true volume) and amplitude (in cm) across institutions per sphere size and motion tested (minus signs mean an underestimation).
| Motion | Sphere radius (mm) | Volume deviations (% of true volume) |
Amplitude deviations (cm) | |||
|---|---|---|---|---|---|---|
| End of inspiration | End of expiration | Mid-ventilation | Average CT | |||
| I: 15 mm/3 s | 7.5 | 14.1% | 2.2% | 16.2% | 43.2% | −0.08 |
| (−5.6, 40.8) | (−8.4, 20.2) | (3.3, 35.4) | (22.1, 66.4) | (−0.25, −0.01) | ||
| 12.5 | 7.7% | 1.1% | 11.3% | 41.4% | −0.08 | |
| (−2.3, 33.9) | (−1.6, 5.6) | (3.5, 36.0) | (26.3, 62.4) | (−0.26, −0.01) | ||
| II: 15 mm/6 s | 7.5 | 3.8% | 1.0% | 11.2% | 12.4% | −0.18 |
| (−98.7, 39.7) | (−1.5, 6.0) | (−33.8, 29.9) | (−33.8, 36.3) | (−0.60, 0.01) | ||
| 12.5 | 21.5% | 2.1% | 13.2% | 31.8% | −0.13 | |
| (−3.5, 68.5) | (−1.5, 3.5) | (0.1, 28.9) | (13.7, 45.9) | (−0.28, −0.01) | ||
| III: 25 mm/4 s | 7.5 | 21.3% | −1.0% | 21.4% | 22.8% | −0.14 |
| (−13.8, 64.8) | (−12.9, 5.9) | (−3.9, 51.1) | (−2.1, 44.6) | (−0.35, −0.02) | ||
Fig. 2.
Coronal views of the phantom with the 7.5 mm radius sphere and the 15 mm/6 sec motion II at (a) static (b) end of expiration phase and (c) end of inspiration phase.
The smallest volume deviations were found at the end of expiration, for each sphere size and each motion tested. The end of expiration provided a statistically more accurate volume than the end of inspiration and the mid-ventilation phase (see Table 3). There was one exception: For the small sphere animated with motion II: A = 15 mm, t = 6 s period the end of expiration was not significantly better than the end of inspiration (p = 0.073) and the mid-ventilation (p = 0.054).
Table 3.
P-values of the Wilcoxon test between the volume deviations observed in each chosen phase.
| Motion | Sphere radius (mm) | End of inspiration/End of expiration | Mid-ventilation/End of expiration | End of inspiration/Mid-ventilation |
|---|---|---|---|---|
| I: 15 mm/3 s | 7.5 | P < 0.05 | P < 0.05 | p > 0.05 |
| II: 15 mm/6 s | p > 0.05 | p > 0.05 | p > 0.05 | |
| III: 25 mm/4 s | P < 0.05 | P < 0.05 | p > 0.05 | |
| I: 15 mm/3s | 12.5 | P < 0.05 | P < 0.05 | p > 0.05 |
| II: 15 mm/6s | P < 0.05 | P < 0.05 | p > 0.05 |
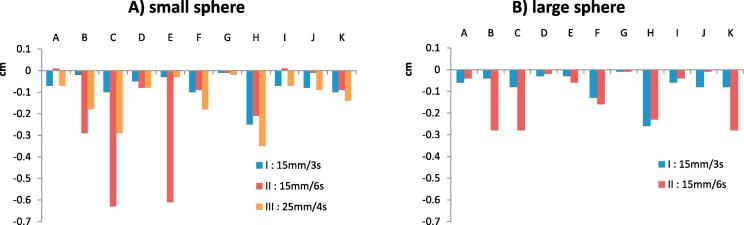
3.2. Amplitude deviations
The amplitude of motion was underestimated in most cases (Fig. 3). Two outliers exhibited deviations near 6 mm from the known trajectory length. As previously displayed in Fig. 2, the deviation observed for institution E was caused by a disappearance of the cranial part of the sphere volume at the end of the inspiration. The second outlier was found for institution C. A sphere was discernible. However, due to residual motion within the bin the sphere HU were inferior to the auto delineation HU threshold set on the 3DCT image. This resulted in an inaccurate centre of mass. Over all institutions the amplitude deviations ranged from −6 mm to +0.1 mm (Table 2).
Fig. 3.
Deviation of the peak to peak amplitude (minus signs mean an underestimation of the amplitude).
Pitch settings ranged from 0.041 to 0.16. Institution J did not adapt the pitch based on the respiratory period. Institutions C, E and F used a too high pitch (>pmax) for the respiratory period of 6 s. Nonetheless these non-optimal set-ups did not translate into significant (p = 0.42) positional inaccuracies, although institution E indeed performed worse for the 6 s period motion II than for respectively the 3 s and 4 s period motions I and III for both sphere sizes. On the contrary institution F presented a smaller deviation for the 6 s motion II than for the 3 s period motion I. As only five datasets were acquired with a pitch larger than the maximum pitch, a statistically relevant test to compare these datasets to the datasets not violating the maximum pitch was not feasible. Furthermore, no correlation could be found between scanner brands and both positional accuracy or volume accuracy.
4. Discussion
The purpose of this study was to provide an overview of the 4DCT acquisition methods and achievable accuracy of image tumour volumes in institutions participating in the trial.
Average volume deviations found in our study for the 7.5 mm sphere were 13% at end of inspiration, 1% at end of expiration and 16% at mid-ventilation. For the larger 12.5 mm sphere these deviations were respectively 15%, 2% and 12%. Therefore, the study confirmed that the expiration phase is the most accurate phase to define the shape and volume of the GTV as the variations in volume shown here exceed the tumour shape deformations occurring during normal free breathing [15]. Amplitude deviations were generally within 2 mm but underestimations of >6 mm were observed. Other segmentation methods might have led to slightly different results, as fixed HU thresholds would tend to underestimate the volume of a mobile lesion on certain phases of 4DCT relative to static CT, while HU thresholds relative to the maximum HU within a phase would tend to overestimate the volume relative to static CT. However, we do not think this would influence the conclusions about which phase can be used best to delineate the sphere. The phases with the largest volume deviations also showed the most deviations from depicting a spherical volume (visual inspection of all images, this was not further quantified). Auto-delineation could in clinical practice lead to worse results as the tumour edge is not defined by a step function in HU differences as is the case in our experiment. Especially for tumours attached to the chest wall or mediastinum, auto-delineation might not lead to acceptable results.
One might argue that the poor results found in some institutions could result from phantom misalignment or timing artefacts between the imaging time and the motion time line. Concerning phantom misalignment, we verified in the CT images itself that the phantom was properly aligned, as the phantom included some reference marks. Timing artefacts cannot be ruled out. Due to the already very extensive 3-day site visit program in which this investigation was included, it was not possible to perform repeat measurements. As timing artefacts, also can have an effect during clinical practice, the deviations found in this study do reflect what can occur in clinical practice. However, in order to determine in more detail how to further optimise scan parameters, repeat measurements per scanner should be performed. This was outside of the scope of this study.
In this study, we only investigated regular breathing patterns in a simple, commercially available phantom and different results may be expected for irregular breathing enhancing for example the differences between amplitude and phase based sorting reconstructions. This setup was chosen to be able to compare our results to previous studies and to be able to also use the same phantom to perform 2D dose measurements. Amplitudes tested are larger than deviations generally seen in clinical practice therefore our results might overemphasize the importance of the observed variations.
As the research preformed and reported here was only sponsored and agreed upon after the criteria for trial participation were determined, the results could not be used as credentialing criteria for the trial. As part of 4DCT credentialing for clinical trials, it would also be of interest to ask the participants about their 4DCT training for staff, the implementation of any patient coaching techniques, or the workflows for motion assessment to support motion management decisions. These elements could all have an important impact on the clinically observed 4DCT quality.
Institutions were asked to scan the phantom according to their own protocol, thus several parameters differed between institutions. This restricted our possibilities to correlate the result to single scan parameters. Such extensive testing including rescanning was outside of the scope of the study and would have added unacceptable time to the already extensive site-visit. However, we have observed that some institutions decreased their tube current without adjustment of their pitch which might not be optimal.
Romero et al. [16] published a phantom study on 4DCT image quality employing a sinusoidal motion which does not represent normal breathing accurately. They found volume deviations on simulated 4DCT images at maximum 3% for a ten mm radius sphere and <4% for a five mm radius sphere. They looked at inhale, exhale and mid-ventilation phases. CT images were simulated using an AquilionTM LB 16-row CT scanner (ToshibaTM) with an RPM system (VarianTM). In comparison, this study used the Lujan function with n = 3 to generate the breathing motion, which better resembles clinical conditions. An observation made by Romero was that an increase of the exposure (mAs), lead to a consequent decrease of the noise in the reconstructed image, thus to a more accurate auto-contouring and therefore a better volume determination. This could not be observed in our study, probably because the exposure was not tested separately and its influence was obscured by the influence of the other varying parameters.
Hurkmans et al. [10] performed a similar study to the one presented in this article using the same breathing period and amplitude as used in this study, with two spherical targets of 15 mm and 30 mm diameter, in eight institutions in The Netherlands. They looked at the following phases; end of inspiration, end of expiration, mid-ventilation and maximum intensity projection. They found an average volume overestimation over the institutions tested for the small sphere of 0.10 cc which is similar to our findings. The amplitude deviation was on average 1–2 mm with deviations up to 5.5 mm, which is also in the range of our findings.
Dou et al. [17] have performed a simulation study taking the acquisition parameters of a Philips Big Bore (rotation 0.5 s, pitch 0.06) and a Siemens Somaton Open (rotation 0.5 s, pitch 0.1). Simulated tumour size and motion were more often underestimated when scanning data at the higher pitch of 0.1. Our data does not show this correlation, but this might be due to the small dataset acquired.
The large variations found among the institutions indicates that further improvements in 4DCT imaging and use for treatment planning are possible.
We therefore recommend each institution to perform a 4DCT phantom study using different amplitudes and (breathing) frequencies before clinical use of the 4DCT scanner. Optimally, this would also include recorded irregular patient breathing patterns. Institutions should use pitch values that do not violate the pitch maximum value which is dependent on the patient’s breathing frequency. It is also advised to quantify the observed amplitude underestimations and take this into consideration when determining CTV to PTV margins. Finally, when using tracking techniques, we recommend delineation of the GTV in the exhale phase for tumours exhibiting large motion causing such motion artefacts that tumour volume determination in other phases is hampered. The GTV can then be propagated to other phases.
In conclusion, our results show that significant differences in 4DCT scanning performance still remain among institutions.
The data presented here together with already published data can be used as a benchmark for other institutions aiming to evaluate their scan techniques. Implementation of improved 4DCT QA procedures within institutions is warranted.
Acknowledgments
Acknowledgements
All the people from the centres visited which helped in the preparation and the execution of the RTQA visit are gratefully acknowledged for all their time and effort.
Coreen Corning from the EORTC is acknowledged for the coordination of the data management between the EORTC and the visited institutions.
During the conduct of the study a PhD grand from Elekta was received.
Conflict of Interest Statement
No conflict of interest to disclose
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2018.10.003.
Contributor Information
Marie Lambrecht, Email: marie.lambrecht@catharinaziekenhuis.nl.
Jan-Jakob Sonke, Email: j.sonke@nki.nl.
Ursula Nestle, Email: ursula.nestle@mariahilf.de.
Heike Peulen, Email: heike.peulen@catharianaziekenhuis.nl.
Damien C. Weber, Email: Damien.weber@psi.ch.
Marcel Verheij, Email: m.verheij@nki.nl.
Coen W. Hurkmans, Email: coen.hurkmans@catharinaziekenhuis.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guckenberger M., Andratschke N., Dieckmann K., Hoogeman M.S., Hoyer M., Hurkmans C. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11–17. doi: 10.1016/j.radonc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Clements N., Kron T., Franich R., Dunn L., Roxby P., Aarons Y. The effect of irregular breathing patterns on internal target volumes in four-dimensional CT and cone-beam CT images in the context of stereotactic lung radiotherapy. Med Phys. 2013;40 doi: 10.1118/1.4773310. [DOI] [PubMed] [Google Scholar]
- 3.Persson G.F., Nygaard D.E., Brink C., Jahn J.W., Munck af Rosenschöld P., Specht L. Deviations in delineated GTV caused by artefacts in 4DCT. Radiother Oncol. 2010;96:61–66. doi: 10.1016/j.radonc.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Li H., Noel C., Garcia-Ramirez J., Low D., Bradley J., Robinson C. Clinical evaluations of an amplitude-based binning algorithm for 4DCT reconstruction in radiation therapy. Med Phys. 2012;39:922–932. doi: 10.1118/1.3679015. [DOI] [PubMed] [Google Scholar]
- 5.Bernatowicz K., Keall P., Mishra P., Knopf A., Lomax A., Kipritidis J. Quantifying the impact of respiratory-gated 4D CT acquisition on thoracic image quality: a digital phantom study: respiratory-gated 4D CT acquisition. Med Phys. 2014;42:324–334. doi: 10.1118/1.4903936. [DOI] [PubMed] [Google Scholar]
- 6.Pollock S., Kipritidis J., Lee D., Bernatowicz K., Keall P. The impact of breathing guidance and prospective gating during thoracic 4DCT imaging: an XCAT study utilizing lung cancer patient motion. Phys Med Biol. 2016;61:6485. doi: 10.1088/0031-9155/61/17/6485. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D., Lamb J., White B., Jani S., Gaudio S., Lee P. A Novel Fast Helical 4D-CT Acquisition Technique to Generate Low-Noise Sorting Artifact-Free Images at User-Selected Breathing Phases. Int J Radiat Oncol. 2014;89:191–198. doi: 10.1016/j.ijrobp.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolthaus J.W.H., Sonke J.-J., van Herk M., Damen E.M.F. Reconstruction of a time-averaged midposition CT scan for radiotherapy planning of lung cancer patients using deformable registrationa) Med Phys. 2008;35:3998–4011. doi: 10.1118/1.2966347. [DOI] [PubMed] [Google Scholar]
- 9.Werner R., Hofmann C., Mücke E., Gauer T. Reduction of breathing irregularity-related motion artifacts in low-pitch spiral 4D CT by optimized projection binning. Radiat Oncol. 2017;12:100. doi: 10.1186/s13014-017-0835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurkmans C.W., van Lieshout M., Schuring D., van Heumen M.J.T., Cuijpers J.P., Lagerwaard F.J. Quality Assurance of 4D-CT Scan Techniques in Multicenter Phase III Trial of Surgery Versus Stereotactic Radiotherapy (Radiosurgery or Surgery for Operable Early Stage (Stage 1A) Non–Small-Cell Lung Cancer [ROSEL] Study) Int J Radiat Oncol. 2011;80:918–927. doi: 10.1016/j.ijrobp.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Adebahr S., Collette S., Shash E., Lambrecht M., Le Pechoux C., Faivre-Finn C. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol. 2015;88:20150036. doi: 10.1259/bjr.20150036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambrecht M., Melidis C., Sonke J.-J., Adebahr S., Boellaard R., Verheij M. Lungtech, a phase II EORTC trial of SBRT for centrally located lung tumours – a clinical physics perspective. Radiat Oncol. 2016;11:7. doi: 10.1186/s13014-015-0567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lujan A.E., Larsen E.W., Balter J.M., Haken R.K.T. A method for incorporating organ motion due to breathing into 3D dose calculations. Med Phys. 1999;26:715–720. doi: 10.1118/1.598577. [DOI] [PubMed] [Google Scholar]
- 14.Hilgers G., Nuver T., Minken A. Helical 4D CT pitch management for the Brilliance CT Big Bore in clinical practice. J Appl Clin Med Phys. 2015;16:389–398. doi: 10.1120/jacmp.v16i3.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J., Lei P., Shekhar R., Li H., Suntharalingam M., D’Souza W.D. Do Tumors in the Lung Deform During Normal Respiration? An Image Registration Investigation. Int J Radiat Oncol. 2009;75:268–275. doi: 10.1016/j.ijrobp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Romero R., Castro-Tejero P. The influence of respiratory motion on CT image volume definition. Med Phys. 2014;41 doi: 10.1118/1.4866889. [DOI] [PubMed] [Google Scholar]
- 17.Dou T.H., Thomas D.H., O’Connell D., Bradley J.D., Lamb J.M., Low D.A. Technical Note: simulation of 4DCT tumor motion measurement errors. Med Phys. 2015;42:6084–6089. doi: 10.1118/1.4931416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.