Highlights
-
•
This study suggests a PQM methodology for breast cancer radiotherapy evaluation.
-
•
The risk/benefit balance estimation includes tumor biology and smoking status.
-
•
Smoking status influenced risk/benefit balance for different treatment techniques.
-
•
Survival benefit dominated for all patients with high-risk breast cancer.
-
•
Survival benefit for smokers with low- or intermediate- risk cancer was not seen.
Keywords: Breast cancer, Three-dimensional conformal radiotherapy, Volumetric-modulated arc therapy, Intensity-modulated arc therapy, Risk assessment
Abstract
Background/purpose
Tumor biology and patient smoking status have clear effects on the benefit of breast radiotherapy. This study developed treatment evaluation strategies that integrated dosimetry, tumor aggressiveness and smoking status for patients undergoing hypo-fractionated whole breast irradiation with simultaneous integrated boost.
Materials/methods
The evaluation method Plan Quality Metrics (PQM) was adapted for breast cancer. Radiotherapy (RT) benefit was assessed for three levels of tumor aggressiveness; RT risk was estimated using mean dose to organs at risk and published Excess Relative Risk per Gy data for lung cancer and cardiac mortality for smokers and non-smokers. Risk for contralateral breast cancer was also evaluated. PQM and benefit/risk was applied to four patient groups (n = 10 each). Plans using 3D conformal radiotherapy (3DCRT), 3DCRT plus intensity-modulated radiation therapy (IMRT), 3DCRT plus volumetric modulated arc therapy (VMAT) and VMAT were evaluated for each patient.
Results
3DCRT-IMRT hybrid planning resulted in higher PQM score (median 87.0 vs. 3DCRT 82.4, p < 0.01), better dose conformity, lower doses to the heart, lungs and contralateral breast. Survival benefit was most predominant for patients with high-risk breast cancer (>7% and >4.5% gain for non-smokers and smokers). For smokers with intermediate- or low-risk breast cancer, RT induced mortality risk dominated for all techniques. When considering the risk of local recurrence, RT benefitted also smokers (>5% and >2% for intermediate- and low-risk cancer).
Conclusions
PQM methodology was suggested for breast cancer radiotherapy evaluation. Further validation is needed. RT was beneficial for all patients with high risk of recurrence. A survival benefit for smokers with low or intermediate risk of recurrence could not be confirmed.
1. Introduction
Individualization of radiation therapy of breast cancer is an issue of high priority and interest towards improving treatment outcome. Optimization and differentiation of the benefit/harm balance for the breast cancer patients based on clinical evidence is desirable. A meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) [1] summarized the benefits of breast cancer radiotherapy based on 17 randomized trials, where patients were stratified into three groups based on cancer severity. Reduction in recurrence rates and mortality were reported for each group. The results suggested that different treatment strategies may be appropriate for different patients, depending on tumor aggressiveness and biology as well as on patient comorbidities and smoking habits.
Quantitative data about differences in potential harm of the radiation treatment are reported in the meta-analysis by Taylor et al. [2] including 75 trials. Excess rate ratios (ERRs) per Gy were determined for lung cancer and cardiac mortality. The ERRs were further applied to population-based mortality rates, and the cumulative risk of lung cancer and cardiac mortality for smokers and non-smokers who underwent radiotherapy were presented. Contralateral breast cancer (CBC) was also included in the analysis without an ERR estimate.
Risk evaluation is directly dependent on the dose to the organs at risk. Hence, the accuracy of risk prediction may be improved by determining specific dose levels for the side being treated. Furthermore, dose levels should be representative for currently available beam delivery techniques reflecting the latest technical advances in the accelerator performance. Treatment planning studies involving static, dynamic beam delivery as well as their combinations have been reported [3], [4], [5], [6], [7]. The dosimetric characteristics of the resulting plans may vary since there are no practical recommendations about plan generation and optimization. Risk prediction based on dose levels achievable at the clinic is of importance, in particular for the more complex breast irradiation with simultaneously integrated boost (SIB).
The choice of planning strategy and optimal plan might be a complex task due to the large number of parameters that should be considered. In a study to assist in the plan evaluation process for prostate cancer patients, a scoring method termed Plan Quality Metrics (PQM) was developed for 125 patients [8]. Adjusted PQM (APQM) was applied to rank 80 clinical plans of prostate cancer patients [9]. PQM was also used to determine the most optimal plan for gastric mucosa-associated lymphoid tissue lymphoma [10]. Selection of treatment plan evaluation criteria and method parameters are not valid for other diagnoses and need to be derived for breast cancer treatment.
National guidelines on radiation treatment usually include general recommendations about optimization and differentiation of the benefit/harm balance for individual breast cancer patients without specifying how this should be done. Clinical factors are currently not taken into consideration during plan evaluation. In the Passos project [11], long term personalized risks were calculated for different treatment plans, taking smoking status into consideration but not radiotherapy benefit depending on tumor aggressiveness. To our knowledge, the current study was warranted due to the lack of reports using individual biological factors and smoking status for treatment plan evaluation of breast cancer radiotherapy.
The aim of this work was to develop treatment strategies that integrated dosimetry, tumor aggressiveness and smoking status, balancing risks and benefits for individual patients undergoing hypo-fractionated whole breast irradiation with simultaneous integrated boost.
2. Materials and methods
2.1. Personalized benefit/risk assessment
The net gain in survival was estimated by approximating the benefit of radiotherapy as well as the excess risk due to exposing organs at risk for each patient. Three levels of tumor aggressiveness were taken into account as specified in the supplementary webappendix of the EBCTCG meta-analysis [1], i.e. high-risk, intermediate-risk, and low-risk breast cancer. The 10-year benefit of radiotherapy for any first recurrence and the 15-year benefit of radiotherapy for breast cancer mortality was used as estimated in the supplementary webappendix of the EBCTCG-meta-analysis [1]. It was assumed that the absolute effect of radiotherapy vs. no radiotherapy would be persistent after 10 years when analyzing recurrences and would be persistent after 15 years when analyzing mortality.
The excess mortality risk for ischemic heart disease (IHD) and lung cancer was estimated utilizing ERR per Gy from Taylor et al. [2] (0.041/Gy for IHD and 0.11/Gy for lung cancer) as well as the baseline cumulative risk of lung cancer death and IHD death for a smoker and a lifelong non-smoker receiving no radiotherapy. For CBC, the ERR per Gy mean dose used was 0.43 [12]. Incidence data for CBC was obtained by extrapolating data for non-irradiated breast cancer patients from the Sweden Breast Cancer Group (SweBCG) 91-RT trial [13]. Extrapolated averaged breast cancer survival data from population-based registers were used to obtain background CBC mortality [14]. The risk for CBC was considered independent of smoking status. The model used a delay in radiation-induced CBC incidence of 10 years [15]. By using these data and applying the mean doses to the organs at risk from different planning techniques, a risk/benefit assessment for breast cancer patients treated at age 50 with RT and followed until 80 years of age was performed for non-smokers as well as smokers for high-, intermediate-, and low-risk breast cancer.
2.2. Target delineation and dose planning
The study analyzed the CT scans of patients who were previously treated by breast-conserving surgery followed by radiotherapy. There were four groups, each with 10 patients: Group 1, CT scan during deep inspiration breath-hold (DIBH) and a left-sided target volume; Group 2, CT scan in free breathing (FB) and a left-sided target volume; Group 3, CT scan in FB and a left-sided target volume with the lumpectomy cavity located in the lower inner quadrant; and Group 4, CT scan in FB and a right-sided target volume. Contours were drawn according to the ESTRO guidelines [16] using a 12-mm planning target volume (PTV) margin to the lumpectomy clinical target volume (CTV), 5 mm in the transversal plane, and 6 mm in the craniocaudal direction to the breast CTV. All target structures were cropped 5 mm below the body surface. The prescription dose was 40 Gy to the breast and 48 Gy to the lumpectomy cavity in 15 fractions (2.67 and 3.2 Gy/fx) according to ARM II in the Radiation Therapy Oncology Group (RTOG) 1005 protocol [17]. For delineation of organs at risk the SweBCG radiotherapy guidelines were used [18]. For heart delineation this guidelines use Feng et al. [19].
Treatment planning was performed for a Varian TrueBeam linear accelerator using the dose calculation algorithm AAA in Varian Eclipse v. 13.6. For each patient four plans based on different techniques were designed (Supp. Fig. 1):
-
•
3D conformal radiotherapy (3DCRT): A conventional plan with tangential static fields focusing on the breast volume and with fields focusing on the boost volume from favorable gantry angles. Photon beams of 6 MV were used for the main tangential fields, and 6 or 15 MV beams were used for eventual supplemental fields.
-
•
3DCRT plus intensity-modulated radiation therapy (IMRT): A hybrid plan with about a 70% dose to the breast PTV from conventional fields of 6 MV photons (as a base plan in the optimization) and the rest of the dose from four IMRT fields [3]. Two IMRT fields covered the entire breast PTV, and two only covered the boost PTV from favorable gantry angles.
-
•
3DCRT plus volumetric modulated arc therapy (VMAT): A hybrid plan consisting of two tangential static photon fields of 6 MV delivering about an 80% dose to the breast PTV and 2–4 VMAT fields with partial arcs of 90° providing the rest of the dose.
-
•
VMAT: A plan with four partial arcs of 90° (the same angles as in the 3DCRT-VMAT plan).
IMRT and VMAT optimization was carried out in the Photon Optimizer 13.6 with 6 MV beams. The RTOG 1005 protocol dose-volume criteria specified for two levels, Per Protocol and Variation Acceptable [17], were utilized to generate the plans. To avoid interplanner variability, all plans were made by one experienced dosimetrist who optimized the plans according to the RTOG 1005 criteria (Supp. Table 1) to maintain target coverage while sparing organs at risk and normal tissue as much as possible. In the case of the 3DCRT-VMAT and VMAT plans the external and target structures were expanded 10 mm towards the external direction of the body in the optimization to account for respiration and possible swelling of the breast [20]. After optimization the plans were calculated on the original CT data. The feasibility of VMAT during DIBH has been previously presented elsewhere [21], [22].
2.3. Plan evaluation
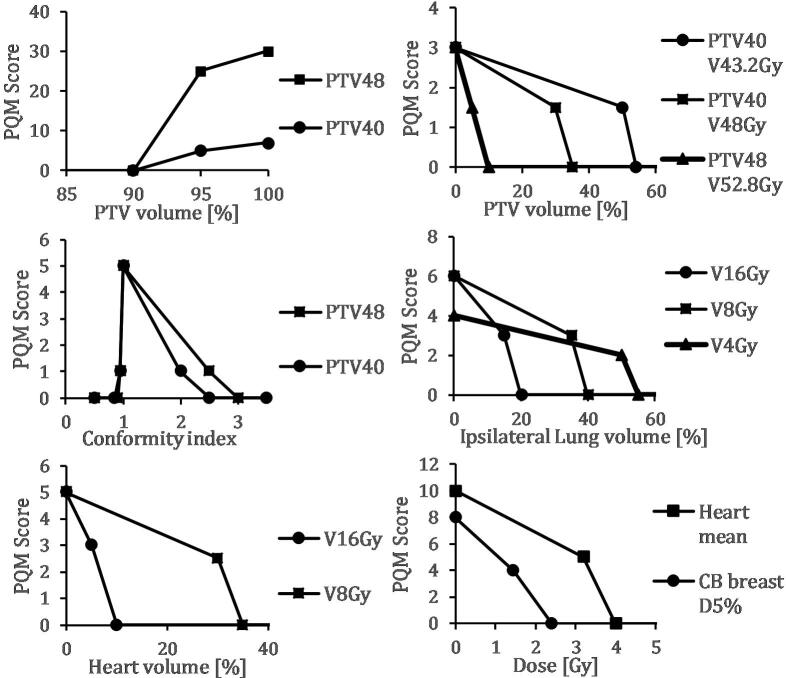
The RTOG 1005 protocol parameters were used when analyzing the dose-volume histograms (DVHs) obtained for the different plans. All plans were compared to 3DCRT, the technique that is currently used at Sahlgrenska University Hospital (Gothenburg, Sweden). A PQM strategy was used to evaluate plan quality and variation for the different treatment techniques [8]. In PQM, scores are assigned to different plan parameters based on how well they comply with the dose-volume constraints, and criteria that are considered biologically more important are weighted more heavily than less important criteria. In our PQM model, the score levels were based on selected RTOG 1005 constraints for Variation Acceptable and Per Protocol (Supp. Table 1). Zero points were given if the parameter failed to reach Variation Acceptable. A linear interpolation between Variation Acceptable until the level for Per Protocol performance was then used and further a linear interpolation from the level of Per Protocol until optimal dose. Different sub-metrics were differently weighted depending on priority in national breast radiotherapy guidelines [18], [23]. This resulted in points for 14 sub-metrics as shown in Fig. 1. The sum of all points gives the PQM score.
Fig. 1.
PQM score levels and intra-level interpolation for the 14 sub-metric components for left-sided breast cancer. All sub-metrics are based on selected constraints in RTOG 1005 (Supp. Table 1) and have three levels: Variation Acceptable, Per Protocol, and Ideal. PTVxx VyyGy denotes the PTVxx volume receiving up to yy Gy. PTV40 is the breast PTV volume prescribed to 40 Gy and PTV48 the lumpectomy PTV volume prescribed to 48 Gy.
2.4. Statistical analysis
The results are presented as the medians for the different parameters together with the corresponding interquartile range. Any statistically significant differences between the different techniques and 3DCRT were tested using the Wilcoxon signed-rank test in Stata version 15 (StataCorp, College Station, TX, USA). P-value <0.05 was considered to indicate statistical significance.
3. Results
Median breast PTV and lumpectomy PTV V95% coverage was above 96% across all groups and techniques (range 96.2%–99.8%) (Supp. Tables 2–5). PTV V43.2Gy were similar for the hybrid and VMAT plans (within a difference of 2% for groups 1, 2 and 4 and 4% for Group 3) and superior to the corresponding conventional plans (a median up to 14.5% lower). Similar behavior were seen for V48Gy and conformity indexes. Doses to organs at risk were generally lower for 3DCRT-IMRT compared to the other techniques, especially compared to plans involving VMAT. Although the 3DCRT-IMRT hybrid technique generated good results in the DVH comparisons, the monitor units (MUs) were higher than for the other techniques (Supp. Table 6). The volume outside the breast PTV that receives 90% of the prescribed dose (i.e. V36Gy), was lowest for the VMAT technique (median 190 cm3) and highest for the 3DCRT technique (428 cm3).
Plans that used the 3DCRT-IMRT technique had the highest total median PQM score (87.0) averaged over the four patient groups, followed by 3DCRT-VMAT (83.5), 3DCRT (82.4) and VMAT (75.7) (Table 1). The relative performance of the four techniques tended to persist even for the individual plans (Supp. Fig. 2). The robustness of the PQM score was tested by changing the weights of the sub-metrics, which resulted in slightly different scores, but preserved the relative order of the planning techniques (data not shown).
Table 1.
PQM scores for the indicated groups and techniques. The p-values presented refer to the comparison of 3DCRT and the given technique.
| Group | 3DCRT | 3DCRT-IMRT |
3DCRT-VMAT |
VMAT |
||||
|---|---|---|---|---|---|---|---|---|
| Mdn (IQR) | Mdn (IQR) | p-val. | Mdn (IQR) | p-val. | Mdn (IQR) | p-val. | ||
| PQM | 1 | 83.0 (82.0–84.0) | 87.6 (86.9–89.7) | <0.01 | 86.0 (85.6–86.5) | 0.03 | 81.6 (79.4–83.9) | 0.28 |
| 2 | 81.7 (81.2–82.9) | 84.4 (84.0–86.5) | <0.01 | 81.0 (79.9–83.1) | 0.96 | 78.0 (75.5–80.5) | 0.03 | |
| 3 | 80.2 (79.1–80.8) | 84.5 (83.7–86.2) | <0.01 | 79.3 (77.0–81.6) | 0.72 | 75.8 (74.2–77.8) | 0.02 | |
| 4 | 85.4 (83.4–86.5) | 89.1 (88.7–90.5) | <0.01 | 86.4 (84.5–87.3) | 0.44 | 83.0 (80.5–86.8) | 0.03 | |
| Total | 82.4 (80.6–84.3) | 87.0 (84.4–88.7) | <0.01 | 83.5 (79.9–86.1) | 0.11 | 79.5 (75.7–83.2) | <0.01 | |
Abbreviations: Mdn = median. IQR = interquartile range.
Median mean doses to the organs at risk that influenced the risk calculation were lower by up to 0.5, 0.7 and 1.6 Gy for heart, lungs and contralateral breast for the 3DCRT and 3DCRT-IMRT techniques compared to 3DCRT-VMAT and VMAT for all patient groups (Table 2). Consequently, the risks from radiotherapy according to the ERR per Gy model were lowest for a 50 years old patient followed until 80 years of age for a 3DCRT-IMRT treatment (Supp. Table 7). For non-smokers, the differences in elevated risk for cardiac and lung cancer mortality were generally low, between 0.0% and 0.2% in all the techniques. Greater differences were observed for smokers, especially for the risk of lung cancer death (up to 1.1% difference between 3DCRT-IMRT and VMAT; range 2.4% to 3.5% for the group with the highest risks). The mortality risk from CBC was also higher for the VMAT technique. For tumors in the lower inner quadrant, i.e. with the tumor bed near the heart (Group 3), the difference was up to 0.8% compared to 3DCRT and 3DCRT-IMRT (range <0.1%–0.8%).
Table 2.
The mean doses for the heart, lungs, and contralateral breast for the indicated groups and techniques. The p-values presented refer to the comparison of 3DCRT and the given technique.
| Group | 3DCRT | 3DCRT-IMRT |
3DCRT-VMAT |
VMAT |
||||
|---|---|---|---|---|---|---|---|---|
| Mdn (IQR) | Mdn (IQR) | p-val. | Mdn (IQR) | p-val. | Mdn (IQR) | p-val. | ||
| Heart, Gy | 1 | 1.0 (0.9–1.3) | 1.0 (0.9–1.2) | 0.01 | 1.3 (1.1–1.5) | <0.01 | 1.4 (1.3–2.3) | <0.01 |
| 2 | 2.5 (1.6–2.6) | 2.2 (1.5–2.4) | <0.01 | 2.6 (2.0–2.8) | <0.01 | 2.6 (2.3–3.0) | 0.09 | |
| 3 | 2.5 (1.6–2.8) | 2.4 (1.7–2.5) | <0.01 | 2.8 (2.2–2.9) | <0.01 | 2.9 (2.7–3.1) | 0.01 | |
| 4 | 0.3 (0.2–0.3) | 0.3 (0.2–0.3) | <0.01 | 0.6 (0.5–0.8) | <0.01 | 0.8 (0.7–0.9) | <0.01 | |
| Lungs, Gy | 1 | 2.3 (2.1–2.4) | 2.0 (1.7–2.2) | <0.01 | 2.6 (2.3–2.8) | <0.01 | 3.0 (2.7–3.5) | <0.01 |
| 2 | 2.4 (2.0–2.7) | 2.2 (1.8–2.5) | <0.01 | 2.6 (2.2–2.9) | 0.03 | 2.7 (2.2–3.3) | 0.03 | |
| 3 | 2.4 (1.9–2.7) | 2.3 (1.8–2.4) | <0.01 | 2.5 (2.1–2.9) | <0.01 | 2.8 (2.2–3.1) | <0.01 | |
| 4 | 3.1 (2.5–3.3) | 2.6 (2.3–2.9) | <0.01 | 3.5 (3.4–3.6) | <0.01 | 3.8 (3.3–4.0) | <0.01 | |
| CB, Gy | 1 | 0.1 (0.0–0.2) | 0.1 (0.0–0.1) | 0.01 | 0.5 (0.4–0.6) | <0.01 | 0.7 (0.6–1.2) | <0.01 |
| 2 | 0.1 (0.0–0.1) | 0.1 (0.0–0.1) | <0.01 | 0.6 (0.3–0.6) | <0.01 | 1.3 (0.9–1.5) | <0.01 | |
| 3 | 0.1 (0.0–0.2) | 0.1 (0.0–0.1) | <0.01 | 0.7 (0.3–0.8) | <0.01 | 1.7 (1.0–1.9) | <0.01 | |
| 4 | 0.1 (0.0–0.2) | 0.1 (0.0–0.1) | 0.02 | 0.5 (0.3–0.7) | <0.01 | 0.9 (0.6–1.0) | <0.01 | |
Abbreviations: Mdn = median. IQR = interquartile range. CB = contralateral breast.
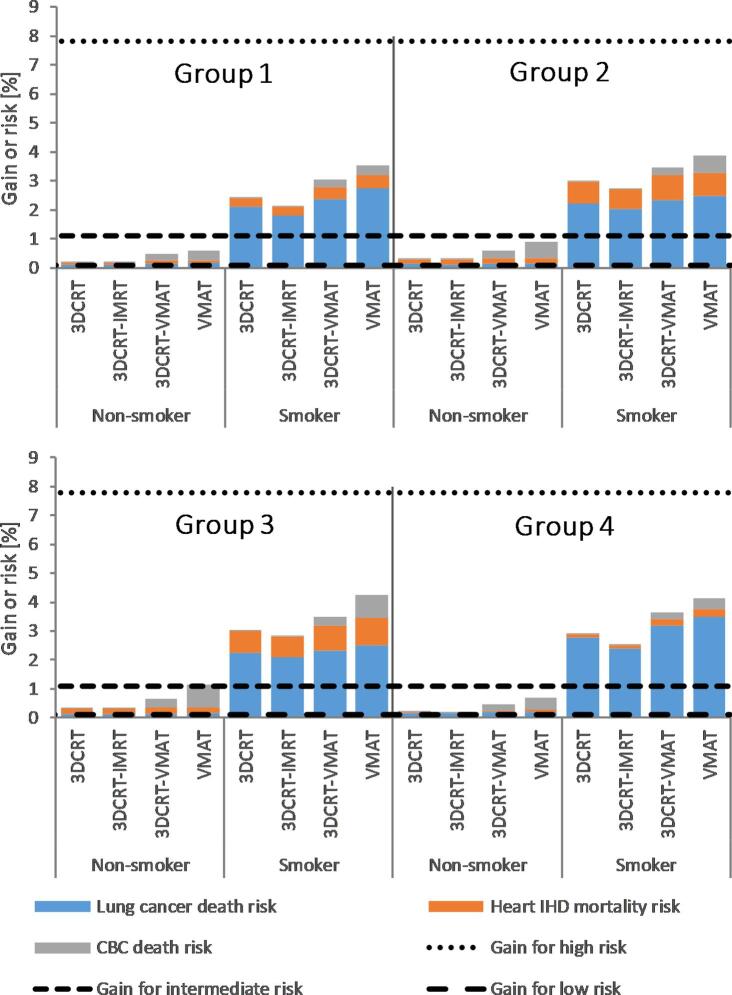
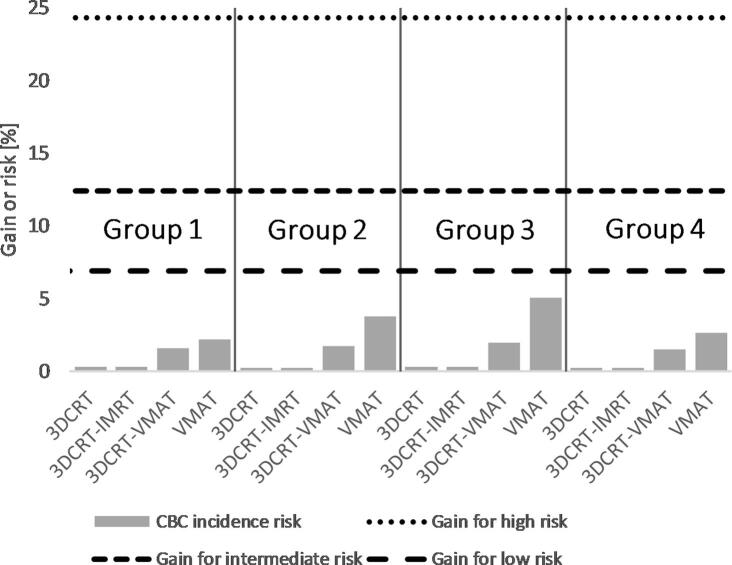
When survival benefit from radiotherapy for the three risk groups is compared with the estimated additional mortality risk from lung cancer, IHD and CBC due to radiotherapy (Fig. 2), both the survival benefit and the total mortality risk estimates were within 1.5% for all techniques for non-smokers with low- and intermediate-risk breast cancer. For smokers, there were larger differences between the techniques, but the net results regarding mortality (survival benefit minus the sum of the mortality risks) were negative for all techniques in the intermediate- and low-risk breast cancer groups. Largest negative net results were obtained for the VMAT technique; up to 4% and 3% for low- and intermediate-risk breast cancer. When the risk of local recurrence of breast cancer was considered and compared to the estimated risk of radiation-induced CBC (Fig. 3), there was a clear benefit of radiotherapy even for those groups. In the high-risk group, the survival benefit of radiotherapy outweighed the risk for all techniques regardless of smoking status.
Fig. 2.
Risk/benefit balance for the different techniques for all groups (Group 1: left-sided under DIBH, Group 2: left-sided in FB, Group 3: left-sided in FB with the lumpectomy cavity only located in the lower inner quadrant and Group 4: right-sided in FB). The horizontal dotted lines indicate the estimated mortality reduction due to radiotherapy for patients with different tumor aggressiveness; the columns show the estimated increased mortality risk from lung cancer, ischemic heart disease and contralateral breast cancer due to radiotherapy.
Fig. 3.
Risk reduction of any recurrence from radiotherapy for the different risk groups (horizontal lines) compared to elevated risk for radiation-induced contralateral breast cancer in the patient groups (Group 1: left-sided under DIBH, Group 2: left-sided in FB, Group 3: left-sided in FB with the lumpectomy cavity only located in the lower inner quadrant and Group 4: right-sided in FB). Contralateral breast cancer was determined to be independent of smoking status.
4. Discussion
By using PQM, the treatment plan could be evaluated with just one score rather than comparing different DVH parameters. The small difference between the PQM scores for the different techniques may have been related to the dominance of target coverage in the total score, as suggested by all major guidelines.
In the intermediate- and low-risk groups, the net result of negative survival for smokers may suggest that these groups should not receive radiotherapy. But considering the increase in the risk of recurrence and the fact that quitting smoking greatly reduces the incidence risk [2], radiotherapy could still be an option for those patients and emphasize the need to encourage patients to quit smoking. The differences in mortality due to lung cancer, cardiac events, or CBC between the different techniques were generally small, especially for non-smokers. This implies that many techniques may be acceptable, but since this patient group is large, even a small difference would increase the number of deaths.
Furthermore, our results suggest that of the techniques we studied, a hybrid of conventional and IMRT fields was the most appropriate SIB technique for treating breast cancer patients after breast-conserving surgery. This plan combined good conformity with a restricted dose bath outside the target. However, the monitor units (MU) used were considerably higher than for other techniques, which may increase the dose uncertainty due to more leaking photons between the MLC leafs. The superiority of the VMAT plans in terms of dose conformity outside the breast target volumes indicates a lower risk of breast pain and skin toxicity [24], and this could justify the use of this technique for a non-smoker, with a low dose to the other organs at risk.
Another study that looked at the risk of IHD in women after radiotherapy for breast cancer found an increased risk of a major coronary event of 7.4% /Gy [25]. The ERRs were higher compared to those used in this study (4.1% per Gy) due to the fact that the endpoint was different (cardiac event instead of cardiac mortality). Using this ERR instead, the estimated risk for the left-sided groups would increase by 0.1–0.2% for non-smokers and up to 0.6–0.8% for smokers for all techniques.
The mean doses to the heart and lungs were generally lower than those reported in the literature as being representative of modern techniques [2]. Probably, this is not due to different delineation of organs at risks, since similar guidelines were used as in other studies. One possible explanation may be that all plans in the current study were constructed by an experienced dosimetrist, which may indicate need for standardization of plan optimization, or possibly automation in generating plans [26], [27].
A limitation of this study is the relative low number of included patients and compared plans. Summarizing many different scores into one PQM score may hide some relevant information and thus more validation of the breast PQM score is necessary. Further, the risk calculations do not take other comorbidities such as diabetes, chronic obstructive pulmonary disease, hypertension or other chronical diseases into account, which may also influence the risk from radiotherapy. In the estimation of the risk/benefit balance, the resulting mortality risk is assumed to be represented by the sum of the mortality risks from lung cancer, IHD and CBC due to radiotherapy. This is an approximation and in general, when several risk factors are present, the combined mortality may be smaller or greater than the sum of the individual mortality rates, or may be nearly the same as the sum [28].
In summary, radiotherapy was found to be beneficial regarding survival for non-smokers and smokers with a high risk of recurrence. We could not confirm a survival benefit for smokers with a low or intermediate risk of recurrence when the risk for mortality caused by lung cancer, cardiac events or CBC was considered. However, the benefit of radiotherapy became more pronounced when the risk of recurrence was taken into account. Use of PQM allowed the identification of a treatment evaluation strategy integrating several dose constraints. The PQM may be further developed to adjust the dose constraint depending on tumor aggressiveness and patient comorbidity. The integration of tumor biology and patient comorbidity in plan evaluation may lead to a more individualized treatment plan optimization. More studies to confirm and evaluate the usefulness of PQM are needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was financed with grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-716711) and from the King Gustav the V Jubilee Clinic Foundation (2017:130). The study was approved by the regional ethics committee (Dnr 030-15).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.08.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor C., Correa C., Duane F.K., Aznar M.C., Anderson S.J., Bergh J. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith S.K., Estoesta R.P., Kader J.A., Martin D., Claridge-Mackonis E.R., Toohey J.M. Hybrid intensity-modulated radiation therapy (IMRT) simultaneous integrated boost (SIB) technique versus three-dimensional (3D) conformal radiotherapy with SIB for breast radiotherapy: a planning comparison. J Radiother Pract. 2016;15:131–142. doi: 10.1017/s146039691600008x. [DOI] [Google Scholar]
- 4.Viren T., Heikkila J., Myllyoja K., Koskela K., Lahtinen T., Seppala J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol. 2015;10:79. doi: 10.1186/s13014-015-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucko E., Jeulink M., Meijnen B., Slotman B., Verbakel W. OC-0084: Hybrid RapidArc for breast with locoregional lymph node irradiation spares more normal tissue. Radiother Oncol. 2016;119(Suppl 1):S40. doi: 10.1016/S0167-8140(16)31333-0. [DOI] [Google Scholar]
- 6.Chen G.P., Liu F., White J., Vicini F.A., Freedman G.M., Arthur D.W. A planning comparison of 7 irradiation options allowed in RTOG 1005 for early-stage breast cancer. Med Dosim. 2015;40:21–25. doi: 10.1016/j.meddos.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aly M.M., Glatting G., Jahnke L., Wenz F., Abo-Madyan Y. Comparison of breast simultaneous integrated boost (SIB) radiotherapy techniques. Radiat Oncol. 2015;10:139. doi: 10.1186/s13014-015-0452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelms B.E., Robinson G., Markham J., Velasco K., Boyd S., Narayan S. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2:296–305. doi: 10.1016/j.prro.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Fusella M., Scaggion A., Pivato N., Rossato M.A., Zorz A., Paiusco M. Efficiently train and validate a RapidPlan model through APQM scoring. Med Phys. 2018;45:2611–2619. doi: 10.1002/mp.12896. [DOI] [PubMed] [Google Scholar]
- 10.Choi S.H., Park S.H., Lee J.J.B., Baek J.G., Kim J.S., Yoon H.I. Combining deep-inspiration breath hold and intensity-modulated radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma: dosimetric evaluation using comprehensive plan quality indices. Radiat Oncol. 2019;14:59. doi: 10.1186/s13014-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eidemuller M., Simonetto C., Kundrat P., Ulanowski A., Shemiakina E., Guthlin D. Long-term health risk after breast-cancer radiotherapy: overview of passos methodology and software. Radiat Prot Dosimetry. 2019;183:259–263. doi: 10.1093/rpd/ncy219. [DOI] [PubMed] [Google Scholar]
- 12.Johansen S., Danielsen T., Olsen D.R. Estimated risk for secondary cancer in the contra-lateral breast following radiation therapy of breast cancer. Acta Oncol. 2008;47:391–396. doi: 10.1080/02841860701846152. [DOI] [PubMed] [Google Scholar]
- 13.Killander F., Karlsson P., Anderson H., Mattsson J., Holmberg E., Lundstedt D. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur J Cancer. 2016;67:57–65. doi: 10.1016/j.ejca.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Socialstyrelsen and Cancerfonden (Swedish National Board of Welfare and The Swedish Cancer Society): Cancer i Siffror 2018 (in swedish). Stockholm: Socialstyrelsen; 2018.
- 15.Tokunaga M., Norman J.E., Jr., Asano M., Tokuoka S., Ezaki H., Nishimori I. Malignant breast tumors among atomic bomb survivors, Hiroshima and Nagasaki, 1950–74. J Natl Cancer Inst. 1979;62:1347–1359. doi: 10.1093/jnci/62.6.1347. [DOI] [PubMed] [Google Scholar]
- 16.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Sola A.B. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118:205–208. doi: 10.1016/j.radonc.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 17.RTOG Foundation inc. RTOG 1005 – A phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer, https://www.rtog.org/clinicaltrials/protocoltable/studydetails.aspx?action=openFile&FileID=9366/; 2014 [accessed 17 aug 2018].
- 18.SWEBCG. Guidelines from the Swedish Breast Cancer Group (in Swedish), http://www.swebcg.se/vardprogram/; 2018 [accessed 17 aug 2018].
- 19.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgia N., Antonella F., Alessandro C., Eugenio V., Luca C. Planning strategies in volumetric modulated arc therapy for breast. Med Phys. 2011;38:4025–4031. doi: 10.1118/1.3598442. [DOI] [PubMed] [Google Scholar]
- 21.Jensen C.A., Roa A.M.A., Johansen M., Lund J.A., Frengen J. Robustness of VMAT and 3DCRT plans toward setup errors in radiation therapy of locally advanced left-sided breast cancer with DIBH. Phys Med. 2018;45:12–18. doi: 10.1016/j.ejmp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Koivumaki T., Fogliata A., Zeverino M., Boman E., Sierpowska J., Moeckli R. Dosimetric evaluation of modern radiation therapy techniques for left breast in deep-inspiration breath-hold. Phys Med. 2018;45:82–87. doi: 10.1016/j.ejmp.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 23.DBCG. Guidelines from the Danish Breast Cancer Cooperative Group (in Danish), http://www.dbcg.dk; 2016 [accessed 17 aug 2018].
- 24.De Rose F., Fogliata A., Franceschini D., Navarria P., Villa E., Iftode C. Phase II trial of hypofractionated VMAT-based treatment for early stage breast cancer: 2-year toxicity and clinical results. Radiat Oncol. 2016;11:120. doi: 10.1186/s13014-016-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 26.van Duren-Koopman M.J., Tol J.P., Dahele M., Bucko E., Meijnen P., Slotman B.J. Personalized automated treatment planning for breast plus locoregional lymph nodes using Hybrid RapidArc. Pract Radiat Oncol. 2018;8:332–341. doi: 10.1016/j.prro.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim H., Kwak J., Jung J., Jeong C., Yoon K., Lee S.W. Automated field-in-field (FIF) plan framework combining scripting application programming interface and user-executed program for breast forward IMRT. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818810391. 1533033818810391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milano A.F., Singer R.B. Mortality in co-morbidity (II)–excess death rates derived from a follow-up study on 10,025 subjects divided into 4 groups with or without depression and diabetes mellitus. J Insur Med. 2007;39:160–166. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.