Abstract
Background & purpose
Clinical introduction of magnetic resonance (MR)-guided radiotherapy involves treatment planning while taking into account machine-specific characteristics. Our aim was to investigate the feasibility of high-quality MR-linac treatment planning for an MR-linac and to benchmark MR-linac plan quality (IMRT) against current clinical practice (VMAT).
Materials & methods
Data of eight rectal and eight prostate cancer patients, who received radiotherapy on a conventional CBCT-integrated linac, were selected. Clinically acquired CTs and associated delineations of target volumes and organs-at-risk (OARs) were used for MR-linac treatment planning in Monaco. To investigate treatment planning software bias ‘quasi MR-linac plans’ were generated in Pinnacle3 by mimicking MR-linac specific beam characteristics. MR-linac, quasi MR-linac, and clinical plans were compared and differences in target and OAR doses assessed. Differences in plan complexity were determined by the number of segments and monitor units.
Results
Compared to clinical plans, MR-linac plans showed a statistically significant decrease in plan homogeneity, an increase in PTV Dmean (prostate: 0.6 Gy; rectum: 0.8 Gy) and D1% (prostate: 1.9 Gy; rectum: 2.0 Gy), and increases in OAR dose. Quasi MR-linac plans were comparable to MR-linac plans with respect to OAR dose and plan homogeneity. For rectal cancer an increase was seen in PTV Dmean (0.12 Gy) and D1% (0.5 Gy) compared to regular MR-linac plans. All created plans were clinically equivalent to current clinical practice.
Conclusions
This study demonstrates the feasibility of creating high-quality MR-linac treatment plans. The results supported the clinical introduction of an MR-linac.
1. Introduction
Radiotherapy aims to deliver the prescribed dose to the tumor while minimizing organ at risk (OAR) dose. Advanced radiotherapy techniques such as intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) allow highly conformal dose distributions, but also require adequate image guidance for correct dose delivery. Currently, verification of patient positioning and adaptation on anatomical changes is mainly based on cone beam computed tomography (CBCT). However, the present CBCT imaging quality limits the advancements in online treatment adaptation.
The availability of in-room magnetic resonance (MR) imaging enables advanced image-guided adaptive radiotherapy (IGART) strategies and might allow for margin reduction and dose escalation strategies. Therefore, different initiatives have integrated MR scanners with radiotherapy delivery systems [1], [2], [3], [4]. It allows radiotherapy dose delivery while simultaneously acquiring MR images with excellent soft-tissue contrast and therefore has the potential to accurately correct for inter- and intra-fractional anatomical changes.
Clinical introduction of MR-guided radiotherapy with an MR-linac involves treatment planning using a dedicated treatment planning system (TPS) for both initial treatment planning and online plan adaptation. Compared to treatment planning for a conventional linac, MR-linac treatment planning is challenging mainly due to the design of the machine. Besides the presence of a magnetic field, several MR-linac-specific beam and collimator characteristics need to be taken into account during treatment planning. A VMAT dose delivery technique is not yet available and instead a step-and-shoot IMRT dose delivery technique is used. In order to fulfill all MR-linac specific requirements without compromising on plan quality, dedicated treatment planning techniques need to be developed and resulting plans need to be validated.
A previous study investigated MR-linac and conventional treatment planning for prostate cancer patients and found comparable plan quality from a clinical perspective [5]. The aim of this study was to investigate MR-linac treatment planning strategies and compare MR-linac plan quality with our current clinical practice for prostate and also rectum tumor sites. In addition, MR-linac characteristics were incorporated in a conventional TPS in order to assess the contribution of MR-linac specific characteristics and TPS operator experience on plan quality.
2. Materials & methods
2.1. The MR-linac system
The Elekta Unity MR-linac (Elekta AB, Stockholm, Sweden) consists of a linac (Elekta AB, Stockholm, Sweden) with a nominal beam energy of 7 MV and an integrated wide-bore 1.5 T MR scanner (Philips, Best, the Netherlands) [6], [7]. The linac is mounted on a ring gantry and allows for continuous rotation. The multileaf collimator (MLC) consists of 160 interdigitating leaves to shape the flattening-filter-free (FFF) beam. The MLC orientation is fixed at 270°, resulting in a cranial-caudal leaf travel direction. The source-axis distance (SAD) of 143.5 cm results in an effective leaf width of 0.71 cm at isocenter. The maximum radiation field size at isocenter is 57 cm in the lateral direction and 22 cm in the cranial-caudal direction [8].
The MR-linac couch contains dense rails resulting in large dose attenuation and steep dose attenuation gradients. For increased delivery robustness, these gantry angles (i.e. 100°–140° and 220°–260°) need to be avoided. The MR-linac contains a cryostat-pipe which treatment beams likewise need to avoid (i.e. 8°–18°).
2.2. Patient data
Data of eight rectal and eight prostate cancer patients recently treated on a conventional CBCT-integrated linac were included in this retrospective treatment planning study. Due to the limited radiation field size, cases were selected on the requirement that the planning target volume (PTV) size in cranial-caudal direction was smaller than 20 cm. These sites were chosen for this study as they will be the first sites treated on the MR-linac at our clinic. For each included patient, the planning CT and associated delineated structures (i.e., target volumes and OARs) acquired for clinical treatment planning as well as the clinically approved treatment plan used for dose delivery were available. PTVs for the selected rectal cancer patients range from 266 to 1368 cm3 (median: 1159 cm3). For the prostate cancer patients a simultaneous integrated boost (SIB) strategy with two dose levels was used. The lower dose volumes (PTV57.8Gy) range from 92 to 191 cm3 (median: 139 cm3), the higher dose volumes (PTV64.6Gy) range from 81 to 173 cm3 (median: 112 cm3) in volume.
2.3. Clinical treatment planning
Clinical treatment planning for a conventional linac was performed using Pinnacle3 9.10 (Philips, Best, the Netherlands). According to departmental protocols, clinical plans for both rectal cancer (25 × 2.0 Gy) and prostate cancer (19 × 3.4 Gy) used a VMAT delivery technique. Plan optimization objectives were individually optimized in order to minimize OAR dose while maintaining PTV dose constraints. For rectal cancer patients, a single dose level treatment technique was used. Prostate cancer patients were treated using SIB with prescribed doses of 57.8 Gy and 64.6 Gy. Table 1 summarizes the relevant clinical treatment planning details for both rectal cancer and prostate cancer. Clinical rectal (prostate) plans were rescaled so that the PTV (PTV64.6Gy) V95% equals 99.3% of the prescribed dose. Table 2 lists the predefined acceptance criteria that were used. According to department protocols, final approval was obtained after plan reviewing by an independent radiation therapist and a radiation oncologist. Clinical plans were delivered on either an Elekta Versa HD system (Elekta AB, Stockholm, Sweden) or an Elekta Synergy system (Elekta AB, Stockholm, Sweden).
Table 1.
Treatment planning details. Settings used for the three planning setups. Abbreviations used: FF(F) = flattening filter (free), CW = clockwise, CCW = counter clockwise, GPUMCD = graphics processing unit Monte Carlo dose, NA = not available. (* Gantry angles adapted to avoid high-density hip prostheses).
| Clinical treatment planning | MR-Linac treatment planning | Quasi MR-Linac treatment planning | |
|---|---|---|---|
| Treatment technique | |||
| Rectal cancer | single dose level | single dose level | single dose level |
| Prostate cancer | simultaneous integrated boost | simultaneous integrated boost | simultaneous integrated boost |
| Fractionation | |||
| Rectal cancer | 25 × 2.0 Gy | 25 × 2.0 Gy | 25 × 2.0 Gy |
| Prostate cancer | 19 × 3.40 Gy | 19 × 3.40 Gy | 19 × 3.40 Gy |
| Dose delivery technique | dual-arc VMAT | 9-beam IMRT | 9-beam IMRT |
| Rectal cancer | 178°-182° CCW; 182°-178° CW | 200°, 270°, 300°, 330°, 0°, 30°, 60°, 90°, 160° | 200°, 270°, 300°, 330°, 0°, 30°, 60°, 90°, 160° |
| Prostate cancer (N = 6) | 140°-220° CCW; 220°-140° CW | 210°, 270°, 300°, 330°, 0°, 30°, 60°, 90°, 150° | 210°, 270°, 300°, 330°, 0°, 30°, 60°, 90°, 150° |
| Prostate cancer (N = 2)* | 182°-42° CCW; 42°-182° CW | 210°, 270°, 300°, 330°, 0°, 20°, 40°, 60°, 150° | 210°, 270°, 300°, 330°, 0°, 20°, 40°, 60°, 150° |
| Beam energy | 10 MV | 7 MV | 6 MV |
| Beam flattening filter | Yes (FF) | No (FFF) | No (FFF) |
| Source-axis distance | 100.0 cm | 143.5 cm | 143.5 cm |
| Magnetic field | 0.0 T | 1.5 T | 0.0 T |
| Plan isocenter | PTV (rectum) or PTV64.6Gy (prostate) center of mass | MR-Linac isocenter | MR-Linac isocenter |
| Collimator angle | 20° | 270° | 270° |
| Multi leaf collimator | |||
| Rectal cancer (N = 4) | 160 (Agility) | 160 (Agility) | 160 (Agility) |
| Rectal cancer (N = 4) | 80 (MLCi) | 160 (Agility) | 160 (Agility) |
| Prostate cancer (N = 4) | 160 (Agility) | 160 (Agility) | 160 (Agility) |
| Prostate cancer (N = 4) | 80 (MLCi) | 160 (Agility) | 160 (Agility) |
| Treatment planning software | Pinnacle39.10 | Monaco 5.4 | Pinnacle3 9.10 |
| Dose engine | Collapsed cone | Monte Carlo (GPUMCD) | Collapsed cone |
| Monte Carlo uncertainty | NA | 1% | NA |
| Dose grid | |||
| Rectal cancer | 4.0 mm | 3.0 mm | 3.0 mm |
| Prostate cancer | 4.0 mm | 3.0 mm | 3.0 mm |
Table 2.
Acceptance and evaluation criteria used for treatment planning. EXT-PTV2cm indicates the patient volume with the PTV, along with an additional 2 cm margin, removed. EXT indicates the patient volume. EQD2 Dmax stands for the maximum dose corrected to 2 Gy fractions.
| Acceptance criteria | Evaluation criteria | |
|---|---|---|
| Rectal cancer | ||
| PTV | V95% > 99%, D1% < 107% | Dmean, D1% |
| Bladder | Dmean < 45 Gy, Dmax* < 65 Gy | |
| Bowel area | Dmax* < 68 Gy | Dmean |
| External | Dmean | |
| EXT-PTV2cm | Dmean, D1% | |
| Spinal cord | Dmax* < 50 Gy | |
| Prostate cancer | ||
| PTV64.6Gy | V95% > 99%, D1% < 107% | Dmean, D1% |
| PTV57.8Gy | V95% > 99% | V95% |
| Anal sphincter | Dmean < 37 Gy | Dmean |
| Rectum | V54Gy < 35%, V62Gy < 10% | Dmean |
| Femur (individual) | EQD2 Dmax* < 50 Gy | |
| External | Dmean | |
| EXT-PTV2cm | Dmean, D1% | |
Dmax replaced with D1% for MR-linac plans to suppress effects of Monte Carlo noise.
2.4. MR-linac treatment planning
MR-linac treatment plans were generated using Monaco 5.4 (Elekta AB, Stockholm, Sweden), a version of the Monaco TPS developed for MR-linac treatment planning. Relevant plan characteristics can be found in Table 1. Monaco 5.4 allows for dose optimization and calculation in the presence of a magnetic field [9]. Target and OAR dose criteria, as well as margins, are identical to those for the clinical plans (Table 2). All MR-linac plans were created using a 9-beam step-and-shoot IMRT technique. MR-linac plan optimization was started using a predefined set of objectives and objective values were individually optimized to achieve PTV coverage while minimizing OAR dose (Supplementary material). MR-linac dose calculation was performed on a uniform 3 mm dose grid with an overall 1% Monte Carlo statistical uncertainty. Rectal (prostate) plans were rescaled so that the PTV (PTV64.6Gy) V95% equals 99.3% of the prescribed dose. MR-linac treatment plans were accepted when all MR-linac acceptance criteria were fulfilled (Table 2). Final plan approval was obtained after plan reviewing by two independent radiation oncologists. Also, radiation oncologists judged the MR-linac plans on clinical equivalence to current clinical practice.
2.5. Quasi MR-linac treatment planning
Quasi MR-linac plans were generated using Pinnacle3, but incorporated comparable MR-linac characteristics (Table 1). Besides a small beam energy difference, the cryostat-induced additional beam attenuation and the absence of a magnetic field, all MR-linac specific characteristics were included during quasi MR-linac treatment planning. For both rectal cancer and prostate cancer, quasi MR-linac plans were created using a 9-beam step-and-shoot IMRT technique with beam configurations identical to MR-linac plans. To achieve PTV coverage and minimized OAR dose, plan optimization objectives were individually optimized. Rectal (prostate) plans were rescaled so that the PTV (PTV64.6Gy) V95% equals 99.3% of the prescribed dose and approved when all clinical acceptance criteria were fulfilled (Table 2).
2.6. Data analysis
MR-linac plans were compared with clinical plans using dose-volume histogram (DVH) parameters. Since all plans were normalized to identical target coverage (V95%), the mean dose (Dmean) and the near-maximum dose (D1%) of the PTV (rectum) or the PTV64.6Gy (prostate), are measures of the homogeneity of the dose distribution in the target. Dose to surrounding OARs was evaluated using criteria defined in local protocols (Table 2). Integral dose differences were determined by calculating the mean dose to the patient (EXT Dmean). Given the applied plan normalization, the Dmean and D1% of the patient excluding the 2.0 cm uniformly expanded PTV (EXT – PTV2cm) were determined to verify dose fall-off differences. To verify plan quality differences induced by MR-linac specific characteristics (TPS observer experience), DVH parameter values of clinical plans (MR-linac plans) and quasi MR-linac plans were compared.
For the prostate plans the normal tissue complication probability (NTCP) was calculated for grade 2 and higher late rectal bleeding toxicity using the QUANTEC-recommended Lyman-Kutcher-Burman model with parameters n = 0.09, m = 0.13, and TD50 = 76.9 Gy [10], [11]. The generalized equivalent uniform dose used for this model was corrected for the fractionation via the linear-quadratic model using a parameter of . For the rectal plans the NTCP was calculated for at least grade 2 acute gastrointestinal toxicity using a Logit model with parameters k = 3.2 and V50 = 410 cc [12].
In addition, the complexity of treatment plans was assessed by the number of monitor units (MU) and the number of segments. To distinguish between differences induced by MR-linac specific characteristics and TPS operator experience bias, MR-linac plans and quasi MR-linac plans were compared. Statistical significance of differences was evaluated using a Wilcoxon signed-ranks test (p < 0.05).
3. Results
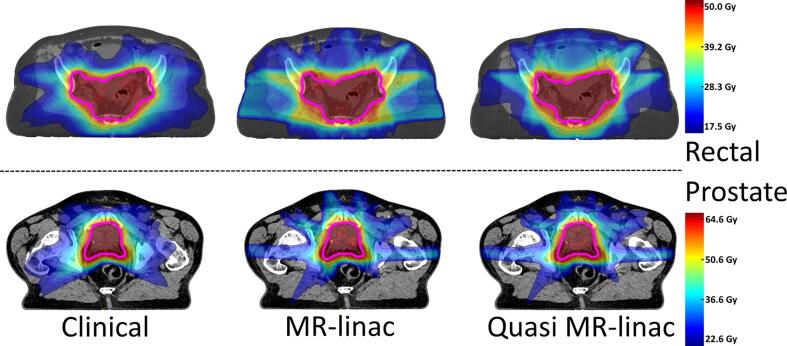
All created MR-linac plans and quasi MR-linac plans fulfilled the clinical acceptance criteria. Furthermore, MR-linac plans were considered clinically equivalent to current clinical plans. Fig. 1 shows an example of dose distributions for the clinical plan, MR-linac plan and quasi MR-linac plan of a rectal cancer patient and a prostate cancer patient.
Fig. 1.
Dose distributions. Colorwash map examples of dose distributions corresponding to current clinical practice (left), an MR-linac plan (middle), and a quasi MR-linac plan (right) for a rectal cancer patient (top) and a prostate cancer patient (bottom). The primary PTV is delineated in pink. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
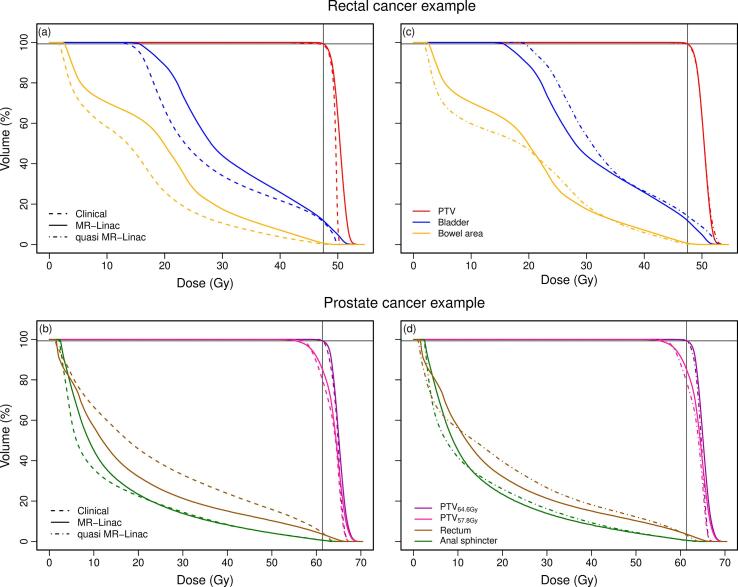
A minimal decrease in plan homogeneity was found for MR-linac plans compared to current clinical practice for all included patients (Table 3). In rectal cancer, MR-linac treatment planning resulted in an increased median PTV Dmean and PTV D1% of 0.83 Gy (p = 0.01) and 2.0 Gy (p = 0.01), respectively. MR-linac plans for prostate cancer showed an increased median PTV64.6Gy Dmean and D1% compared to clinical plans of 0.62 Gy (p = 0.03) and 1.9 Gy (p = 0.01), respectively. In addition, we observed a minimal median decrease of 0.34% in PTV57.8Gy V95% (p = 0.01). Furthermore, a limited increase in OAR dose was found for MR-linac plans compared to current clinical practice (Table 3). Compared to clinical plans, rectal cancer MR-linac plans resulted in an increased median Dmean to the bladder and bowel area of 2.9 Gy (p = 0.03) and 4.0 Gy (p = 0.01), respectively. In prostate cancer, MR-linac plans showed a median increase in Dmean for rectum and anal sphincter of respectively 0.75 Gy (p = 0.12) and 0.67 Gy (p = 0.58). As an example, Fig. 2(a and b) shows DVHs of PTV and OARs for two typical patients.
Table 3.
Dosimetric results. Median (min – max) DVH parameter differences between MR-linac plans compared to current clinical practice and MR-linac plans compared to quasi MR-linac plans. Positive values indicate higher DVH parameters for the MR-linac plans. Statistical significant difference (Wilcoxon p < 0.05) is indicated by *. Also listed are the median (min – max) DVH parameters for the clinical plans.
| MR-linac – Clinical | MR-Linac – Quasi MR-Linac | Clinical | |
|---|---|---|---|
| Rectal cancer | |||
| PTV Dmean (Gy) | 0.8 (0.2–1.3)* | −0.1 (−0.3–0.6) | 49.5 (49.2–50.0) |
| PTV D1% (Gy) | 2.0 (1.1–2.4)* | −0.5 (−1.9–0.7) | 50.6 (50.0–51.4) |
| Bladder Dmean (Gy) | 2.9 (−0.5–6.0)* | −1.7 (−2.9–0.8)* | 29.1 (11.3–37.7) |
| Bowel area Dmean (Gy) | 4.1 (2.1–5.9)* | 1.1 (−1.1–3.3) | 16.9 (11.6–26.3) |
| EXT Dmean (Gy) | 2.4 (1.1–2.9)* | 1.4 (0.7–2.2)* | 18.2 (12.1–21.1) |
| EXT-PTV2cm Dmean (Gy) | 2.5 (1.1–3.2)* | 1.7 (1.1–2.7)* | 12.4 (8.80–14.2) |
| EXT-PTV2cm D1% (Gy) | 4.2 (2.1–6.8)* | 1.9 (0.2–4.3)* | 29.5 (26.2–31.2) |
| Gastrointestinal grade >=2 acute toxicity NTCP (%) | 0.013 (−0.61–0.41) | −0.010 (−4.02–0.0061) | 0.08 (0.00–21.2) |
| Prostate cancer | |||
| PTV64.6Gy Dmean (Gy) | 0.6 (−0.1–1.3)* | 0.5 (0.1–1.2)* | 64.6 (64.2–64.8) |
| PTV64.6Gy D1% (Gy) | 1.9 (0.5–2.4)* | 1.6 (0.8–3.0)* | 66.5 (65.6–66.8) |
| PTV57.8Gy V95% (%) | −0.3 (−0.6–−0.2)* | −0.4 (−0.8–−0.2)* | 100 (99.1–100) |
| Rectum Dmean (Gy) | 0.8 (−2.5–3.7) | −1.2 (−2.9–0.6)* | 25.3 (12.7–29.7) |
| Anal sphincter Dmean (Gy) | 0.7 (−2.0–−0.9) | 0.3 (−1.1–2.5) | 12.3 (3.80–31.3) |
| EXT Dmean (Gy) | −1.7 (−2.7–−1.4)* | −1.4 (−2.5–−1.1)* | 7.45 (5.70–8.20) |
| EXT-PTV2cm Dmean (Gy) | −1.2 (−2.0–−0.9)* | −1.0 (−1.9–−0.7)* | 5.56 (4.50–5.90) |
| EXT-PTV2cm D1% (Gy) | 0.2 (−2.4–2.7) | −0.7 (−1.3–2.5) | 29.8 (28.1–32.4) |
| Late rectal bleeding grade >= 2 toxicity NTCP (%) | 0.17 (−2.31–1.81) | −0.46 (−1.19–0.50) | 10.4 (3.88–13.7) |
Fig. 2.
DVH curves. For one rectal cancer patient (top) and one prostate cancer patient (bottom), DVH curves of planned dose distributions for the PTV and OARs based on VMAT plans for clinical treatment planning versus step-and-shoot IMRT MR-linac treatment planning (left), and step-and-shoot IMRT MR-linac treatment planning versus step-and-shoot IMRT quasi MR-linac treatment planning (right). The intersection of the black lines indicates PTV (rectum) or PTV64.6Gy (prostate) V95% = 99.3% of prescribed dose.
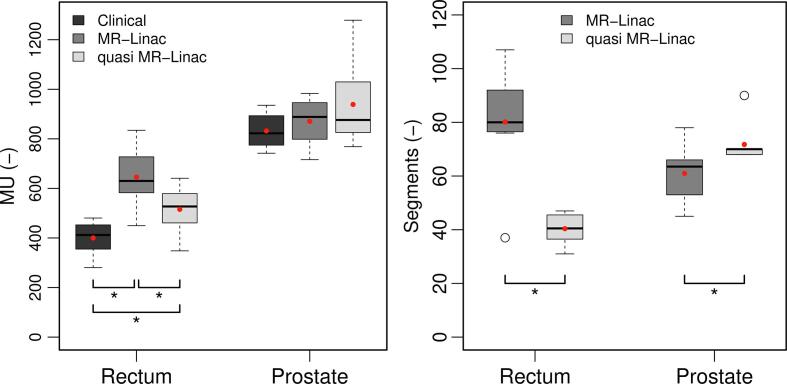
For both tumor sites, the small differences in EXT Dmean between MR-linac plans and clinical plans indicated a limited difference in integral dose. Moreover, the decrease in dose fall-off for rectal cancer MR-linac plans was limited (Table 3). However, MR-linac treatment planning (IMRT) required on average 63% (p = 0.01) and 6% (p = 0.48) more MUs compared to clinical practice (VMAT) for rectal cancer and prostate cancer, respectively (Fig. 3).
Fig. 3.
Plan complexity. Boxplots of the number of MUs (left) and the number of segments (right) over all plans of all patients. Note that clinical plans used VMAT and therefore have no well-defined number of segments. Boxes represent upper and lower quartiles (IQR), the band inside the box the median value, the red dot the mean value, open dots the outliers, and the whiskers the highest (lowest) value within 1.5 IQR of the upper (lower) quartile. Statistical significant difference (Wilcoxon p < 0.05) between adjacent boxplots is indicated by *. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The NTCP differences for rectal bleeding (prostate plans) and gastrointestinal toxicity (rectum plans) between MR-linac and clinical plans were found to be small and not statistically significant (Table 3).
Quasi MR-linac treatment planning resulted in plans comparable with MR-linac plans in terms of plan homogeneity and dose to OARs (Table 3). In rectal cancer, a minimal increase in PTV Dmean and D1% was found for quasi MR-linac plans and differences in OAR dose were limited. A typical example of DVHs for both tumor sites is shown in Fig. 2(c and d). However, large differences in plan complexity were found between MR-linac plans and quasi MR-linac plans indicated by differences in number of segments and MUs (Fig. 3). For rectal cancer MR-linac plans, the number of MUs and segments was on average 26% (p = 0.01) and 96% (p = 0.01) higher than for quasi MR-linac plans. In prostate cancer, quasi MR-linac plans required on average 15% more segments (p = 0.05) and 5% more MUs (p = 0.58) compared to MR-linac (Fig. 3).
4. Discussion
In this study we investigated the feasibility of creating high-quality MR-linac treatment plans and determined the influence of MR-linac specific machine characteristics on plan quality. For rectal cancer and prostate cancer, MR-linac plans were created, approved using clinical acceptance criteria by radiation oncologists and benchmarked against current clinical practice. In addition, quasi MR-linac plans were generated to distinguish between plan quality differences induced by MR-linac specific machine characteristics and TPS user experience. Given the differences in machine characteristics, some minor degradation in plan quality was found for MR-linac plans compared to current clinical practice. Moreover, the limited differences between MR-linac plans and quasi MR-linac plans indicated minimal plan quality differences induced by TPS observer experience and the presence of a magnetic field.
Several studies have been conducted on the potential benefit of MR-guided radiotherapy using an MR-linac system [13], [14]. However, only a few of them focused on MR-linac treatment planning strategies by investigating the consequences of machine characteristics on plan quality [15].
Differences between MR-linac plans and clinical plans are mainly due to a larger washout of the low dose region and an increase in target inhomogeneity. This leads to changes in OAR dose in the low-dose region and around the near-maximum. The increased dose in the low-dose region is due to the use of the 7 MV beam of the MR-linac instead of our conventional choice for pelvic cases of 10 MV beams. The larger SAD also contributes to the increased dose in the low-dose region. However, investigating the contribution of individual characteristics on dose distributions by varying single characteristics is beyond the clinical scope of this study. The effects of different MLCs used for dose delivery in clinical practice were not studied due to the limited sample size.
Compared to clinical plans, the increased mean dose (median 4 Gy) to the bowel area for MR-linac plans in rectal cancer was independently judged by two radiation oncologists to be not clinically relevant. Also, the calculated NTCP values for at least grade 2 acute gastrointestinal toxicity showed no statistically significant difference and supported the judgement of the two radiation oncologists.
Our institute has limited experience with treatment planning in Monaco for systems other than the MR-linac, since our clinical practice is Pinnacle3. Thus, to have a fair comparison with our clinical practice, and minimize TPS bias, quasi MR-linac plans were created by incorporating comparable MR-linac characteristics in Pinnacle3. However, the additional beam hardening caused by the cryostat, or the present magnetic field, were not included in these plans and are therefore limitations of our study.
Although planned dose distributions were comparable, between the MR-linac plans generated in Monaco and the quasi MR-linac plans generated in Pinnacle3, considerable differences in plan complexity were found. For rectum plans the MR-linac plans used more MUs and segments than the quasi MR-linac plans, for prostate plans the situation is reversed. The sequencing algorithms implemented in both TPSs are different (i.e. Monaco: Smart Sequencing [16]; Pinnacle3: Direct Machine Parameter Optimization [17]) and the segmentation outcome is therefore likely to be different. Furthermore, the increase in MUs for MR-linac and quasi MR-linac plans compared to clinical plans might partly be due the non-flattened beam profile of the MR-linac beam in combination with the increased number of off-axis apertures caused by the fixed MR-linac isocenter.
The MR-linac plans were judged by two independent radiation oncologists to be clinically equivalent to our current clinical practice. Unfortunately the dosimetric accuracy of the MR-linac plans could not be assessed since the MR-linac beam was not available at the time of study. However, the differences between delivered and planned doses were found to be small in literature [13]. The effects of a potential decreased margin size owing to the better soft tissue contrast of MRI compared to the regularly used CBCT imaging for online patient position verification and the availability of daily plan adaptation were not considered in this study.
Similar studies on MR-guided radiotherapy on the Elekta Unity and the ViewRay systems report clinical equivalence [15], [18]. An increase in the number of MUs for MR-linac plans compared to clinical plans was noted as well in the quoted MR-linac study [15].
In conclusion, this study demonstrated the ability of creating high-quality and clinically equivalent MR-linac treatment plans for rectal cancer and prostate cancer. Given the differences in machine characteristics, some minor differences in plan quality were found between MR-linac plans and current clinical practice. Furthermore, a limited contribution of TPS bias on MR-linac plan quality was determined. In general, these results support the clinical introduction of treatment planning for the MR-linac.
Acknowledgments
Acknowledgements
The authors would like to thank Pieter Pronk (NKI-AvL) for defining the treatment machine for quasi MR-linac treatment planning in the Pinnacle3 treatment planning system. We would also like to thank Luc Moonen (NKI-AvL) for the additional clinical evaluation of the prostate plans, and Luc Dewit (NKI-AvL) for the additional clinical evaluation of the rectum plans.
Conflict of interest
NKI-AvL is part of the Elekta Atlantic MR-linac Research Consortium and we acknowledge financial and technical support from Elekta AB (Stockholm, Sweden) under a research agreement. Our department receives software license fees from Elekta AB. Part of this research was funded from these license fees.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.04.004.
Contributor Information
Agustinus J.A.J. van de Schoot, Email: s.vd.schoot@nki.nl.
Wouter van den Wollenberg, Email: w.vd.wollenberg@nki.nl.
Casper Carbaat, Email: c.carbaat@nki.nl.
Peter de Ruiter, Email: p.d.ruiter@nki.nl.
Marlies E. Nowee, Email: m.nowee@nki.nl.
Floris Pos, Email: f.pos@nki.nl.
Baukelien van Triest, Email: b.v.triest@nki.nl.
Jan-Jakob Sonke, Email: j.sonke@nki.nl.
Tomas M. Janssen, Email: t.janssen@nki.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mutic S., Dempsey J.F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Keall P.J., Barton M., Crozier S. The Australian magnetic resonance imaging-linac program. Semin Radiat Oncol. 2014;24:203–206. doi: 10.1016/j.semradonc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Fallone B.G. The rotating biplanar linac-magnetic resonance imaging system. Semin Radiat Oncol. 2014;24:200–202. doi: 10.1016/j.semradonc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Lagendijk J.J.W., Raaymakers B.W., van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24:207–209. doi: 10.1016/j.semradonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen R.L., Hansen C.R., Dahlrot R.H., Bertelsen A.S., Hansen O., Brink C. Plan quality for high-risk prostate cancer treated with high field magnetic resonance imaging guided radiotherapy. Phys Imaging Radiat Oncol. 2018;7:1–8. doi: 10.1016/j.phro.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagendijk J.J.W., Raaymakers B.W., Raaijmakers A.J.E., Overweg J., Brown K.J., Kerkhof E.M. MRI/linac integration. Radiother Oncol. 2008;86:25–29. doi: 10.1016/j.radonc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Raaymakers B.W., Lagendijk J.J.W., Overweg J., Kok J.G.M., Raaijmakers A.J.E., Kerkhof E.M. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54:N229–N237. doi: 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]
- 8.Chuter R.W., Whitehurst P., Choudhury A., van Herk M., McWilliam A. Technical Note: Investigating the impact of field size on patient selection for the 1.5T MR-linac. Med Phys. 2017;44:5667–5671. doi: 10.1002/mp.12557. [DOI] [PubMed] [Google Scholar]
- 9.Hissoiny S., Raaijmakers A.J.E., Ozell B., Després P., Raaymakers B.W. Fast dose calculation in magnetic fields with GPUMCD. Phys Med Biol. 2011;56:5119–5129. doi: 10.1088/0031-9155/56/16/003. [DOI] [PubMed] [Google Scholar]
- 10.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lui M., Moiseenko V., Agranovich A., Karvat A., Kwan W., Saleh Z.H. Normal tissue complication probability (NTCP) modeling of late rectal bleeding following external beam radiotherapy for prostate cancer: a test of the QUANTEC-recommended NTCP model. Acta Oncol. 2016;49:1040–1044. doi: 10.3109/0284186X.2010.509736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roeske J.C., Bonta D., Mell L.K., Lujan A.E., Mundt A.J. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–207. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Raaymakers B.W., Jürgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A.N.T.J., van Asselen B. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 14.Bainbridge H.E., Menten M.J., Fast M.F., Nill S., Oelfke U., McDonald F. Treating locally advanced lung cancer with a 1.5 T MR-Linac: effects of the magnetic field and irradiation geometry on conventionally fractionated and isotoxic dose-escalated radiotherapy. Radiother Oncol. 2017;125:280–285. doi: 10.1016/j.radonc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menten M.J., Fast M.F., Nill S., Kamerling C.P., McDonald F., Oelfke U. Lung stereotactic body radiotherapy with an MR-linac – quantifying the impact of the magnetic field and real-time tumor tracking. Radiother Oncol. 2016;119:461–466. doi: 10.1016/j.radonc.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements M., Schupp N., Tattersall M., Brown A., Larson R. Monaco treatment planning system tools and optimization processes. Med Dosim. 2018;43:106–117. doi: 10.1016/j.meddos.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Hardemark B, Liander A, Rehbinder H, Lof J. P3IMRT – Direct machine parameter optimization. Pinnacle3 White Paper.
- 18.Saenz D.L., Paliwal B.R., Bayouth J.E. A dose homogeneity and conformity evaluation between ViewRay and pinnacle-based linear accelerator IMRT treatment plans. J Med Phys. 2014;39:64–70. doi: 10.4103/0971-6203.131277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.